Low-Intensity Extracorporeal Shock Wave Therapy Promotes Bladder Regeneration and Improves Overactive Bladder Induced by Ovarian Hormone Deficiency from Rat Animal Model to Human Clinical Trial
Abstract
1. Introduction
2. Results
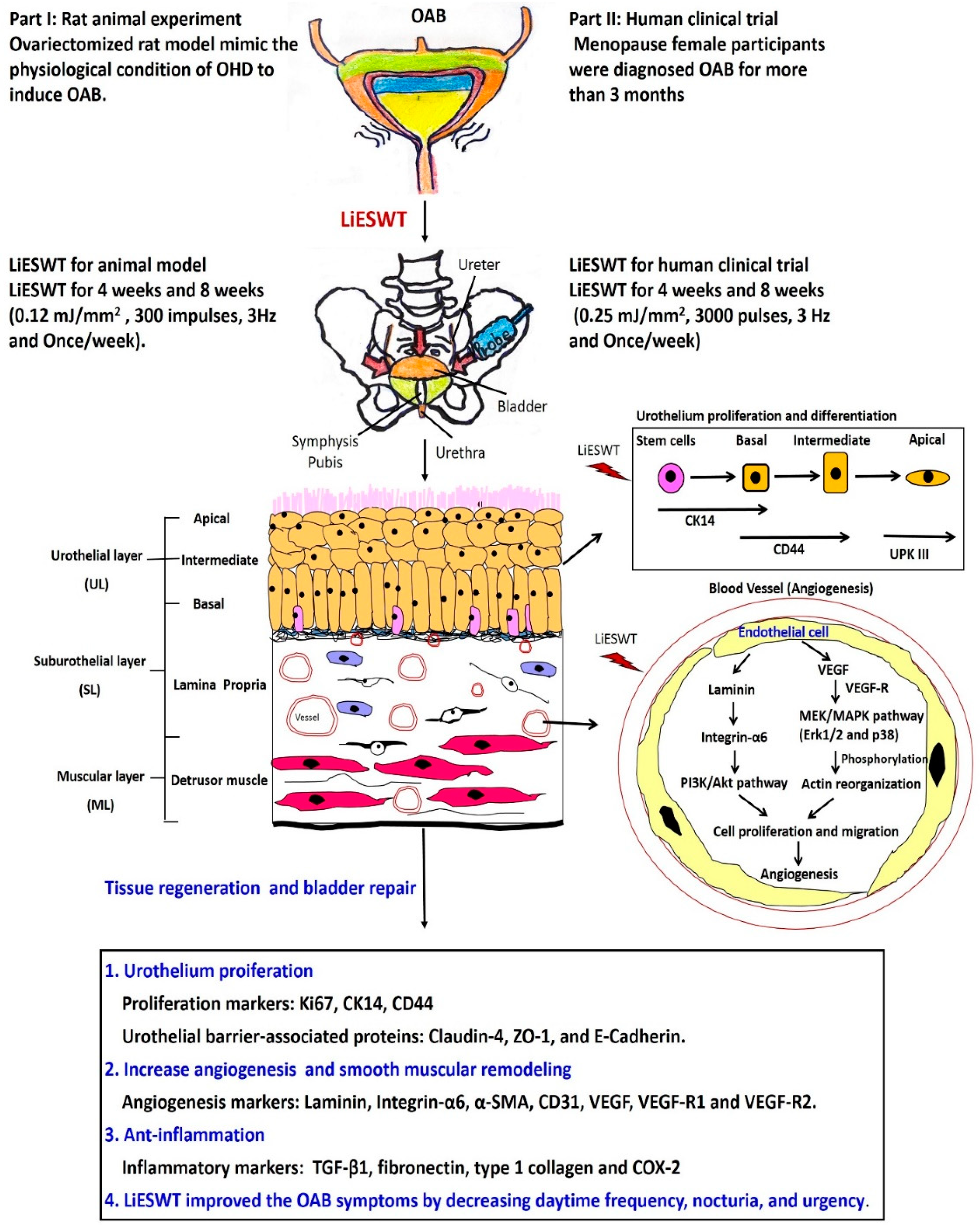
2.1. Part I: OAB Animal Model Experiment
2.1.1. Serum Estradiol Concentration Reduced after Bilateral Ovariectomy (OVX)
2.1.2. Physical Characteristics
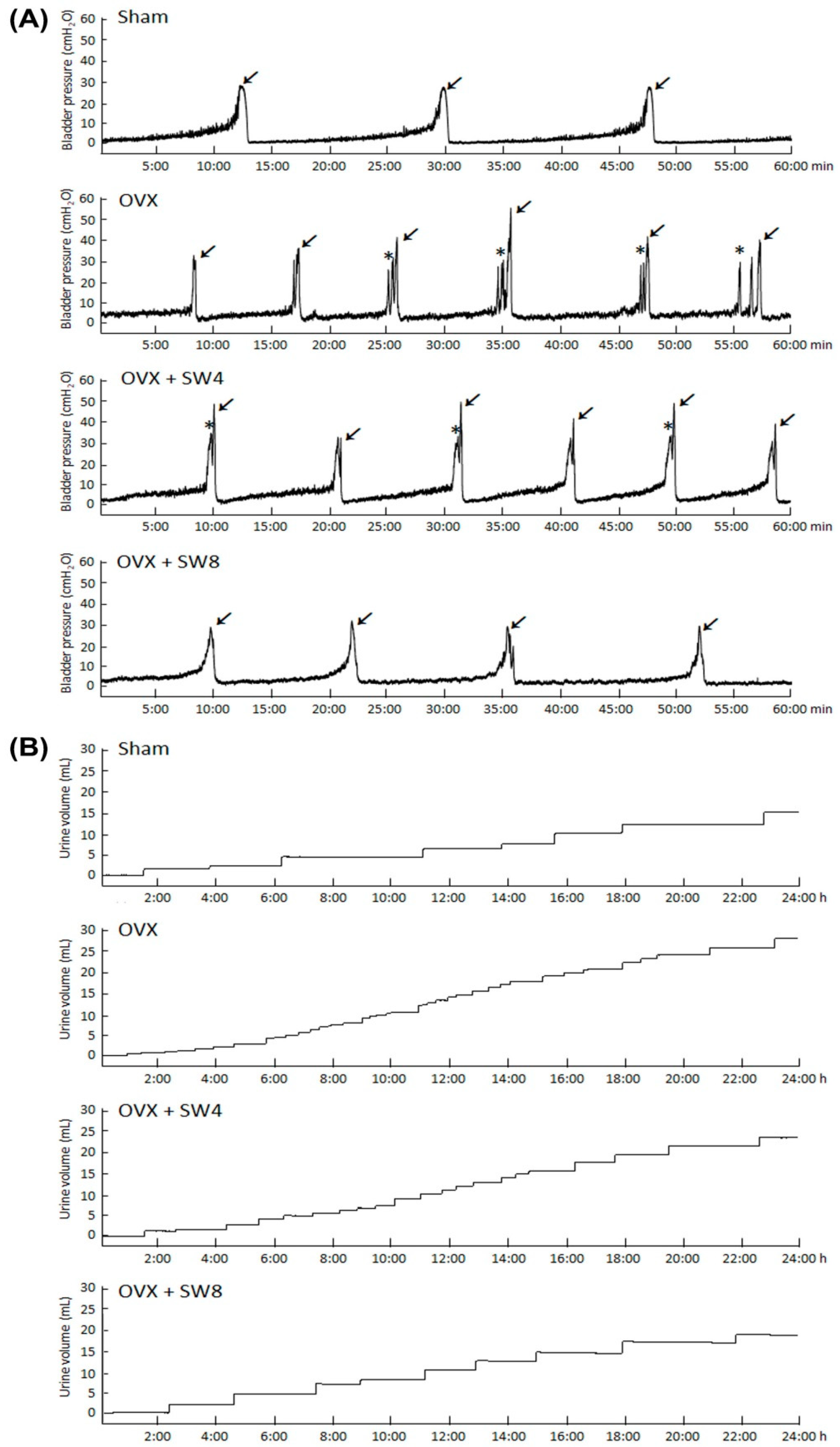
2.1.3. LiESWT Treatment Ameliorated Bladder Overactivity
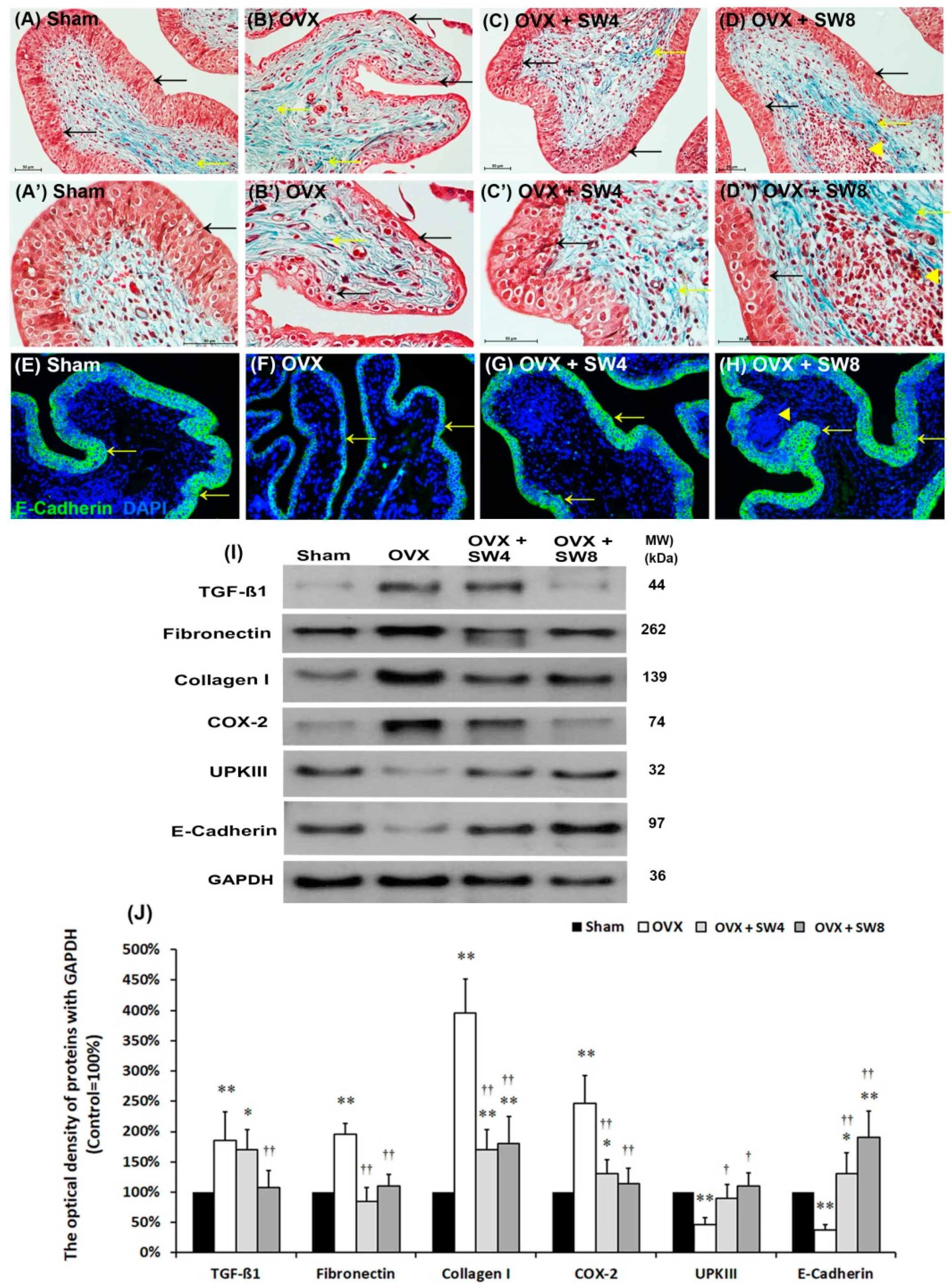
2.1.4. Effects of LiESWT on OVX-Induced Bladder Interstitial Fibrosis
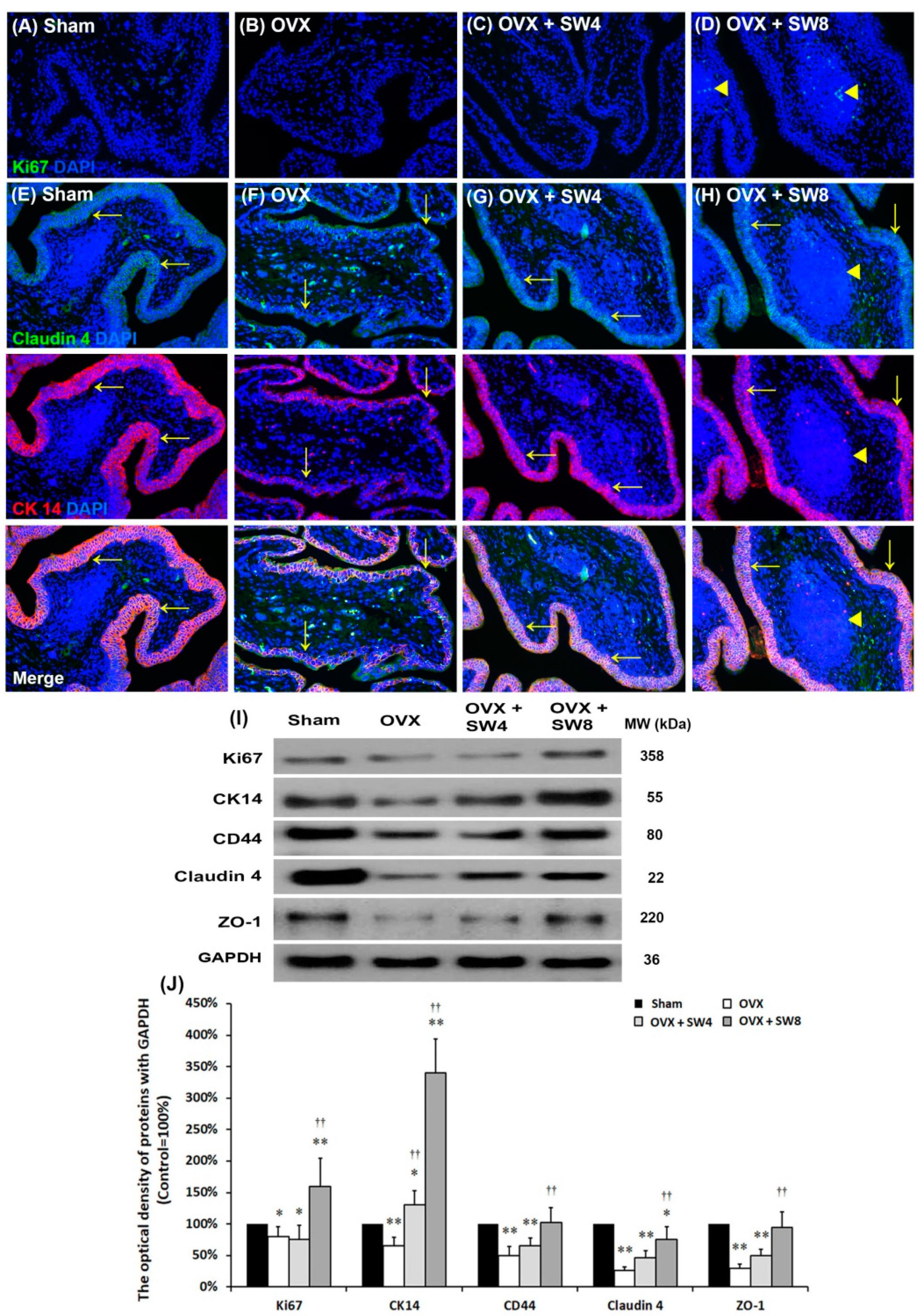
2.1.5. LiESWT Improved OVX-Induced Pathological Alteration
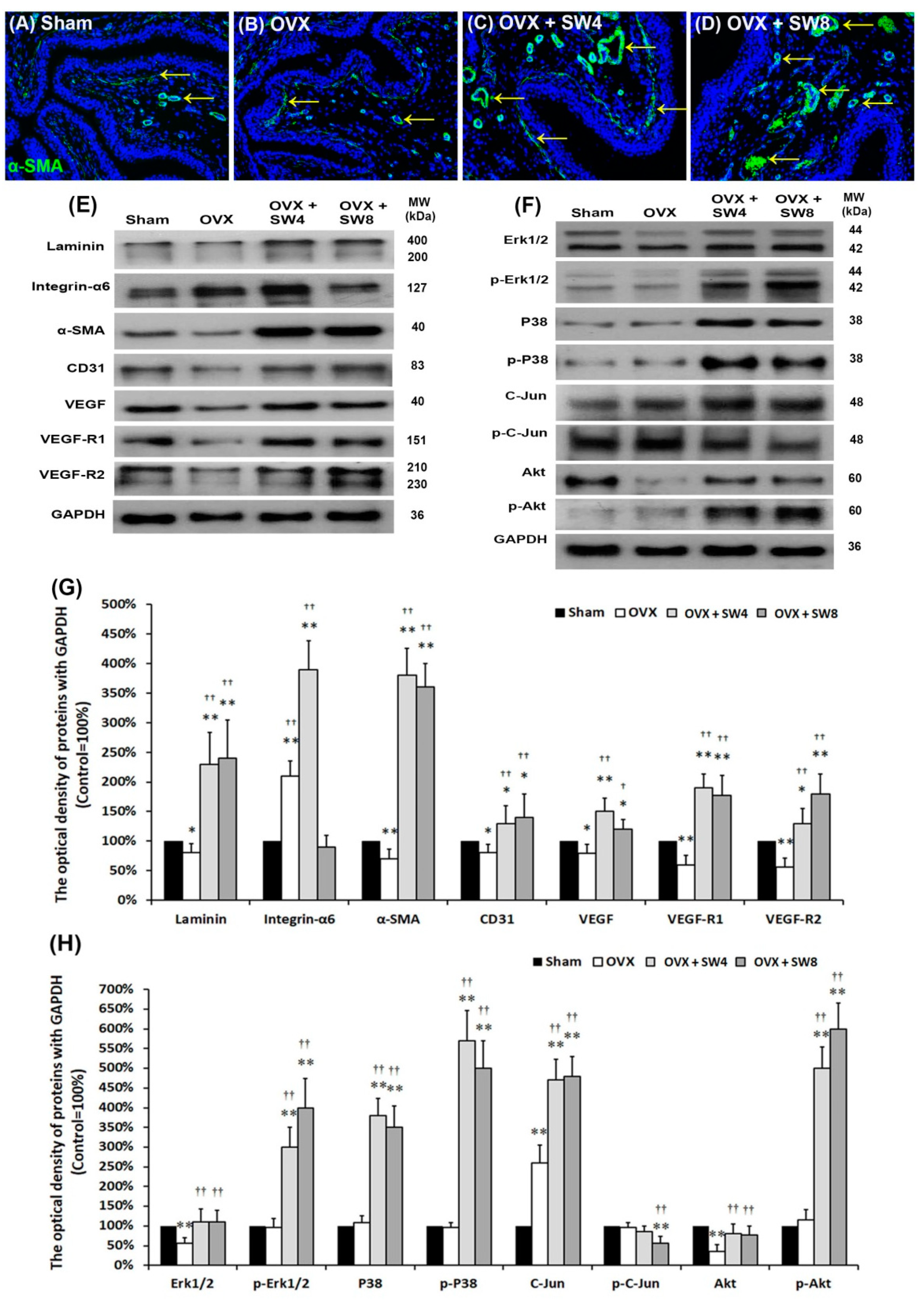
2.1.6. LiESWT Altered Bladder Angiogenic Remodeling
2.1.7. Proposed Potential Mechanism for Regulating Angiogenic Remodeling Triggered by LiESWT Contributed to the Pathogenesis of OHD
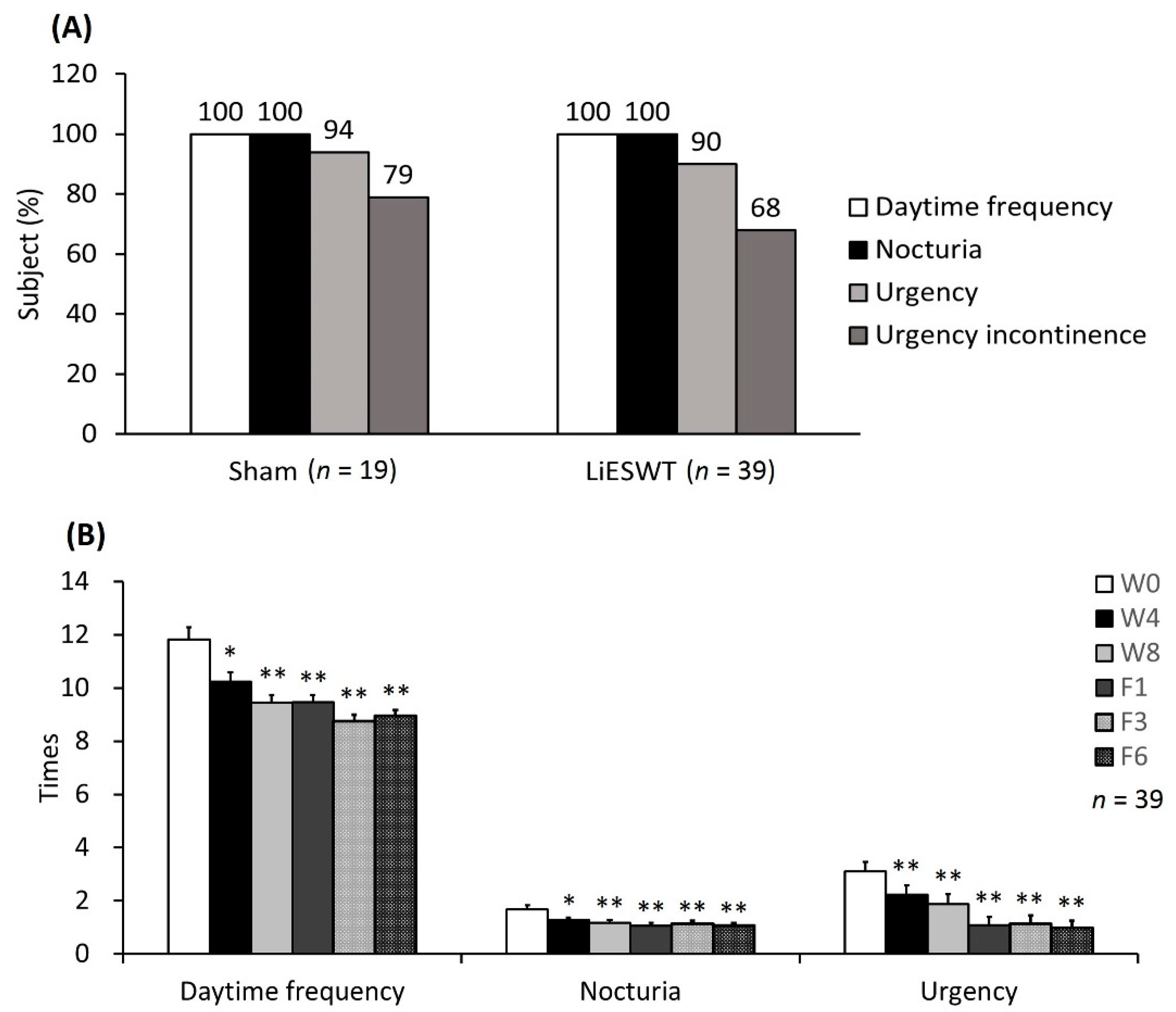
2.2. Part II: Human Clinical Trial
2.2.1. Baseline Characteristics
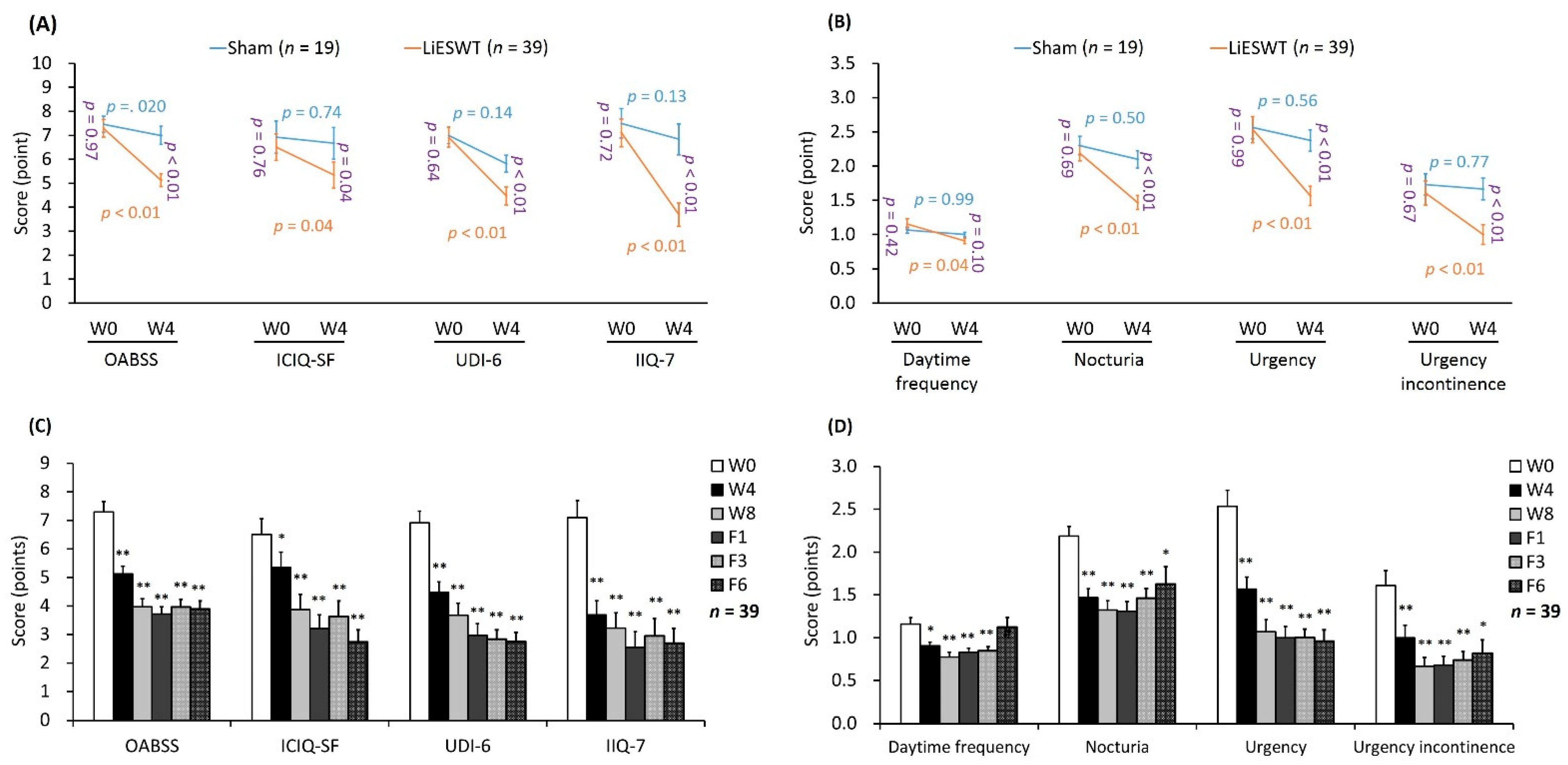
2.2.2. Primary and Secondary End Points
2.2.3. Safety of LiESWT Treatment
2.2.4. A Proposed Diagram for the Therapeutic Effect of LiESWT on Altering Bladder Angiogenesis and Improving OHD-Induced OAB
3. Discussion
4. Materials and Methods
4.1. Part I: Animal experiment
4.1.1. Experimental Design of Animal Model and LiESWT Protocol
4.1.2. LiESWT Treatment for Animal Model
4.1.3. Evaluation of Estradiol Level
4.1.4. Isovolumetric Cystometrograms (CMGs)
4.1.5. Physiological Metabolic Cage for Tracing Voiding Behavior Studies
4.1.6. Histological Study by Masson’s Trichrome Stain
4.1.7. Protein Isolation and Western Blot Analysis
4.1.8. Immunofluorescence Staining for the Localization of Protein Expression
4.1.9. Statistical Analysis
4.2. Part II: Human Clinical Trial
4.2.1. Design for Human Clinical Trial
4.2.2. Procedure and Medical Information of LiESWT
4.2.3. Physical and Serum Biochemical Indicators
4.2.4. Outcome Measurements and Therapeutic Efficacy Assessment for LiESWT
4.2.5. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hunskaar, S.; Burgio, K.; Diokno, A.; Herzog, A.R.; Hjalmas, K.; Lapitan, M.C. Epidemiology and natural history of urinary incontinence in women. Urology 2003, 62, 16–23. [Google Scholar] [CrossRef]
- Goldstein, I.; Dicks, B.; Kim, N.N.; Hartzell, R. Multidisciplinary overview of vaginal atrophy and associated genitourinary symptoms in postmenopausal women. Sex Med. 2013, 1, 44–53. [Google Scholar] [CrossRef]
- Nappi, R.E.; Kokot-Kierepa, M. Women’s voices in the menopause: Results from an international survey on vaginal atrophy. Maturitas 2010, 67, 233–238. [Google Scholar] [CrossRef]
- Nappi, R.E.; Kokot-Kierepa, M. Vaginal Health: Insights, Views & Attitudes (VIVA)—Results from an international survey. Climacteric 2012, 15, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Iosif, C.S.; Bekassy, Z. Prevalence of genito-urinary symptoms in the late menopause. Acta Obs. Gynecol Scand. 1984, 63, 257–260. [Google Scholar] [CrossRef]
- Bodner-Adler, B.; Alarab, M.; Ruiz-Zapata, A.M.; Latthe, P. Effectiveness of hormones in postmenopausal pelvic floor dysfunction-International Urogynecological Association research and development-committee opinion. Int. Urogynecol. J. 2020, 31, 1577–1582. [Google Scholar] [CrossRef]
- Cheng, C.L.; Li, J.R.; Lin, C.H.; de Groat, W.C. Positive association of female overactive bladder symptoms and estrogen deprivation: A nationwide population-based cohort study in Taiwan. Medicine 2016, 95, e4107. [Google Scholar] [CrossRef] [PubMed]
- Weiderpass, E.; Baron, J.A.; Adami, H.O.; Magnusson, C.; Lindgren, A.; Bergström, R.; Correia, N.; Persson, I. Low-potency oestrogen and risk of endometrial cancer: A case-control study. Lancet 1999, 353, 1824–1828. [Google Scholar] [CrossRef]
- Robinson, D.; Toozs-Hobson, P.; Cardozo, L. The effect of hormones on the lower urinary tract. Menopause Int. 2013, 19, 155–162. [Google Scholar] [CrossRef]
- Teng, J.; Wang, Z.Y.; Jarrard, D.F.; Bjorling, D.E. Roles of estrogen receptor alpha and beta in modulating urothelial cell proliferation. Endocr. Relat. Cancer 2008, 15, 351–364. [Google Scholar] [CrossRef]
- Imamov, O.; Yakimchuk, K.; Morani, A.; Schwend, T.; Wada-Hiraike, O.; Razumov, S.; Warner, M.; Gustafsson, J.-A. Gustafsson, Estrogen receptor beta-deficient female mice develop a bladder phenotype resembling human interstitial cystitis. Proc. Natl. Acad. Sci. USA 2007, 104, 9806–9809. [Google Scholar] [CrossRef] [PubMed]
- Long, C.Y.; Liu, C.M.; Hsu, S.C.; Wu, C.H.; Wang, C.L.; Tsai, E.M. A randomized comparative study of the effects of oral and topical estrogen therapy on the vaginal vascularization and sexual function in hysterectomized postmenopausal women. Menopause 2006, 13, 737–743. [Google Scholar] [CrossRef] [PubMed]
- Juan, Y.S.; Chuang, S.M.; Long, C.Y.; Chen, C.H.; Levin, R.M.; Liu, K.M.; Huang, C.H. Neuroprotection of green tea catechins on surgical menopause-induced overactive bladder in a rat model. Menopause 2012, 19, 346–354. [Google Scholar] [CrossRef] [PubMed]
- Juan, Y.S.; Chuang, S.M.; Lee, Y.L.; Long, C.Y.; Wu, T.H.; Chang, W.C.; Levin, R.M.; Liu, K.M.; Huang, C.H. Green tea catechins decrease oxidative stress in surgical menopause-induced overactive bladder in a rat model. BJU Int. 2012, 110, e236–e244. [Google Scholar] [CrossRef]
- Hass, M.A.; Nichol, P.; Lee, L.; Levin, R.M. Estrogen modulates permeability and prostaglandin levels in the rabbit urinary bladder. Prostaglandins Leukot Essent Fat. Acids 2009, 80, 125–129. [Google Scholar] [CrossRef]
- Lin, A.D.; Mannikarottu, A.; Kogan, B.A.; Whitbeck, C.; Chichester, P.; Leggett, R.E.; Levin, R.M. Estrogen induces angiogenesis of the female rabbit bladder. J. Endocrinol. 2006, 190, 241–246. [Google Scholar] [CrossRef]
- Lin, A.D.; Levin, R.; Kogan, B.; Whitbeck, C.; Chichester, P.; Sokol, R.; Mannikarottu, A. Estrogen induced functional hypertrophy and increased force generation of the female rabbit bladder. Neurourol Urodyn. 2006, 25, 473–479. [Google Scholar] [CrossRef] [PubMed]
- Lin, A.D.; Levin, R.M.; Kogan, B.A.; Whitbeck, C.; Leggett, R.E.; Kearns, C.; Mannikarottu, A. Alteration of contractile and regulatory proteins in estrogen-induced hypertrophy of female rabbit bladder. Urology 2006, 68, 1139–1143. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.L.; Lin, K.L.; Wu, B.N.; Chuang, S.M.; Wu, W.J.; Lee, Y.C.; Ho, W.T.; Juan, Y.S. Epigallocatechin-3-gallate alleviates bladder overactivity in a rat model with metabolic syndrome and ovarian hormone deficiency through mitochondria apoptosis pathways. Sci. Rep. 2018, 8, 5358. [Google Scholar] [CrossRef]
- Aikawa, K.; Sugino, T.; Matsumoto, S.; Chichester, P.; Whitbeck, C.; Levin, R.M. The effect of ovariectomy and estradiol on rabbit bladder smooth muscle contraction and morphology. J. Urol. 2003, 170, 634–637. [Google Scholar] [CrossRef]
- Losordo, D.W.; Isner, J.M. Estrogen and angiogenesis: A review. Arter. Thromb Vasc. Biol. 2001, 21, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Przydacz, M.; Golabek, T.; Dudek, P.; Lipinski, M.; Chlosta, P. Prevalence and bother of lower urinary tract symptoms and overactive bladder in Poland, an Eastern European Study. Sci. Rep. 2020, 10, 19819. [Google Scholar] [CrossRef]
- Haylen, B.T.; de Ridder, D.; Freeman, R.M.; Swift, S.E.; Berghmans, B.; Lee, J.; Monga, A.; Petri, E.; Rizk, D.E.; Sand, P.K.; et al. International Urogynecological, S. International Continence, An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for female pelvic floor dysfunction. Neurourol. Urodyn. 2010, 29, 4–20. [Google Scholar] [CrossRef] [PubMed]
- Lapitan, M.C.; Chye, P.L. The epidemiology of overactive bladder among females in Asia: A questionnaire survey. Int. Urogynecol. J. Pelvic Floor Dysfunct. 2001, 12, 226–231. [Google Scholar] [CrossRef]
- Soliman, Y.; Meyer, R. Baum, Falls in the Elderly Secondary to Urinary Symptoms. Rev. Urol. 2016, 18, 28–32. [Google Scholar]
- Krause, M.; Wheeler, T.L., 2nd; Snyder, T.E.; Richter, H.E. Local Effects of Vaginally Administered Estrogen Therapy: A Review. J. Pelvic Med. Surg. 2009, 15, 105–114. [Google Scholar] [CrossRef]
- Zimmermann, R.; Cumpanas, A.; Miclea, F.; Janetschek, G. Extracorporeal shock wave therapy for the treatment of chronic pelvic pain syndrome in males: A randomised, double-blind, placebo-controlled study. Eur. Urol. 2009, 56, 418–424. [Google Scholar] [CrossRef]
- Moayednia, A.; Haghdani, S.; Khosrawi, S.; Yousefi, E.; Vahdatpour, B. Long-term effect of extracorporeal shock wave therapy on the treatment of chronic pelvic pain syndrome due to non bacterial prostatitis. J. Res. Med. Sci. 2014, 19, 293–296. [Google Scholar]
- Guu, S.J.; Geng, J.H.; Chao, I.T.; Lin, H.T.; Lee, Y.C.; Juan, Y.S.; Liu, C.C.; Wang, C.J.; Tsai, C.C. Efficacy of Low-Intensity Extracorporeal Shock Wave Therapy on Men With Chronic Pelvic Pain Syndrome Refractory to 3-As Therapy. Am. J. Mens Health 2018, 12, 441–452. [Google Scholar] [CrossRef] [PubMed]
- Yuan, P.; Ma, D.; Zhang, Y.; Gao, X.; Liu, Z.; Li, R.; Wang, T.; Wang, S.; Liu, J.; Liu, X. Efficacy of low-intensity extracorporeal shock wave therapy for the treatment of chronic prostatitis/chronic pelvic pain syndrome: A systematic review and meta-analysis. Neurourol. Urodyn. 2019, 38, 1457–1466. [Google Scholar] [CrossRef]
- Guu, S.J.; Liu, C.C.; Juan, Y.S.; Li, C.C.; Tsai, C.C. The 12-month follow-up of the low-intensity extracorporeal shockwave therapy in the treatment of patients with chronic pelvic pain syndrome refractory to 3-As medications. Aging Male 2020, 23, 793–800. [Google Scholar] [CrossRef]
- Chung, E.; Wang, J. A state-of-art review of low intensity extracorporeal shock wave therapy and lithotripter machines for the treatment of erectile dysfunction. Expert Rev. Med. Dev. 2017, 14, 929–934. [Google Scholar] [CrossRef]
- Chung, E.; Cartmill, R. Evaluation of clinical efficacy, safety and patient satisfaction rate after low-intensity extracorporeal shockwave therapy for the treatment of male erectile dysfunction: An Australian first open-label single-arm prospective clinical trial. BJU Int. 2015, 115 (Suppl. 5), 46–49. [Google Scholar] [CrossRef]
- Clavijo, R.I.; Kohn, T.P.; Kohn, J.R.; Ramasamy, R. Effects of Low-Intensity Extracorporeal Shockwave Therapy on Erectile Dysfunction: A Systematic Review and Meta-Analysis. J. Sex. Med. 2017, 14, 27–35. [Google Scholar] [CrossRef]
- Dong, L.; Chang, D.; Zhang, X.; Li, J.; Yang, F.; Tan, K.; Yang, Y.; Yong, S.; Yu, X. Effect of Low-Intensity Extracorporeal Shock Wave on the Treatment of Erectile Dysfunction: A Systematic Review and Meta-Analysis. Am. J. Mens Health 2019, 13, 1557988319846749. [Google Scholar] [CrossRef]
- Sokolakis, I.; Hatzichristodoulou, G. Clinical studies on low intensity extracorporeal shockwave therapy for erectile dysfunction: A systematic review and meta-analysis of randomised controlled trials. Int. J. Impot. Res. 2019, 31, 177–194. [Google Scholar] [CrossRef]
- Myojo, M.; Ando, J.; Uehara, M.; Daimon, M.; Watanabe, M.; Komuro, I. Feasibility of Extracorporeal Shock Wave Myocardial Revascularization Therapy for Post-Acute Myocardial Infarction Patients and Refractory Angina Pectoris Patients. Int. Heart J. 2017, 58, 185–190. [Google Scholar] [CrossRef][Green Version]
- Lee, Y.C.; Chuang, S.M.; Lin, K.L.; Chen, W.C.; Lu, J.H.; Chueh, K.S.; Shen, M.C.; Liu, L.W.; Long, C.Y.; Juan, Y.S. Low-Intensity Extracorporeal Shock Wave Therapy Ameliorates the Overactive Bladder: A Prospective Pilot Study. Biomed. Res. Int. 2020, 2020, 9175676. [Google Scholar] [CrossRef]
- Long, C.Y.; Lin, K.L.; Lee, Y.C.; Chuang, S.M.; Lu, J.H.; Wu, B.N.; Chueh, K.S.; Ker, C.R.; Shen, M.C.; Juan, Y.S. Therapeutic effects of Low intensity extracorporeal low energy shock wave therapy (LiESWT) on stress urinary incontinence. Sci. Rep. 2020, 10, 5818. [Google Scholar] [CrossRef] [PubMed]
- Chuang, Y.C.; Meng, E.; Chancellor, M.; Kuo, H.C. Pain reduction realized with extracorporeal shock wave therapy for the treatment of symptoms associated with interstitial cystitis/bladder pain syndrome-A prospective, multicenter, randomized, double-blind, placebo-controlled study. Neurourol. Urodyn. 2020, 39, 1505–1514. [Google Scholar] [CrossRef]
- Chen, Y.T.; Yang, C.C.; Sun, C.K.; Chiang, H.J.; Chen, Y.L.; Sung, P.H.; Zhen, Y.Y.; Huang, T.H.; Chang, C.L.; Chen, H.H.; et al. Extracorporeal shock wave therapy ameliorates cyclophosphamide-induced rat acute interstitial cystitis though inhibiting inflammation and oxidative stress-in vitro and in vivo experiment studies. Am. J. Transl. Res. 2014, 6, 631–648. [Google Scholar]
- Wang, H.S.; Oh, B.S.; Wang, B.; Ruan, Y.; Zhou, J.; Banie, L.; Lee, Y.C.; Tamaddon, A.; Zhou, T.; Wang, G.; et al. Low-intensity extracorporeal shockwave therapy ameliorates diabetic underactive bladder in streptozotocin-induced diabetic rats. BJU Int. 2018, 122, 490–500. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.C.; Hsieh, T.J.; Tang, F.H.; Jhan, J.H.; Lin, K.L.; Juan, Y.S.; Wang, H.S.; Long, C.Y. Therapeutic effect of Low intensity Extracorporeal Shock Wave Therapy (Li-ESWT) on diabetic bladder dysfunction in a rat model. Int. J. Med. Sci. 2021, 18, 1423–1431. [Google Scholar] [CrossRef]
- Gruenwald, I.; Appel, B.; Vardi, Y. Low-intensity extracorporeal shock wave therapy—A novel effective treatment for erectile dysfunction in severe ED patients who respond poorly to PDE5 inhibitor therapy. J. Sex. Med. 2012, 9, 259–264. [Google Scholar] [CrossRef]
- Cohen, J.M.; Fagin, A.P.; Hariton, E.; Niska, J.R.; Pierce, M.W.; Kuriyama, A.; Whelan, J.S.; Jackson, J.L.; Dimitrakoff, J.D. Therapeutic intervention for chronic prostatitis/chronic pelvic pain syndrome (CP/CPPS): A systematic review and meta-analysis. PLoS ONE 2012, 7, e41941. [Google Scholar] [CrossRef]
- Van Voorhis, B.J. Genitourinary symptoms in the menopausal transition. Am. J. Med. 2005, 118 (Suppl. 12B), 47–53. [Google Scholar] [CrossRef]
- Blakeman, P.J.; Hilton, P.; Bulmer, J.N. Oestrogen and progesterone receptor expression in the female lower urinary tract, with reference to oestrogen status. BJU Int. 2000, 86, 32–38. [Google Scholar] [CrossRef]
- Ning, N.; Lin, G.; Lue, T.F.; Lin, C.S. Effects of estrogen, raloxifene, and levormeloxifene on the expression of Rho-kinase signaling molecules in urethral smooth muscle cells. Urology. 2010, 76, 1517.e6–1517.e11. [Google Scholar] [CrossRef][Green Version]
- Dobberfuhl, A.D.; Schuler, C.; Leggett, R.E.; De, E.J.B.; Levin, R.M. Estrogen replacement is protective to the effect of in vitro hypoxia on female rabbit bladder and pelvic floor contractile response. Investig. Clin. Urol. 2020, 61, 432–440. [Google Scholar] [CrossRef]
- Gee, E.; Milkiewicz, M.; Haas, T.L. p38 MAPK activity is stimulated by vascular endothelial growth factor receptor 2 activation and is essential for shear stress-induced angiogenesis. J. Cell Physiol. 2010, 222, 120–126. [Google Scholar] [CrossRef]
- Fan, N.; Zhang, M.; Xu, K.; Ke, X.; Ding, Y.; Wang, D.; Liu, Y.; Ning, Y.; Deng, X.; He, H. Serum level of vascular endothelial growth factor decreased in chronic ketamine abusers. Drug Alcohol Depend. 2015, 152, 57–61. [Google Scholar] [CrossRef]
- Zhu, G.Q.; Jeon, S.H.; Bae, W.J.; Choi, S.W.; Jeong, H.C.; Kim, K.S.; Kim, S.J.; Cho, H.J.; Ha, U.S.; Hong, S.H.; et al. Efficient Promotion of Autophagy and Angiogenesis Using Mesenchymal Stem Cell Therapy Enhanced by the Low-Energy Shock Waves in the Treatment of Erectile Dysfunction. Stem Cells Int. 2018, 2018, 1302672. [Google Scholar] [CrossRef]
- Hassanpour, M.; Rezabakhsh, A.; Pezeshkian, M.; Rahbarghazi, R.; Nouri, M. Distinct role of autophagy on angiogenesis: Highlights on the effect of autophagy in endothelial lineage and progenitor cells. Stem Cell Res. Ther. 2018, 9, 305. [Google Scholar] [CrossRef]
- Hayashi, D.; Kawakami, K.; Ito, K.; Ishii, K.; Tanno, H.; Imai, Y.; Kanno, E.; Maruyama, R.; Shimokawa, H.; Tachi, M. Low-energy extracorporeal shock wave therapy enhances skin wound healing in diabetic mice: A critical role of endothelial nitric oxide synthase. Wound Repair Regen. 2012, 20, 887–895. [Google Scholar] [CrossRef]
- Kuo, Y.R.; Wang, C.T.; Wang, F.S.; Chiang, Y.C.; Wang, C.J. Extracorporeal shock-wave therapy enhanced wound healing via increasing topical blood perfusion and tissue regeneration in a rat model of STZ-induced diabetes. Wound Repair Regen. 2009, 17, 522–530. [Google Scholar] [CrossRef]
- Dumfarth, J.; Zimpfer, D.; Vogele-Kadletz, M.; Holfeld, J.; Sihorsch, F.; Schaden, W.; Czerny, M.; Aharinejad, S.; Wolner, E.; Grimm, M. Prophylactic low-energy shock wave therapy improves wound healing after vein harvesting for coronary artery bypass graft surgery: A prospective, randomized trial. Ann. Thorac. Surg. 2008, 86, 1909–1913. [Google Scholar] [CrossRef]
- Kuo, Y.R.; Wang, C.T.; Wang, F.S.; Yang, K.D.; Chiang, Y.C.; Wang, C.J. Extracorporeal shock wave treatment modulates skin fibroblast recruitment and leukocyte infiltration for enhancing extended skin-flap survival. Wound Repair Regen. 2009, 17, 80–87. [Google Scholar] [CrossRef]
- Huang, T.H.; Sun, C.K.; Chen, Y.L.; Wang, C.J.; Yin, T.C.; Lee, M.S.; Yip, H.K. Shock Wave Enhances Angiogenesis through VEGFR2 Activation and Recycling. Mol. Med. 2017, 22, 850–862. [Google Scholar] [CrossRef]
- Weihs, A.M.; Fuchs, C.; Teuschl, A.H.; Hartinger, J.; Slezak, P.; Mittermayr, R.; Redl, H.; Junger, W.G.; Sitte, H.H.; Runzler, D. Shock wave treatment enhances cell proliferation and improves wound healing by ATP release-coupled extracellular signal-regulated kinase (ERK) activation. J. Biol. Chem. 2014, 289, 27090–27104. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Zhou, J.; Banie, L.; Reed-Maldonado, A.B.; Ning, H.; Lu, Z.; Ruan, Y.; Zhou, T.; Wang, H.S.; Oh, B.S.; et al. Low-intensity extracorporeal shock wave therapy promotes myogenesis through PERK/ATF4 pathway. Neurourol. Urodyn. 2018, 37, 699–707. [Google Scholar] [CrossRef]
- Wang, H.J.; Lee, W.C.; Tyagi, P.; Huang, C.C.; Chuang, Y.C. Effects of low energy shock wave therapy on inflammatory moleculars, bladder pain, and bladder function in a rat cystitis model. Neurourol. Urodyn. 2017, 36, 1440–1447. [Google Scholar] [CrossRef]
- Mariotto, S.; de Prati, A.C.; Cavalieri, E.; Amelio, E.; Marlinghaus, E.; Suzuki, H. Extracorporeal shock wave therapy in inflammatory diseases: Molecular mechanism that triggers anti-inflammatory action. Curr. Med. Chem. 2009, 16, 2366–2372. [Google Scholar] [CrossRef]
- Jiang, D.; Liang, J.; Fan, J.; Yu, S.; Chen, S.; Luo, Y.; Prestwich, G.D.; Mascarenhas, M.M.; Garg, H.G.; Quinn, D.A.; et al. Regulation of lung injury and repair by Toll-like receptors and hyaluronan. Nat. Med. 2005, 11, 1173–1179. [Google Scholar] [CrossRef]
- Mondalek, F.G.; Fung, K.M.; Yang, Q.; Wu, W.; Lu, W.; Palmer, B.W.; Frimberger, D.C.; Greenwood-Van Meerveld, B.; Hurst, R.E.; Kropp, B.P.; et al. Temporal expression of hyaluronic acid and hyaluronic acid receptors in a porcine small intestinal submucosa-augmented rat bladder regeneration model. World J. Urol. 2015, 33, 1119–1128. [Google Scholar] [CrossRef]
- Kaya, G.; Rodriguez, I.; Jorcano, J.L.; Vassalli, P.; Stamenkovic, I. Selective suppression of CD44 in keratinocytes of mice bearing an antisense CD44 transgene driven by a tissue-specific promoter disrupts hyaluronate metabolism in the skin and impairs keratinocyte proliferation. Genes Dev. 1997, 11, 996–1007. [Google Scholar] [CrossRef]
- Zhang, X.; Yan, X.; Wang, C.; Tang, T.; Chai, Y. The dose-effect relationship in extracorporeal shock wave therapy: The optimal parameter for extracorporeal shock wave therapy. J. Surg. Res. 2014, 186, 484–492. [Google Scholar] [CrossRef] [PubMed]
- Wu, A.K.; Zhang, X.; Wang, J.; Ning, H.; Zaid, U.; Villalta, J.D.; Wang, G.; Banie, L.; Lin, G.; Lue, T.F. Treatment of stress urinary incontinence with low-intensity extracorporeal shock wave therapy in a vaginal balloon dilation induced rat model. Transl. Androl. Urol. 2018, 7, S7–S16. [Google Scholar] [CrossRef] [PubMed]
- Chuang, Y.C.; Huang, T.L.; Tyagi, P.; Huang, C.C. Urodynamic and Immunohistochemical Evaluation of Intravesical Botulinum Toxin A Delivery Using Low Energy Shock Waves. J. Urol. 2016, 196, 599–608. [Google Scholar] [CrossRef] [PubMed]
- Juan, Y.S.; Lee, Y.L.; Long, C.Y.; Wong, J.H.; Jang, M.Y.; Lu, J.H.; Wu, W.J.; Huang, Y.S.; Chang, W.C.; Chuang, S.M. Translocation of NF-kappaB and expression of cyclooxygenase-2 are enhanced by ketamine-induced ulcerative cystitis in rat bladder. Am. J. Pathol. 2015, 185, 2269–2285. [Google Scholar] [CrossRef]
- Liu, K.M.; Chuang, S.M.; Long, C.Y.; Lee, Y.L.; Wang, C.C.; Lu, M.C.; Lin, R.J.; Lu, J.H.; Jang, M.Y.; Wu, W.J.; et al. Ketamine-induced ulcerative cystitis and bladder apoptosis involve oxidative stress mediated by mitochondria and the endoplasmic reticulum. Am. J. Physiol. Renal Physiol. 2015, 309, F318–F331. [Google Scholar] [CrossRef] [PubMed]
- Chuang, S.M.; Liu, K.M.; Li, Y.L.; Jang, M.Y.; Lee, H.H.; Wu, W.J.; Chang, W.C.; Levin, R.M.; Juan, Y.S. Dual involvements of cyclooxygenase and nitric oxide synthase expressions in ketamine-induced ulcerative cystitis in rat bladder. Neurourol. Urodyn. 2013, 32, 1137–1143. [Google Scholar] [CrossRef] [PubMed]
- Homma, Y.; Yoshida, M.; Seki, N.; Yokoyama, O.; Kakizaki, H.; Gotoh, M.; Yamanishi, T.; Yamaguchi, O.; Takeda, M.; Nishizawa, O. Symptom assessment tool for overactive bladder syndrome—Overactive bladder symptom score. Urology 2006, 68, 318–323. [Google Scholar] [CrossRef] [PubMed]
- Ozberk, S.S.; Gundogar, H.; Ozkaya, M.; Taner, I.L.; Erciyas, K. The effect of photobiomodulation therapy on nonsurgical periodontal treatment in patients with type 2 diabetes mellitus: A randomized controlled, single-blind, split-mouth clinical trial. Lasers Med. Sci. 2019, 35, 497–504. [Google Scholar] [CrossRef] [PubMed]
- Rosario, J.L. Relief from Back Pain Through Postural Adjustment: A Controlled Clinical Trial of the Immediate Effects of Muscular Chains Therapy (MCT). Int. J. Ther. Massage Bodyw. 2014, 7, 2–6. [Google Scholar] [CrossRef][Green Version]
| Variable | Sham | OVX | OVX + SW4 | OVX + SW8 |
|---|---|---|---|---|
| No. Rats | 8 | 8 | 8 | 8 |
| Physical Indicators | ||||
| Serum estradiol conc. (pg/mL) before treatment | 32.17 ± 1.41 | 32.33 ± 1.52 | 31.75 ± 1.29 | 32.35 ± 0.53 |
| Serum estradiol conc. (pg/mL) after treatment | 33.64 ± 3.66 | 16.53 ± 1.28 ** | 15.59 ± 0.99 ** | 15.65 ± 1.36 ** |
| Water intake (mL/24 h) | 41.25 ± 14.36 | 35.50 ± 5.81 | 36.17 ± 5.31 | 32.33 ± 7.74 |
| Urine output (mL/24 h) | 20.08 ± 3.34 | 13.53 ± 6.78 | 18.12 ± 9.19 | 18.06 ± 7.36 |
| Body weight (g) | 400.60 ± 39.41 | 555.13 ± 85.95 ** | 524.86 ± 56.74 ** | 537.88 ± 75.65 ** |
| Bladder weight (mg) | 175.00 ± 19.79 | 163.33 ± 10.33 | 158.57 ± 2.37 | 205.00 ± 25.88 † |
| The ratio of bladder weight (mg)/body weight (g) | 0.46 ± 0.07 | 0.32 ± 0.02 * | 0.33 ± 0.10 * | 0.41 ± 0.10 |
| Urodynamic Parameters | ||||
| Frequency (No. voids/1 h) | 3.60 ± 0.89 | 6.83 ± 2.71 * | 4.29 ± 1.25 † | 3.50 ± 0.84 †† |
| Peak micturition pressure (cm H2O) | 25.58 ± 2.94 | 35.56 ± 5.73 * | 25.53 ± 7.60 | 23.51 ± 4.41 |
| Voided volume (mL) | 2.82 ± 0.80 | 1.46 ± 0.36 ** | 2.15 ± 0.69 | 2.92 ± 0.65 †† |
| No. of non-voiding contractions between micturition (No. voids/h) | 0.00 ± 0.00 | 3.17 ± 1.60 ** | 1.00 ± 1.73 †† | 0.33 ± 0.52 †† |
| Parameter | Sham | LiESWT | p Value | Normal Range |
|---|---|---|---|---|
| Physical parameter | ||||
| Female age (years) | 60.79 ± 1.98 | 59.05 ± 1.21 | 0.44 | |
| Height (cm) | 159.35 ± 1.03 | 157.94 ± 0.80 | 0.30 | |
| Weight (kg) | 57.64 ± 1.83 | 60.69 ± 1.77 | 0.29 | |
| BMI (kg/m2) | 22.69 ± 0.66 | 24.28 ± 0.61 | 0.12 | 18.5–26 |
| Waistline (cm) | 81.34 ± 2.36 | 85.96 ± 1.88 | 0.15 | |
| Systolic pressure (mmHg) | 118.26 ± 3.89 | 123.92 ± 2.33 | 0.20 | 100–120 |
| Diastolic pressure (mmHg) | 69.74 ± 2.28 | 73.63 ± 1.71 | 0.19 | 60–80 |
| MAP (mmHg) | 85.91 ± 2.68 | 89.94 ± 1.67 | 0.20 | 70–110 |
| Serum parameter | ||||
| HbA1C (%) | 5.76 ± 0.07 | 5.75 ± 0.09 | 0.95 | 4–6 |
| AC sugar (mg/dL) | 103.00 ± 7.67 | 101.44 ± 1.81 | 0.61 | 65–109 |
| BUN (mg/dL) | 12.27 ± 0.97 | 13.01 ± 0.70 | 0.55 | 8–20 |
| Creatinine (mg/dL) | 0.73 ± 0.03 | 0.71 ± 0.02 | 0.65 | 0.44–1.03 |
| GOT(AST) (IU/L) | 23.16 ± 1.27 | 24.03 ± 0.83 | 0.56 | 10–42 |
| GPT(ALT) (IU/L) | 22.41 ± 2.43 | 23.05 ± 1.48 | 0.80 | 10–40 |
| Triglycerides (mg/dL) | 104.84 ± 10.57 | 111.26 ± 8.80 | 0.66 | 35–160 |
| Cholesterol (mg/dL) | 207.11 ± 7.93 | 208.95 ± 6.37 | 0.87 | 140–200 |
| HDL (mg/dL) | 66.14 ± 3.80 | 58.22 ± 2.32 | 0.07 | 29–85 |
| LDL (mg/dL) | 115.78 ± 6.62 | 122.76 ± 4.43 | 0.38 | 0–130 |
| Parameter | Sham | LiESWT | ||||||
|---|---|---|---|---|---|---|---|---|
| W0 | W4 | W0 | W4 | W8 | F1 | F3 | F6 | |
| 3-day urinary diary record | ||||||||
| Intake (mL) | 2048.4 ± 69.4 | 2043.1 ± 46.1 | 2029.6 ± 74.6 | 1914.0 ± 54.4 | 1912.9 ± 45.5 | 1851.1 ± 50.8 | 1800.2 ± 56.3 | 1921.4 ± 57.2 |
| Output (mL) | 2070.9 ± 36.3 | 2016.4 ± 55.7 | 2017.4 ± 76.5 | 1977.1 ± 73.1 | 1932.6 ± 71.6 | 1897.9 ± 69.2 | 1922.9 ± 76.7 | 1928.8 ± 66.1 |
| Average voided volume (mL) | 186.8 ± 4.8 | 195.7 ± 4.5 | 186.8 ± 7.6 | 195.5 ± 6.2 | 213.2 ± 6.5 ** | 213.6 ± 7.1 ** | 218.4 ± 7.9 ** | 209.0 ± 7.6 * |
| FBC (mL) | 344.3 ± 6.1 | 350.2 ± 6.4 | 338.1 ± 11.2 | 343.7 ± 11.2 | 376.0 ± 11.8 ** | 378.9 ± 14.8 ** | 356.7 ± 11.5 * | 352.7 ± 14.7 |
| Daytime frequency (times) | 11.38 ± 0.33 | 11.09 ± 0.30 | 11.83 ± 0.46 | 10.24 ± 0.35 *,† | 9.45 ± 0.28 ** | 9.47 ± 0.27 ** | 8.76 ± 0.25 ** | 8.96 ± 0.21 ** |
| Nocturia (times) | 1.73 ± 0.12 | 1.51 ± 0.11 | 1.68 ± 0.14 | 1.27 ± 0.10 *,† | 1.17 ± 0.11 ** | 1.07 ± 0.10 ** | 1.14 ± 0.10 ** | 1.06 ± 0.10 ** |
| Urgency (times) | 2.90 ± 0.23 | 2.69 ± 0.24 | 3.10 ± 0.35 | 2.22 ± 0.36 **,† | 1.87 ± 0.38 ** | 1.08 ± 0.30 ** | 1.14 ± 0.30 ** | 0.97 ± 0.26 ** |
| Uroflowmetry data | ||||||||
| Voided urine volume (mL) | 321.3 ± 17.6 | 339.0 ± 16.4 | 314.8 ± 14.6 | 369.6 ± 14.1 ** | 392.5 ± 17.6 ** | 380.7 ± 14.0 ** | 362.8 ± 14.2 ** | 359.7 ± 13.1 * |
| Qmax (mL/s) | 25.30 ± 1.54 | 26.65 ± 1.18 | 24.21 ± 1.09 | 27.58 ± 1.43 * | 28.35 ± 1.15 * | 28.09 ± 1.39 * | 26.98 ± 0.90 * | 26.98 ± 1.14 * |
| PVR (mL) | 42.79 ± 4.58 | 44.00 ± 4.66 | 46.67 ± 5.27 | 35.06 ± 4.63 *,† | 31.01 ± 4.94 ** | 21.45 ± 1.97 ** | 26.62 ± 2.97 ** | 24.16 ± 2.92 ** |
| OABSS score (points) | ||||||||
| Daytime frequency | 1.07 ± 0.04 | 1.00 ± 0.03 | 1.22 ± 0.07 | 0.86 ± 0.04 * | 0.77 ± 0.05 ** | 0.83 ± 0.05 ** | 0.85 ± 0.05 ** | 1.13 ± 0.11 |
| Nocturia | 2.30 ± 0.14 | 2.10 ± 0.13 | 2.19 ± 0.11 | 1.47 ± 0.10 **,†† | 1.32 ± 0.11 ** | 1.31 ± 0.11 ** | 1.46 ± 0.11 ** | 1.63 ± 0.20 * |
| Urgency | 2.56 ± 0.16 | 2.38 ± 0.16 | 2.53 ± 0.19 | 1.57 ± 0.14 **,†† | 1.07 ± 0.14 ** | 1.00 ± 0.13 ** | 1.00 ± 0.10 ** | 0.96 ± 0.13 ** |
| Urgency incontinence | 1.73 ± 0.16 | 1.67 ± 0.16 | 1.61 ± 0.18 | 1.00 ± 0.14 **,†† | 0.67 ± 0.10 ** | 0.68 ± 0.10 ** | 0.74 ± 0.10 ** | 0.82 ± 0.15 * |
| Inclusion Criteria | Exclusion Criteria |
|---|---|
| 1. Menopause female participants aged 40–75 years who were diagnosed with overactive bladder (OAB) for more than 3 months. | 1. Urinary tract infection detected at screening, and recurrent urinary tract infections (more than 3 episodes in the past 3 months). |
| 2. OAB symptoms included daytime frequency of micturition ≥ 8 times, and nocturia, urgency or urgency incontinence ≥ 1 times. | 2. Chronic urinary inflammation (interstitial cystitis, urethral syndrome, or painful bladder syndrome). |
| 3. Patients could understand and follow the instructions and were able to complete the questionnaire. | 3. Neuropathic diseases. |
| 4. Patients with OAB symptom did not take antimuscarinic or ß3 agonist. | 4. Lower urinary tract surgery within last 6 months. |
| 5. OAB patient with antimuscarinic or ß3 agonist treatment could also be included after 3 months of medication withdrawal | 5. Significant bladder outflow obstruction. |
| 6. Signature of informed consent form. | 6. Urinary catheterization. |
| 7. Drug or nondrug treatments of OAB in the previous 3 months. | |
| 8. Perineal operations, intravesical injection, irradiation, shockwave or electrostimulation in the past 12 months. | |
| 9. History of urolithiasis or urologic malignancy. | |
| 10. Gross hematuria. | |
| 11. Comorbidities associated to OAB (diabetes mellitus, spinal cord injury, stroke, or neurogenic diseases). | |
| 12. Severe cardiopulmonary disease, liver or renal dysfunction. | |
| 13. Previous pelvic radiation therapy. | |
| 14. Coagulopathy. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, K.-L.; Lu, J.-H.; Chueh, K.-S.; Juan, T.-J.; Wu, B.-N.; Chuang, S.-M.; Lee, Y.-C.; Shen, M.-C.; Long, C.-Y.; Juan, Y.-S. Low-Intensity Extracorporeal Shock Wave Therapy Promotes Bladder Regeneration and Improves Overactive Bladder Induced by Ovarian Hormone Deficiency from Rat Animal Model to Human Clinical Trial. Int. J. Mol. Sci. 2021, 22, 9296. https://doi.org/10.3390/ijms22179296
Lin K-L, Lu J-H, Chueh K-S, Juan T-J, Wu B-N, Chuang S-M, Lee Y-C, Shen M-C, Long C-Y, Juan Y-S. Low-Intensity Extracorporeal Shock Wave Therapy Promotes Bladder Regeneration and Improves Overactive Bladder Induced by Ovarian Hormone Deficiency from Rat Animal Model to Human Clinical Trial. International Journal of Molecular Sciences. 2021; 22(17):9296. https://doi.org/10.3390/ijms22179296
Chicago/Turabian StyleLin, Kun-Ling, Jian-He Lu, Kuang-Shun Chueh, Tai-Jui Juan, Bin-Nan Wu, Shu-Mien Chuang, Yung-Chin Lee, Mei-Chen Shen, Cheng-Yu Long, and Yung-Shun Juan. 2021. "Low-Intensity Extracorporeal Shock Wave Therapy Promotes Bladder Regeneration and Improves Overactive Bladder Induced by Ovarian Hormone Deficiency from Rat Animal Model to Human Clinical Trial" International Journal of Molecular Sciences 22, no. 17: 9296. https://doi.org/10.3390/ijms22179296
APA StyleLin, K.-L., Lu, J.-H., Chueh, K.-S., Juan, T.-J., Wu, B.-N., Chuang, S.-M., Lee, Y.-C., Shen, M.-C., Long, C.-Y., & Juan, Y.-S. (2021). Low-Intensity Extracorporeal Shock Wave Therapy Promotes Bladder Regeneration and Improves Overactive Bladder Induced by Ovarian Hormone Deficiency from Rat Animal Model to Human Clinical Trial. International Journal of Molecular Sciences, 22(17), 9296. https://doi.org/10.3390/ijms22179296






