Abstract
Indigenous communities across the globe, especially in rural areas, consume locally available plants known as Traditional Food Plants (TFPs) for their nutritional and health-related needs. Recent research shows that many TFPs are highly nutritious as they contain health beneficial metabolites, vitamins, mineral elements and other nutrients. Excessive reliance on the mainstream staple crops has its own disadvantages. Traditional food plants are nowadays considered important crops of the future and can act as supplementary foods for the burgeoning global population. They can also act as emergency foods in situations such as COVID-19 and in times of other pandemics. The current situation necessitates locally available alternative nutritious TFPs for sustainable food production. To increase the cultivation or improve the traits in TFPs, it is essential to understand the molecular basis of the genes that regulate some important traits such as nutritional components and resilience to biotic and abiotic stresses. The integrated use of modern omics and gene editing technologies provide great opportunities to better understand the genetic and molecular basis of superior nutrient content, climate-resilient traits and adaptation to local agroclimatic zones. Recently, realizing the importance and benefits of TFPs, scientists have shown interest in the prospection and sequencing of TFPs for their improvements, cultivation and mainstreaming. Integrated omics such as genomics, transcriptomics, proteomics, metabolomics and ionomics are successfully used in plants and have provided a comprehensive understanding of gene-protein-metabolite networks. Combined use of omics and editing tools has led to successful editing of beneficial traits in several TFPs. This suggests that there is ample scope for improvement of TFPs for sustainable food production. In this article, we highlight the importance, scope and progress towards improvement of TFPs for valuable traits by integrated use of omics and gene editing techniques.
1. Introduction
As per Food and Agriculture Organization (FAO) estimates, the global population is expected to reach nine billion by 2050 and the world will have to produce 50% more food than we produce today to feed the burgeoning population [1]. However, increasing the food production of the currently available crops on available land is a challenging task [2]. This challenge is further limited by several factors such as excessive reliance on a limited number of industrialized crops, decreasing land for agriculture and global climate change [2]. Several factors such as desertification and conversion of agricultural lands for non-agricultural activities also pose a major threat to the global food-producing systems [3]. State of the World’s Plants Report 2016 estimated the existence of more than 391,000 species of vascular plants on this planet [4]. This report further estimated that approximately 30,000 species have at least one documented use and more than 5000 of them provide food to humans [5]. It is reported that nearly 2500 species of plants belonging to more than 160 families have undergone domestication throughout the world [6]. Surprisingly, despite having a huge diversity of vascular food plants, the world relies on only a limited number of approximately 15 major crops for 70 percent of food and nutritional requirements that were domesticated by our ancestors more than 10,000 years ago [7,8]. Of the 15 major crops, more than 50 percent of the calories come from five cereal crops, namely wheat, rice, millet, sorghum and maize [7,9]. Excessive reliance on a limited number of mainstream domesticated crops for nutritional requirements has been flagged as an important issue in the global fight against food insecurity and in ensuring global food security to achieve zero hunger by 2030 as envisaged in the Agenda 2030 of Sustainable Development Goals [10]. Furthermore, the current widespread cultivation of uniform domesticated varieties carries huge risks of crop failures and significant reduction in yield as they are more vulnerable to biotic (pathogen and pests) and abiotic stresses (due to global climate change) [11]. It has been estimated that the rise in global mean temperatures may result in a reduction in significant yields of several crops currently in use such as wheat, rice, maize and soybean [12,13]. However, the effect of global climate change is perceived differently by different varieties/crops, and in different regions of the world [14,15]. It further necessitates the identification of the local species/varieties that are used in different agro-climatic regions of the world [16]. Therefore, identification of new crops and varieties with superior nutrition content suitable to the local agro-climatic zones is an important agenda for plant scientists [16,17].
A number of recent studies have pointed towards the exploration and exploitation of traditionally used food crops (TFPs) for nutritional food security and their mainstreaming [18,19,20]. TFPs can act as supplementary diets and also as emergency foods in times of pandemics or when the global supply chains are disrupted due to man-made or natural disasters. A traditional food crop is an indigenous crop species that is native to a particular region of the world or was introduced from another place long ago and due to its use for generations, it has become a part of the culture of that particular community or region [21,22]. Several local indigenous communities of the world still use and rely on such traditional crops which were in use for centuries but are currently neglected, underutilized, restricted to particular geographical locations and are not in mainstream use [23]. Nevertheless, recent years have seen increased preference of consumers towards these ancient traditional varieties and there is an increased focus on the reintroduction and mainstreaming of such traditionally used ancient food crop varieties [24,25,26,27]. Considering the nutritional, economic and agricultural importance of TFPs and their use as future climate-resilient crops, it is important to explore the application of the modern omics technologies for dissection of molecular mechanisms governing those traits [28]. Furthermore, the extensive exploitation of genetic diversity is required to address the vulnerability of crop plants due to the narrow genetic base [29]. Modern technologies can be used to characterize the crop germplasm collections to be used for better and sustainable food production and supply; for example, Milner et al. [30] and Langridge and Waugh [31] evaluated more than 20,000 wild and domesticated barley genotypes with the aid of genotyping and informatics technologies and demonstrated the scope of exploitation of genetic resources in crop improvement [30,31].
This review article discusses the potential use of various omics technologies for understanding the genetic makeup, proteomes, metabolomes, ionomes and nutritional composition of TFPs. This review also provides details about the use of available genomics information from model crops and its potential in translational research of TFPs. We further discussed a detailed futuristic outline of integrated use of omics and gene editing technologies for rapid improvement/domestication of TFPs.
2. Importance of Traditional Food Plants
2.1. Diversity of Traditional Food Plants across the Globe
Incidences of crop failures triggered by climate change and pathogens are expected to rise in the future [32]. We have already experienced such crop failures in the past; for example, over-dependence on the potato and the attack of Phytophthora infestans resulted in the Irish famine which led to starvation, widespread deaths and emigrations to the other parts of the world [33]. Southern leaf blight of corn in the United States is another example of the risks of a single crop or one type of crop carrying pathogens [34]. There are several other examples of major crop failures from across the world, indicating the potential risks inherent to the cultivation of less diversified and uniform crops [35,36]. The uniform varieties are most likely to be destroyed simultaneously with the evolution of resistant pathogens or with climate change unless region-specific strategies and preventive measures are in place [37]. This leads to widespread hunger, malnutrition, migration and may even lead to civil wars [11,38]. Therefore, the existence of diversity in food plants is crucial and required for the breeding of improved varieties for desirable traits such as stress resistance and nutritional superiority [39,40,41]. It is also desirable to ensure healthy, sustainable food security, to reduce the impacts of diseases and climate change and to improve the stability of food production [42,43]. Minor TFPs have so far largely been ignored and much attention is not given to them for their role in sustainable food security because of certain undesirable characteristics and their restricted geographical availability [44]. However, recent years have seen an increased interest in the revival of the traditional plants and the food systems that are based on the TFPs [43,44,45,46]. Efforts across the globe are ongoing to diversify the currently cultivated basket of food crops, to provide more options to the farmers to grow crops and to the consumers for diversifying their food menu [47]. Large amounts of fragmented ethnobotanical data on TFPs are available from various countries [48]. Several studies have performed their nutritional and stress-related analysis and results from these studies suggest the potential roles of TFP diversity in fighting against the hidden hunger of the world by ensuring global food security [49].
The diversification of nutritionally rich and stress-resilient traditional, orphan and underutilized crops can help to achieve the goal of a zero-hunger world as envisaged in the United Nations Sustainable Development Goals (SDGs), which specifically propose to end hunger, achieve food security, improve nutrition and promote sustainable agriculture globally by 2030 [50,51]. However, extensive research is needed on TFPs to integrate them into global food systems [51]. It is necessary to understand consumption barriers as well as production constraints [52]. Although TFPs are very important for food security [53], many of them produce relatively lesser yields due to the lack of selection of improved traits. They are also not cultivated on a large scale because of unfavorable policies for their promotion [54]. However, several initiatives have recently been taken that are focused on the promotion of TFPs and improvement of their traits with the aid of genetic and genomic tools [54]. For example, African Orphan Crops Consortium (AOCC) is involved in the sequencing of 101 orphan crops and their integration into African food production-consumption systems [55]. The AOCC is a global partnership dedicated to the genome-enabled advancement of 101 African orphan crops that have superior nutrient and adaptive characteristics [52,56]. The consortium is aimed to elucidate reference genomes of 101 species for exploring genetic diversity. AOCC is an important model that can be adopted in other parts of the world especially to those areas which have rich diversity of TFPs [52]. Similarly, there exists an independent international organization named Crops For the Future (CFF) which aims to promote and facilitate the use of underutilized, neglected and orphan crops and their integration into human diets. The mission of CFF includes increasing the knowledge base of neglected crops, advocating policies related to promotion of neglected crops and spreading awareness about the relevance of neglected crops for rural livelihoods [57]. The Food and Agricultural Organization of the United Nations is also taking initiatives for the promotion of neglected crops by collaborating with agencies such as the International Council for Research in Agroforestry (ICRAF) [58]. Therefore, for attaining sustainability of food production, collective efforts are required to advance the research and development on TFPs [54].
2.2. Traditional Food Plants Possess Important Nutritional Traits
Experimental evidence suggests that ancient TFPs have certain important nutritional and stress-resilient traits that can be exploited to reduce global hunger and malnutrition under increasing global climate change [59]. TFPs are promising future crops considering their multiple benefits to the farmers, consumers and the environment as well [44,59,60,61]. Traditional crops that are used generation after generation are mostly consumed in a particular region by the local communities for nutritional and therapeutic purposes [62,63]. Several studies have experimentally proven that a number of traditional crops are highly rich in nutritional components, and many of them are resilient to several stresses [19,64]. Some of the examples are the fruit of Elaeagnus umbellata, which have ten times higher quantity of lycopene in their fruit than tomato [65], and Chenopodium quinoa, which has higher mineral content than maize and barley, including calcium, magnesium, iron, copper, potassium, phosphorus and zinc [66]. Even though they have multiple benefits, the lack of domestication and their cultivation being limited to geographical boundaries hinders their integration into large-scale production systems [23]. Although TFPs possess several important traits, some are also burdened with certain undesirable traits [44]. For example, there are some TFPs with antinutritional components which are harmful when consumed [67]. Therefore, it becomes necessary to remove undesirable traits before they are made available for extensive cultivation and consumption [44]. Prior knowledge of the undesirable traits and the genes governing them is also crucial and we can employ modern gene editing tools to get rid of them. Therefore, rapid domestication of TFPs using gene editing tools is an effective solution for this problem [68]. Redomestication of crops for their wild traits that could be lost due to domestication is another important strategy to access the lost gene pools [69].
2.3. Traditional Food Plants Show Varying Degrees of Tolerance to Stresses
FAO has emphasized four important dimensions that determine the food security of a country, region or population viz. enough availability of food, sufficient access to food, food utilization and stability of the first three dimensions [45,70]. Availability of food means enough production of a particular food and its seamless distribution to consumers [42,70]. Sufficient food access indicates economic affordability or freedom to access sufficient food and sufficient allocation of the food resources [71,72]. The third component indicates bio-assimilation of the food that is eaten [70]. The fourth and the last components indicate seamless and sustainable availability of access to and utilization of the food resources [45,71]. The disturbance in the stability of the three dimensions would eventually result in the food insecurity of a region, country or population [73]. Ensuring the food security of a growing population in the future is going to be a challenging task [74]. Various factors affect the components of a healthy and secure food system [45,70]. The production of food is already limited by several factors such as global climate change, biotic and abiotic resources and loss of genetic resources [75]. The sustainable food supply (first component) is disrupted by various factors such as pandemics, wars, natural disasters, droughts, climate change and excessive rainfall [76,77]. Sufficient access to food (second component) is limited by factors such as poverty, food price rises, unemployment, low per capita income and poor market access [78]. If the food is not biologically utilized in the body, it may lead to widespread disease or malnourishment [79]. Therefore, the stability of all three components over time is essential for ensuring sustainable global food security [73]. One of the most important factors that contributes towards the disruption of the stability of the three dimensions of food security is climate change, its associated negative impacts, biotic and abiotic stresses. Such disruptions may result in widespread food insecurity across the globe [78]. A number of studies have reported that climate change and stresses pose serious threats to the growth and reproduction of crop plants and reduce their yields by affecting various processes in the cells [77,80]. Excessive threats of failures that the currently cultivated crops face across the globe necessitate identification of the new climate-resilient crops, and the diversification of the crops [14]. Several studies have also indicated the identification and cultivation of climate-resilient food crops with biotic and abiotic stress tolerance traits [77]. Therefore, there is a larger consensus among various stakeholders about the urgency to identify and promote climate-resilient crops that possess abiotic stress tolerance. Interestingly, a large number of TFPs are adapted to the region of their origin, have huge regional importance to the regional local communities [81], show considerable resilience to climate change and can perform better even under unfavorable environmental conditions [19]. Traditional food plants are more climate-resilient and disease- and pest-resistant, and can survive in harsh environmental conditions [82]. Cultivation of traditional food plants is in congruence with the four important dimensions of food security as defined by FAO [44] (Figure 1). The traditional food systems based on traditional food plants are also resilient and sustainable. The food production, supply and consumption must be sustainable and resilient during times of natural calamities, civil wars or during pandemics when the supply chains are threatened. The current definition of food security therefore also includes sustainability and resilience. The traditional foods and the food systems based on them are sustainable and resilient to such situations. The promotion of climate-resilient, underutilized food crops along with modern crop varieties will be important for stable food production systems, especially under fluctuating environmental conditions [83]. A non-exhaustive list of TFPs with their nutritional and stress-resilient traits is presented in Table 1.
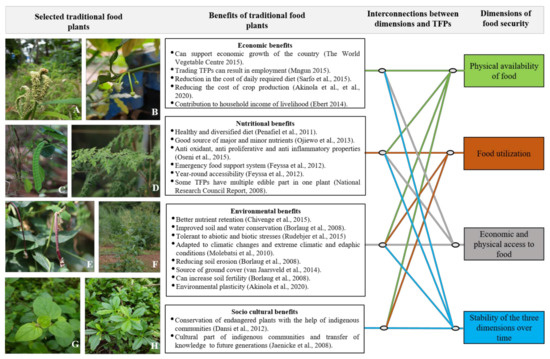
Figure 1.
The congruence of traditional food plants with four dimensions of food security [44,58]. Examples of few traditional food plants: (A) Eleusine coracana (L.) Gaertn., (B) Garcinia madruno (Kunth) Hammel., (C) Canavalia ensiformis (L.) DC., (D) Moringa oleifera Lam., (E) Vigna unguiculata L. (Walp), (F) Amaranthus palmeri S. Watson, (G) Boerhavia diffusa L. and (H) Talinum triangulare (Jacq.).

Table 1.
Diverse traditional food plants grown across the globe, their uses and nutritional importance.
2.4. Traditional Food Plants Ensure Stable and Sustainable Food Security
Stability of food supply, access to food and food utilization over time is important for a healthy food system and ensuring food security [42,45,70]. If concerted efforts are not taken in the immediate future to revive and conserve them, they may disappear from the global food menu [25,213,214]. This will contribute to the loss of genetic diversity and resources important for breeding the nutritionally superior and climate-resilient varieties [215,216,217]. Therefore, it becomes necessary to enhance our focus from the model and select domesticated crops towards less-consumed and neglected traditional crops that hold promising potential in alleviating global hunger and ensuring food security [218]. There is an increasing interest among scientists in the exploitation of TFPs, understanding their genetic basis of important traits and further improvement. However, breeding improved varieties that are nutritionally superior and climate-resilient requires a complete understanding of the genetic and molecular basis of such traits [219]. Recent technological advancements in the high throughput omics approaches provide opportunities to dissect the genetic and molecular basis of nutritional and stress tolerance-related traits. Integration of multi-omics tools such as genomics, transcriptomics, metabolomics, proteomics and ionomics can help us comprehensively investigate the gene–protein–metabolite networks of nutrition, climate resilience and other traits [220]. In an interconnected, interdependent and globalized world, several countries are involved in bilateral and multilateral trades in food and food-related products [221]. Situations such as global pandemics, wars and physical disruptions to logistics can disrupt global food supply chains, resulting in global, regional or local food insecurity endangering a large population [222]. Currently, COVID-19 has threatened multilateral and bilateral trades between nations [223]. The supply of food from one country to another is severely affected [224]. Some countries which are excessively dependent on the import of food grains are the most affected due to COVID-19 [225]. Such pandemic-related disruptions in food security can be averted if foods are locally grown and made available for the local populations [226]. Additionally, the cultivation of local varieties promotes local agriculture and conserves the biodiversity of the local agroecosystems [227]. It has also been argued that consumption of locally grown foods may be advantageous over long-distance foods, as locally harvested foods are almost available in less time to the consumers and their freshness ensures that they are of better nutritional quality [228]. The promotion of TFPs will also promote the role of local communities in maintaining and managing local genetic diversity for sustainable food and nutritional security [227].
2.5. Traditional Food Plants Provide Alternative Sources of Income to the Farmers and Unorganized Workers
In addition to having a key role in subsistence agriculture, as a source of food and medicine during shortages of food supply, they provide livelihood opportunities to rural communities [229,230]. Therefore, TFPs simultaneously act as a source of income for local communities. Among vegetables, Cleome, Amaranthus, Corchorus and Vigna spp. and fruit trees such as Azanza garckeana, Adansonia digitata, Sclerocarya birrea, Strychnos spinosa, Vangueria infausta and Grewia spp. are the major TFPs of Botswanan communities, providing them with income [231]. They grow naturally and the local women and children sell such crops or their products in formal and informal markets. This helps them raise their income—it may not be significant but can at least help them fulfill daily needs [231,232]. Cruz-Garcia and Price [233] reported that in the case of the poorest northeast region of Thailand, the sale of traditional food plants constitutes an important household income strategy to deal with situations of stress. Traditional crops such as Eleusine coracana (finger millet), Vigna radiata (green gram), Coix lacryma-jobi (Job’s tears), Lens culinaris (lentils), Vigna radiata (mungbean), Sesamum indicum (sesame), Glycine max (local soybean), Ipomoea batatas (sweet potato) and Dioscorea spp (yam) are the main source of income for poor and marginal farmers from East and South Asia [234]. In South Africa, traditional food plants are a vital source of income for indigenous communities [235], and in West Africa, the survival of small farmers in tribal communities is completely dependent on traditional food plants [236]. Secondary products of the TFPs are also highly marketable. For example, the malt produced from Panicum sumatrense (little millet) provides good incomes in India [237]. The processing of little millet led to generations of employment in the villages and increased the income of the rural folks significantly [196]. In India, it was reported that TFPs are a good source for increasing the incomes as well as improving the nutritional security of community people through the sale of several items such as ethnic millet papad, chakli, fermented breakfast food paddu and other novel foods prepared using little millet [238]. Islam et al. [239] reported that the poorest families in the Kurigram district of Bangladesh depend heavily on TFPs, especially in times of famine. Considering these limited studies, it can be stated that TFPs act as an alternative source of income for poor farmers and other poor communities including indigenous communities.
3. Multi-Omics Tools to Dissect Nutritional and Stress-Related Traits for Ensuring Sustainable Global Food Security
Being traditionally and culturally important, TFPs are used across the globe for nutritional purposes by a large proportion of the population [59]. However, due to selective breeding, the yield and quality of TFPs is not up to the mark, and modern technologies can be used to improve yield and quality traits [240]. Advanced crop improvement tools can be implemented effectively to have a clear understanding of complex molecular machinery governing growth, development, nutrients, other quality traits and stress responses in TFPs [241]. The recent advancements and revolutions in omics technologies allow large-scale investigations of organisms at the gene, genome, metabolome, ionome and proteome levels at a faster rate within a relatively shorter period of time [242]. The chromosomal organization, sequence polymorphism and genome structure of the plants can be studied by using structural genomics tools and by developing genetic and physical maps of genomic regions controlling a particular trait of an organism [243]. Further, functional genomics technologies enable the understanding of the functions of genes regulating these traits [26,243]. Transcriptomics allows the study of the expression of total mRNA in a cell, tissue or in an organism under a given condition [244]. Transcriptomics also enable the identification of the transcripts and their correlation with the phenotypic data provides opportunities to decipher gene–trait relationships [244]. With the advancements in next-generation high-throughput sequencing technologies and the availability of advanced bioinformatics tools, it is easier to analyze large datasets including sequence alignment, annotation and expression profiling of genes [245]. Establishing a correlation of transcript abundance with the proteins and metabolites accumulation is slightly challenging because of the post-translational protein modifications and the regulation of metabolites by complex enzymatic pathways [246]. The quantitative and qualitative measurement of protein metabolite content is attained with the help of proteomics and metabolomics approaches [247]. Similarly, the complete mineral and elemental composition of a plant species can be understood with ionomics tools, and the integration of other omics tools such as genomics, proteomics and transcriptomics can help to establish the link between the elemental composition, transport and storage with the genes regulating various processes [248]. Omics tools are therefore very important for the discovery of the genes controlling a particular trait of interest in a crop plant [249,250].
In the past two decades, we have seen an increased number of plants being sequenced [251]. Arabidopsis was the first model plant to be sequenced and it has provided significant insights about the various processes in the plants. Completion of its sequencing took several years [252]. However, innovations and improvements in the sequencing technologies have made it possible to sequence large and complex genomes in a shorter period of time at lower costs [253]. Therefore, many of the genomes of major crops have been recently sequenced within a relatively shorter span of time [254]. Many studies have focused on the genome sequences of the model crop plants, but recently we have also seen the application of omics technologies to non-model crops [255,256]. To date, whole genomes of more than 328 vascular plant species (comprising 323 angiosperms, 5 gymnosperms and 3 lycophytes), 3 non-vascular land plant species (2 mosses and 2 liverworts) and 60 green algae have been sequenced [257]. Genome sequencing technologies provide a holistic overview of the various genetic components of an organism [258]. Whole-genome sequencing studies of plants have led to the identification of thousands of genes and other regulatory elements controlling the traits [259]. The integration of the low-cost sequencing technologies with computational bioinformatics tools and high throughput phenotyping technologies can enhance the identification of genes that govern important agronomic traits relevant to the production of food and its quality [260,261]. The results of multi-omics studies provide a holistic overview of the various genes, proteins, metabolomes and ionomes of the organisms. Therefore, the convergence of multi-omics technologies provides an important opportunity to accelerate the task of identification of genes that control agronomically relevant traits in plants, including traditional food plants, and speed up improvement programs using both conventional breeding as well as modern revolutionary CRISPR/Cas9-mediated and other gene editing technologies [28]. Figure 2 provides an overview of the application of omics and gene editing tools to the traditional food plants for their improvement.
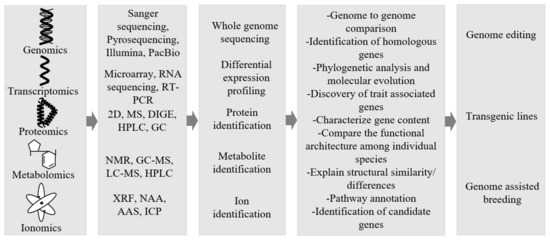
Figure 2.
Multi-omics approaches to improve traditional food plants. Candidate genes governing important traits can be identified by combining the data from genomics, transcriptomics, proteomics, metabolomics and ionomics. Manipulations of candidate genes by various techniques to generate improved varieties [31,245,261].
The extension of integrative omics tools including whole genome sequencing to decipher the genetic and molecular basis of nutritional and stress-related traits in TFPs is not only crucial but also urgently required [31,37,255,262,263,264,265,266,267]. Table 2 presents some TFPs where omics tools have been applied successfully for the comprehensive dissection of important traits. The following subsections explain some important plants where omics tools have helped to understand the genomic basis of important traits in TFPs.

Table 2.
Overview of use of omics tools to identify genes/proteins/ions regulating important traits in traditional food plants.
4. Examples of Application of Multi-Omics Tools to Traditional Food Plants
4.1. Lysine Biosynthesis in Amaranthus
One of the most important TFPs, also known as pseudocereal, is Amaranthus which belongs to the family Amaranthaceae. The genus Amaranthus comprises nearly 70 species [382,383]. A. caudatus, A. cruentus and A. hypochondriacus are three important species of Amaranthus that are traditionally consumed worldwide [384]. It is estimated that species of Amaranthus were domesticated nearly 8000 years ago in Central and South America and they sustained the Inca and the Aztec civilizations for several thousand years [385]. Unfortunately, the consumption of amaranth has reduced in modern times and only recently has there been an increased consumption of this species [386]. The growing interest in the consumption of amaranth has risen due to its unique nutritional composition [387]. Amaranthus is unique in its lysine content (5.19 g/16 g N) which has been found to be even more than that of milk [319]. This unique nutritional composition and resilience to a wide range of environmental conditions have led to its categorization as an important future, alternative wonder crop [388,389]. Amaranths are very important for another reason: they are C4 crops rather than most of the protein-yielding legume crops which are C3 plants [321]. Being C4, Amaranthus can perform better even at elevated temperatures when compared with the C3 species [321]. The nutritional and stress-resilient traits of amaranth have advantages which will definitely contribute to nutritional security. As the global temperature is rising, it is expected that such crops will provide more nutritional security to the growing population under elevated temperatures. Therefore, understanding the genetic basis of the nutritional and stress-resilient traits of Amaranthus is necessary. Lysine is important amino acid for human health, but unfortunately it is a limited in cereals, and this can be supplemented by consuming high-lysine-containing A. hypochondriacus. Sunil et al. [321] sequenced the genome and transcriptome of A. hypochondriacus and reported 24,829 protein-coding genes. This study further provided important details about the genes involved in the biosynthesis of betalains and lysine content [321]. The draft genomes of A. tuberculatus, A. hybridus and A. palmeri species were also reported recently by Montgomery et al. [390]. Taken together, these results will further enhance our genomic understanding of amaranths and trait manipulation.
4.2. Transcriptional Regulation of Anti-Nutritional Saponins in Chenopodium quinoa
Quinoa (Chenopodium quinoa) is an excellent nutritious grain that is designated as an important alternative future crop to improve global food security. Many genetic resources are not available for its improvement [391]. Jarvis et al. [392] reported the assembly of the reference genome sequence of quinoa. The genome sequencing has led to the identification of the transcription factor which may regulate the production of saponins, the anti-nutritional triterpenoids compounds synthesized in quinoa seeds. This is an important step towards establishing genetic resources for quinoa improvement [392]. Recently, Golicz et al. [393] performed genome-wide identification and analysis of orthologous genes of the Arabidopsis thaliana flowering genes in quinoa and provided important information about genes that controls vernalization, photoperiod, flowering and gibberellin biosynthesis pathway. The study further provided insights about the orphan genes that are unique to quinoa. This information is valuable as it will help to facilitate further programs aimed at quinoa improvement.
4.3. Genetic Mechanism of Stress Tolerance in Manihot esculenta
Cassava (Manihot esculenta) is a crop that is adapted to marginal soil conditions and erratic rainfall and is rich in carbohydrates and protein content [316]. Rabbi et al. [312] identified markers associated with the nutritional traits and have performed a genome-wide association mapping and identified candidate genes for carotenoid (phytoene synthase) and starch biosynthesis (UDP-glucose pyrophosphorylase and sucrose synthase) through this study. The transcriptomics study performed by Siirwat et al. [310] resulted in the identification of genes responsible for starch biosynthesis and revealed the mechanism behind the stress responses of cassava. Several transcriptomic studies on cassava have helped in unraveling the mechanisms of tolerance to various stresses. Utsumi et al. [314] reported upregulation of nearly 1300 genes during drought stress. The expression of Cu/Zn superoxide dismutase and catalase together during cold and drought stress improved drought and cold stress tolerance in cassava [394]. Lokko et al. [315] characterized heat stress transcription factors such as A3 (heat-shock transcription factor 21) and ATHB12 (a homeobox-leucine zipper protein) drought stress. In the same study, they reported expression of dehydration tolerance-related transcription factors such as Early response to dehydration (ERD1), Responsive to dehydration 19 (RD19) and Responsive to dehydration 22 precursor (RD22) at the time of drought stress [315]. An et al. [317] reported drought-induced Di19-like protein during drought stress with the aid of proteomic tools. Wang et al. [395] reported the draft genome sequences of a cassava wild ancestor and a domesticated variety of cassava. This study led to the identification of gene models specific to the wild and domesticated varieties.
4.4. Genetic Dissection of Pathogen Resistance and the Early Fruit Development and Evolution in Physalis
The genus Physalis (groundcherry) belongs to the Solanaceae family. Several members of the Solanaceae family are important sources of food, spice and medicine. Physalis ixocarpa, P. peruviana, P. pubescens are underutilized berries that have many essential minerals such as potassium and vitamins such as Vitamin C [396]. They are also well known for the phenolic compounds which provide excellent antioxidant activity [397]. Much information on Physalis is not available and it is necessary to broaden the information about its nutritional content and other properties [340]. Garzón-Martínez et al. [398] studied the leaf transcriptome of Physalis peruviana and identified genes responsible for major biological processes and molecular functions. This study provided candidate genes responsible for resistance against diseases caused by viruses, fungi and bacteria. Even though tomato and ground cherry are in the same family, Physalis possess modified calyx, which is absent in Solanum. Gao et al. [399] studied the floral transcriptome of Physalis for the first time and identified some candidate genes causing variations accounting for the early fruit development and evolution in Physalis floridana and compared with Solanum pimpinellifolium. They reported a total of 14,536 single-copy orthologous gene pairs between them. It was revealed that the distinction between Solanum and Physalis was because of nine types of genetic variations that were differentially expressed either in trend or dosage at the flower–fruit transition between the two.
4.5. Detection of Genes Regulating Uptake and Storage of Micronutrients in Traditional Food Plants
Plants are an important source of a large number of mineral ions. Minerals and trace elements in optimal levels are very important for the growth and development of a plant and such minerals are a very important part of the human diet [400]. Plants acquire elements from the soil, fertilizers and manures. Therefore, soil type and composition influence the nutrient composition of the plants [401], and large-scale cultivation/adoption of TFPs in other regions may result in the change in their nutrient composition. However, studies by Akinola et al. [44] suggested that TFPs can increase the soil fertility (e.g., traditional legume species through nitrogen fixation). The cultivation of diverse traditional food plants also increases the soil organic matter than uniform crop cultivation [44]. TFPs can also be cultivated with low input on the marginal lands. Uptake of micronutrients from the soil and further transport within the plants is facilitated by several transporter proteins [402]. There are several metals that are toxic to plants as well as humans when consumed in higher concentrations. For example, excessive accumulation of aluminum, lead, zinc and cadmium results in metal toxicity which can harm the plants, and at times may result in the death of the plants, as well [403,404]. Their entry into the food web is also problematic as it may lead to serious health issues for humans. Therefore, quantitative determination of the total composition of such minerals and metals in edible plants is important for ensuring the safety of humans [405]. The total mineral and element composition of an organism has been termed as an “ionome” [248,406]. Ionome profiling of plants belonging to different species, collected from various habitats and cultivated in different soils can inform us about the fundamental differences in the total ionome composition [407]. Minerals such as sulfur, nitrogen and phosphorus are essential components of several metabolites, whereas trace metals such as zinc, copper, iron and manganese are essential components of several proteins [408]. Therefore, minerals and trace metals also regulate the composition of metabolites and proteins within the plants and perform important biological functions [408]. High-throughput techniques such as inductively coupled plasma mass spectroscopy (ICP-MS), inductively coupled optical emission spectrometry, X-ray fluorescence, neutron activation and atomic absorption spectroscopy analysis are nowadays employed to profile complete ionomes of plants [406]. Genomic technologies have enabled the identification of a large number of transporter genes and even gene families from model plants that facilitate mineral and metal uptake and transport in the plants [409]. A large number of indigenous communities still rely on TFPs, and the mineral and metal composition of TFPs greatly influences their health and well-being [410]. For example, an analysis of mineral and heavy metal contents of traditionally important aquatic plants of Tripura, India, was carried out by [411] using atomic absorption spectroscopy. Several other new studies have recently tried to investigate the nutritional composition of TFPs, which will have huge implications on future crop improvement and breeding strategies. For example, nutrient and antinutrient composition analyses of Launaea cornuta, Vigna vexillata, Momordica foetida and Basella alba performed by Chacha et al. [412] showed that they are rich in vitamin A, B1, B2, B3 and C and minerals such as Ca, Fe, Mg and Zn. The rich sources of micronutrients in the underutilized crops promise their capacity to abolish hidden hunger in the future. Combining results of ionomics with genomics can help in the detection of genes responsible for the accumulation of mineral elements in plants [413]. For example, Pasha et al. [282] uncovered the molecular mechanism behind the nutritional quality of Moringa plant parts. They reported genes responsible for mineral content including, vacuolar iron transporters (VIT), calreticulin for calcium storage, zinc transporters and magnesium transporters inside different tissues. Similarly, several calcium transporters such as calcium ATPase, calcium exchanger (CaX), calcium-dependent protein kinase (CDPKs) and calcium-binding proteins (CBPs) of Eleusine coracana (L.) were identified by Nirgude et al. [269] and Kumar et al. [271] with the aid of high throughput genomics tools. The identification of the plants with higher amounts of essential minerals and their genes would further enhance our understanding of the TFPs.
4.6. Unraveling the Mechanism behind High Amount of α-Linolenic Acid and Salinity Tolerance in Portulaca oleracea
Purslane (Portulaca oleracea) belongs to the Portulacaceae family. It is a highly nutritious vegetable with several medicinal properties [414]. It has been recognized as the richest source of a-linolenic acid, essential omega-3 and 6 fatty acids, ascorbic acid, glutathione, alpha-tocopherol and beta-carotene [415]. Because of exceptional quantities of omega-3 fatty acids in purslane, there is a growing interest to introduce this as an important vegetable crop [415]. Purslane is also considered as a future powerful biosaline food crop that can grow under various environmental stresses such as salinity, nutrient deficiency, heat and drought [416]. Liu et al. [339] quantified the fatty acid and β-carotene content of purslane with the aid of HPLC and GC. They reported 1.5–2.5 mg/g of fatty acid from leaves, as well as 0.6–0.9 mg/g and 80–170 mg/g from stems and seeds, respectively. Its leaves contain about 60% of α-linolenic acid (C18:3ω3) of total fatty acids. The β-carotene content in its leaves was recorded between 22 to 30 mg/g fresh mass. The first metabolite profile of P. oleracea was performed by Farag and Shakour [338] by using ultra-performance liquid chromatography-mass spectrometry on three taxa and recognized hundreds of metabolites including amino acids, phenolic compounds, alkaloids and fatty acids which indicate their nutritive and health benefits. Besides having an extraordinary nutrient profile, Portulaca shows excellent tolerance towards salinity stress and drought stress. The transcriptome sequencing and metabolome analysis of P. oleracea regarding salinity tolerance were conducted by Xing et al. [417]. They reported that genes of photosynthesis and aquaporins were depressed during salinity treatment which indicates the inhibition of photosynthesis and water uptake during salinity stress. However, the expression of L-3-cyanoalanine synthase/cysteine synthase and cyanoalanine synthase were elevated. Higher content of pyrophosphate, D-galacturonic acid and elaidic acid was detected in salinity-tolerant plants that positively regulate glycolysis, energy supply and integrity of cell membrane. These studies regarding nutritional profiling and genes that regulate the tolerance to salinity are important for further improvement programs.
4.7. Higher Accumulation of Lycopene in Elaeagnus
Silverberry (Elaeagnus) belongs to the Elaeagnaceae family which is recognized as an important fruit crop used widely because of the presence of high lycopene content in the berries, which is ten times higher than tomatoes, especially in the species E. umbellata. [65]. The berries are well known for their high ascorbic acid, protein and magnesium content, as well as drought tolerance and adaptation to a variety of moisture and edaphic conditions [418]. The proteomic study of E. umbellata with special emphasis on fruit quality traits was performed and the quantity of soluble sugar, organic acids, lycopene and total protein content was analyzed [361]. The expression of the phytoene synthase (EutPSY) gene was found to be correlated with the higher accumulation of lycopene in E. umbellata, suggesting its importance [360]. The results suggest that the EutPSY gene could be considered as a target for increasing the lycopene content in other fruits and hence increase their quality.
4.8. Nutritional Composition of Dioscorea, a Neglected Staple Tuber Crop of the Indigenous Communities
Yam (Dioscorea) is one of the oldest tuber crops harvested from the wild in the tropical regions throughout the world and acts as a chief food item for a number of indigenous groups [332]. Yam is the main source of diosgenin-steroid which is effective against neurodegenerative diseases [419]. It is also an effective nutritional supplement with a high amount of protein. There are about 600 Dioscorea species, but only seven contribute to the human diet in the tropics [420]. Despite its wide utility, this tuber crop remains orphaned and its genomic and proteomic information is not available in detail [421]. Recently, little progress on genomic studies of Dioscorea have been reported. Nakayasu et al. [422] performed comparative transcriptome analysis of high-saponin-containing yams, i.e., D. esculenta and D. cayenensis, and low-saponin-containing D. alata for understanding biosynthesis of steroidal saponins and identified the β-glucosidase (DeF26G1) gene to be responsible for higher accumulation of saponins in D. esculenta. The first report of genome-wide characterization of Dioscorea taxon was reported in D. zingiberensis by Zhou et al. [423] where they identified 4935 genes, 81 tRNAs, 661 miRNAs and 69 rRNAs. Transcriptome profiling of D. alata led to the identification of several thousand unigenes, some of them code for enzymes involved in the flavonoid biosynthesis pathway. The study further found the upregulation of several genes such as flavanone 3-hydroxylase (F3H), chalcone isomerase (CHS), dihydroflavonol 4-reductase (DFR), leucoanthocyanidin dioxygenase (LDOX), flavonoid 3′-monooxygenase (F3′H) and flavonol 3-O-glucosyltransferase (UF3GT) in the tubers of purple flesh cultivar compared to white flesh cultivar [397]. Price et al. [332] performed whole metabolite profiling of yam and identified 152 metabolites. They developed biochemical phenotyping of accessions of the yam varieties through a large-scale metabolomic study. The integration with other omics studies can be used for yam breeding programs.
4.9. Transcriptional Basis of Lipid Biosynthesis in Salvia, a Wonder Seed for the 21st Century
Some species of the genus Salvia such as S. columbariae, S. hispanica and S. polystachya are commonly known as chia and are consumed for their seeds which have multiple nutritional and medicinal benefits [424,425]. Chia seeds are rich in insoluble fiber, high omega-3 and omega-6 fatty acids, α-linolenic acid, linoleic acid, proteins, amino acids, antioxidants and minerals [426,427]. Because of their high nutritive value, chia is known as the “seed for the first 21st century” [426]. The seeds of chia also contain metabolites that show anti-cancer, anti-inflammatory, antioxidant, anti-blood clotting and antidiabetic activities. The seeds have also been found to show action against cardiovascular diseases and hypertension [427,428,429,430]. The transcriptomic study of wild and cultivated accessions of S. hispanica suggests the genetic basis of oil and protein content accumulation in chia seeds [431]. The study has also identified several transcription factors such as AP2/EREBP202 and simple sequence repeat (SSRs) markers which would be helpful for breeding or in translational genomics programs. The transcription factor AP2/EREBP is known to regulate the expression of genes related to fatty acid biosynthesis [431]. Transcriptome analysis of chia seeds from its different developmental stages has further identified important candidate genes such as monoacylglycerol acyltransferase (MGAT), Acyl-CoA desaturase 1 (OLE1), diacylglycerol acyltransferase (DGAT1, 2 and 3), phospholipid:diacylglycerol acyltransferase (PDAT), Thiolase and Desaturase, responsible for lipid biosynthesis and oil accumulation [432].
4.10. The Adansonia digitata Contains More Vitamin C Than Oranges
The Adansonia digitata L. is commonly known as African baobab and belongs to the family Malvaceae. It is a very important tree with multiple benefits and is a source of traditional food in Saharan countries [433]. Additionally, it is also a source of medicine, fiber and income for rural communities [434,435]. Almost all its parts can be consumed and it contains high vitamin C content as compared to oranges [434]. Using microsatellite loci, Chládová et al. [435] suggested huge genetic diversity among its populations. However, further research is needed to understand the genetic basis of the higher accumulation of vitamin C and other important compounds that make it a wonder tree.
5. Integrating Omics and Gene Editing Tools for Improvement/Domestication of Traditional Food Plants
A lot of information is available on the genetic regulation of yield, nutritional quality and stress-related traits of several model domesticated crops [436,437,438]. The genetic and genomic analysis of many domesticated crops such as maize, tomato, rice, sorghum and wheat have led to the identification of several genes/QTLs that regulate domestication traits [436,437,439,440]. Some of the important domesticated crops, their relative traditional crops and the genes regulating domestication traits are shown in Table 3. The results of genomics and other omics research have provided fundamental clues about the genetic regulation of important traits [441]. The knowledge obtained using omics approaches can be used for crop improvement programs such as the development of nutritionally superior, disease-resistant and stress-tolerant crops with high yields [241]. The integration of genomics with gene editing tools is now possible, and allows editing of important genes with greater precision, accuracy and rapid pace [442]. Finding plants with desirable traits and having superior traits is an important step [443]. The plants with one or more of the desirable traits such as superior nutritional composition, high yields and biotic and abiotic stress tolerance should be given priority [444]. With the development of both genomics tools and bioinformatics pipelines, it is now easier to identify the genetic variation in wild species, which can be utilized for the transfer of traits to accelerate adaptive introgression in crops, as well as de novo domestication of wild relatives and landraces [83]. Since much genomic information is available on domesticated crops and other model plants, it is now possible to directly translate this information to the non-model TFPs for their rapid improvement by using various gene editing tools such as mega nucleases, Zinc Finger Nucleases (ZFNs), Transcriptional activator-like Effector Nucleases (TALENs) and Clustered Regularly Interspaced Short Palindromic Repeat-Associated Protein 9 (CRISPR-Cas9) [445,446,447,448]. Among the several gene editing tools, CRISPR-Cas9 has been one of the most important and popular gene editing tools and has attracted considerable attention from crop scientists [20,215,443,447,449,450]. The CRISPR-Cas9 editing has increased possibilities for genome modification and enables metabolic engineering, biofortification and crop improvement [443,444,449]. Several attempts for improving various traits such as yield and stress tolerance in several crops have been exercised using CRISPR/Cas [443].

Table 3.
Genes governing major domestication traits in crop plants related to traditional food plants [437].
The CRISPR-Cas9 mediated gene editing is based on the guidance of short RNA sequences termed as guide RNAs which are designed to complement target DNA [451]. The target DNA is cleaved by a Cas endonuclease that results in a single or double-strand breaks in the DNA [451], followed by ligation of the DNA by the endogenous repair mechanisms [452,453,454]. In case of gene editing of less-studied plants, for the identification of particular traits and related genes, homologous genes from extensively studied plants such as model plants are used. The genetic information from the domesticated species can be translated to the traditional food plants. (See next section for the example of translation of genetic information from Solanum lycopersicum and S. pimpinellifolium to Physalis pruinosa.) With the help of databases such as the National Center for Biotechnology Information (NCBI), identification of target genes for their construction of sgRNA by comparison with a homologous sequence is possible [455]. Software are used for the construction of plasmid that carries Cas9, gRNA and reporter genes along with their promoter [456]. Cas-Designer is good software for this purpose [456]. For delivering the construct Cas9-gRNA-Reporter, several methods such as agroinfiltration and electroporation can be used [457]. After delivery, induction of precise breaks at target sequences takes place at the target site. Endogenous machinery of cells repairs the breaks by non-homologous end joining (NHEJ) in the absence of a homologous repair template that results in insertions/deletions (indels) that disrupt/change/edit the target sequence or homology directed recombination (HDR) by providing a homologous repair template thereby inducing genomic mutations at the target locations [447,458]. For the validation of CRISPR/Cas9, editing the construct pCas9-gRNA-reporter is introduced into nodal explants after tissue culture using the Agrobacterium-mediated transformation method. After the regeneration of successful transformed plants, phenotypic and genotyping (using RT-PCR) and screening help to check the mutation effect [455]. A generalized workflow involving various steps in genome editing for improved varieties is presented in Figure 3.
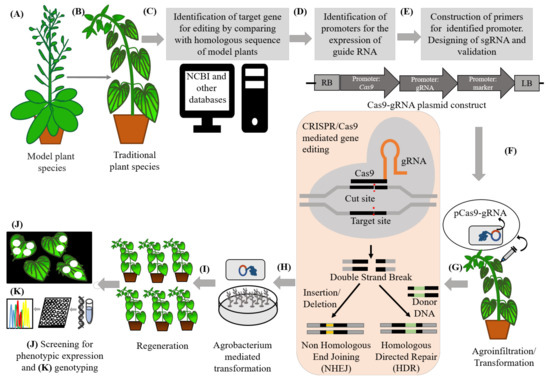
Figure 3.
General workflow of CRISPR/Cas9 based gene editing in neglected crops for their improvement. (A) Extensively studied model plant species chosen for the ease of identification of homologous genes governing particular traits. (B) Underutilized, orphan or neglected traditional plants with undesirable traits can be edited for trait improvement, and biotic and abiotic stress tolerance. (C) Identification of target gene(s) for construction of sgRNA by comparing with a homologous sequence of model plants using databases such as the National Center for Biotechnology Information. (D) Identification of promoters for the expression of guide RNA. (E) Construction of plasmid carrying Cas9, gRNA and reporter gene in the promoter region with software Cas-Designer. (F) Agroinfiltration on young leaves with Agrobacterium harboring the construct Cas9-gRNA-Reporter. (G) Induction of precise breaks at the target sequence site(s). Endogenous machinery of cells repairs the breaks by non-homologous end joining (NHEJ) in the absence of a homologous repair template resulting insertions/deletions (indels) that disrupt/change/edit the target sequence or homology directed recombination by providing a homologous repair template, thereby inducing genomic mutations at the target locations. Other than CRISPR/Cas9 zinc finger nucleases (ZNF), mega nucleases and transcription activator-like effector nucleases (TALEN) are also used for gene editing, but the feasibility of CRISPR/Cas9 is greater when compared with other methods. (H) Validation of the efficiency of CRISPR/Cas9 for targeted mutagenesis in stable transgenic plants. The construct pCas9-gRNA-reporter introduced into nodal-explants after tissue culture using the Agrobacterium-mediated transformation method. (I) Regeneration of stable transgenic plants. (J) Screening of the regenerated plants for the mutated effect by checking their phenotypes. (K) Genotyping or putative transgenic plants containing Cas9 confirmed by PCR analysis [447,455,458].
6. Recent Successful Examples of Gene Editing and Translational Genomics in Traditional Food Plants
TFPs with many beneficial traits are important for a sustainable food system. Physalis pruinosa (groundcherry) is a traditionally important plant consumed in various parts of the world for its important nutritional properties [340,511]. Huge inter- and intraspecific diversity of Physalis is available in the world, but it is not cultivated or consumed on a larger scale because of its certain undesirable traits such as extensive growth habit, smaller fruits and fruit dropping because of an abscission [446]. It is a relative of the tomato as both of them belong to the family Solanaceae and they share common genetic architecture with the same chromosome number of 12. Since both are from the family Solanaceae, and we know a lot about the genetic regulation of various traits in tomato, it is easy to translate genetic information from the model tomato to the non-model traditionally important crop, groundcherry for its improvement using gene editing tools [441,446]. Gene editing tools can be used to rid of undesirable traits from ground cherries. On these lines, a study was carried out by Lemmon et al. [446] and they obtained very successful gene-edited crops with improved characters in groundcherry. The undesirable characteristics of Physalis are similar to the wild ancestor of the tomato, S. pimpinellifolium, which underwent domestication in its traits leading to modern-day S. lycopersicum. Using gene editing, Lemmon et al. [446] targeted repressors of the florigen pathway to increase flower numbers and delimit flowering time, both on primary and axillary shoots. They performed a knockout of classical SELF PRUNING (SP) genes that control determinate and indeterminate growth habits of the plant. The results led to extreme compactness in P. pruinosa. Another knockout of the florigen repressor, SELF PRUNING 5G (SP5G), resulted in increased axillary flowering and fruit density. Targeting of the shoot apical meristem size-regulating gene CLAVATA resulted in increased flower meristem size, additional flower organs and conversion of small two-loculer fruit into larger three-loculer fruit, as illustrated in Figure 4 [446]. This study has opened up new hopes and possibilities for the rapid improvement and fast domestication of traditional orphan and wild crops. Many other groups around the globe are now focusing on editing the genes in non-model crops based on genetic and genomic information obtained from model crops [68,441]. The gene editing tools are particularly employed with an aim to increase quality, enhance yields, improve biotic and abiotic stress resistance and expand geographical ranges of cultivation of traditional orphan crops [446]. However, TFPs have not undergone intensive selection for domestication [512]. Thus, traditional orphan crops are less productive and unsuitable for cultivation at larger agricultural scales [52]. Similar studies can be undertaken and the information from omics studies can be combined with gene editing tools to other TFPs. A similar approach can be extended to wild edible species for de novo domestication [68]. The de novo domestication of wild plants is considered as an important solution for designing customized crops for the future [68]. By unleashing the multiplexing ability of CRISPR/Cas9 technology, multiple targets can be modified simultaneously in an efficient way by pyramiding multiple beneficial traits [452]. Taken together, the results of these studies suggest that the gene editing tools are a valuable tool to target homologs of domestication genes in traditional food plants quickly [20].
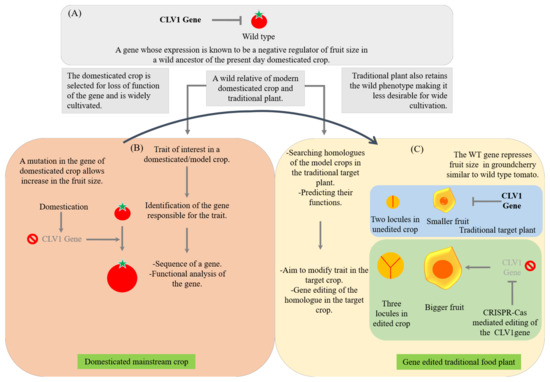
Figure 4.
Example of rapid improvement of a traditional orphan food crop for larger fruits. The genomics information obtained from the tomato (a domesticated crop) genome sequencing and the functional analysis of the genes is directly translated to the traditional crop, groundcherry (a traditional food plant) [446]. A wild type gene CLV1 which is a negative regulator of fruit size in the domesticated tomato (A), a mutation during domestication has occurred in this gene leading to the formation of a bigger fruit size of domesticated tomato (B) and its homologue in a traditional food plant, groundcherry is targeted for gene editing for its improvement for bigger fruits (C).
Gene editing has led to several revolutions in the field of crop improvement and it has been realized in several major crops and other plants such as tomato, maize, tobacco, grapevine, apple, opium poppy, cucumber and cotton for important traits and the results obtained are impressive [513,514,515,516,517]. Zsögön et al. [445] engineered S. pimpinellifolium (wild) using CRISPR/Cas9 and their several traits were altered that resulted in superior gene-edited S. pimpinellifolium than the S. lycopersicum. In 2014, CRISPR/Cas9 gene editing was successfully applied to tomato and citrus. Some successful cases of CRISPR/Cas9 fruit trait improvement are cucumber, apple, grape (2016), watermelon (2017), kiwifruit, banana, cacao, strawberry, papaya and groundcherry [449]. Other examples of successful gene editing using CRISPR/Cas9 include trait improvement of grain number, grain size, panicle architecture of rice [518,519], grain length, weight of wheat [520], seed oil composition (high oleic and low polyunsaturated fatty acids) of flax [521], late-flowering in soybean [522], reduced zein protein in maize [523]. Most of the successful works, however, are reported in major crops, and efforts are needed to improve and mainstream TFPs with the aid of genome editing tools and integrative genomics approaches. Examples of successful gene editing in crops to date are included in Table 4. Varshney et al. [524] explained the success story of translational genomics of the grain legume crops chickpea (Cicer arietinum), common bean (Phaseolus vulgaris), groundnut (Arachis hypogaea), pigeon pea (Cajanus cajan) and soybean (Glycine max) for their drought tolerance and pathogen resistance by multiple QTLs or genes from model legume Medicago truncatula. Ji et al. [525] attempted gene editing using CRISPR/Cas9 in Cowpea (Vigna unguiculata) which is also an important traditional food plant because of its symbiotic nitrogen fixation capability. Recently Syombua et al. [455] introduced a CRISPR/Cas9-based genome editing system for underutilized yam Dioscorea alata with improved genetic transformation, which can lead to trait improvement in yam. By the establishment of an efficient CRISPR/Cas9 editing protocol, Syombua et al. [455] suggested that it is possible to remove undesirable traits of Dioscorea alata such as poor seed set and non-synchronous flowering. Considering the importance of gene editing technology and its application in successfully editing genes of several crops for improved varieties and the beginning of editing traditional orphan crops, future studies aiming at the extension of this technology will lead to the mainstreaming of many TFPs. It will lead to diversification of the food basket of people across the globe, reducing excessive reliance on a select number of crops.

Table 4.
Examples of gene editing in major and few minor crops.
7. Challenges to Translational Genomics Using Gene Editing Technology/Tools
Although considerable progress has been achieved in the field of translational genomics particularly with the aid of gene editing tool CRISPR/Cas9 [552], there are also a number of important challenges. Several traits are quantitatively controlled and require multiple genes. Therefore, to produce desired phenotypes in the edited crops, we need to edit multiple genes [450]. Further, genomic information of many traditional food plants is not available. Another important challenge is that it is not easy to create precise modifications in DNA sequences. However, several gene editing strategies such as replicons, base editors and targeted nonhomologous insertions provide efficient precise gene editing in plants [457]. The unavailability of efficient delivery methods for gene editing reagents (DNA plasmid, mRNA (Cas9 + sgRNA), Ribonucleoprotein (RNP)) is another challenge [457]. Several other challenges such as ethical issues and technical bottlenecks are discussed elsewhere (see [450,552,553,554]).
8. Conclusions and Future Perspectives
Many TFPs have been a part of human civilizations since ancient times. Different parts of the plants are consumed by humans from generation to generation in different geographical areas of the world. They are unique as they possess various nutritional components and abiotic stress tolerance-related traits. Several studies have shown that some TFPs such as quinoa, millet, cassava and amaranth show tolerance to multiple abiotic stresses. The nutritional composition of many TFPs is also incredible, with a variety of health benefits and pharmacological values. Multi-omics tools have been applied to several TFPs for unraveling the basis of important traits. The availability of genome sequence information of relatives can be directly translated to many TFPs using several tools including CRISPR/Cas-mediated gene editing. Many TFPs are grown regionally and have regional importance. Therefore, they have undergone some level of domestication, and if they have to be domesticated and cultivated at a large scale, it is essential to get rid of undesirable traits that burden these crops. Since they are subjected to a certain level of domestication, tweaking a few genes using gene editing technologies will make them cultivable at a large scale, as evidenced by studies on groundcherry by Lemmon et al. [446]. The reintroduction of improved traditional crops into the current food systems will help diversify the food basket of the public, giving more options. Identification and mainstreaming of traditional food plants having higher nutritional and micro-nutritional values will help eradicate hidden hunger, which is prevalent due to the deficiency of the micronutrients in diets [51,555]. One of the issues linked to mainstreaming TFPs is their increased demand in food-secure regions due to scientific studies and increased popularity suggesting their health benefits, as well as increases in their prices. The increased prices could increase the income of the local farmers and the communities that rely on them. The increased demand for traditional foods also means increased opportunities in the entire supply chain from production, distribution and marketing to delivery for consumption. However, the increased prices may be beyond the purchasing capacity of the poor farmers and consumers in the producing regions. This increased popularity and increased demand has led to skyrocketing prices of quinoa in the Andes, Bolivia, and as a result, local farmers have resorted to non-traditional foods [556]. This situation has led to a situation where growers are in a dilemma of whether to have traditional foods or non-traditional foods. Another study by Enrico Avitabile in collaboration with the FAO suggests that increased prices of quinoa led to increased economic power of the local farmers in Bolivia [557]. He argues that although it led to overall reduction in the domestic consumption among the rural population, the increased incomes increased their economic freedom to access richer diets. Similar situations may also arise with similar TFPs if they become more popular and their increased demand elsewhere affects food and nutritional security in the regions where such traditional crops are produced. That will be an unhealthy situation and steps must be taken to ensure that the real producers of TFPs also consume healthy traditional foods for their own nutritional security, and not just remain producers.
Author Contributions
A.K.: conceptualization, supervision, investigation, writing—original draft preparation, review and editing, critical suggestions and improvement, visualization. T.A. and S.K.: writing—original draft preparation, visualization. S.S. (Sajana Sreedharan): writing—original draft preparation, review and editing, visualization. S.S.C.: writing—original draft preparation, review and editing, critical suggestions and improvement. S.R.C., S.S. (Sonam Singh) and Y.P.L.: writing—review and editing. N.R.: conceptualization, writing— original draft preparation, writing—review and editing, critical suggestions and improvement, visualization. All authors contributed significantly to this article. All authors have read and agreed to the published version of the manuscript.
Funding
Ministry of Agriculture, Food and Rural Affairs (MAFRA), Ministry of Oceans and Fisheries (MOF), Rural Development Administration (RDA) and Korea Forest Services (KFS), Republic of Korea for Golden Seed Project (213006-05-5-SB110).
Acknowledgments
Ajay Kumar acknowledges the Central University of Kerala for extending the support towards this study. Yong Pyo Lim acknowledges the Ministry of Agriculture, Food and Rural Affairs (MAFRA), Ministry of Oceans and Fisheries (MOF), Rural Development Administration (RDA) and Korea Forest Services (KFS), Republic of Korea for Golden Seed Project (213006-05-5-SB110).
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| AOCC | African Orphan Crops Consortium |
| Cas9 | CRISPR-Associated Protein 9 |
| CFF | Crops For the Future |
| CRISPR-Cas9 | Clustered Regularly Interspaced Short Palindromic Repeat-Associated Protein 9 |
| DGE | Differential Gene Expression |
| DNA | Deoxyribonucleic Acid |
| FAO | Food and Agricultural Organization |
| GC | Gas Chromatography |
| gRNA | Guide ribonucleic Acid |
| HDR | Homology Directed Recombination |
| HPLC | High Performance Liquid Chromatography |
| ICP-MS | Inductively Coupled Plasma Mass Spectroscopy |
| ICRAF | International Council for Research in Agroforestry |
| mRNA | Messenger Ribonucleic Acid |
| NCBI | National Center for Biotechnology Information |
| NHEJ | Non-Homologous End Joining |
| PEG | Poly Ethylene Glycol |
| sgRNA | Single Guide Ribonucleic Acid |
| RNA | Ribonucleic Acid |
| RT-PCR | Real-Time Polymerase Chain Reaction |
| QTLs | Quantitative Trait Locus |
| TALENs | Transcriptional Activator-Like Effector Nucleases |
| TFPs | Traditional Food Plants |
| Trex2 | Three prime Repair Exonuclease 2 |
| ZFNs | Zinc Finger Nucleases |
References
- FAO. Proceedings of the Expert Meeting on How to Feed the World in 2050; Food and Agriculture Organization: Rome, Italy, 2009. [Google Scholar]
- Godfray, H.C.J.; Beddington, J.R.; Crute, I.R.; Haddad, L.; Lawrence, D.; Muir, J.F.; Pretty, J.; Robinson, S.; Thomas, S.M.; Toulmin, C. Food Security: The Challenge of Feeding 9 Billion People. Science 2010, 327, 812–818. [Google Scholar] [CrossRef]
- Beddington, J.R.; Asaduzzaman, M.; Fernández, A.; Clark, M.E.; Guillou, M.; Jahn, M.M.; Erda, L.; Mamo, T.; Van, B.N.; Nobre, C.A.; et al. Achieving Food Security in the Face of Climate Change: Summary for Policy Makers from the Commission on Sustainable Agriculture and Climate Change; CGIAR Research Program on Climate Change, Agriculture and Food Security: Wageningen, The Netherlands, 2011. [Google Scholar]
- Dhyani, A. Plants of the World—Diverse, Fascinating and Threatened; Science Reporter, NISCAIR-CSIR India: Delhi, India, 2020; Volume 57, p. 3. Available online: http://nopr.niscair.res.in/handle/123456789/54100 (accessed on 7 April 2021).
- Willis, K.J. State of the World’s Plants. Available online: https://stateoftheworldsplants.org/ (accessed on 7 June 2021).
- Meyer, R.S.; DuVal, A.E.; Jensen, H.R. Patterns and Processes in Crop Domestication: An Historical Review and Quantitative Analysis of 203 Global Food Crops. New Phytol. 2012, 196, 29–48. [Google Scholar] [CrossRef]
- Ross-Ibarra, J.; Morrell, P.L.; Gaut, B.S. Plant Domestication, a Unique Opportunity to Identify the Genetic Basis of Adaptation. Proc. Natl. Acad. Sci. USA 2007, 104, 8641–8648. [Google Scholar] [CrossRef]
- Pimentel, D.; Jackson, W.; Bender, M.; Pickett, W. Perennial Grains—An Ecology of New Crops. Interdiscip. Sci. Rev. 1986, 11, 42–49. [Google Scholar] [CrossRef]
- Hawtin, G.; Collins, W. Conserving and Using Crop Plant Biodiversity in Agroecosystems. In Biodiversity in Agroecosystems; Collin, W.W., Quaslet, C.O., Eds.; CRC Press: Boca Raton, FL, USA, 1998; pp. 267–281. [Google Scholar]
- Hambrey, J. The 2030 Agenda and the Sustainable Development Goals: The Challenge for Aquaculture Development and Management, FAO Fisheries and Aquaculture Circular. Available online: http://www.fao.org/policy-support/tools-and-publications/resources-details/en/c/1153661/ (accessed on 24 June 2020).
- Thrupp, L. Linking Agricultural Biodiversity and Food Security: The Valuable Role of Agrobiodiversity for Sustainable Agriculture. Int. Aff. 2000, 76, 283–297. [Google Scholar] [CrossRef]
- Zhao, C.; Liu, B.; Piao, S.; Wang, X.; Lobell, D.; Huang, Y.; Huang, M.; Yao, Y.; Bassu, S.; Ciais, P.; et al. Temperature Increase Reduces Global Yields of Major Crops in Four Independent Estimates. Proc. Natl. Acad. Sci. USA 2017, 114, 9326–9331. [Google Scholar] [CrossRef] [PubMed]
- Tigchelaar, M.; Battisti, D.; Naylor, R.; Ray, D. Future Warming Increases Probability of Globally Synchronized Maize Production Shocks. Proc. Natl. Acad. Sci. USA 2018, 115, 6644–6649. [Google Scholar] [CrossRef]
- Byg, A.; Salick, J. Local Perspectives on a Global Phenomenon—Climate Change in Eastern Tibetan Villages. Tradit. Peoples Clim. Change 2009, 19, 156–166. [Google Scholar] [CrossRef]
- Kotir, J. Climate Change and Variability in Sub-Saharan Africa: A Review of Current and Future Trends and Impacts on Agriculture and Food Security. Environ. Dev. Sustain. 2011, 13, 587–605. [Google Scholar] [CrossRef]
- Speranza, C.I. Resilient Adaptation to Climate Change in African Agriculture, 54th ed.; German Development Institute: Bonn, Germany, 2010. [Google Scholar]
- Padulosi, S.; Bhag, M.; Bala, R.S.; Shanthakumar, G.; Yenagi, N.; Dutta, M. Food Security and Climate Change: Role of Plant Genetic Resources of Minor Millets. Indian J. Plant Genet. Resour. 2009, 22, 1–16. [Google Scholar]
- Hughes, J. Just Famine Foods? What Contributions Can Underutilized Plants Make to Food Security? Acta Hortic. 2009, 806, 39–48. [Google Scholar] [CrossRef]
- Jarvis, D.I.; Hodgkin, T.; Sthapit, B.R.; Fadda, C.; Lopez-Noriega, I. An Heuristic Framework for Identifying Multiple Ways of Supporting the Conservation and Use of Traditional Crop Varieties within the Agricultural Production System. Crit. Rev. Plant Sci. 2011, 30, 125–176. [Google Scholar] [CrossRef]
- Wolter, F.; Schindele, P.; Puchta, H. Plant Breeding at the Speed of Light: The Power of CRISPR/Cas to Generate Directed Genetic Diversity at Multiple Sites. BMC Plant Biol. 2019, 19, 176. [Google Scholar] [CrossRef]
- Maundu, P.M. The Status of Traditional Vegetable Utilization in Kenya. In Proceedings of the IPGRI International Workshop on Genetic Resources of Traditional Vegetables in Africa: Conservation and Use 29–31 August 1995, Guarino, Nairobi, Kenya. Institute of Plant Genetics and Crop Plant Research, Gatersleben/International Plant Genetic Resources Institute, Rome, Italy, 1998, L. Ed.: ICRAF-HQ; Volume 16, pp. 66–75.
- Muthoni, J.; Nyamongo, D. Traditional Food Crops and Their Role in Food and Nutritional Security in Kenya. J. Agric. Food Inf. 2010, 11, 36–50. [Google Scholar] [CrossRef]
- Adhikari, L.; Hussain, A.; Rasul, G. Tapping the Potential of Neglected and Underutilized Food Crops for Sustainable Nutrition Security in the Mountains of Pakistan and Nepal. Sustainability 2017, 9, 291. [Google Scholar] [CrossRef]
- Longin, C.F.H.; Würschum, T. Back to the Future—Tapping into Ancient Grains for Food Diversity. Trends Plant Sci. 2016, 21, 731–737. [Google Scholar] [CrossRef] [PubMed]
- Dwivedi, S.L.; Lammerts van Bueren, E.T.; Ceccarelli, S.; Grando, S.; Upadhyaya, H.D.; Ortiz, R. Diversifying Food Systems in the Pursuit of Sustainable Food Production and Healthy Diets. Trends Plant Sci. 2017, 22, 842–856. [Google Scholar] [CrossRef] [PubMed]
- Muthamilarasan, M.; Singh, N.; Prasad, M. Multi-omics Approaches for Strategic Improvement of Stress Tolerance in Underutilized Crop Species: A Climate Change Perspective. Adv. Genet. 2019, 103, 1–38. [Google Scholar] [CrossRef]
- Adhikari, L.; Tuladhar, S.; Hussain, A.; Aryal, K. Are Traditional Food Crops Really ‘Future Smart Foods? ’ A Sustainability Perspective. Sustainability 2019, 11, 5236. [Google Scholar] [CrossRef]
- Banerjee, R.; Kumar, G.V.; Kumar, S.P.J. OMICS–Based Approaches in Plant Biotechnology; John Wiley & Sons: Hoboken, NJ, USA, 2019. [Google Scholar]
- Esquinas-Alcázar, J. Science and Society: Protecting Crop Genetic Diversity for Food Security: Political, Ethical and Technical Challenges. Nat. Rev. Genet. 2005, 6, 946–953. [Google Scholar] [CrossRef] [PubMed]
- Milner, S.; Jost, M.; Taketa, S.; Mazón, E.; Himmelbach, A.; Oppermann, M.; Weise, S.; Knüpffer, H.; Basterrechea Salido, M.; König, P.; et al. Genebank Genomics Highlights the Diversity of a Global Barley Collection. Nat. Genet. 2019, 51, 319–326. [Google Scholar] [CrossRef]
- Langridge, P.; Waugh, R. Harnessing the Potential of Germplasm Collections. Nat. Genet. 2019, 51, 200–201. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Zavaglia, A.; Mejuto, J.C.; Simal-Gandara, J. Mitigation of Emerging Implications of Climate Change on Food Production Systems. Food Res. Int. 2020, 134, 1–12. [Google Scholar] [CrossRef]
- Gráda, C.Ó. Black ’47 and Beyond; The Great Irish Famine in History, Economy, and Memory, Ed.; Princeton University Press: Princeton, NJ, USA, 1999. [Google Scholar]
- Bruns, H. Southern Corn Leaf Blight: A Story Worth Retelling. Agron. J. 2017, 109, 1218–1224. [Google Scholar] [CrossRef]
- Risch, S.; Andow, D.; Altieri, M. Agroecosystem Diversity and Pest Control: Data, Tentative Conclusions, and New Research Directions. Environ. Entomol. 1983, 12, 625–629. [Google Scholar] [CrossRef]
- Altieri, M. Altieri, M. A Monocultures and their impacts on biodiversity. In Red Sugar, Green Deserts: Latin American Report on Monocultures and Violations of the Human Rights to Adequate Food and Housing, to Water, to Land and to Territory; FIAN International: Heidelberg, Germany, 2009; pp. 67–76. [Google Scholar]
- Turner, M.; Calder, W.; Cumming, G.; Hughes, T.; Jentsch, A.; LaDeau, S.; Lenton, T.; Shuman, B.; Turetsky, M.; Ratajczak, Z.; et al. Climate Change, Ecosystems and Abrupt Change: Science Priorities. Philos. Trans. R. Soc. B Biol. Sci. 2020, 375, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Tomiyama, J.-M.; Takagi, D.; Kantar, M. The Effect of Acute and Chronic Food Shortage on Human Population Equilibrium in a Subsistence Setting. Agric. Food Secur. 2020, 9, 1–12. [Google Scholar] [CrossRef]
- Ciaccia, C.; Testani, E.; Roccuzzo, G.; Stefano, C. The Role of Agrobiodiversity in Sustainable Food Systems Design and Management. In Genetic Diversity in Horticultural Plants. Sustainable Development and Biodiversity; Springer: Berlin/Heidelberg, Germany, 2019; Volume 22, pp. 245–271. [Google Scholar]
- Chaudhary, P.; Bhatta, S.; Aryal, K.; Joshi, B.; Gauchan, D. Threats, Drivers and Conservation Imperative of Agrobiodiversity. J. Agric. Environ. 2020, 21, 44–61. [Google Scholar]
- Choi, H.-K. Translational Genomics and Multi-Omics Integrated Approaches as a Useful Strategy for Crop Breeding. Genes Genom. 2019, 41, 133–146. [Google Scholar] [CrossRef]
- El Bilali, H.; Callenius, C.; Strassner, C.; Probst, L. Food and Nutrition Security and Sustainability Transitions in Food Systems. Food Energy Secur. 2018, 8, 1–20. [Google Scholar] [CrossRef]
- Negi, G.C.S.; Samal, P.; Kuniyal, J.C.; Sharma, R.; Dhyani, P.P. Impacts of Climate Change on Western Himalayan Mountain Ecosystems: An Overview. Trop. Ecol. 2012, 53, 345–356. [Google Scholar]
- Akinola, R.; Pereira, L.M.; Mabhaudhi, T.; de Bruin, F.-M.; Rusch, L. A Review of Indigenous Food Crops in Africa and the Implications for More Sustainable and Healthy Food Systems. Sustainability 2020, 12, 3493. [Google Scholar] [CrossRef]
- Gregory, P.; Mayes, S.; Chai, H.H.; Jahanshiri, E.; Julkifle, A.; Kuppusamy, G.; Kuan, H.; Lin, T.; Massawe, F.; Syaheerah, T.; et al. Crops For the Future (CFF): An Overview of Research Efforts in the Adoption of Underutilised Species. Planta 2019, 250, 1–10. [Google Scholar] [CrossRef]
- Hanafiah, N.M.; Mispan, M.S.; Lim, P.E.; Baisakh, N.; Cheng, A. The 21st Century Agriculture: When Rice Research Draws Attention to Climate Variability and How Weedy Rice and Underutilized Grains Come in Handy. Plants 2020, 9, 365. [Google Scholar] [CrossRef] [PubMed]
- Agulanna, F.T. The Role of Indigenous and Underutilized Crops in The Enhancement of Health and Food Security in Nigeria. Afr. J. Biomed. Res. 2020, 23, 305–312. [Google Scholar]
- Borelli, T.; Hunter, D.; Padulosi, S.; Amaya, N.; Meldrum, G.; de Oliveira Beltrame, D.M.; Samarasinghe, G.; Wasike, V.W.; Güner, B.; Tan, A.; et al. Local Solutions for Sustainable Food Systems: The Contribution of Orphan Crops and Wild Edible Species. Agronomy 2020, 10, 231. [Google Scholar] [CrossRef]
- Conti, M.V.; Campanaro, A.; Coccetti, P.; De Giuseppe, R.; Galimberti, A.; Labra, M.; Cena, H. Potential Role of Neglected and Underutilized Plant Species in Improving Women’s Empowerment and Nutrition in Areas of Sub-Saharan Africa. Nutr. Rev. 2019, 77, 817–828. [Google Scholar] [CrossRef]
- FAO and the 17 Sustainable Development Goals. Sustainable Development Knowledge Platform. Available online: https://sustainabledevelopment.un.org/index.php?page=view&type=400&nr=2205&menu=1515 (accessed on 7 June 2021).
- Dawson, I.K.; Hendre, P.; Powell, W.; Sila, D.; McMullin, S.; Simons, T.; Revoredo-Giha, C.; Odeny, D.A.; Barnes, A.P.; Graudal, L.; Working Paper, No.; et al. 276 ed.; World Agroforestry United Nations; World Agroforestry Centre: Niarobi, Kenya, 2018. [Google Scholar] [CrossRef]
- Jamnadass, R.; Mumm, R.H.; Hale, I.; Hendre, P.; Muchugi, A.; Dawson, I.K.; Powell, W.; Graudal, L.; Yana-Shapiro, H.; Simons, A.J.; et al. Enhancing African Orphan Crops with Genomics. Nat. Genet. 2020, 52, 356–360. [Google Scholar] [CrossRef] [PubMed]
- Ramdwar, M.; Siew, N. Strategic Approaches to Food Security in Developing Countries. In Agricultural Development and Food Security in Developing Nations; Ganpat, W.G., Ed.; IGI Global: Hershey, PA, USA, 2017; pp. 197–221. [Google Scholar]
- Tadele, Z. Orphan Crops: Their Importance and the Urgency of Improvement. Planta 2019, 250, 677–694. [Google Scholar] [CrossRef]
- Hendre, P.; Muchugi, A.; Chang, Y.; Fu, Y.; Song, Y.; Liu, M.; Liao, X.; Liu, H.; Song, B.; Xu, X.; et al. Generation of Open-Source Genomics Resources for African Orphan Tree Crops by African Orphan Crops Consortium (AOCC), a Public-Private Partnership for Promoting Food and Nutritional Security in Africa. Acta Hortic. 2020, 615–622. [Google Scholar] [CrossRef]
- Yssel, A.; Kao, S.-M.; Peer, V. Sterck ORCAE-AOCC: A Centralized Portal for the Annotation of African Orphan Crop Genomes. Genes 2019, 10, 950. [Google Scholar] [CrossRef]
- Department for International Development. Crops for the Future Strategic Plan 2009–2013. Available online: https://www.gov.uk/research-for-development-outputs/crops-for-the-future-strategic-plan-2009-2013 (accessed on 7 June 2021).
- FAO. Promoting Neglected and Underutilized Crop Species. Available online: http://www.fao.org/news/story/en/item/1032516/icode/ (accessed on 7 June 2021).
- Maundu, M.P.; Ngugi, W.G.; Kabuye, H.S.C. Traditional Food Plants of Kenya; National Museums of Kenya: Nairobi, Kenya, 1999. [Google Scholar]
- Campbell, J. Development, Global Change and Traditional Food Security in Pacific Island Countries. Reg. Environ. Change 2014, 15, 1–12. [Google Scholar] [CrossRef]
- Shelef, O.; Weisberg, P.; Provenza, F. The Value of Native Plants and Local Production in an Era of Global Agriculture in: Mirás-Avalos & Baveye 2018 Agroecosystems Facing Global Climate Change The Search for Sustainability. Front. Plant Sci. 2019, 8, 2069. [Google Scholar] [CrossRef]
- Rajapaksha, U. Traditional Food Plants in Sri Lanka; Hector Kobbekaduwa Agrarian Research and Training Institute: Colombo, Sri Lanka, 1998. [Google Scholar]
- Kristbergsson, K.; Oliveira, J. Traditional foods: General and consumer aspects. In Integrating Food Science and Engineering Knowledge into the Food Chain; Kristbergsson, K., Ed.; Springer: New York, NY, USA, 2016; pp. 85–86. [Google Scholar]
- Molina, M.; Tardío, J.; Aceituno-Mata, L.; Morales, R.; Reyes-García, V.; Pardo-de-Santayana, M. Weeds and Food Diversity: Natural Yield Assessment and Future Alternatives for Traditionally Consumed Wild Vegetables. J. Ethnobiol. 2014, 34, 44–67. [Google Scholar] [CrossRef]
- Gamba, G.; Donno, D.; Mellano, M.G.; Riondato, I.; De Biaggi, M.; Randriamampionona, D.; Beccaro, G.L. Phytochemical Characterization and Bioactivity Evaluation of Autumn Olive (Elaeagnus umbellata Thunb.) Pseudo drupes as Potential Sources of Health-Promoting Compounds. Appl. Sci. 2020, 10, 4354. [Google Scholar] [CrossRef]
- Kozioł, M.J. Chemical Composition and Nutritional Evaluation of Quinoa (Chenopodium quinoa Willd.). J. Food Compos. Anal. 1992, 5, 35–68. [Google Scholar] [CrossRef]
- Mwanri, W.A.; Mamboleo, F.T.; Msuya, M.J.; Gowele, F.V. Oxalate, Phytate and Nitrate Content in African Nightshade, Spider Plant and Amaranths at Different Stages of Maturity. Afr. J. Food Sci. 2018, 12, 316–322. [Google Scholar] [CrossRef]
- Sedbrook, J.C.; Phippen, W.B.; Marks, M.D. New Approaches to Facilitate Rapid Domestication of a Wild Plant to an Oilseed Crop: Example Pennycress (Thlaspi arvense L.). Plant Sci. Int. J. Exp. Plant Biol. 2014, 227, 122–132. [Google Scholar] [CrossRef] [PubMed]
- Mueller, N.; Fritz, G.; Patton, P.; Carmody, S.; Horton, E. Growing the Lost Crops of Eastern North America’s Original Agricultural System. Nat. Plants 2017, 3, 1–5. [Google Scholar] [CrossRef]
- Peng, W.; Berry, E. The Concept of Food Security. In Encyclopedia of Food Security and Sustainability, 1st ed.; Ferranti, P., Berry, E., Jock, A., Eds.; Elsevier Science & Technology: Amsterdam, The Netherlands, 2018; pp. 1–7. [Google Scholar]
- Schmidhuber, J.; Tubiello, F.N. Global Food Security under Climate Change. Proc. Natl. Acad. Sci. USA 2007, 104, 19703–19708. [Google Scholar] [CrossRef]
- Van Berkum, S.; Ruben, R. The Food System Approach: Sustainable Solutions for a Sufficient Supply of Healthy Food; Wageningen Economic Research: Memorandum 2018-064; Wageningen University & Research: Wageningen, The Netherlands, 2018. [Google Scholar]
- Ashby, S.; Kleve, S.; McKechnie, R.; Palermo, C. Measurement of the Dimensions of Food Insecurity in Developed Countries: A Systematic Literature Review. Public Health Nutr. 2016, 19, 2887–2896. [Google Scholar] [CrossRef] [PubMed]
- Abdulkadyrova, M.A.; Dikinov, A.H.; Tajmashanov, H.È.; Shidaev, L.A.; Shidaeva, E.A. Global Food Security Problems in the Modern World Economy. Int. J. Environ. Sci. Educ. 2016, 11, 5320–5330. [Google Scholar]
- Dresselhaus, T.; Hückelhoven, R. Biotic and Abiotic Stress Responses in Crop Plants. Agronomy 2018, 8, 267. [Google Scholar] [CrossRef]
- Porter, J.; Semenov, M. Crop Response to Climatic Variation. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 2005, 360, 2021–2035. [Google Scholar] [CrossRef] [PubMed]
- Dhankher, O.P.; Foyer, C.H. Climate Resilient Crops for Improving Global Food Security and Safety. Plant Cell Environ. 2018, 41, 877–884. [Google Scholar] [CrossRef]
- Scholes, R.; Biggs, R. Ecosystem Services in Southern Africa: A Regional Assessment; Council for Scientific and Industrial Research: Pretoria, South Africa, 2004. [Google Scholar]
- Chen, Y.; Michalak, M.; Agellon, L.B. Importance of Nutrients and Nutrient Metabolism on Human Health. Yale J. Biol. Med. 2018, 91, 95–103. [Google Scholar] [PubMed]
- Houghton, J.E.T.; Ding, Y.; Griggs, D.; Noguer, M.; van der Linden, P.; Dai, X.; Maskell, M.; Johnson, C. Climate Change 2001: The Scientific Basis. In Climate Change 2001: The Scientific Basis. Contribution of Working Group I to the Third Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2001; Volume 881. [Google Scholar]
- Ebert, A.W. Potential of Underutilized Traditional Vegetables and Legume Crops to Contribute to Food and Nutritional Security, Income and More Sustainable Production Systems. Sustainability 2014, 6, 319–335. [Google Scholar] [CrossRef]
- Berkelaar, D.; The Importance of Indegenous Food Plants. Echo Community. Available online: https://www.echocommunity.org/en/resources/a118dadf-50d6-492c-a19f-948a23c93e83 (accessed on 12 October 2020).
- Zhang, F.; Batley, J. Exploring the Application of Wild Species for Crop Improvement in a Changing Climate. Curr. Opin. Plant Biol. 2020, 56, 218–222. [Google Scholar] [CrossRef]
- Schnyder, H.; Seo, S.; Rademacher, I.F.; Kühbauch, W. Spatial Distribution of Growth Rates and of Epidermal Cell Lengths in the Elongation Zone during Leaf Development in Lolium perenne L. Planta 1990, 181, 423–431. [Google Scholar] [CrossRef]
- Mishra, S.; Mohanara, S.K. Dash. Review on Cleome gynandra. Int. J. Res. Pharm. Chem. 2011, 1, 681–689. [Google Scholar]
- Rao, A.P.; Rajendrudu, G. Net Photosynthetic Rate in Relation to Leaf Anatomical Characteristics of C3, C3-C4 and C4 Dicotyledons. Proc. Indian Acad. Sci. Plant Sci. 1989, 99, 529–538. [Google Scholar]
- Kumar, U.D.J.; Saraswathy, R.; Rama Das, V.S. Differential Performance of Cleome gynandra L. (C4) and Cleome speciosa L. (C3) under Water Stress and Recovery. Environ. Exp. Bot. 1984, 24, 305–310. [Google Scholar] [CrossRef]
- Bamidele, O.; Akinnuga, A.M.; Olorunfemi, J.O.; Tony, O.A.; Oparaji, C.K.; Ezeigbo, N. Effects of Aqueous Extract of Basella alba Leaves on Haematological and Biochemical Parameters in Albinorats. Afr. J. Biotechnol. 2010, 9, 6952–6955. [Google Scholar] [CrossRef]
- Adhikari, R.; Kumar, H.N.N; Shruthi, S.D. A Review on Medicinal Importance of Basella alba L. Int. J. Pharm. Sci. Drug Res. 2012, 4, 110–114. [Google Scholar]
- Deshmukh, S.; Gaikwad, D. A Review of the Taxonomy, Ethnobotany, Phytochemistry and Pharmacology of Basella alba (Basellaceae). J. Appl. Pharm. Sci. 2014, 4, 153–165. [Google Scholar] [CrossRef]
- Murevanhema, Y.Y.; Jideani, V.A. Potential of Bambara Groundnut (Vigna subterranea (L.) Verdc) Milk as a Probiotic Beverage-a Review. Crit. Rev. Food Sci. Nutr. 2013, 53, 954–967. [Google Scholar] [CrossRef] [PubMed]
- Adebowale, K.O.; Adeniyi Afolabi, T.; Lawal, O.S. Isolation, Chemical Modification and Physicochemical Characterisation of Bambarra Groundnut (Voandzeia subterranean) Starch and Flour. Food Chem. 2002, 78, 305–311. [Google Scholar] [CrossRef]
- Omoikhoje, S.O. Assessment of the Nutritive Value of Bambara Groundnut as Influenced by Cooking Time. Livest. Res. Rural Dev. 2008, 20, 55–60. [Google Scholar]
- Aberoumand, A.; Deokule, S.S. Chemical Analysis and Nutritional Value of Chlorophytum comosum: A Plant Food in Iran. J. Med. Food Plants 2009, 1, 87–91. [Google Scholar]
- Schippers, R.R. African Indigenous Vegetables: An Overview of the Cultivated Species; Natural Resources Institute/ACP-EU Technical Centre for Agricultural and Rural Cooperation: Chatham, UK, 2002. [Google Scholar]
- Lee, C.-F.; Fan, C.-W.; Chiang, N.-N.; Chang, H.-C.; Chen, C.; Huang, Y.-S.; Wang, H.-Y.; Lin, W.-C.; Chen, F.-A. Protective Effect of Corchorus capsularis L. Leaves on Ethanol-Induced Acute Gastric Mucosal Lesion in Rats. J. Vet. Med. Sci. 2019, 81, 1636–1642. [Google Scholar] [CrossRef]
- Bhartiya, A.; Aditya, J.; Kant, L. Nutritional and Remedial Potential of an Underutilized Food Legume Horsegram (Macrotyloma uniflorum): A Review. J. Anim. Plant Sci. 2015, 25, 908–920. [Google Scholar]
- Campbell, C.G.; Heller, J.; Engels, J. Buckwheat. Fagopyrum Esculentum Moench, 19th ed.; International Plant Genetic Resources Institute: Rome, Italy, 1997. [Google Scholar]
- Cartea, M.E.; Francisco, M.; Soengas, P.; Velasco, P. Phenolic Compounds in Brassica Vegetables. Molecules 2011, 16, 251–280. [Google Scholar] [CrossRef] [PubMed]
- Grubben, G.J.H.; Denton, O.A. Plant Resources of Tropical Africa; Netherlands/Backhuys Publishers: Wageningen, The Netherlands, 2004. [Google Scholar]
- Temesgen, M.; Retta, N. Nutritional Potential, Health and Food Security Benefits of Taro Colocasia esculenta (L.): A Review. Open Food Sci. J. 2015, 36, 23–30.
- Kaushal, P.; Kumar, V.; Sharma, H. Utilization of Taro (Colocasia esculenta): A Review. J. Food Sci. Technol. 2013, 52, 27–40. [Google Scholar] [CrossRef]
- Khalafalla, M.M.; Daffalla, H.M.; Abdellatef, E.; Agabna, E.; El-Shemy, H.A. Establishment of an in Vitro Micropropagation Protocol for Boscia senegalensis (Pers.) Lam. Ex Poir. J. Zhejiang Univ. Sci. 2011; 12. [Google Scholar] [CrossRef]
- Kim, T.R.; Pastuszyn, A.; Vanderjagt, D.J.; Glew, R.S.; Millson, M.; Glew, R.H. The Nutritional Composition of Seeds From Boscia senegalensis (Dilo) from the Republic of Niger. J. Food Compos. Anal. 1997, 10, 73–81. [Google Scholar] [CrossRef]
- FAO Food and Nutrition Paper (FAO). Traditional Food Plants. A Resource Book for Promoting the Exploitation and Consumption of Food Plants in Arid, Semi-Arid and Sub-Humid Lands of Eastern Africa; FAO: Rome, Italy, 1998. [Google Scholar]
- Adewale, D.; Odoh, N. A Review on Genetic Resources, Diversity and Agronomy of African Yam Bean (Sphenostylis stenocarpa (Hochst. Ex A. Rich.) Harms): A Potential Future Food Crop. Sustain. Agric. Res. 2012; 2. [Google Scholar] [CrossRef]
- Adegboyega, T.T.; Abberton, M.T.; AbdelGadir, A.H.; Dianda, M.; Maziya-Dixon, B.; Oyatomi, O.A.; Ofodile, S.; Babalola, O.O. Evaluation of Nutritional and Antinutritional Properties of African Yam Bean (Sphenostylis stenocarpa (Hochst Ex. A. Rich.) Harms.) Seeds. J. Food Qual. 2020; 11. [Google Scholar] [CrossRef]
- Okoli, B.E.; Mgbeogu, C.M. Fluted Pumpkin, Telfairia occidentalis: West African Vegetable Crop. Econ. Bot. 1983, 37, 145–149. [Google Scholar] [CrossRef]
- Glew, R.H.; Laabes, E.P.; Presley, J.M.; Schulze, J.; Andrews, R.; Wang, Y.-C.; Chang, Y.-C.; Chuang, L.-T. Fatty Acid, Amino Acid, Mineral and Antioxidant Contents of Acha (Digitaria exilis) Grown on the Jos Plateau, Nigeria. Int. J. Nutr. Metab. 2013, 5, 1–8. [Google Scholar] [CrossRef]
- Istifanus, M.F.; Agbo, E.B. Nutritional and Health Benefits of Acha (Digitaria exilis) in the Human Diet—A Review. Niger. Food J. 2016, 34, 72–78. [Google Scholar] [CrossRef]
- Jideani, I.A. Traditional and Possible Technological Uses of Digitaria exilis (Acha) and Digitaria iburua (Iburu): A Review. Plant Foods Hum. Nutr. Dordr. Neth. 1999, 54, 363–374. [Google Scholar] [CrossRef] [PubMed]
- Abukutsa-Onyango, M. Response of Slenderleaf (Crotalaria brevidens Benth) to Inorganic Nitrogen Application. Afr. J. Food Agric. Nutr. Dev. 2007, 7, 1–10. [Google Scholar]
- Ajibesin, K. Dacryodes edulis (G. Don) H.J. Lam: A Review on Its Medicinal, Phytochemical and Economical Properties. Res. J. Med. 2011; 5. [Google Scholar] [CrossRef]
- Stadlmayr, B.; Charrondiere, U.; Eisenwagen, S.; Jamnadass, R.; Kehlenbeck, K. Review: Nutrient Composition of Selected Indigenous Fruits from Sub-Saharan Africa. J. Sci. Food Agric. 2013, 93, 2627–2636. [Google Scholar] [CrossRef] [PubMed]
- Ene-Obong, H.; Igile, G.; Ekpo, A.; Egbung, E.; Agbo, M. Variations in the Nutrients and Bioactive Compounds of Different Accessions of the West African Pear (Dacryodes edulis): Implications for Dietary Intake Assessment and Health. J. Food Compos. Anal. 2019, 79, 80–86. [Google Scholar] [CrossRef]
- Nuga, O.O.; Ofodile, E.A.U. Potentials of Treculia africana Decne—An Endangered Species of Southern Nigeria. J. Agric. Soc. Res. 2010, 10, 91–99. [Google Scholar] [CrossRef]
- Okafor, J.C.; Okolo, H.C. Potentials and Some Indigenous Fruit Trees of Nigeria. In Proceedings of the 5th Annual Conference on Forestry Association of Nigeria, Jos, Nygeria, 1–6 December 1974. [Google Scholar]
- Thakur, G.S.; Bag, M.; Sanodiya, B.S.; Bhadouriya, P.; Debnath, M.; Prasad, G.B.K.S.; Bisen, P.S. Momordica balsamina: A Medicinal and Neutraceutical Plant for Health Care Management. Curr. Pharm. Biotechnol. 2009, 10, 667–682. [Google Scholar] [CrossRef]
- Flyman, M.V.; Afolayan, A.J. Proximate and Mineral Composition of the Leaves of Momordica balsamina L.: An under-Utilized Wild Vegetable in Botswana. Int. J. Food Sci. Nutr. 2007; 58. [Google Scholar] [CrossRef]
- Gebauer, J.; El-Siddig, K.; Ebert, G. Baobab (Adansonia digitata L.): A Review on a Multipurpose Tree with Promising Future in the Sudan. Gartenbauwissenschaft 2002, 67, 155–160.
- Yazzie, D.; VanderJagt, D.J.; Pastuszyn, A.; Okolo, A.; Glew, R.H. The Amino Acid and Mineral Content of Baobab (Adansonia digitata L.) Leaves. J. Food Compos. Anal. 1994; 7. [Google Scholar] [CrossRef]
- Lusepani, N.E. Reproductive Biology and Utilisation of Berchemia discolor (Klotzsch) Hemsley (Rhamnaceae). Ph.D. Dissertation, Stellenbosch University, Stellenbosch, South Africa, 1999. [Google Scholar]
- Udosen, E.O.; Udok, U.E.; Unuigbe, O.S. The Comparison of the Nutrient Compositions of Lasianthera africana and Hejnsia crinita. J. Food Biochem. 1999, 23, 571–576. [Google Scholar] [CrossRef]
- Lepcha, P.; Egan, A.; Doyle, J.; Narayana, N.S. A Review on Current Status and Future Prospects of Winged Bean (Psophocarpus tetragonolobus) in Tropical Agriculture. Plant Foods Hum. Nutr. 2017, 72, 1–11. [Google Scholar] [CrossRef]
- Amoo, I.A.; Adebayo, O.; Oyeleye, A. Chemical Evaluation of Winged Beans (Psophocarpus tetragonolobus), Pitanga Cherries (Eugenia uniflora) and Orchid Fruit (Orchid Fruit Myristica). Afr. J. Food Agric. Nutr. Dev. 2011, 6, 1–12. [Google Scholar] [CrossRef]
- Misra, P.S.; Misra, G.; Prakash, D.; Tripathi, R.D.; Chaudhary, A.R.; Mishra, P.N. Assay of Some Nutritional and Antinutritional Factors in Different Cultivars of Winged Bean (Psophocarpus tetragonolobus (L.) DC) Seeds. Plant Foods Hum. Nutr. 1987; 36. [Google Scholar] [CrossRef]
- Jaffe, W.G.; Korte, R. Nutritional Characteristics of the Winged Bean (Psophocarpus tetragonolobus) in Rats (A Little Known Crop Presently Cultivated in Parts of South East Asia, Some Parts of Africa, and Mostly in Papua New Guinea). Nutr. Rep. Int. USA 1976, 14, 449–455. [Google Scholar]
- Ticona, L.N.A.; Pérez, V.T.; Benito, P.B. Local/Traditional Uses, Secondary Metabolites and Biological Activities of Mashua (Tropaeolum tuberosum Ruíz & Pavón). J. Ethnopharmacol. 2020, 247, 112–152. [Google Scholar] [CrossRef]
- María Elena, J.H.; Yamilet Irene, G.G.; Iván, Y.G.; Migdalia, M.M. Chemical Study and Determination of the Antioxidant Activity of Three Varieties Tropaeolum tuberosum (Mashua). Am. J. Plant Sci. 2019, 10, 2279–2297. [Google Scholar] [CrossRef][Green Version]
- Campos, D.; Chirinos, R.; Gálvez Ranilla, L.; Pedreschi, R. Bioactive Potential of Andean Fruits, Seeds, and Tubers. In Advances in Food and Nutrition Research; Michael Eskin N., A., Ed.; Elsevier, 2018; Vol. 84, pp. 287–343 ISBN 9780128149904.
- Flores, H.; Walker, T.; Guimarães, R.; Bais, H.; Vivanco, J. Andean Root and Tuber Crops: Underground Rainbows. HortScience 2003, 38, 161–167. [Google Scholar] [CrossRef]
- Ojansivu, I.; Ferreira, C.L.; Salminen, S. Yacon, A New Source of Prebiotic Oligosaccharides with a History of Safe Use. Trends Food Sci. Technol. 2011, 22, 40–46. [Google Scholar] [CrossRef]
- Lachman, J.; Fernández, E.; Orsák, M. Yacon [Smallanthus sonchifolia (Poepp. et Endl.) H. Robinson] Chemical Composition and Use—A Review. Plant Soil Environ. 2003; 49. [Google Scholar] [CrossRef]
- Repo-Carrasco-Valencia, R.; Acevedo de La Cruz, A.; Icochea Alvarez, J.C.; Kallio, H. Chemical and Functional Characterization of Kañiwa (Chenopodium pallidicaule) Grain, Extrudate and Bran. Plant Foods Hum. Nutr. Dordr. Neth. 2009, 64, 94–101. [Google Scholar] [CrossRef]
- White, P.L.; Alvistur, E.; Dias, C.; Visas, E.; White, H.S.; Collazos, C. Nutrient Content and Protein Quality of Quinua and Caflihua, Edible Seed Products of the Andes Mountains. J. Agric. Food Chem. 1955, 3, 531–534. [Google Scholar] [CrossRef]
- Gross, R.; Koch, F.; Malaga, I.; de Miranda, A.F.; Schoeneberger, H.; Trugo, L.C. Chemical Composition and Protein Quality of Some Local Andean Food Sources. Food Chem. 1989, 34, 25–34. [Google Scholar] [CrossRef]
- Maass, B.; Knox, M.; Chinnegowda, V.; Angessa, T.T.; Ramme, S.; Pengelly, B.C. Lablab purpureus-A Crop Lost for Africa? Trop. Plant Biol. 2010, 3, 123–135. [Google Scholar] [CrossRef]
- Engle, L.M.; Altoveros, N.C. Collection, Conservation and Utilization of Indigenous Vegetables. Proceedings of World Vegetable Center, a Workshop on Collection, Conservation and Utilization of Indigenous Vegetables, Shanhua, Taiwan, 16–18 August 1999; Mecozzi, M., Ed.; AVRDC Publication: Tainan, Taiwan, 2000. [Google Scholar]
- Naeem, M.; Aftab, T.; Khan, M.M. Hyacinth Bean (Lablab purpureus L.)—An Underutilised Crop with Future Potential. Sci. Hortic. 2020; 12. [Google Scholar] [CrossRef]
- Mariod, A.A.; Abdelwahab, S.I. Sclerocarya birrea (Marula), An African Tree of Nutritional and Medicinal Uses: A Review. Food Rev. Int. 2012, 28, 375–388. [Google Scholar] [CrossRef]
- Behera, A.; Kumar, S.; Jena, P.K. A Review on Amorphophallus Species: Important Medicinal Wild Food Crops of Odisha. Int. J. Pharm. Life Sci. 2014, 5, 3512–3516. [Google Scholar]
- Tripathi, A.; Chitra, V.; Sheikh, D.; Mohale, D.; Dewani, A. Immunomodulatory Activity of the Methanol Extract of Amorphophallus campanulatus (Araceae) Tuber. Trop. J. Pharm. Res. 2010, 9, 451–454. [Google Scholar] [CrossRef][Green Version]
- Acosta, O.; Pérez, A.M.; Vaillant, F. Chemical Characterization, Antioxidant Properties, and Volatile Constituents of Naranjilla (Solanum quitoense Lam.) Cultivated in Costa Rica. Arch. Latinoam. Nutr. 2009, 59, 88–94.
- Kubmarawa, D.; Magomya, A.M.; Yebpella, G.G.; Adedayo, S.A. Nutrient Content and Amino Acid Composition of the Leaves of Cassia tora and Celtis integrifolia. Int. Res. J. Biochem. Bioinforma. 2011, 1, 222–225. [Google Scholar]
- Shukla, S.; Kumar, A.; Terrence, M.; Yusuf, J.; Singh, V.; Mishra, M. The Probable Medicinal Usage of Cassia tora: An Overview. OnLine J. Biol. Sci. 2013, 13, 109–125. [Google Scholar] [CrossRef]
- Li, J.-W.; Fan, L.-P.; Ding, S.-D.; Ding, X.-L. Nutritional Composition of Five Cultivars of Chinese Jujube. Food Chem. 2007, 103, 454–460. [Google Scholar] [CrossRef]
- Guil-Guerrero, J.; Delgado, A.; González, M.; Isasa, M.E. Fatty Acids and Carotenes in Some Ber (Ziziphus jujuba Mill) Varieties. Plant Foods Hum. Nutr. Dordr. Neth. 2004, 59, 23–27. [Google Scholar] [CrossRef] [PubMed]
- Lim, K.T. Edible Medicinal and Non-Medicinal Plants: Volume 1, Fruits; Springer: Dordrecht, The Netherlands, 2012. [Google Scholar]
- Marcone, M.F.; Jahaniaval, F.; Aliee, H.; Kakuda, Y. Chemical Characterization of Achyranthes bidentata Seed. Food Chem. 2003, 81, 7–12. [Google Scholar] [CrossRef]
- Shen, R.; Yang, S.; Zhao, G.; Shen, Q.; Diao, X. Identification of Carotenoids in Foxtail Millet (Setaria italica) and the Effects of Cooking Methods on Carotenoid Content. J. Cereal Sci. 2015, 61, 86–93. [Google Scholar] [CrossRef]
- Yadav, A.K. Phalsa: A Potential New Small Fruit for Georgia. In Perspectives on New Crops and New Uses; Janik, J., Ed.; ASHS Press: Alexandria, Egypt, 1999; pp. 348–352. [Google Scholar]
- Khan, R.; Asghar, W.; Khalid, N.; Nazir, W.; Farooq, M.; Ahmed, I.; Syed, Q.A. Phalsa (Grewia asiatica L) Fruit Berry a Promising Functional Food Ingredient: A Comprehensive Review. J. Berry Res. 2019, 9, 1–15. [Google Scholar] [CrossRef]
- Venthodika, A.; Chhikara, N.; Mann, S.; Garg, M.K.; Sofi, S.A.; Panghal, A. Bioactive Compounds of Aegle marmelos L., Medicinal Values and Its Food applications: A Critical Review. Phytother. Res. 2021; 35. [Google Scholar] [CrossRef]
- Singh, A.; Sharma, P.C.; Kumar, A.; Meena, M.D.; Sharma, D.K. Genotypic Differences for Salt Tolerance in Bael (Aegle marmelos) Cultivars. Indian J. Agric. Sci. 2018, 88, 435–441. [Google Scholar]
- Jayakumar, K.; Muthuraman, B. Traditional Uses and Nutrient Status of Indian Native Plant Fruit (Carissa carandas Linn.). World Sci. News 2018, 96, 217–224.
- Dalal, R.P.S. ; Navjot; Thakur, A.; Singh, A. Nutritional Value of Karonda (Carissa carandas Linn.)—A Non-Conventional Fruit under Semi-Arid Condition of Punjab. Indian J. Agrofor. 2020, 12, 102–104.
- Rodrigues, B.; Souza, B.; Nogueira, R.; Mauro, E.; Santos, M. Tolerance to Water Deficit in Young Trees of Jackfruit and Sugar Apple. Rev. Cienc. Agron. 2010, 41, 245–252. [Google Scholar] [CrossRef]
- Ranasinghe, R.A.S.N.; Maduwanthi, S.D.T.; Marapana, R.A.U.J. Nutritional and Health Benefits of Jackfruit (Artocarpus heterophyllus Lam.): A Review. Int. J. Food Sci. 2019; 12. [Google Scholar] [CrossRef]
- Busch, J.M.; Sangketkit, C.; Savage, G.P.; Martin, R.J.; Halloy, S.; Deo, B. Nutritional Analysis and Sensory Evaluation of Ulluco (Ullucus tuberosus Loz) Grown in New Zealand. J. Sci. Food Agric. 2000, 80, 2232–2240. [Google Scholar] [CrossRef]
- Lim, T. Arracacia xanthorrhiza. In Edible Medicinal and Non Medicinal Plants; Lim, T.K., Ed.; Springer: Cham, Switzerland, 2015; pp. 361–366. [Google Scholar]
- Manner, H.; Buker, R.; Smith, V.; Ward, D.; Elevitch, C. Species Profiles for Pacific Island Agroforestry; Permanent Agriculture Resources: New York, NY, USA, 2006. [Google Scholar]
- Chan-Blanco, Y.; Vaillant, F.; Mercedes Perez, A.; Reynes, M.; Brillouet, J.-M.; Brat, P. The Noni Fruit (Morinda citrifolia L.): A Review of Agricultural Research, Nutritional and Therapeutic Properties. Biodivers. Nutr. 2006; 19. [Google Scholar] [CrossRef]
- Ekanayake, S.; Jansz, E.; Nair, B. Literature Review of an Underutilized Legume: Canavalia gladiata L. Plant Foods Hum. Nutr. Dordr. Neth. 2000, 55, 305–321. [Google Scholar] [CrossRef]
- Popoola, J.; Ojuederie, O.; Omonhinmin, C.; Adegbite, A. Neglected and Underutilized Legume Crops: Improvement and Future Prospects. In Recent Advances in Grain Crops Research; IntechOpen: London, UK; 2019; ISBN 978-1-78985-449-7. [Google Scholar]
- Mohan, V.R.; Janardhanan, K. The Biochemical Composition and Nutrient Assessment of Less Known Pulses of the Genus Canavalia. Int. J. Food Sci. Nutr. 1994, 45, 255–262. [Google Scholar] [CrossRef]
- Eastwood, R.J.; Hughes, C.E. Lupinus mutabilis. Curtis’s Bot. Mag. 2018, 35, 134–148. [CrossRef]
- Carvajal-Larenas, F.E.; Linnemann, A.R.; Nout, M.J.R.; Koziol, M.; van Boekel, M.A.J.S. Lupinus mutabilis: Composition, Uses, Toxicology, and Debittering. Crit. Rev. Food Sci. Nutr. 2016, 56, 1454–1487. [Google Scholar] [CrossRef]
- Gulisano, A.; Alves, S.; Martins, J.N.; Trindade, L.M. Genetics and Breeding of Lupinus mutabilis: An Emerging Protein Crop. Front. Plant Sci. 2019, 10, 1385–1385. [Google Scholar] [CrossRef]
- Vijayvargia, P.; Vijayvergia, R. A Review on Limonia acidissima l.: Multipotential Medicinal Plant. Int. J. Pharm. Sci. Rev. Res. 2014, 28, 191–195.
- Ratnayake, S.S.; Kumar, L.; Kariyawasam, C.S. Neglected and Underutilized Fruit Species in Sri Lanka: Prioritisation and Understanding the Potential Distribution under Climate Change. Agronomy 2020, 10, 34. [Google Scholar] [CrossRef]
- Meghwal, P.; Singh, A. Lasoda or Gonda (Cordia myxa L.). In Lasoda or Gonda (Cordia myxa L.), Ghos, S.,N., Ed.; Jaya Publishing House: New Delhi, India, 2015; pp. 247–253. [Google Scholar]
- Singh, A.; Uppal, G. A Review on Carissa carandas Phytochemistry, Ethnopharmacology, and Micropropagation as Conservation Strategy. Asian J. Pharm. Clin. Res. 2015, 8, 26–30. [Google Scholar]
- Arif, M.; Kamal, M.; Jawaid, T. Carissa carandas Linn. (Karonda): An Exotic Minor Plant Fruit with Immense Value in Nutraceutical and Pharmaceutical Industries. Asian J. Biomed. Pharm. Sci. 2016, 6, 14–19.
- Muhammad, I.; Zhao, J.; Dunbar, D.C.; Khan, I.A. Constituents of Lepidium meyenii ‘Maca. 2002; 59. [Google Scholar] [CrossRef]
- Peres, N.d.S.L.; Bortoluzzi, L.C.P.; Marques, L.L.M.; Formigoni, M.; Fuchs, R.H.B.; Droval, A.A.; Cardoso, F.A.R. Medicinal Effects of Peruvian Maca (Lepidium meyenii): A Review. Food Funct. 2020, 11, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Lim, T.K. Pastinaca sativa. In Edible Medicinal and Non Medicinal Plants; Lim, T.K., Ed.; Springer: Dordrecht, The Netherlands, 2015; pp. 417–428. [Google Scholar]
- Tutin, T.G. Umbellifers of the British Isles; Botanical Society of the British Isles: London, UK, 1980. [Google Scholar]
- Boakye, A.A.; Wireko-Manu, F.D.; Oduro, I.; Ellis, W.O.; Gudjónsdóttir, M.; Chronakis, I.S. Utilizing Cocoyam (Xanthosoma sagittifolium) for Food and Nutrition Security: A Review. Food Sci. Nutr. 2018, 6, 703–713. [Google Scholar] [CrossRef]
- Miller, C.D. Food Values of Poi, Taro, and Limu, Periodicals Service Co: Hudson, NY, USA, 1971; p. 25.
- Nyman, L.P.; Gonzales, C.J.; Arditti, J. In-Vitro Selection for Salt Tolerance of Taro (Colocasia esculenta var antiquorum). Ann. Bot. 1983, 51, 229–236. [Google Scholar] [CrossRef]
- Rai, S.; Wahile, A.; Mukherjee, K.; Saha, B.P.; Mukherjee, P.K. Antioxidant Activity of Nelumbo nucifera (Sacred Lotus) Seeds. J. Ethnopharmacol. 2006, 104, 322–327. [Google Scholar] [CrossRef]
- Shad, M.; Nawaz, H.; Siddique, F.; Zahra, J.; Mushtaq, A. Nutritional and Functional Characterization of Seed Kernel of Lotus (Nelumbo nucifera): Application of Response Surface Methodology. Food Sci. Technol. Res. 2013, 19, 163–172. [Google Scholar] [CrossRef]
- Nohara, S.; Kimura, M. Growth Characteristics of Nelumbo nucifera Gaertn. in Response to Water Depth and Flooding. Ecol. Res. 1997, 12, 11–20. [Google Scholar] [CrossRef]
- Manikandan, S.; Lakshmanan, G.M.; Chandran, C. Phytochemical Screening and Evaluation of Tuber Extract of Plectranthus rotundifolius Spreng. By GC-MS and FTIR Spectrum Analysis. Eur. J. Herb. Med. 2016, 4, 36–40.
- Sethuraman, G.; Nizar, M.; Nadia, F.; Syaheerah, T.; Jahanshiri, E.; Gregory, P.; Azam-Ali, S. Nutritional Composition of Black Potato (Plectranthus rotundifolius (Poir.) Spreng.) (Synonym: Solenostemon rotundifolius). Int. J. Sci. Eng. Res. 2020, 11, 1145–1150.
- Priya, M.H.; Anbuselvi, S. Physico Chemical Analysis of Plectranthus rotundifolius. J. Chem. Pharm. Res. 2013, 5, 12–14. [Google Scholar]
- Hidalgo, A.; Brandolini, A. Nutritional Properties of Einkorn Wheat (Triticum monococcum L.). J. Sci. Food Agric. 2014, 94, 601–612.
- Prażak, R. Salt Tolerance of Triticum monococcum L., T. dicoccum (Schrank) Schubl., T. durum Desf. and T. aestivum L. Seedlings. J. Appl. Genet. 2001, 42, 289–292.
- Dhanavath, S.; Prasada Rao, U.J.S. Nutritional and Nutraceutical Properties of Triticum dicoccum Wheat and Its Health Benefits: An Overview. J. Food Sci. 2017, 82, 2243–2250. [Google Scholar] [CrossRef] [PubMed]
- Zaharieva, M.; Ayana, N.G.; Hakimi, A.A; Misra, S.C.; Monneveux, P. Cultivated Emmer Wheat (Triticum dicoccon Schrank), An Old Crop with Promising Future: A Review. Genet. Resour. Crop Evol. 2010, 57, 937–962. [Google Scholar] [CrossRef]
- Zlatica, K.; Jolana, K. Nutritional Value and Baking Application of Spelt Wheat. Acta Sci. Pol. Technol. Aliment. 2008, 7, 5–14. [Google Scholar]
- Ruibal-Mendieta, N.L.; Delacroix, D.L.; Mignolet, E.; Pycke, J.-M.; Marques, C.; Rozenberg, R.; Petitjean, G.; Habib-Jiwan, J.-L.; Meurens, M.; Quetin-Leclercq, J.; et al. Spelt (Triticum aestivum Ssp. spelta) as a Source of Breadmaking Flours and Bran Naturally Enriched in Oleic Acid and Minerals but Not Phytic Acid. J. Agric. Food Chem. 2005; 53. [Google Scholar] [CrossRef]
- Burgos, M.; Messmer, M.; Stamp, P.; Schmid, J.E. Flooding Tolerance of Spelt (Triticum spelta L.) Compared to Wheat (Triticum aestivum L.)—A Physiological and Genetic Approach. 2001. [Google Scholar] [CrossRef]
- Chandra, D.; Chandra, S. ; Pallavi; Sharma, A.K. Review of Finger Millet (Eleusine coracana (L.) Gaertn): A Power House of Health Benefiting Nutrients. Food Sci. Hum. 2016; 5. [Google Scholar] [CrossRef]
- Hittalmani, S.; Mahesh, H.B.; Shirke, M.D.; Biradar, H.; Uday, G.; Aruna, Y.R.; Lohithaswa, H.C.; Mohanrao, A. Genome and Transcriptome Sequence of Finger Millet (Eleusine coracana (L.) Gaertn.) Provides Insights into Drought Tolerance and Nutraceutical Properties. BMC Genom. 2017; 18. [Google Scholar] [CrossRef]
- Devi, P.B.; Vijayabharathi, R.; Sathyabama, S.; Malleshi, N.G.; Priyadarisini, V.B. Health Benefits of Finger Millet (Eleusine coracana L.) Polyphenols and Dietary Fiber: A Review. J. Food Sci. Technol. 2011; 51. [Google Scholar] [CrossRef]
- Kalinova, J.; Moudry, J. Content and Quality of Protein in Proso Millet (Panicum miliaceum L.) Varieties. Plant Foods Hum. Nutr. Dordr. Neth. 2006; 61. [Google Scholar] [CrossRef]
- Zhang, Y. Comparative Analysis of Proso Millet (Panicum miliaceum L.) Leaf Transcriptomes for Insight into Drought Tolerance Mechanisms. BMC Plant Biol. 2019, 19, 397.
- Jukanti, A.K.; Gowda, C.L.L.; Rai, K.N.; Manga, V.K.; Bhatt, R.K. Crops That Feed the World 11. Pearl Millet (Pennisetum glaucum L.): An Important Source of Food Security, Nutrition and Health in the Arid and Semi-Arid Tropics. Food Secur. 2016; 8. [Google Scholar] [CrossRef]
- Sade, F.Q. Proximate, Antinutritional Factors and Functional Properties of Processed Pearl Millet (Pennisetum glaucum). J. Food Technol. 2009, 7, 92–97. [Google Scholar]
- Onyango, C.; Ochanda, S.; Mwasaru, M.; Ochieng, J.; Mathooko, F.; Kinyuru, J. Effects of Malting and Fermentation on Anti-Nutrient Reduction and Protein Digestibility of Red Sorghum, White Sorghum and Pearl Millet. J. Food Res. 2013, 2, 41–49. [Google Scholar] [CrossRef]
- Newman, Y.; Jennings, E.D.; Vendramini, J.; Blount, A. Pearl Millet (Pennisetum glaucum): Overview and Management; Institute of Food and Agricultural Sciences, University of Florida: Gainesville, FL, USA, 2020; Available online: https://edis.ifas.ufl.edu/publication/AG347 (accessed on 21 March 2021).
- Rzedowski, J. The Northern Limit of Tropical Rain Forests in Continental North America. Vegetatio 1963, 11, 173–198. [Google Scholar] [CrossRef]
- Subiria-Cueto, R.; Larqué-Saavedra, A.; Reyes-Vega, M.L.; de la Rosa, L.A.; Santana-Contreras, L.E.; Gaytán-Martínez, M.; Vázquez-Flores, A.A.; Rodrigo-García, J.; Corral-Avitia, A.Y.; Núñez-Gastélum, J.A.; et al. Brosimum alicastrum Sw. (Ramón): An Alternative to Improve the Nutritional Properties and Functional Potential of the Wheat Flour Tortilla. 2019; 8. [Google Scholar] [CrossRef]
- Taylor, M.B.; Tuia, V.S. Breadfruit in the Pacific Region. Acta Hortic. 2007, 757, 43–50. [Google Scholar] [CrossRef]
- Tukura, B.W.; Obliva, O. Proximate and Nutritional Compositions of Breadfruit (Artocarpus altilis) Seeds. IOSR J. Environ. Sci. 2015, 9, 68–73. [Google Scholar] [CrossRef]
- Encalada, S.V.; Campos, M.R.S. Mucuna pruriens Fiber: Nutritional, Functional And Biological Properties. Food Sci. Tech. 2020, 41, 120–126. [Google Scholar] [CrossRef]
- Lampariello, L.R.; Cortelazzo, A.; Guerranti, R.; Sticozzi, C.; Valacchi, G. The Magic Velvet Bean of Mucuna pruriens. J. Tradit. Complement. Med. 2012, 2, 331–339. [Google Scholar] [CrossRef]
- Rodrigues, A.P.; Pereira, G.A.; Tomé, P.H.F.; Arruda, H.S.; Eberlin, M.N.; Pastore, G.M. Chemical Composition and Antioxidant Activity of Monguba (Pachira aquatica) Seeds. Food Res. Int. 2019, 121, 880–887. [Google Scholar] [CrossRef]
- Oliveira, J.T.A.; Vasconcelos, I.; Bezerra, L.C.N.M.; Silveira, S.B.; Monteiro-Moreira, A.; Moreira, R. Composition and Nutritional Properties of Seeds from Pachira aquatica Aubl, Sterculia striata St Hil et Naud and Terminalia catappa Linn. Food Chem. 2000, 70, 185–191. [Google Scholar] [CrossRef]
- Orwa, C.; Mutua, C.; Kindt, R.; Jamnadass, R.; Anthony, S. Strychnos cocculoides. Available online: http://apps.worldagroforestry.org/treedb/AFTPDFS/Strychnos_cocculoides.PDF (accessed on 2 March 2021).
- Ngadze, R.T.; Linnemann, A.R.; Nyanga, L.K.; Fogliano, V.; Verkerk, R. Local Processing and Nutritional Composition of Indigenous Fruits: The Case of Monkey Orange (Strychnos Spp.) from Southern Africa. Food Rev. Int. 2017; 33. [Google Scholar] [CrossRef]
- Maikhuri, R.K.; Semwal, R.L.; Rao, K.S.; Nautiyal, S.; Saxena, K.G. Eroding Traditional Crop Diversity Imperils the Sustainability of Agricultural Systems in Central Himalaya. Curr. Sci. 1997, 73, 777–782. [Google Scholar] [CrossRef]
- Muthamilarasan, M.; Prasad, M. Small Millets for Enduring Food Security Amidst Pandemics. Trends Plant Sci. 2020, 26, 1–8. [Google Scholar] [CrossRef]
- Zsögön, A.; Cermak, T.; Voytas, D.; Peres, L. Genome Editing as a Tool to Achieve the Crop Ideotype and de novo Domestication of Wild Relatives: Case Study in Tomato. Plant Sci. 2016, 256, 120–130. [Google Scholar] [CrossRef]
- Hammer, K.; Arrowsmith, N.; Gladis, T. Agrobiodiversity with Emphasis on Plant Genetic Resources. Sci. Nat. 2003, 90, 241–250. [Google Scholar] [CrossRef] [PubMed]
- Ogwu, M.; Osawaru, M.; Ahana, C. Challenges in Conserving and Utilizing Plant Genetic Resources (PGR). Int. J. Genet. Mol. Biol. 2014, 6, 16–23. [Google Scholar] [CrossRef]
- Mabhaudhi, T.; Chibarabada, T.P.; Chimonyo, V.G.P.; Murugani, V.G.; Pereira, L.M.; Sobratee, N.; Govender, L.; Slotow, R.; Modi, A.T. Mainstreaming Underutilized Indigenous and Traditional Crops into Food Systems: A South African Perspective. Sustainability 2019, 11, 172. [Google Scholar] [CrossRef]
- Moose, S.; Mumm, R. Molecular Plant Breeding as the Foundation for 21st Century Crop Improvement. Plant Physiol. 2008, 147, 969–977. [Google Scholar] [CrossRef] [PubMed]
- Fukushima, A.; Kusano, M.; Redestig, H.; Arita, M.; Saito, K. Integrated Omics Approaches in Plant Systems Biology. Curr. Opin. Chem. Biol. 2009, 13, 532–538. [Google Scholar] [CrossRef] [PubMed]
- Friel, S.; Hattersley, L.; Snowdon, W.; Thow, A.-M.; Lobstein, T.; Sanders, D.; Barquera, S.; Mohan, S.; Hawkes, C.; Kelly, B.; et al. Monitoring the Impacts of Trade Agreements on Food Environments. Obes. Rev. Off. J. Int. Assoc. Study Obes. 2013, 14, 120–134. [Google Scholar] [CrossRef] [PubMed]
- Grote, U. Can We Improve Global Food Security? A Socio-Economic and Political Perspective. Food Secur. 2014, 6, 187–200. [Google Scholar] [CrossRef]
- Glauber, J.; Laborde Debucquet, D.; Martin, W.; Vos, R. COVID-19: Trade Restrictions Are Worst Possible Response to Safeguard Food Security. Available online: https://ebrary.ifpri.org/digital/collection/p15738coll2/id/133833/ (accessed on 7 June 2021).
- Laborde, D.; Martin, W.; Swinnen, J.; Vos, R. COVID-19 Risks to Global Food Security. Science 2020, 369, 500–502. [Google Scholar] [CrossRef]
- Espitia, A.; Rocha, N.; Ruta, M. COVID-19 and Food Protectionism: The Impact of the Pandemic and Export Restrictions on World Food Markets; Policy Research Working Paper; No. 9253; Social Science Research Network: Rochester, NY, USA; World Bank, Washington, DC, USA, 2020; Available online: https://openknowledge.worldbank.org/handle/10986/33800 (accessed on 9 March 2021).
- Béné, C. Resilience of Local Food Systems and Links to Food Security—A Review of Some Important Concepts in the Context of COVID-19 and Other Shocks. Food Secur. 2020, 12, 805–822. [Google Scholar] [CrossRef] [PubMed]
- Paudyal, A.; Regmi, B. Climate Change and Agrobiodiversity in Nepal: Opportunities to Include Agrobiodiversity Maintenance to Support Nepal’s National Adaptation Programme of Action; A Report Prepared by LI-BIRD for the Platform for Agrobiodiversity Research in Collaboration with FAO and Bioversity International; FAO: Rome, Italy, 2009. [Google Scholar]
- Coelho, F.C.; Coelho, E.M.; Egerer, M. Local Food: Benefits and Failings Due to Modern Agriculture. Sci. Agric. 2018, 75, 84–94. [Google Scholar] [CrossRef]
- Sheil, D.; Wunder, S. The Value of Tropical Forest to Local Communities: Complications, Caveats, and Cautions. Ecol. Soc. 2002, 6, 1–15. [Google Scholar] [CrossRef]
- Legwaila, G.M.; Mojeremane, W.; Madisa, M.; Mmolotsi, R.; Rampart, M. Potential of Traditional Food Plants in Rural Household Food Security in Botswana. J. Hortic. For. 2011, 3, 171–177. [Google Scholar] [CrossRef]
- Nesamvuni, C.; Steyn, N.; Potgieter, M. Nutritional Value of Wild, Leafy Plants Consumed by the Vhavenda. South Afr. J. Sci. 2001, 97, 51–54. [Google Scholar]
- Kadu, C.A.C.; Imbuga, M.; Jamnadass, R.; Dawson, I.K. Genetic Management of Indigenous Fruit Trees in Southern Africa: A Case Study of Sclerocarya birrea Based on Nuclear and Chloroplast Variation. South Afr. J. Bot. 2006, 72, 421–427. [Google Scholar] [CrossRef][Green Version]
- Cruz-Garcia, G.S.; Price, L.L. Gathering of Wild Food Plants in Anthropogenic Environments across the Seasons: Implications for Poor and Vulnerable Farm Households. Ecol. Food Nutr. 2014, 53, 363–389. [Google Scholar] [CrossRef]
- Bourgeois, R. Secondary crops, rural poverty and policy bias. In Proceedings of the Regional Workshop on Rural Prosperity and Secondary Crops: Towards Applied Pro-Poor Research and Policies in Asia and the Pacific, Bogor, Indonesia; 6–9 December, 2005, Bourgeois, R., Svensson, L., Burrows, M., Eds.; UNESCAP-CAPSA. Publisher: Bogor, Indonesia, 2005. [Google Scholar]
- Hart, T. The Significance of African Vegetables in Ensuring Food Security for South Africa’s Rural Poor. Agric. Hum. Values 2011, 28, 321–333. [Google Scholar] [CrossRef]
- Lockett, C.T.; Calvert, C.C.; Grivetti, L.E. Energy and Micronutrient Composition of Dietary and Medicinal Wild Plants Consumed during Drought. Study of Rural Fulani, Northeastern Nigeria. Int. J. Food Sci. Nutr. 2000; 51. [Google Scholar] [CrossRef]
- Amadou, I.; Gbadamosi, O.; Le, G. Millet-Based Traditional Processed Foods and Beverages: A Review. Cereal Foods World 2011, 56, 115–121. [Google Scholar] [CrossRef]
- Yenagi, N.; Handigol, J.; Ravi, S.; Mal, B.; Padulosi, S. Nutritional and Technological Advancements in the Promotion of Ethnic and Novel Foods Using the Genetic Diversity of Minor Millets in India. Indian J. Plant Genet. Resour. 2010, 23, 82–86. [Google Scholar]
- Islam, M.; Das, P.R.; Salehin, M.F.; Mahmud, B.; Hasan, M.; Jahan, I.; Seraj, S.; Islam, F.; Khatun, Z.; Chowdhury, A.; et al. A Survey of Non-Conventional Plant Items Consumed During Food Scarcity in Two Randomly Selected Villages of Kurigram District, Bangladesh. Am. Eurasian J. Sustain. Agric. 2011, 5, 233–239. [Google Scholar]
- Bhattacharjee, R. Harnessing Biotechnology for Conservation and Increased Utilization of Orphan Crops. Afr. Technol. Dev. Forum J. 2009, 6, 24–82. [Google Scholar]
- Hu, H.; Scheben, A.; Edwards, D. Advances in Integrating Genomics and Bioinformatics in the Plant Breeding Pipeline. Agriculture 2018, 8, 75. [Google Scholar] [CrossRef]
- Mosa, K.A.; Ismail, A.; Helmy, M. Plant Stress Tolerance: An Integrated Omics Approach; SpringerBriefs in Systems Biology; Springer International Publishing: Cham, Switzerland, 2017. [Google Scholar]
- Yokoyama, S.; Yura, K. Special Issue: Big Data Analyses in Structural and Functional Genomics. J. Struct. Funct. Genom. 2017, 17, 67–70. [Google Scholar] [CrossRef][Green Version]
- Lowe, R.; Shirley, N.; Bleackley, M.; Dolan, S.; Shafee, T. Transcriptomics Technologies. PLoS Comput. Biol. 2017, 13, e1005457. [Google Scholar] [CrossRef]
- Appleby, N.; Edwards, D.; Batley, J. New Technologies for Ultra-High Throughput Genotyping in Plants. In Plant Genomics: Methods and Protocols; Gustafson, J.P., Langridge, P., Somers, D.J., Eds.; Humana Press: Totowa, NJ, USA, 2009; pp. 19–39. [Google Scholar]
- Vu, L.D.; Gevaert, K.; De Smet, I. Protein Language: Post-Translational Modifications Talking to Each Other. Trends Plant Sci. 2018, 23, 1068–1080. [Google Scholar] [CrossRef] [PubMed]
- Eldakak, M.; Milad, S.I.M.; Nawar, A.I.; Rohila, J.S. Proteomics: A Biotechnology Tool for Crop Improvement. Front. Plant Sci. 2013, 4, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Lahner, B.; Gong, J.; Mahmoudian, M.; Smith, E.L.; Abid, K.B.; Rogers, E.E.; Guerinot, M.L.; Harper, J.F.; Ward, J.M.; McIntyre, L.; et al. Genomic Scale Profiling of Nutrient and Trace Elements in Arabidopsis thaliana. Nat. Biotechnol. 2003, 21, 1215–1221. [Google Scholar] [CrossRef]
- Van Emon, J. Omics Revolution in Agricultural Research. J. Agric. Food Chem. 2015, 64, 34–44. [Google Scholar] [CrossRef] [PubMed]
- Benkeblia, N. Omics Technologies and Crop Improvement; Taylor & Francis, Milten Park, Australia, 2014.
- Xia, E.-H.; Tong, W.; Wu, Q.; Wei, S.; Zhao, J.; Zhang, Z.-Z.; Wei, C.-L.; Wan, X.-C. Tea Plant Genomics: Achievements, Challenges and Perspectives. Hortic. Res. 2020, 7, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Ausubel, F.M. Arabidopsis Genome. A Milestone in Plant Biology. Plant Physiol. 2000, 124, 1451–1454. [Google Scholar] [CrossRef]
- Hamilton, J.P.; Buell, C.R. Advances in Plant Genome Sequencing. Plant J. Cell Mol. Biol. 2012, 70, 177–190. [Google Scholar] [CrossRef]
- Michael, T.P.; Jackson, S. The First 50 Plant Genomes. Plant Genome 2013, 6, 1–7. [Google Scholar] [CrossRef]
- Heck, M.; Neely, B.A. Proteomics in Non-Model Organisms: A New Analytical Frontier. J. Proteome Res. 2020, 19, 3595–3606. [Google Scholar] [CrossRef]
- Fabres, P.J. A Multiple “Omics” Approach to Study the Interaction between the Vitis vinifera Transcriptome and Epigenome and the Barossa Valley Terroir. Ph.D. Dissertation, University of Adelaide, Australia, 2020. [Google Scholar]
- Kersey, P. Plant Genome Sequences: Past, Present, Future. Curr. Opin. Plant Biol. 2019, 48, 1–8. [Google Scholar] [CrossRef]
- Pareek, C.S.; Smoczynski, R.; Tretyn, A. Sequencing Technologies and Genome Sequencing. J. Appl. Genet. 2011, 52, 413–435. [Google Scholar] [CrossRef] [PubMed]
- Pryer, K.M.; Schneider, H.; Zimmer, E.A.; Ann Banks, J. Deciding among Green Plants for Whole Genome Studies. Trends Plant Sci. 2002, 7, 550–554. [Google Scholar] [CrossRef]
- Steinwand, M.; Ronald, P. Crop Biotechnology and the Future of Food. Nat. Food 2020, 1, 273–283. [Google Scholar] [CrossRef]
- Mochida, K.; Shinozaki, K. Advances in Omics and Bioinformatics Tools for Systems Analyses of Plant Functions. Plant Cell Physiol. 2011, 52, 2017–2038. [Google Scholar] [CrossRef]
- Lepcha, P.; Kumar, P.R.; Sathyanarayana, N. Exploring Genomics Research in the Context of Some Underutilized Legumes—A Review. In OMICS-Based Approaches in Plant Biotechnology; Banerjee, R., Kumar, G.V., Kumar, S.P.J., Eds.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2019; pp. 1–18. [Google Scholar]
- Khound, R.; Santra, D. Omics for Proso Millet Genetic Improvement. Nucl. India 2020, 63, 241–247. [Google Scholar] [CrossRef]
- Moe, K.T.; Kwon, S.-W.; Park, Y. Trends in Genomics and Molecular Marker Systems for the Development of Some Underutilized Crops. Genes Genom. 2012, 34, 451–456. [Google Scholar] [CrossRef]
- Chang, Y.; Liu, H.; Liu, M.; Liao, X.; Sahu, S.K.; Fu, Y.; Song, B.; Cheng, S.; Kariba, R.; Muthemba, S.; et al. The Draft Genomes of Five Agriculturally Important African Orphan Crops. GigaScience 2019, 8, 1–16. [Google Scholar] [CrossRef]
- Li, Q.; Yan, J. Sustainable Agriculture in the Era of Omics: Knowledge-Driven Crop Breeding. Genome Biol. 2020, 21, 1–5. [Google Scholar] [CrossRef]
- Singh, N.; Rai, V.; Singh, N. Multi-omics Strategies and Prospects to Enhance Seed Quality and Nutritional Traits in Pigeonpea. Nucleus 2020, 21, 1–8. [Google Scholar] [CrossRef]
- Verma, V.; Patel, S. Value Added Products from Nutri-Cereals: Finger Millet (Eleusine coracana). Emir. J. Food Agric. 2012, 25, 169–176. [Google Scholar] [CrossRef]
- Nirgude, M.; Babu, B.; Shambhavi, Y.; Singh, U.; Upadhyaya, H.; Kumar, A. Development and Molecular Characterization of Genic Molecular Markers for Grain Protein and Calcium Content in Finger Millet (Eleusine coracana (L.) Gaertn.). Mol. Biol. Rep. 2014; 41. [Google Scholar] [CrossRef]
- Kumar, A.; Babu, B.; Yadav, S.; Agrawal, P. Allele Mining for Resistance Gene Analogs (RGAs) in Crop Plants: A Special Emphasis on Blast Resistance in Finger Millet (Eleusine coracana L.). Indian J. Genet. Plant Breed. 2016; 76. [Google Scholar] [CrossRef]
- Kumar, A.; Gaur, V.; Goel, A.; Gupta, A. De Novo Assembly and Characterization of Developing Spikes Transcriptome of Finger Millet (Eleusine coracana): A Minor Crop Having Nutraceutical Properties. Plant Mol. Biol. Report. 2014, 33, 905–922. [Google Scholar] [CrossRef]
- Singh, M.; Metwal, M.; Kumar, V.; Kumar, A. Identification and Molecular Characterization of 48 KDa Calcium Binding Protein as Calreticulin from Finger Millet (Eleusine coracana) Using Peptide Mass Fingerprinting and Transcript Profiling. J. Sci. Food Agric. 2015, 96, 672–679. [Google Scholar] [CrossRef]
- Anatala, T.; Gajera, H.; Mandavia, M.; Dave, R.; Vallabhbhai, K.; Golakiya, B.A. Leaf Proteome Alterations in Tolerant Pearl Millet (Pennisetum glaucum L.) Genotype under Water Stress. Int. J. Agric. Environ. Biotechnol. 2015; 8. [Google Scholar] [CrossRef]
- Bandyopadhyay, T.; Muthamilarasan, M.; Prasad, M. Millets for Next Generation Climate-Smart Agriculture. Front. Plant Sci. 2017, 8, 1266. [Google Scholar] [CrossRef]
- Lata, C.; Sahu, P.P.; Prasad, M. Comparative Transcriptome Analysis of Differentially Expressed Genes in Foxtail Millet (Setaria italica L.) during Dehydration Stress. Biochem. Biophys. Res. Commun. 2010. [Google Scholar] [CrossRef]
- Shi, W.; Cheng, J.; Wen, X.; Wang, J.; Shi, G.; Yao, J.; Liyuan, H.; Sun, Q.; Xiang, P.; Yuan, X.; et al. Transcriptomic Studies Reveal a Key Metabolic Pathway Contributing to a Well-Maintained Photosynthetic System under Drought Stress in Foxtail Millet (Setaria italica L.). 2018; 6. [Google Scholar] [CrossRef]
- Sreenivasulu, N.; Miranda, M.; Prakash, H.S.; Wobus, U.; Weschke, W. Transcriptome Changes in Foxtail Millet Genotypes at High Salinity: Identification and Characterization of a PHGPX Gene Specifically up-Regulated by NaCl in a Salt-Tolerant Line. J. Plant Physiol. 2004, 161, 467–477. [Google Scholar] [CrossRef]
- Leone, A.; Fiorillo, G.; Criscuoli, F.; Ravasenghi, S.; Santagostini, L.; Fico, G.; Spadafranca, A.; Battezzati, A.; Schiraldi, A.; Pozzi, F.; et al. Nutritional Characterization and Phenolic Profiling of Moringa oleifera Leaves Grown in Chad, Sahrawi Refugee Camps, and Haiti. Int. J. Mol. Sci. 2015, 16, 18923–18937. [Google Scholar] [CrossRef] [PubMed]
- Bosch, C.H. Moringa oleifera Lam. In Plant Resources of Tropical Africa Vegetables; Grubben, G.J.H., Denton, O.A., Eds.; Backhuys Publishers: Kerkwerve, The Netherlands, 2004. [Google Scholar]
- Tian, Y.; Zeng, Y.; Zhang, J.; Yang, C.; Yan, L.; Wang, X.; Shi, C.; Xie, J.; Dai, T.; Peng, L.; et al. High Quality Reference Genome of Drumstick Tree (Moringa oleifera Lam.), a Potential Perennial Crop. Sci. China Life Sci. 2015; 58. [Google Scholar] [CrossRef]
- Pirrò, S.; Matic, I.; Guidi, A.; Zanella, A.; Gisondi, A.; Cicconi, A.; Bernardini, R.; Colizzi, V.; Canini, A.; Mattei, M.; et al. Identification of microRNAs and Relative Target Genes in Moringa oleifera leaf and callus. Sci. Rep. 2019, 9, 15145. [Google Scholar] [CrossRef] [PubMed]
- Pasha, S.N.; Shafi, K.M.; Joshi, A.G.; Meenakshi, I.; Harini, K.; Mahita, J.; Sajeevan, R.S.; Karpe, S.D.; Ghosh, P.; Nitish, S.; et al. The Transcriptome Enables the Identification of Candidate Genes behind Medicinal Value of Drumstick Tree (Moringa oleifera). Genomics 2020, 112, 621–628. [Google Scholar] [CrossRef] [PubMed]
- Makita, C.S. Metabolomic Exploration of Pharmacologically Relevant Metabolites in Moringa oleifera and Moringa ovalifolia through the Use of UPLC-QTOF-MS and Multivariate Models. Ph.D. Thesis, Faculty of Science, University of the Witwatersrand, Johannesburg, South Africa, 2017. [Google Scholar]
- Fuentes, F.; Martínez, E.; Hinrichsen, P.; Jellen, R.; Maughan, J. Assessment of Genetic Diversity Patterns in Chilean Quinoa (Chenopodium quinoa Willd.) Germplasm Using Multiplex Fluorescent Microsatellite Markers. Conserv. Genet. 2009; 10. [Google Scholar] [CrossRef]
- Nowak, V.; Du, J.; Charrondière, U.R. Assessment of the Nutritional Composition of Quinoa (Chenopodium quinoa Willd.). Food Chem. 2016; 54. [Google Scholar] [CrossRef]
- Aranda, M.; Vega-Galvez, A.; Quispe, I.; Rodriguez, M.; Martínez, E. Nutritional Aspects of Six Quinoa (Chenopodium quinoa Willd.) Ecotypes from the Geographical Areas of Chile. Chil. J. Agric. Res. 2012, 72, 175–181.
- Yasui, Y.; Hirakawa, H.; Oikawa, T.; Toyoshima, M.; Matsuzaki, C.; Ueno, M.; Mizuno, N.; Nagatoshi, Y.; Imamura, T.; Miyago, M.; et al. Draft Genome Sequence of an Inbred Line of Chenopodium quinoa, an Allotetraploid Crop with Great Environmental Adaptability and Outstanding Nutritional Properties. DNA Res. 2016, 23, 215–224. [Google Scholar] [CrossRef]
- Ruiz Carrasco, K.; Maldonado, J.; Biondi, S.; Silva, H. RNA-Seq Analysis of Salt-Stressed Versus Non Salt-Stressed Transcriptomes of Chenopodium quinoa Landrace R49. Genes 2019, 10, 1042. [Google Scholar] [CrossRef]
- Sobota, A.; Swieca, M.; Gesinski, K.; Wirkijowska, A.; Bochnak-Niedźwiecka, J. Yellow-coated Quinoa (Chenopodium quinoa Willd)—Physicochemical, Nutritional, and Antioxidant Properties. J. Sci. Food Agric. 2019, 100, 2035–2042. [Google Scholar] [CrossRef]
- Bose, J.; Rodrigo-Moreno, A.; Lai, D.; Xie, Y.; Shen, W.; Shabala, S. Rapid Regulation of the Plasma Membrane H+-ATPase Activity Is Essential to Salinity Tolerance in Two Halophyte Species, Atriplex lentiformis and Chenopodium quinoa. Ann. Bot. 2015, 115, 481–494. [Google Scholar] [CrossRef]
- Gonçalves, A.; Goufo, P.; Barros, A.; Domínguez-Perles, R.; Trindade, H.; Rosa, E.A.S.; Ferreira, L.; Rodrigues, M. Cowpea (Vigna unguiculata L. Walp), a Renewed Multipurpose Crop for a More Sustainable Agri-Food System: Nutritional Advantages and Constraints. J. Sci. Food Agric. 2016; 96. [Google Scholar] [CrossRef]
- Jayathilake, C.; Visvanathan, R.; Deen, A.; Bangamuwage, R.; Jayawardana, B.C.; Nammi, S.; Liyanage, R. Cowpea: An Overview on Its Nutritional Facts and Health Benefits. J. Sci. Food Agric. 2018, 98, 4793–4806. [Google Scholar] [CrossRef]
- Domínguez-Perles, R.; Machado, N.; Abraão, A.S.; Carnide, V.; Ferreira, L.; Rodrigues, M.; Rosa, E.A.D.S.; Barros, A.I.R.N.A. Chemometric Analysis on Free Amino Acids and Proximate Compositional Data for Selecting Cowpea (Vigna unguiculata L.) Diversity. J. Food Compos. Anal. 2016; 53. [Google Scholar] [CrossRef]
- Ehlers, J.D.; Hall, A.E. Cowpea (Vigna unguiculata L. Walp.). Field Crops Res. 1997; 53. [Google Scholar] [CrossRef]
- Amorim, L.L.B.; Ferreira-Neto, J.R.C.; Bezerra-Neto, J.P.; Pandolfi, V.; de Araújo, F.T.; da Silva Matos, M.K.; Santos, M.G.; Kido, E.A.; Benko-Iseppon, A.M. Cowpea and Abiotic Stresses: Identification of Reference Genes for Transcriptional Profiling by QPCR. Plant Methods 2018, 14, 1–17. [Google Scholar] [CrossRef]
- Goufo, P.; Moutinho-Pereira, J.M.; Jorge, T.F.; Correia, C.M.; Oliveira, M.R.; Rosa, E.A.S.; António, C.; Trindade, H. Cowpea (Vigna uunguiculata L. Walp.) Metabolomics: Osmoprotection as a Physiological Strategy for Drought Stress Resistance and Improved Yield. Front. Plant Sci. 2017; 8. [Google Scholar] [CrossRef]
- Hashiguchi, A.; Zhu, W.; Tian, J.; Komatsu, S. Proteomics and Metabolomics-Driven Pathway Reconstruction of Mung Bean for Nutraceutical Evaluation. BBA-Proteins Proteom. 2017. [Google Scholar] [CrossRef]
- Akundabweni, L.S.M.; Maina, D.; Akundabweni, L. Ionomic Variation Characterization in African Leafy Vegetables for Micronutrients Using XRF and HPLC. Afr. J. Food Agric. Nutr. Dev. 2011, 10, 4320–4339. [Google Scholar] [CrossRef]
- Tang, D.; Dong, Y.; Guo, N.; Li, L.; Ren, H. Metabolomic Analysis of the Polyphenols in Germinating Mung Beans (Vigna rradiata) Seeds and Sprouts. J. Sci. Food Agric. 2014, 94, 1639–1647. [Google Scholar] [CrossRef]
- Haider, M.; Hussain, M.; Farooq, M.; Nawaz, A. Zinc Nutrition for Improving the Productivity and Grain Biofortification of Mungbean. J. Soil Sci. Plant Nutr. 2020, 20, 1321–1325. [Google Scholar] [CrossRef]
- Kangama, C.; Xu, R. Introduction of Sorghum (Sorghum bicolor (L.) Moench) into China. Afr. J. Biotechnol. 2005, 4, 575–579.
- Rhodes, D.H.; Hoffmann, L.; Rooney, W.L.; Ramu, P.; Morris, G.P.; Kresovich, S. Genome-Wide Association Study of Grain Polyphenol Concentrations in Global Sorghum (Sorghum bbicolor (L.) Moench) Germplasm. J. Agric. Food Chem. 2014; 62. [Google Scholar] [CrossRef]
- Kulamarva, A.G.; Sosle, V.R.; Raghavan, G.S.V. Nutritional and Rheological Properties of Sorghum. Int. J. Food Prop. 2009, 12, 55–69. [Google Scholar] [CrossRef]
- Kaplan, M.; Kale, H.; Kardes, Y.M.; Karaman, K.; Kahraman, K.; Yılmaz, M.F.; Temizgül, R.; Akar, T. Characterization of Local Sorghum (Sorghum bbicolor L.) Population Grains in Terms of Nutritional Properties and Evaluation by GT Biplot Approach. 2020; 72. [Google Scholar] [CrossRef]
- Johnson, S.M.; Lim, F.-L.; Finkler, A.; Fromm, H.; Slabas, A.R.; Knight, M.R. Transcriptomic Analysis of Sorghum bicolor Responding to Combined Heat and Drought Stress. BMC Genom. 2014, 15, 456–456. [Google Scholar] [CrossRef] [PubMed]
- Buchanan, C.D.; Lim, S.; Salzman, R.A.; Kagiampakis, I.; Morishige, D.T.; Weers, B.D.; Klein, R.R.; Pratt, L.H.; Cordonnier-Pratt, M.-M.; Klein, P.E.; et al. Sorghum bicolor’s Transcriptome Response to Dehydration, High Salinity and ABA. Plant Mol. Biol. 2005, 58, 699–720. [Google Scholar] [CrossRef] [PubMed]
- Ogbaga, C.; Stepien, P.; Dyson, B.; Rattray, N.; Ellis, D.; Goodacre, R.; Johnson, G. Biochemical Analyses of Sorghum Varieties Reveal Differential Responses to Drought. PLoS ONE 2016, 11, e0154423. [Google Scholar] [CrossRef]
- Swamy, A.K.; Alam, S.; Sengupta, N.; Sarina, R. Differential Proteomic Analysis of Salt Stress Response in Sorghum bbicolor Leaves. Environ. Exp. Bot. 2011, 71, 321–328. [Google Scholar] [CrossRef]
- Ferraro, V.; Piccirillo, C.; Tomlins, K.; Pintado, M.E. Cassava (Manihot esculenta Crantz) and Yam (Dioscorea Spp.) Crops and Their Derived Foodstuffs: Safety, Security and Nutritional Value. Crit. Rev. Food Sci. Nutr. 2016; 56. [Google Scholar] [CrossRef]
- Siriwat, W.; Kalapanulak, S.; Suksangpanomrung, M.; Netrphan, S.; Meechai, A.; Saithong, T. Transcriptomic Data Integration Inferring the Dominance of Starch Biosynthesis in Carbon Utilization of Developing Cassava Roots. Procedia Comput. Sci. 2012, 11, 96–106. [Google Scholar] [CrossRef][Green Version]
- Salvador, E.; Steenkamp, V.; Mccrindle, C. Production, Consumption and Nutritional Value of Cassava (Manihot esculenta, Crantz) in Mozambique: An Overview. J. Agric. Biotechnol. Sustain. Dev. 2014, 6, 29–38. [Google Scholar] [CrossRef]
- Rabbi, I.; Udoh, L.; Wolfe, M.; Parkes, E.; Gedil, M.; Dixon, A.; Ramu, P.; Jannink, J.-L.; Kulakow, P. Genome-Wide Association Mapping of Correlated Traits in Cassava: Dry Matter and Total Carotenoid Content. Plant Genome 2017, 10, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Feng, R.J.; Ren, M.Y.; Lu, L.F.; Peng, M.; Guan, X.; Zhou, D.B.; Zhang, M.Y.; Qi, D.F.; Li, K.; Tang, W.; et al. Involvement of Abscisic Acid-Responsive Element-Binding Factors in Cassava (Manihot esculenta) Dehydration Stress Response. Sci. Rep. 2019, 9, 12661. [Google Scholar] [CrossRef]
- Utsumi, Y.; Tanaka, M.; Morosawa, T.; Kurotani, A.; Yoshida, T.; Mochida, K.; Matsui, A.; Umemura, Y.; Ishitani, M.; Shinozaki, K.; et al. Transcriptome Analysis Using a High-Density Oligomicroarray under Drought Stress in Various Genotypes of Cassava: An Important Tropical Crop. DNA Res. 2012, 19, 335–345. [Google Scholar] [CrossRef]
- Lokko, Y.; Anderson, J.V.; Rudd, S.; Raji, A.; Horvath, D.; Mikel, M.A.; Kim, R.; Liu, L.; Hernandez, A.; Dixon, A.G.O.; et al. Characterization of an 18,166 EST Dataset for Cassava (Manihot esculenta Crantz) Enriched for Drought-Responsive Genes. Plant Cell Rep. 2007, 26, 1605–1618. [Google Scholar] [CrossRef]
- Wei, Y.; Shi, H.; Xia, Z.; Tie, W.; Ding, Z.; Yan, Y.; Wang, W.; Hu, W.; Li, K. Genome-Wide Identification and Expression Analysis of the WRKY Gene Family in Cassava. Front. Plant Sci. 2016, 7, 1–18. [Google Scholar] [CrossRef]
- An, F.; Li, G.; Li, Q.; Li, K.; Carvalho, L.; Ou, W.; Chen, S. The Comparatively Proteomic Analysis in Response to Cold Stress in Cassava Plantlets. Plant Mol. Biol. Report. 2016, 34, 1095–1110. [Google Scholar] [CrossRef]
- O’Brien, G.K.; Price, M.L. Amaranth: Grain and Vegetable Types; Echo Technical Note: Bangui, Central Africa, 2008. [Google Scholar]
- Bressani, R. The Proteins of Grain Amaranth. Food Rev. Int. 1989, 5, 13–38. [Google Scholar] [CrossRef]
- Alegbejo, J. Nutritional Value and Utilization of Amaranthus (Amaranthus spp.)—A Review. Bayero J. Pure Appl. Sci. 2014; 6. [Google Scholar] [CrossRef]
- Sunil, M.; Hariharan, A.K.; Nayak, S.; Gupta, S.; Nambisan, S.; Gupta, R.; Panda, B.; Choudhary, B.; Srinivasan, S. The Draft Genome and Transcriptome of Amaranthus hypochondriacus: A C4 Dicot Producing High-Lysine Edible Pseudo-Cereal. DNA Res. 2014, 21, 585–602. [Google Scholar] [CrossRef]
- Huerta-Ocampo, J.Á.; Briones-Cerecero, E.P.; Mendoza-Hernández, G.; De León-Rodríguez, A.; Barba de la Rosa, A.P. Proteomic Analysis of Amaranth (Amaranthus hypochondriacus L.) Leaves under Drought Stress. Int. J. Plant Sci. 2009. [Google Scholar] [CrossRef]
- Lokhande, V.; Nikam, T.; Penna, S. Sesuvium portulacastrum (L.) L. A Promising Halophyte: Cultivation, Utilization and Distribution in India. Genet. Resour. Crop Evol. 2009; 56. [Google Scholar] [CrossRef]
- Zeng, H.-C.; Deng, L.-H.; Zhang, C.-F. Cloning of Salt Tolerance-Related CDNAs from the Mangrove Plant Sesuvium portulacastrum L. J. Integr. Plant Biol. 2006, 48, 952–957. [Google Scholar] [CrossRef]
- Fan, W.; Zhang, Z.; Zhang, Y. Cloning and Molecular Characterization of Fructose-1,6-Bisphosphate Aldolase Gene Regulated by High-Salinity and Drought in Sesuvium portulacastrum. Plant Cell Rep. 2009, 28, 975–984. [Google Scholar] [CrossRef] [PubMed]
- Venkatesalu, V.; Kumar, R.R.; Chellappan, K.P. Growth and Mineral Distribution of Sesuvium portulacastrum l., a Salt Marsh Halophyte, under Sodium Chloride Stress. Commun. Soil Sci. Plant Anal. 1994; 25. [Google Scholar] [CrossRef]
- Mohanraj, R.; Sivasankar, S. Sweet Potato (Ipomoea batatas [L.] Lam)—A Valuable Medicinal Food: A Review. J. Med. 2014; 17. [Google Scholar] [CrossRef]
- Tao, X.; Gu, Y.-H.; Wang, H.-Y.; Zheng, W.; Li, X.; Zhao, C.-W.; Zhang, Y.-Z. Digital Gene Expression Analysis Based on Integrated de Novo Transcriptome Assembly of Sweet Potato (Ipomoea batatas (L.) Lam). 2012; 7. [Google Scholar] [CrossRef]
- Wang, A.; Li, R.; Ren, L.; Gao, X.; Zhang, Y.; Ma, Z.; Ma, D.; Luo, Y. A Comparative Metabolomics Study of Flavonoids in Sweet Potato with Different Flesh Colors (Ipomoea batatas (L.) Lam). Food Chem. 2018. [Google Scholar] [CrossRef]
- Teow, C.C.; Truong, V.; McFeeters, R.F.; Thompson, R.L.; Pecota, K.V.; Yencho, G.C. Antioxidant Activities, Phenolic and β-Carotene Contents of Sweet Potato Genotypes with Varying Flesh Colours. Food Chem. 2007, 103, 829–838. [Google Scholar] [CrossRef]
- Luo, Y.; Reid, R.; Freese, D.; Li, C.; Watkins, J.; Shi, H.; Zhang, H.; Loraine, A.; Song, B.-H. Salt Tolerance Response Revealed by RNA-Seq in a Diploid Halophytic Wild Relative of Sweet Potato. Sci. Rep. 2017, 7, 1–13. [Google Scholar] [CrossRef]
- Price, E.J.; Bhattacharjee, R.; Lopez-Montes, A.; Fraser, P.D. Metabolite Profiling of Yam (Dioscorea Spp.) Accessions for Use in Crop Improvement Programmes. Metabolomics Off. J. Metab. Soc. 2017; 13. [Google Scholar] [CrossRef]
- Padhan, B.; Panda, D. Potential of Neglected and Underutilized Yams (Dioscorea Spp.) for Improving Nutritional Security and Health Benefits. Front. Pharmacol. 2020; 11. [Google Scholar] [CrossRef]
- Sugihara, Y.; Darkwa, K.; Yaegashi, H.; Natsume, S.; Shimizu, M.; Abe, A.; Hirabuchi, A.; Ito, K.; Oikawa, K.; Oli, M.T.; et al. Genome Analyses Reveal the Hybrid Origin of the Staple Crop White Guinea Yam (Dioscorea rotundata). Proc. Natl. Acad. Sci. USA 2020, 117, 31987–31992. [Google Scholar] [CrossRef]
- Iranshahy, M.; Javadi, B.; Iranshahi, M.; Jahanbakhsh, S.P.; Mahyari, S.; Hassani, F.V.; Karimi, G. A Review of Traditional Uses, Phytochemistry and Pharmacology of Portulaca oleracea L. J. Ethnopharmacol. 2017, 205, 158–172. [Google Scholar] [CrossRef]
- Petropoulos, S.; Karkanis, A.; Martins, N.; Ferreira, I.C.F.R. Phytochemical Composition and Bioactive Compounds of Common Purslane (Portulaca oleracea L.) as Affected by Crop Management Practices. Trends Food Sci. Technol. 2016; 55. [Google Scholar] [CrossRef]
- Adams, S. Purslane Eyed as a Rich Food Source. Agric. Res. 1992, 40, 20–21. [Google Scholar]
- Farag, M.A.; Shakour, Z.T.A. Metabolomics Driven Analysis of 11 Portulaca Leaf Taxa as Analysed via UPLC-ESI-MS/MS and Chemometrics. Phytochemistry 2019, 161, 117–129. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Howe, P.; Zhou, Y.-F.; Xu, Z.-Q.; Hocart, C.; Zhang, R. Fatty Acids and β-Carotene in Australian Purslane (Portulaca oleracea) Varieties. J. Chromatogr. A 2000, 893, 207–213. [Google Scholar] [CrossRef]
- Shenstone, E.; Lippman, Z.; Van Eck, J. A Review of Nutritional Properties and Health Benefits of Physalis Species. Plant Foods Hum. Nutr. Dordr. Neth. 2020, 75, 316–325. [Google Scholar] [CrossRef] [PubMed]
- Dello-Russo, R. Climatic Stress in the Middle Rio Grande Valley of New Mexico: An Evaluation of Changes in Foraging Behaviors During the Late Archaic / Basketmaker II Period. Ph.D. Thesis, University of New Mexico, Albuquerque, NM, USA, 1999. [Google Scholar]
- Kindscher, K.; Long, Q.; Corbett, S.; Bosnak, K.; Loring, H.; Cohen, M.; Timmermann, B. The Ethnobotany and Ethnopharmacology of Wild Tomatillos, Physalis longifolia Nutt., and Related Physalis Species: A Review. Econ. Bot. 2012; 66. [Google Scholar] [CrossRef]
- Yu, Y.; Chen, X.; Zheng, Q. Metabolomic Profiling of Carotenoid Constituents in Physalis peruviana During Different Growth Stages by LC-MS/MS Technology. J. Food Sci. 2019, 84, 3608–3613. [Google Scholar] [CrossRef]
- Kambhar, S.V. Rumex vesicarius L. (Polygonaceae): An Overview. J. Glob. Ecol. Environ. 2014, 1, 11–14.
- El-Hawary, S.A.; Sokkar, N.M.; Ali, Z.Y.; Yehia, M.M. A Profile of Bioactive Compounds of Rumex vesicarius L. J. Food Sci. 2011, 76, 1195–1202. [Google Scholar] [CrossRef] [PubMed]
- Chippindale, C. Before Scotland: The Story of Scotland before History—Alistair Moffat. J. R. Anthropol. Inst. 2006, 12, 679–680. [Google Scholar] [CrossRef]
- Enescu, C.; Durrant, T.; de Rigo, D.; Caudullo, G. Corylus avellana in Europe: Distribution, Habitat, Usage and Threats. Eur. Atlas For. Tree Species 2016, 54, 86–87. [Google Scholar]
- Alasalvar, C.; Shahidi, F.; Liyanapathirana, C.M.; Ohshima, T. Turkish Tombul Hazelnut (Corylus avellana L.). 1. Compositional Characteristics. J. Agric. Food Chem. 2003; 51. [Google Scholar] [CrossRef]
- Köksal, A.; Artik, N.; Şimşek, A.; Gunes, N. Nutrient Composition of Hazelnut (Corylus avellana L.) Varieties Cultivated in Turkey. Food Chem. 2006; 99. [Google Scholar] [CrossRef]
- Cristofori, V.; Ferramondo, S.; Bertazza, G.; Bignami, C. Nut and Kernel Traits and Chemical Composition of Hazelnut (Corylus avellana L.) Cultivars. J. Sci. Food Agric. 2008; 88. [Google Scholar] [CrossRef]
- Ahmad, M. ; Gul-Zaffar; Dar, Z.; Habib, M. A Review on Oat (Avena sativa L.) as a Dual-Purpose Crop. Sci. Res. 2014; 9. [Google Scholar] [CrossRef]
- Ishida, Y.; Hiei, Y.; Komari, T. High-Efficiency Transformation Techniques. In Applications of Genetic and Genomic Research in Cereals; Miedaner, T., Korzun, V., Eds.; Woodhead Publishing: Sawston, UK, 2019; pp. 97–120. [Google Scholar]
- Ibrahim, M.S.; Ahmad, A.; Sohail, A.; Asad, M.J. Nutritional and Functional Characterization of Different Oat (Avena sativa L.) Cultivars. Int. J. Food Prop. 2020; 23. [Google Scholar] [CrossRef]
- Foresman, B.J.; Oliver, R.E.; Jackson, E.W.; Chao, S.; Arruda, M.P.; Kolb, F.L. Genome-Wide Association Mapping of Barley Yellow Dwarf Virus Tolerance in Spring Oat (Avena sativa L.). 2016; 11. [Google Scholar] [CrossRef]
- Flores, H.E.; Galston, A.W. Osmotic Stress-Induced Polyamine Accumulation in Cereal Leaves: I. Physiological Parameters of the Response. Plant Physiol. 1984, 75, 102–109. [Google Scholar] [CrossRef]
- Pathak, R.; Thakur, V.; Gupta, R.K. Nutritional Analysis of Cereal Bars Formulated Using Morinda citrifolia and Bacopa monnieri. J. Pharmacogn. Phytochem. 2018, 7, 1546–1549. [Google Scholar]
- Prabhudas, S.K.; Natarajan, P. De Novo Assembly of Transcriptome and Draft Chloroplast Genome from RNAseq Data of Bacopa monnieri L. (Bramhi). Can. J. Biotechnol. 2017; 1. [Google Scholar] [CrossRef]
- Debnath, M. Responses of Bacopa monnieri to Salinity and Drought Stress in Vitro. J. Med. Plants Res. 2008, 2, 347–351. [Google Scholar] [CrossRef]
- Fordham, I.; Clevidence, B.; Wiley, E.; Zimmerman, R. Fruit of Autumn Olive: A Rich Source of Lycopene. HortScience 2001, 36, 1136–1137. [Google Scholar] [CrossRef]
- Wang, T.; Hou, Y.; Hu, H.; Wang, C.; Weilin, Z.; Li, H.; Cheng, Z.; Yang, L. Functional Validation of Phytoene Synthase and Lycopene ε-Cyclase Genes for High Lycopene Content in Autumn Olive Fruit (Elaeagnus umbellata). J. Agric. Food Chem. 2020, 68, 11503–11511. [Google Scholar] [CrossRef]
- Wu, M.-C.; Hu, H.-T.; Yang, L.; Yang, L. Proteomic Analysis of Up-Accumulated Proteins Associated with Fruit Quality during Autumn Olive (Elaeagnus umbellata) Fruit Ripening. J. Agric. Food Chem. 2011, 59, 577–583. [Google Scholar] [CrossRef]
- Sengupta, S.; Majumder, A.L. Insight into the Salt Tolerance Factors of a Wild Halophytic Rice, Porteresia coarctata: A Physiological and Proteomic Approach. Planta 2009, 229, 911–929. [Google Scholar] [CrossRef]
- Ghosh, R.; Mitra, A. Effect of Salinity on Nutritional Value of Saltmarsh Grass (Porteresia coarctata) from Gangetic Delta, Northeast Coast of India. Indian J. Geo-Mar. Sci. 2015, 44, 1043–1052.
- Khalil, J.; Sawaya, W.N.; Hyder, S.Z. Nutrient Composition of Atriplex Leaves Grown in Saudi Arabia. J. Range Manag. USA 1986, 39, 104–107. [Google Scholar] [CrossRef]
- Ohsako, T.; Ohnishi, O. Intra- and Interspecific Phylogeny of Wild Fagopyrum (Polygonaceae) Species Based on Nucleotide Sequences of Noncoding Regions in Chloroplast DNA. Am. J. Bot. 2000, 87, 573–82. [Google Scholar] [CrossRef]
- Pongrac, P.; Vogel-Mikuš, K.; Potisek, M.; Kovačec, E.; Budič, B.; Kump, P.; Regvar, M.; Kreft, I. Mineral and Trace Element Composition and Importance for Nutritional Value of Buckwheat Grain, Groats, and Sprouts. In Molecular Breeding and Nutritional Aspects of Buckwheat, Zhou, M., Kreft, I., Woo, S.-H., Chrungoo, N., Wieslander, G., Eds.; Academic Press: Cambridge, MA, USA, 2016; pp. 261–271. [Google Scholar]
- Logacheva, M.; Kasianov, A.; Vinogradov, D.; Samigullin, T.; Gelfand, M.; Makeev, V.; Penin, A. De Novo Sequencing and Characterization of Floral Transcriptome in Two Species of Buckwheat (Fagopyrum). BMC Genom. 2011, 12, 1–37. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Khound, R.; Santra, M.; Santra, D.K. Beyond Bird Feed: Proso Millet for Human Health and Environment. Agriculture 2019, 9, 64. [Google Scholar] [CrossRef]
- Zou, C.; Li, L.; Miki, D.; Li, D.; Tang, Q.; Xiao, L.; Rajput, S.; Deng, P.; Peng, L.; Jia, W.; et al. The Genome of Broomcorn Millet. Nat. Commun. 2019, 10, 436. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Kwon, S.-J.; Yu, J.-H.; Sarker, K.; Cho, K.; Moon, Y.-J.; Jung, T.-W.; Park, C.-H.; Woo, S.-H. Comparison of Protein Profiles of Proso Millet (Panicum miliaceum) Seeds of Various Korean Cultivars. Korean J. Crop Sci. 2017, 62, 40–50. [Google Scholar] [CrossRef]
- Kim, J.K.; Park, S.-Y.; Yeo, Y.; Cho, H.S.; Kim, Y.B.; Bae, H.; Park, C.H.; Lee, J.-H.; Park, S.U. Metabolic Profiling of Millet (Paniciim miliaceum) Using Gas Chromatography—Time-of-Flight Mass Spectrometry (GC-TOFMS) for Quality Assessment. Plant Omics 2013, 6, 73–80. [Google Scholar]
- Gao, Q.-H.; Wu, C.-S.; Wang, M. The Jujube (Ziziphus jujuba Mill.) Fruit: A Review of Current Knowledge of Fruit Composition and Health Benefits. J. Agric. Food Chem. 2013; 61. [Google Scholar] [CrossRef]
- Chang, X; Sun, J. , Liu, L., He, W. Transcriptome Analysis of Differentially Expressed Genes in Wild Jujube Seedlings under Salt Stress. J. Am. Soc. Hortic. Sci. 2020, 1, 1–12.
- Yang, L.; Jin, J.; Fan, D.; Hao, Q.; Niu, J. Transcriptome Analysis of a Jujube (Ziziphus jujuba Mill.) Cultivar Response to Heat Stress. 2021. [Google Scholar] [CrossRef]
- San, B.; Yildirim, A. Phenolic, Alpha-Tocopherol, Beta-Carotene and Fatty Acid Composition of Four Promising Jujube (Ziziphus jujuba Miller) Selections. J. Food Compos. Anal. 2010, 23, 706–710. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, L.; Liu, Z.; Zhao, Z.; Zhao, J.; Wang, Z.; Zhou, G.; Liu, P.; Liu, M. Transcriptome and Metabolome Profiling Unveil the Mechanisms of Ziziphus jujuba Mill. Peel Coloration. Food Chem. 2019, 312, 125903. [Google Scholar] [CrossRef]
- Ramírez, F. Notes about Lulo (Solanum quitoense Lam.): An Important South American Underutilized Plant. Genet. Resour. Crop Evol. 2021; 68. [Google Scholar] [CrossRef]
- Gade, D.W. Ethnobotany of Cañihua (Chenopodium pallidicaule), Rustic Seed Crop of the Altiplano. Econ. Bot. 1970, 24, 55–61. [Google Scholar] [CrossRef]
- Martin, I. Fruits for the Future. 8. Monkey Orange. Strychnos cocculoides. By C. K. Mwamba. Southampton, UK: Southampton Centre for Underutilised Crops (2006), pp. 98, available free on request to national scientists of developing countries. ISBN 0854328416 Exp. Agric. 2007; 43. [Google Scholar] [CrossRef]
- Sebastin, R.; Lee, G.A.; Lee, K.J.; Shin, M.J.; Cho, G.T.; Lee, J.R.; Ma, K.H.; Chung, J.W. The Complete Chloroplast Genome Sequences of Little Millet (Panicum sumatrense Roth ex Roem. and Schult.) (Poaceae). Mitochondrial DNA Part B Resours. 2018. 3, 719-720. [CrossRef]
- Das, R.; Pradhan, S.; Parida, A. De-Novo Transcriptome Analysis Unveils Differentially Expressed Genes Regulating Drought and Salt Stress Response in Panicum sumatrense. Sci. Rep. 2020, 10, 1–14. [Google Scholar] [CrossRef]
- Amaranthus in Flora of North America @ Efloras. Available online: http://www.efloras.org/florataxon.aspx?flora_id=1&taxon_id=101257 (accessed on 7 June 2021).
- Das, S. Amaranthus: A Promising Crop of Future; Springer: Cham, Switzerland, 2016. [Google Scholar]
- Joshi, D.C.; Sood, S.; Hosahatti, R.; Kant, L.; Pattanayak, A.; Kumar, A.; Yadav, D.; Stetter, M.G. From Zero to Hero: The Past, Present and Future of Grain Amaranth Breeding. Theor. Appl. Genet. 2018, 131, 1807–1823. [Google Scholar] [CrossRef]
- Das, S. Domestication, Phylogeny and Taxonomic Delimitation in Underutilized Grain Amaranthus (Amaranthaceae)—A Status Review. Feddes Repert. 2012, 123, 273–282. [Google Scholar] [CrossRef]
- Alemayehu, F.R.; Bendevis, M.A.; Jacobsen, S.-E. The Potential for Utilizing the Seed Crop Amaranth (Amaranthus Spp.) in East Africa as an Alternative Crop to Support Food Security and Climate Change Mitigation. J. Agron. Crop Sci. 2015. [Google Scholar] [CrossRef]
- Venskutonis, R.; Kraujalis, P. Nutritional Components of Amaranth Seeds and Vegetables: A Review on Composition, Properties, and Uses. Compr. Rev. Food Sci. Food Saf. 2013, 12, 381–412. [Google Scholar] [CrossRef] [PubMed]
- Tucker, J.B. Amaranth: The Once and Future Crop. BioScience 1986, 36, 9–13. [Google Scholar] [CrossRef]
- Rastogi, D.A.; Shukla, S. Amaranth: A New Millennium Crop of Nutraceutical Values. Crit. Rev. Food Sci. Nutr. 2013, 53, 109–125. [Google Scholar] [CrossRef]
- Montgomery, J.S.; Giacomini, D.; Waithaka, B.; Lanz, C.; Murphy, B.P.; Campe, R.; Lerchl, J.; Landes, A.; Gatzmann, F.; Janssen, A.; et al. Draft Genomes of Amaranthus tuberculatus, Amaranthus hybridus, and Amaranthus palmeri. Genome Biol. Evol. 2020, 12, 1988–1993. [Google Scholar] [CrossRef]
- Chevarria-Lazo, M.; Bazile, D.; dessauw, D.; Louafi, S.; Trommetter, M.; Hocdé, H. Quinoa and the exchange of genetic resources: Improving the regulation systems. In State of the Art Report on Quinoa around the World in 2013; FAO Regional Office for Latin America and the Caribbean: Rome, Italy, 2015; pp. 83–105. [Google Scholar]
- Jarvis, D.E.; Ho, Y.S.; Lightfoot, D.J.; Schmöckel, S.M.; Li, B.; Borm, T.J.A.; Ohyanagi, H.; Mineta, K.; Michell, C.T.; Saber, N.; et al. The Genome of Chenopodium quinoa. Nature 2017, 542, 307–312. [Google Scholar] [CrossRef] [PubMed]
- Golicz, A.A.; Steinfort, U.; Arya, H.; Singh, M.B.; Bhalla, P.L. Analysis of the Quinoa Genome Reveals Conservation and Divergence of the Flowering Pathways. Funct. Integr. Genom. 2020, 20, 245–258. [Google Scholar] [CrossRef]
- Xu, J.; Duan, X.; Yang, J.; Beeching, J.R.; Zhang, P. Coupled Expression of Cu/Zn-Superoxide Dismutase and Catalase in Cassava Improves Tolerance against Cold and Drought Stresses. Plant Signal. Behav. 2013, 8, e24525. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Feng, B.; Xiao, J.; Xia, Z.; Xincheng, Z.; Li, P.; Zhang, W.; Wang, Y.; Møller, B.; Zhang, P.; et al. Cassava Genome from a Wild Ancestor to Cultivated Varieties. Nat. Commun. 2014, 10, 1–9. [Google Scholar] [CrossRef]
- Briones-Labarca, V.; Giovagnoli-Vicuña, C.; Figueroa-Alvarez, P.; Quispe, I.; Pérez-Won, M. Extraction of β -Carotene, Vitamin C and Antioxidant Compounds from Physalis peruviana (Cape Gooseberry) Assisted by High Hydrostatic Pressure. Food Nutr. Sci. 2013, 4, 109–118. [Google Scholar] [CrossRef]
- Wu, Z.-G.; Jiang, W.; Mantri, N.; Bao, X.-Q.; Chen, S.-L.; Tao, Z.-M. Transciptome Analysis Reveals Flavonoid Biosynthesis Regulation and Simple Sequence Repeats in Yam (Dioscorea alata L.) Tubers. BMC Genom. 2015; 16. [Google Scholar] [CrossRef]
- Garzón-Martínez, G.A.; Zhu, Z.I.; Landsman, D.; Barrero, L.S.; Mariño-Ramírez, L. The Physalis peruviana Leaf Transcriptome: Assembly, Annotation and Gene Model Prediction. BMC Genom. 2012, 13, 151. [Google Scholar] [CrossRef]
- Gao, H.; Li, J.; Wang, L.; Zhang, J.; He, C. Transcriptomic Variation of the Flower-Fruit Transition in Physalis and Solanum. Planta 2020, 252, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Maathuis, F.; Diatloff, E. Roles and Functions of Plant Mineral Nutrients. Methods Mol. Biol. 2013, 953, 1–21. [Google Scholar] [CrossRef]
- Singh, B.; Schulze, D.G.; Soil Minerals and Plant Nutrition. Nature Education Knowledge. Available online: https://www.nature.com/scitable/knowledge/library/soil-minerals-and-plant-nutrition-127881474/ (accessed on 5 July 2021).
- Chrispeels, M.J.; Crawford, N.M.; Schroeder, J.I. Proteins for Transport of Water and Mineral Nutrients across the Membranes of Plant Cells. Plant Cell 1999, 11, 661–676. [Google Scholar] [CrossRef]
- Foy, C.D.; Chaney, R.L.; White, M.C. The Physiology of Metal Toxicity in Plants. Annu. Rev. Plant Physiol. 1978, 29, 511–566. [Google Scholar] [CrossRef]
- Gill, M. Heavy Metal Stress in Plants: A Review. Int. J. Adv. Res. 2014, 2, 1043–1055. [Google Scholar]
- Schilter, B.; Andersson, C.; Anton, R.; Constable, A.; Kleiner, J.; O’Brien, J.; Renwick, A.G.; Korver, O.; Smit, F.; Walker, R.; et al. Guidance for the Safety Assessment of Botanicals and Botanical Preparations for Use in Food and Food Supplements. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2003, 41, 1625–1649. [Google Scholar] [CrossRef]
- Salt, D.; Baxter, I.; Lahner, B. Ionomics and the Study of the Plant Ionome. Annu. Rev. Plant Biol. 2008, 59, 709–33. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.-Y.; Salt, D.E. Plant Ionomics: From Elemental Profiling to Environmental Adaptation. Mol. Plant 2016, 9, 787–797. [Google Scholar] [CrossRef] [PubMed]
- Salt, D.E. Update on Plant Ionomics. Plant Physiol. 2004, 136, 2451–2456. [Google Scholar] [CrossRef]
- Migeon, A.; Blaudez, D.; Wilkins, O.; Montanini, B.; Campbell, M.M.; Richaud, P.; Thomine, S.; Chalot, M. Genome-Wide Analysis of Plant Metal Transporters, with an Emphasis on Poplar. Cell. Mol. Life Sci. 2010, 67, 3763–3784. [Google Scholar] [CrossRef] [PubMed]
- Nandal, U.; Bhardwaj, R.L. The Role of Underutilized Fruits in Nutritional and Economic Security of Tribals: A Review. Crit. Rev. Food Sci. Nutr. 2014, 54, 880–890. [Google Scholar] [CrossRef] [PubMed]
- Bhowmik, S.; Datta, B.K.; Saha, A. Determination of Mineral Content and Heavy Metal Content of Some Traditionally Important Aquatic Plants of Tripura, India Using Atomic Absorption Spectroscopy. J. Agric. Technol. 2012, 8, 1467–1476. [Google Scholar]
- Chacha, J.; Laswai, H. Micronutrients Potential of Underutilized Vegetables and Their Role in Fighting Hidden Hunger. Int. J. Food Sci. 2020, 2020, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Satismruti, K.; Natesan, S.; Sampathrajan, V.; Raja, R.; Muthurajan, R. Plant Ionomics: A Platform for Identifying Novel Gene Regulating Plant Mineral Nutrition. Am. J. Plant Sci. 2013, 447162, 1309–1315. [Google Scholar] [CrossRef]
- Elshamy, M.M.; Heikal, Y.M.; Bonanomi, G. Phytoremediation Efficiency of Portulaca oleracea L. Naturally Growing in Some Industrial Sites, Dakahlia District, Egypt. 2019. [Google Scholar] [CrossRef]
- Amirul Alam, M.; Juraimi, A.S.; Rafii, M.Y.; Hamid, A.A.; Kamal Uddin, M.; Alam, M.Z.; Latif, M.A. Genetic Improvement of Purslane (Portulaca oleracea L.) and Its Future Prospects. Mol. Biol. Rep. 2014; 41. [Google Scholar] [CrossRef]
- Kumar, A.; Sreedharan, S.; Achigan-Dako, E.G.; Singh, P.; Ramchiary, N. Improvement of a Traditional Orphan Food Crop, Portulaca oleracea L. (Purslane) Using Genomics for Sustainable Food Security and Climate Resilient Agriculture. Front. Sustain. Food Syst. 2021. [Google Scholar] [CrossRef]
- Xing, J.; Zhao, B.; Dong, J.; Liu, C.; Wen, Z.; Zhu, X.; Ding, H.; He, T.; Yang, H.; Wang, M.; et al. Transcriptome and Metabolome Profiles Revealed Molecular Mechanisms Underlying Tolerance of Portulaca oleracea to Saline Stress. Russ. J. Plant Physiol. 2020, 67, 146–152. [Google Scholar] [CrossRef]
- Patel, S. Plant Genus Elaeagnus: Underutilized Lycopene and Linoleic Acid Reserve with Permaculture Potential. Fruits 2015, 70, 191–199. [Google Scholar] [CrossRef]
- Mustafa, A.; Ahmed, A.; Tantray, A.; Parry, P. Ethnopharmacological Potential and Medicinal Uses of Miracle Herb Dioscorea Spp. J. Ayurvedic Herb. Med. 2018, 4, 79–85. [Google Scholar]
- Akoroda, M.O. Yams: Dioscorea spp. In Genetic Improvement of Vegetable Crops; Kalloo, G., Bergh, B.O., Eds.; Pergamon: Amsterdam, The Netherlands, 1993; pp. 717–733. [Google Scholar]
- Sharma, S.; Deswal, R. Genomic and Proteomic Tools for Understanding Mysterious Protein Dioscorin from Dioscorea Tuber. In; Plant Omics and Crop Breeding, Zargar, S.M., Rai, V., Eds.; Apple Academic Academic Press: Boca Raton, USA, 2016; pp. 97–114. [Google Scholar]
- Nakayasu, M.; Kawasaki, T.; Lee, H.; Sugimoto, Y.; Onjo, M.; Muranaka, T.; Mizutani, M. Identification of Furostanol Glycoside 26-O-β-Glucosidase Involved in Steroidal Saponin Biosynthesis from Dioscorea esculenta. Plant Biotechnol. 2015, 32, 1015–1023. [Google Scholar] [CrossRef]
- Zhou, W.; Li, B.; Li, L.; Ma, W.; Liu, Y.; Feng, S.; Wang, Z. Genome Survey Sequencing of Dioscorea zingiberensis. Genome 2018, 61, 567–574. [Google Scholar] [CrossRef]
- Valdivia, M.; Tecante, A. Chia (Salvia hispanica): A Review of Native Mexican Seed and Its Nutritional and Functional Properties. Adv. Food Nutr. Res. 2015, 75, 53–75. [Google Scholar] [CrossRef]
- Tacer-Caba, Z. The concept of superfoods in diet. In The Role of Alternative and Innovative Food Ingredients and Products in Consumer Wellness; Galanakis, C.M., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 73–101. [Google Scholar]
- Orona-Tamayo, D.; Valverde, M.; Paredes-Lopez, O. Chia-The New Golden Seed for the 21st Century: Nutraceutical Properties and Technological Uses. In Sustainable Protein Sources, 1st ed.; Academic Press: London, UK, 2016; pp. 265–281. [Google Scholar]
- Melo, D.; Machado, T.; Oliveira, M. Chia Seeds: An Ancient Grain Trending in Modern Human Diets. Food Funct. 2019, 10, 3068–3089. [Google Scholar] [CrossRef]
- Hao, D.-C.; Ge, G.-B.; Xiao, P.-G. Anticancer Drug Targets of Salvia Phyto metabolites: Chemistry, Biology and Omics. Curr. Drug Targets 2018, 19, 1–20. [Google Scholar] [CrossRef]
- Ullah, R.; Nadeem, M.; Khalique, A.; Imran, M.; Mehmood, S.; Javid, A.; Hussain, J. Nutritional and Therapeutic Perspectives of Chia (Salvia hispanica L.): A Review. J. Food Sci. Technol. 2015; 53. [Google Scholar] [CrossRef]
- Parker, J.; Schellenberger, A.N.; Roe, A.L.; Oketch-Rabah, H.; Calderón, A.I. Therapeutic Perspectives on Chia Seed and Its Oil: A Review. Planta Med. 2018, 84, 606–612. [Google Scholar] [CrossRef]
- Peláez, P.; Orona-Tamayo, D.; Montes-Hernández, S.; Valverde, M.; Paredes-Lopez, O.; Cibrian, A. Comparative Transcriptome Analysis of Cultivated and Wild Seeds of Salvia hispanica (Chia). Sci. Rep. 2019, 9, 1–11. [Google Scholar] [CrossRef]
- Sreedhar, R.V.; Kumari, P.; Rupwate, S.; Rajasekharan, R.; Srinivasan, M. Exploring Triacylglycerol Biosynthetic Pathway in Developing Seeds of Chia (Salvia hispanica L.): A Transcriptomic Approach. 2015; 10. [Google Scholar] [CrossRef]
- Wiehle, M.; Prinz, K.; Kehlenbeck, K.; Goenster, S.; Mohamed, S.A.; Finkeldey, R.; Buerkert, A.; Gebauer, J. The African Baobab (Adansonia digitata, Malvaceae): Genetic Resources in Neglected Populations of the Nuba Mountains, Sudan. Am. J. Bot. 2014, 101, 1498–1507. [Google Scholar] [CrossRef] [PubMed]
- Rahul, J.; Jain, M.K.; Singh, S.P.; Kamal, R.K. ; Anuradha; Naz, A.; Gupta, A.K.; Mrityunjay, S.K. Adansonia digitata L. (Baobab): A Review of Traditional Information and Taxonomic Description. Asian Pac. J. Trop. Biomed. 2015; 5. [Google Scholar] [CrossRef]
- Chládová, A.; Kalousová, M.; Mandák, B.; Kehlenbeck, K.; Prinz, K.; Šmíd, J.; Van Damme, P.; Lojka, B. Genetic Diversity and Structure of Baobab (Adansonia digitata L.) in Southeastern Kenya. R. Soc. Open Sci. 2019; 6. [Google Scholar] [CrossRef]
- Dillon, S.L.; Shapter, F.M.; Henry, R.J.; Cordeiro, G.; Izquierdo, L.; Lee, L.S. Domestication to Crop Improvement: Genetic Resources for Sorghum and Saccharum (Andropogoneae). Ann. Bot. 2007, 100, 975–989. [Google Scholar] [CrossRef]
- Meyer, R.; Purugganan, M. Evolution of Crop Species: Genetics of Domestication and Diversification. Nat. Rev. Genet. 2013, 14, 840–52. [Google Scholar] [CrossRef] [PubMed]
- Smykal, P.; Nelson, M.; Berger, J.; Wettberg, E. The Impact of Genetic Changes during Crop Domestication. Agronomy 2018, 8, 119. [Google Scholar] [CrossRef]
- Poncet, V.; Robert, T.; Sarr, A.; Gepts, P. Quantitative Trait Locus Analyses of the Domestication Syndrome and Domestication Process. Encycl. Plant Crop Sci. 2004, 1069, 1069–1073. [Google Scholar] [CrossRef]
- Simons, K.; Fellers, J.; Trick, H.; Zhang, Z.; Tai, Y.-S.; Gill, B.; Faris, J. Molecular Characterization of the Major Wheat Domestication Gene Q. Genetics 2006, 172, 547–555. [Google Scholar] [CrossRef]
- Salentijn, E.M.J.; Pereira, A.B.; Angenent, G.C.; Linden, C.G. van der; Krens, F.A.; Smulders, M.J.M.; Vosman, B. Plant Translational Genomics: From Model Species to Crops. Mol. Breed. 2007, 20, 1–13. [Google Scholar] [CrossRef]
- Fraser, P.D.; Aharoni, A.; Hall, R.D.; Huang, S.; Giovannoni, J.J.; Sonnewald, U.; Fernie, A.R. Metabolomics Should Be Deployed in the Identification and Characterization of Gene-Edited Crops. Plant J. Cell Mol. Biol. 2020, 102, 897–902. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Wang, Y.; Zhang, R.; Zhang, H.; Gao, C. CRISPR/Cas Genome Editing and Precision Plant Breeding in Agriculture. Annu. Rev. Plant Biol. 2019, 70, 667–697. [Google Scholar] [CrossRef]
- Fernie, A.R.; Yan, J. De Novo Domestication: An Alternative Route toward New Crops for the Future. Mol. Plant 2019, 12, 615–631. [Google Scholar] [CrossRef]
- Zsögön, A.; Cermak, T.; Naves, E.; Notini, M.; Edel, K.; Weinl, S.; Freschi, L.; Voytas, D.; Kudla, J.; Peres, L. De Novo Domestication of Wild Tomato Using Genome Editing. Nat. Biotechnol. 2018, 36, 1211–1216. [Google Scholar] [CrossRef]
- Lemmon, Z.; Reem, N.; Dalrymple, J.; Soyk, S.; Swartwood, K.; Rodriguez-Leal, D.; Eck, J.; Lippman, Z. Rapid Improvement of Domestication Traits in an Orphan Crop by Genome Editing. Nat. Plants 2018, 4, 766–770. [Google Scholar] [CrossRef]
- Ahmar, S.; Saeed, S.; Khan, M.H.; Ullah Khan, S.; Mora-Poblete, F.; Kamran, M.; Faheem, A.; Maqsood, A.; Rauf, M.; Saleem, S.; et al. A Revolution toward Gene-Editing Technology and Its Application to Crop Improvement. Int. J. Mol. Sci. 2020, 21, 5665. [Google Scholar] [CrossRef] [PubMed]
- Maher, M.; Nasti, R.; Vollbrecht, M.; Starker, C.; Clark, M.; Voytas, D. Plant Gene Editing through de Novo Induction of Meristems. Nat. Biotechnol. 2020, 38, 1–6. [Google Scholar] [CrossRef]
- Wang, T.; Zhang, H.; Zhu, H. CRISPR Technology Is Revolutionizing the Improvement of Tomato and Other Fruit Crops. Hortic. Res. 2019, 6, 1–13. [Google Scholar] [CrossRef]
- Van Eck, J. Applying Gene Editing to Tailor Precise Genetic Modifications in Plants. J. Biol. Chem. 2020, 295, 13267–13276. [Google Scholar] [CrossRef]
- Hille, F.; Charpentier, E. CRISPR-Cas: Biology, Mechanisms and Relevance. Philos. Trans. R. Soc. B Biol. Sci. 2016, 371, 1–16. [Google Scholar] [CrossRef]
- Bortesi, L.; Fischer, R. The CRISPR/Cas9 System for Plant Genome Editing and Beyond. Biotechnol. Adv. 2015, 33, 41–52. [Google Scholar] [CrossRef]
- Zhang, J.-P.; Li, X.-L.; Li, G.-H.; Chen, W.; Arakaki, C.; Botimer, G.; Baylink, D.; Zhang, L.; Wen, W.; Fu, Y.-W.; et al. Efficient Precise Knockin with a Double Cut HDR Donor after CRISPR/Cas9-Mediated Double-Stranded DNA Cleavage. Genome Biol. 2017, 18, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Gupta, D.; Bhattacharjee, O.; Mandal, D.; Sen, M.K.; Dey, D.; Dasgupta, A.; Kazi, T.A.; Gupta, R.; Sinharoy, S.; Acharya, K.; et al. CRISPR-Cas9 System: A New-Fangled Dawn in Gene Editing. Life Sci. 2019, 232, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Syombua, E.D.; Zhang, Z.; Tripathi, J.N.; Ntui, V.O.; Kang, M.; George, O.O.; Edward, N.K.; Wang, K.; Yang, B.; Tripathi, L. A CRISPR/Cas9-Based Genome-Editing System for Yam (Dioscorea Spp.). Plant Biotechnol. J. 2021; 19. [Google Scholar] [CrossRef]
- Park, J.; Bae, S.; Kim, J.-S. Cas-Designer: A Web-Based Tool for Choice of CRISPR-Cas9 Target Sites. Bioinformatics 2015, 31, 4014–4016. [Google Scholar] [CrossRef] [PubMed]
- Atkins, P.A.; Voytas, D.F. Overcoming Bottlenecks in Plant Gene Editing. Genome Stud. Mol. Genet. 2020, 54, 79–84. [Google Scholar] [CrossRef]
- Mushtaq, M.; Sakina, A.; Wani, S.H.; Shikari, A.B.; Tripathi, P.; Zaid, A.; Galla, A.; Abdelrahman, M.; Sharma, M.; Singh, A.K.; et al. Harnessing Genome Editing Techniques to Engineer Disease Resistance in Plants. Front. Plant Sci. 2019, 10, 550. [Google Scholar] [CrossRef] [PubMed]
- Soundararajan, P.; Won, S.; Kim, J. Insight on Rosaceae Family with Genome Sequencing and Functional Genomics Perspective. BioMed Res. Int. 2019, 2019, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Iwata, H.; Gaston, A.; Remay, A.; Thouroude, T.; Jeauffre, J.; Kawamura, K.; Oyant, L.H.-S.; Araki, T.; Denoyes, B.; Foucher, F. The TFL1 Homologue KSN Is a Regulator of Continuous Flowering in Rose and Strawberry. Plant J. Cell Mol. Biol. 2012, 69, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Koskela, E.A.; Mouhu, K.; Albani, M.C.; Kurokura, T.; Rantanen, M.; Sargent, D.J.; Battey, N.H.; Coupland, G.; Elomaa, P.; Hytönen, T. Mutation in TERMINAL FLOWER1 Reverses the Photoperiodic Requirement for Flowering in the Wild Strawberry Fragaria vesca. Plant Physiol. 2012, 159, 1043–1054. [Google Scholar] [CrossRef]
- Ferchichi, S.; Hessini, K.; Dell Aversana, E.; D Amelia, L.; Woodrow, P.; Ciarmiello, L.F.; Fuggi, A.; Carillo, P. Hordeum vulgare and Hordeum maritimum Respond to Extended Salinity Stress Displaying Different Temporal Accumulation Pattern of Metabolites. Funct. Plant Biol. FPB 2018, 45, 1096–1109. [Google Scholar] [CrossRef] [PubMed]
- von Bothmer, R.; Jacobsen, N.; Baden, C.; Jørgensen, R.B.; Linde-Laursen, I. An ecogeographical study of the genus Hordeum. 2nd ed. Systematic and Ecogeographic Studies on Crop Genepools 7. International Plant Genetic Resources Institute: Rome, 1995; pp. 129 ISBN 92-9043-229-2.
- Yu, S.; Long, H.; Deng, G.; Pan, Z.; Liang, J.; Zeng, X.; Tang, Y.; Tashi, N.; Yu, M. A Single Nucleotide Polymorphism of Nud Converts the Caryopsis Type of Barley (Hordeum vulgare L.). Plant Mol. Biol. Report. 2015; 34. [Google Scholar] [CrossRef]
- Komatsuda, T.; Pourkheirandish, M.; He, C.; Azhaguvel, P.; Kanamori, H.; Perovic, D.; Stein, N.; Graner, A.; Wicker, T.; Tagiri, A.; et al. Six-Rowed Barley Originated from a Mutation in a Homeodomain-Leucine Zipper I-Class Homeobox Gene. Proc. Natl. Acad. Sci. 2007, 104, 1424–1429. [Google Scholar] [CrossRef]
- Digel, B.; Tavakol, E.; Verderio, G.; Tondelli, A.; Xu, X.; Cattivelli, L.; Rossini, L.; von Korff, M. Photoperiod-H1 (Ppd-H1) Controls Leaf Size. Plant Physiol. 2016, 172, 405–415. [Google Scholar] [CrossRef]
- Genger, R.; Williams, K.; Raman, H.; Read, B.; Wallwork, H.; Burdon, J.; Brown, A. Leaf Scald Resistance Genes in Hordeum vulgare and Hordeum vulgare Ssp. Spontaneum: Parallels between Cultivated and Wild Barley. Aust. J. Agric. Res. 2003; 54. [Google Scholar] [CrossRef]
- Herrero, E.; Kolmos, E.; Bujdoso, N.; Yuan, Y.; Wang, M.; Berns, M.C.; Uhlworm, H.; Coupland, G.; Saini, R.; Jaskolski, M.; et al. EARLY FLOWERING4 Recruitment of EARLY FLOWERING3 in the Nucleus Sustains the Arabidopsis Circadian Clock. Plant Cell 2012, 24, 428–443. [Google Scholar] [CrossRef] [PubMed]
- Ramsay, L.; Comadran, J.; Druka, A.; Marshall, D.F.; Thomas, W.T.B.; Macaulay, M.; MacKenzie, K.; Simpson, C.; Fuller, J.; Bonar, N.; et al. INTERMEDIUM-C, a Modifier of Lateral Spikelet Fertility in Barley, Is an Ortholog of the Maize Domestication Gene TEOSINTE BRANCHED 1. Nat. Genet. 2011, 43, 169–172. [Google Scholar] [CrossRef]
- Bortolotto, I.M.; Amorozo, M.C.d.M.; Neto, G.G.; Oldeland, J.; Damasceno-Junior, G.A. Knowledge and Use of Wild Edible Plants in Rural Communities along Paraguay River, Pantanal, Brazil. J. Ethnobiol. Ethnomedicine 2015, 11, 46–61. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.; Li, X.; Liu, F.; Sun, X.; Li, C.; Zhu, Z.; Fu, Y.; Cai, H.; Wang, X.; Xie, D.; et al. Control of a Key Transition from Prostrate to Erect Growth in Rice Domestication. Nat. Genet. 2008, 40, 1360–1364. [Google Scholar] [CrossRef]
- Li, C.; Zhou, A.; Sang, T. Rice Domestication by Reducing Shattering. Science 2006, 311, 1936–1939. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Griffith, M.E.; Li, X.; Zhu, Z.; Tan, L.; Fu, Y.; Zhang, W.; Wang, X.; Xie, D.; Sun, C. Origin of Seed Shattering in Rice (Oryza sativa L.). 2007; 20. [Google Scholar] [CrossRef]
- Zhu, B.-F.; Si, L.; Wang, Z.; Zhou, Y.; Zhu, J.; Shangguan, Y.; Lu, D.; Fan, D.; Li, C.; Lin, H.; et al. Genetic Control of a Transition from Black to Straw-White Seed Hull in Rice Domestication. Plant Physiol. 2011, 155, 1301–1311. [Google Scholar] [CrossRef]
- Sweeney, M.T.; Thomson, M.J.; Pfeil, B.E.; McCouch, S. Caught Red-Handed: Rc Encodes a Basic Helix-Loop-Helix Protein Conditioning Red Pericarp in Rice. Plant Cell 2006, 18, 283–294. [Google Scholar] [CrossRef]
- Filiz, E.; Akbudak, M.A. Ammonium Transporter 1 (AMT1) Gene Family in Tomato (Solanum lycopersicum L.): Bioinformatics, Physiological and Expression Analyses under Drought and Salt Stresses. 2020. [Google Scholar] [CrossRef]
- Lee, J.; Park, J.J.; Kim, S.; Yim, J.; An, G. Mutations in the Rice Liguleless Gene Result in a Complete Loss of the Auricle, Ligule, and Laminar Joint. Plant Mol. Biol. 2007, 65, 487–499. [Google Scholar] [CrossRef]
- Baicharoen, A.; Vijayan, R.; Pongprayoon, P. Structural Insights into Betaine Aldehyde Dehydrogenase (BADH2) from Oryza sativa Explored by Modeling and Simulations. Sci. Rep. 2017, 8, 12892. [Google Scholar] [CrossRef] [PubMed]
- Weng, J.; Gu, S.; Wan, X.; Gao, H.; Guo, T.; Su, N.; Lei, C.; Zhang, X.; Cheng, Z.; Guo, X.; et al. Isolation and Initial Characterization of GW5, a Major QTL Associated with Rice Grain Width and Weight. Cell Res. 2008, 18, 1199–1209. [Google Scholar] [CrossRef]
- Merida, A.; Rodriguez-Galan, J.; Vincent, C.; Romero, J. Expression of the Granule-Bound Starch Synthase I (Waxy) Gene from Snapdragon Is Developmentally and Circadian Clock Regulated. Plant Physiol. 1999, 120, 401–410. [Google Scholar] [CrossRef] [PubMed]
- Han, H.; Yang, C.; Zhu, J.; Zhang, L.; Bai, Y.; Li, E.; Gilbert, R.G. Competition between Granule Bound Starch Synthase and Starch Branching Enzyme in Starch Biosynthesis. Rice 2019, 12, 96–96. [Google Scholar] [CrossRef]
- Takano-Kai, N.; Jiang, H.; Kubo, T.; Sweeney, M.; Matsumoto, T.; Kanamori, H.; Padhukasahasram, B.; Bustamante, C.; Yoshimura, A.; Doi, K.; et al. Evolutionary History of a Gene Conferring Grain Length in Rice. Genetics 2009, 182, 1323–1334. [Google Scholar] [CrossRef]
- Takahashi, Y.; Shimamoto, K. Heading Date 1 (Hd1), an Ortholog of Arabidopsis CONSTANS, Is a Possible Target of Human Selection during Domestication to Diversify Flowering Times of Cultivated Rice. Genes Genet. Syst. 2011, 86, 175–182. [Google Scholar] [CrossRef]
- Konishi, S.; Izawa, T.; Lin, S.Y.; Ebana, K.; Fukuta, Y.; Sasaki, T.; Yano, M. An SNP Caused Loss of Seed Shattering during Rice Domestication. Science 2006, 312, 1392–1396. [Google Scholar] [CrossRef] [PubMed]
- Hulbert, S.H.; Richter, T.E.; Axtell, J.D.; Bennetzen, J.L. Genetic Mapping and Characterization of Sorghum and Related Crops by Means of Maize DNA Probes. Proc. Natl. Acad. Sci. USA 1990, 87, 4251–4255. [Google Scholar] [CrossRef] [PubMed]
- Dorweiler, J.E.; Doebley, J. Developmental Analysis of Teosinte Glume Architecture1: A Key Locus in the Evolution of Maize (Poaceae). Am. J. Bot. 1997, 84, 1313–1322. [Google Scholar] [CrossRef] [PubMed]
- Doebley, J.; Stec, A.; Gustus, C. Teosinte Branched1 and the Origin of Maize: Evidence for Epistasis and the Evolution of Dominance. Genetics 1995, 141, 333–346. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Nussbaum-Wagler, T.; Li, B.; Zhao, Q.; Vigouroux, Y.; Faller, M.; Bomblies, K.; Lukens, L.; Doebley, J.F. The Origin of the Naked Grains of Maize. Nature 2005, 436, 714–719. [Google Scholar] [CrossRef]
- Wang, H.; Studer, A.J.; Zhao, Q.; Meeley, R.; Doebley, J.F. Evidence That the Origin of Naked Kernels During Maize Domestication Was Caused by a Single Amino Acid Substitution in Tga1. Genetics 2015, 200, 965–974. [Google Scholar] [CrossRef]
- Yang, X. Study of RAMOSA1 Function during Maize Inflorescence Development. Ph.D. Thesis, Iowa State University, Ames, IA, USA, 2011. [CrossRef][Green Version]
- Hubbard, L.; McSteen, P.; Doebley, J.; Hake, S. Expression Patterns and Mutant Phenotype of Teosinte Branched1 Correlate with Growth Suppression in Maize and Teosinte. Genetics 2002, 162, 1927–1935. [Google Scholar] [CrossRef] [PubMed]
- Gallavotti, A.; Zhao, Q.; Kyozuka, J.; Meeley, R.B.; Ritter, M.K.; Doebley, J.F.; Pè, M.E.; Schmidt, R.J. The Role of Barren Stalk1 in the Architecture of Maize. Nature 2004, 432, 630–635. [Google Scholar] [CrossRef]
- Putterill, J.; Robson, F.; Lee, K.; Simon, R.; Coupland, G. The CONSTANS Gene of Arabidopsis Promotes Flowering and Encodes a Protein Showing Similarities to Zinc Finger Transcription Factors. Cell 1995, 80, 847–857. [Google Scholar] [CrossRef]
- Li, Y.; Xu, M. CCT Family Genes in Cereal Crops: A Current Overview. Crop J. 2017, 5, 449–458. [Google Scholar] [CrossRef]
- Han, J.-J.; Jackson, D.; Martienssen, R. Pod Corn Is Caused by Rearrangement at the Tunicate1 Locus. Plant Cell 2012, 24, 2733–2744. [Google Scholar] [CrossRef]
- James, M.G.; Robertson, D.S.; Myers, A.M. Characterization of the Maize Gene Sugary1, a Determinant of Starch Composition in Kernels. Plant Cell 1995, 7, 417–429. [Google Scholar] [CrossRef]
- Dinges, J.R.; Colleoni, C.; Myers, A.M.; James, M.G. Molecular Structure of Three Mutations at the Maize Sugary1 Locus and Their Allele-Specific Phenotypic Effects. Plant Physiol. 2001, 125, 1406–1418. [Google Scholar] [CrossRef]
- Smartt, J. Evolution of genetic resources. In Grain legumes; Smartt, J., Ed.; Cambridge University Press: Cambridge, 1990; pp. 140–175. [Google Scholar]
- Weeden, N.F. Genetic Changes Accompanying the Domestication of Pisum Sativum: Is There a Common Genetic Basis to the “domestication Syndrome” for Legumes? Ann. Bot. 2007, 100, 1017–1025. [Google Scholar] [CrossRef]
- de Wet, J.M.J.; Oestry-Stidd, L.L.; Cubero, J.I. Origins and Evolution of Foxtail Millets (Setaria Italica). J. Tradit. Agric. Appl. Bot. 1979, 26, 53–64. [Google Scholar] [CrossRef]
- Fukunaga, K.; Kawase, M.; Kato, K. Structural Variation in the Waxy Gene and Differentiation in Foxtail Millet [Setaria italica (L.) P. Beauv.]: Implications for Multiple Origins of the Waxy Phenotype. Mol. Genet. Genom. 2002. [Google Scholar] [CrossRef]
- Seung, D. Amylose in Starch: Towards an Understanding of Biosynthesis, Structure and Function. New Phytol. 2020, 228, 1490–1504. [Google Scholar] [CrossRef]
- Mueller, L.A.; Solow, T.H.; Taylor, N.; Skwarecki, B.; Buels, R.; Binns, J.; Lin, C.; Wright, M.H.; Ahrens, R.; Wang, Y.; et al. The SOL Genomics Network. A Comparative Resource for Solanaceae Biology and Beyond. Plant Physiol. 2005, 138, 1310–1317. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, G.R.; Muños, S.; Anderson, C.; Sim, S.-C.; Michel, A.; Causse, M.; Gardener, B.B.M.; Francis, D.; van der Knaap, E. Distribution of SUN, OVATE, LC, and FAS in the Tomato Germplasm and the Relationship to Fruit Shape Diversity. Plant Physiol. 2011, 156, 275–285. [Google Scholar] [CrossRef] [PubMed]
- Paterson, A.H.; Lander, E.S.; Hewitt, J.D.; Peterson, S.; Lincoln, S.E.; Tanksley, S.D. Resolution of Quantitative Traits into Mendelian Factors by Using a Complete Linkage Map of Restriction Fragment Length Polymorphisms. Nature 1988, 335, 721–726. [Google Scholar] [CrossRef]
- Li, B.; Sun, S.; Gao, X.; Wu, M.; Deng, Y.; Zheng, Q.; Li, X.; Xiao, J.; Ke, Y.; Wang, S. Overexpression a “Fruit-Weight 2.2-like” Gene OsFWL5 Improves Rice Resistance. 2019; 12. [Google Scholar] [CrossRef]
- Wang, S.; Chang, Y.; Ellis, B. Overview of OVATE FAMILY PROTEINS, A Novel Class of Plant-Specific Growth Regulators. Front. Plant Sci. 2016, 7, 417. [Google Scholar] [CrossRef]
- Wu, S.; Xiao, H.; Cabrera, A.; Meulia, T.; van der Knaap, E. SUN Regulates Vegetative and Reproductive Organ Shape by Changing Cell Division Patterns. Plant Physiol. 2011, 157, 1175–1186. [Google Scholar] [CrossRef]
- Rossetto, M.; Jackes, B.; Scott, K.; Henry, R. Intergeneric Relationships in the Australian Vitaceae: New Evidence from CpDNA Analysis. Genet. Resour. Crop Evol. 2001, 48, 307–314. [Google Scholar] [CrossRef]
- Péros, J.-P.; Launay, A.; Berger, G.; Lacombe, T.; This, P. MybA1 Gene Diversity across the Vitis Genus. Genetica 2015, 143, 373–384. [Google Scholar] [CrossRef] [PubMed]
- USDA, GRIN and NRCS DATABASE. Plants. Available online: https://plants.sc.egov.usda.gov/home (accessed on 7 June 2021).
- Dawson, I.; Powell, W.; Hendre, P.; Bančič, J.; Hickey, J.; Kindt, R.; Hoad, S.; Hale, I.; Jamnadass, R. The Role of Genetics in Mainstreaming the Production of New and Orphan Crops to Diversify Food Systems and Support Human Nutrition. New Phytol. 2019, 224, 37–44. [Google Scholar] [CrossRef]
- Svitashev, S.; Young, J.K.; Schwartz, C.; Gao, H.; Falco, S.C.; Cigan, A.M. Targeted Mutagenesis, Precise Gene Editing, and Site-Specific Gene Insertion in Maize Using Cas9 and Guide RNA. Plant Physiol. 2015, 169, 931–945. [Google Scholar] [CrossRef] [PubMed]
- Alagoz, Y.; Gurkok, T.; Zhang, B.; Unver, T. Manipulating the Biosynthesis of Bioactive Compound Alkaloids for Next-Generation Metabolic Engineering in Opium Poppy Using CRISPR-Cas 9 Genome Editing Technology. Sci. Rep. 2016, 6, 30910. [Google Scholar] [CrossRef]
- Chandrasekaran, J.; Brumin, M.; Wolf, D.; Leibman, D.; Klap, C.; Pearlsman, M.; Sherman, A.; Arazi, T.; Gal-On, A. Development of Broad Virus Resistance in Non-Transgenic Cucumber Using CRISPR/Cas9 Technology. Mol. Plant Pathol. 2016, 17, 1140–1153. [Google Scholar] [CrossRef]
- Malnoy, M.; Viola, R.; Jung, M.-H.; Koo, O.-J.; Kim, S.; Kim, J.-S.; Velasco, R.; Nagamangala Kanchiswamy, C. DNA-Free Genetically Edited Grapevine and Apple Protoplast Using CRISPR/Cas9 Ribonucleoproteins. Front. Plant Sci. 2016, 7, 1904. [Google Scholar] [CrossRef] [PubMed]
- Jyoti, A.; Kaushik, S.; Srivastava, V.K.; Datta, M.; Kumar, S.; Yugandhar, P.; Kothari, S.L.; Rai, V.; Jain, A. The Potential Application of Genome Editing by Using CRISPR/Cas9, and Its Engineered and Ortholog Variants for Studying the Transcription Factors Involved in the Maintenance of Phosphate Homeostasis in Model Plants. Semin. Cell Dev. Biol. 2019; s9. [Google Scholar] [CrossRef]
- Jiang, W.; Zhou, H.; Bi, H.; Fromm, M.; Yang, B.; Weeks, D.P. Demonstration of CRISPR/Cas9/SgRNA-Mediated Targeted Gene Modification in Arabidopsis, Tobacco, Sorghum and Rice. Nucleic Acids Res. 2013, 41, 648–657. [Google Scholar] [CrossRef]
- Li, M.; Li, X.; Zhou, Z.; Wu, P.; Fang, M.; Pan, X.; Lin, Q.; Luo, W.; Wu, G.; Li, H. Reassessment of the Four Yield-Related Genes Gn1a, DEP1, GS3, and IPA1 in Rice Using a CRISPR/Cas9 System. Front. Plant Sci. 2016, 7, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Simmonds, J.; Pan, Q.; Davidson, D.; He, F.; Battal, A.; Akhunova, A.; Trick, H.; Uauy, C.; Akhunov, E. Gene Editing and Mutagenesis Reveal Inter-Cultivar Differences and Additivity in the Contribution of TaGW2 Homoeologues to Grain Size and Weight in Wheat. Theor. Appl. Genet. 2018, 131, 2463–2475. [Google Scholar] [CrossRef]
- Jiang, W.Z.; Henry, I.M.; Lynagh, P.G.; Comai, L.; Cahoon, E.B.; Weeks, D.P. Significant Enhancement of Fatty Acid Composition in Seeds of the Allohexaploid, Camelina sativa, Using CRISPR/Cas9 Gene Editing. Plant Biotechnol. J. 2017, 15, 648–657. [Google Scholar] [CrossRef]
- Cai, Y.; Chen, L.; Liu, X.; Guo, C.; Sun, S.; Wu, C.; Jiang, B.; Han, T.; Hou, W. CRISPR/Cas9-Mediated Targeted Mutagenesis of GmFT2a Delays Flowering Time in Soya Bean. Plant Biotechnol. J. 2018, 16, 176–185. [Google Scholar] [CrossRef]
- Qi, W.; Zhu, T.; Tian, Z.; Li, C.; Zhang, W.; Song, R. High-Efficiency CRISPR/Cas9 Multiplex Gene Editing Using the Glycine tRNA-Processing System-Based Strategy in Maize. BMC Biotechnol. 2016, 16, 1–8. [Google Scholar] [CrossRef]
- Varshney, R.; Kudapa, H.; Pazhamala, L.T.; Chitikineni, A.; Thudi, M.; Gaur, P.; Pasupuleti, J.; Fikre, A.; Kimurto, P.; Ellis, N. Translational Genomics in Agriculture: Some Examples in Grain Legumes. Crit. Rev. Plant Sci. 2015, 34, 169–194. [Google Scholar] [CrossRef]
- Ji, J.; Zhang, C.; Sun, Z.; Wang, L.; Duanmu, D.; Fan, Q. Genome Editing in Cowpea Vigna unguiculata Using CRISPR-Cas9. Int. J. Mol. Sci. 2019, 20, 2471. [Google Scholar] [CrossRef]
- Čermák, T.; Baltes, N.J.; Čegan, R.; Zhang, Y.; Voytas, D.F. High-Frequency, Precise Modification of the Tomato Genome. Genome Biol. 2015, 16, 232. [Google Scholar] [CrossRef] [PubMed]
- Nekrasov, V.; Wang, C.; Win, J.; Lanz, C.; Weigel, D.; Kamoun, S. Rapid Generation of a Transgene-Free Powdery Mildew Resistant Tomato by Genome Deletion. Sci. Rep. 2017, 7, 482. [Google Scholar] [CrossRef] [PubMed]
- Butler, N.M.; Baltes, N.J.; Voytas, D.F.; Douches, D.S. Geminivirus-Mediated Genome Editing in Potato (Solanum tuberosum L.) Using Sequence-Specific Nucleases. Front. Plant Sci. 2016; 7. [Google Scholar] [CrossRef]
- Andersson, M.; Turesson, H.; Olsson, N.; Fält, A.-S.; Ohlsson, P.; Gonzalez, M.N.; Samuelsson, M.; Hofvander, P. Genome Editing in Potato via CRISPR-Cas9 Ribonucleoprotein Delivery. Physiol. Plant. 2018, 164, 378–384. [Google Scholar] [CrossRef]
- Shi, J.; Gao, H.; Wang, H.; Lafitte, H.R.; Archibald, R.L.; Yang, M.; Hakimi, S.M.; Mo, H.; Habben, J.E. ARGOS8 Variants Generated by CRISPR-Cas9 Improve Maize Grain Yield under Field Drought Stress Conditions. Plant Biotechnol. J. 2017, 15, 207–216. [Google Scholar] [CrossRef]
- Hu, J.; Li, S.; Li, Z.; Li, H.; Song, W.; Zhao, H.; Lai, J.; Xia, L.; Li, D.; Zhang, Y. A Barley Stripe Mosaic Virus-Based Guide RNA Delivery System for Targeted Mutagenesis in Wheat and Maize. Mol. Plant Pathol. 2019, 20, 1463–1474. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.; Zhang, K.; Chen, K.; Gao, C. Targeted Mutagenesis in Zea Mays Using TALENs and the CRISPR/Cas System. J. Genet. Genom. 2014, 41, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Xu, F.; Liu, L.; Char, S.N.; Ding, Y.; Je, B.I.; Schmelz, E.; Yang, B.; Jackson, D. The Maize Heterotrimeric G Protein β Subunit Controls Shoot Meristem Development and Immune Responses. Proc. Natl. Acad. Sci. USA 2020, 117, 1799–1805. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Gadlage, M.J.; Lafitte, H.R. Superior Field Performance of Waxy Corn Engineered Using CRISPR–Cas9. Nat. Biotechnol. 2020, 38, 579–581. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, X.; Chen, R.; Yang, L.; Fan, K.; Liu, Y.; Wang, G.; Ren, Z.; Liu, Y. Generation of Transgene-Free Semidwarf Maize Plants by Gene Editing of Gibberellin-Oxidase 20-3 Using CRISPR/Cas9. Front. Plant Sci. 2020, 11, 1048. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, X.; Wu, C.; He, Y.; Ma, Y.; Hou, H.; Guo, X.; Du, W.; Zhao, Y.; Xia, L. Engineering Herbicide-Resistant Rice Plants through CRISPR/Cas9-Mediated Homologous Recombination of Acetolactate Synthase. Mol. Plant 2016, 9, 628–631. [Google Scholar] [CrossRef]
- Begemann, M.B.; Gray, B.N.; January, E.; Gordon, G.C.; He, Y.; Liu, H.; Wu, X.; Brutnell, T.P.; Mockler, T.C.; Oufattole, M. Precise Insertion and Guided Editing of Higher Plant Genomes Using Cpf1 CRISPR Nucleases. Sci. Rep. 2017, 7, 11606. [Google Scholar] [CrossRef]
- Li, J.; Zhang, X.; Sun, Y.; Zhang, J.; Du, W.; Guo, X.; Li, S.; Zhao, Y.; Xia, L. Efficient Allelic Replacement in Rice by Gene Editing: A Case Study of the NRT1. 1B Gene. J. Integr. Plant Biol. 2018, 60, 536–540. [Google Scholar] [CrossRef]
- Toda, E.; Koiso, N.; Takebayashi, A.; Ichikawa, M.; Kiba, T.; Osakabe, K.; Osakabe, Y.; Sakakibara, H.; Kato, N.; Okamoto, T. An Efficient DNA- and Selectable-Marker-Free Genome-Editing System Using Zygotes in Rice. Nat. Plants 2019, 5, 363–368. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.; Chen, K.; Li, T.; Zhang, Y.; Wang, Y.; Zhao, Q.; Liu, J.; Zhang, H.; Liu, C.; Ran, Y.; et al. Efficient DNA-Free Genome Editing of Bread Wheat Using CRISPR/Cas9 Ribonucleoprotein Complexes. Nat. Commun. 2017, 8, 14261. [Google Scholar] [CrossRef]
- Murovec, J.; Guček, K.; Bohanec, B.; Avbelj, M.; Jerala, R. DNA-Free Genome Editing of Brassica oleracea and B. rapa Protoplasts Using CRISPR-Cas9 Ribonucleoprotein Complexes. Front. Plant Sci. 2018; 9. [Google Scholar] [CrossRef]
- Hu, B.; Li, D.; Liu, X.; Qi, J.; Gao, D.; Zhao, S.; Huang, S.; Sun, J.; Yang, L. Engineering Non-Transgenic Gynoecious Cucumber Using an Improved Transformation Protocol and Optimized CRISPR/Cas9 System. Mol. Plant 2017, 10, 1575–1578. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Lee, E.; Heo, J.; Kim, D.H.; Chun, H.J.; Kim, M.C.; Bang, W.Y.; Lee, Y.K.; Park, S.J. Rapid Generation of Transgenic and Gene-Edited Solanum nigrum Plants Using Agrobacterium-Mediated Transformation. Plant Biotechnol. Rep. 2020, 14, 497–504. [Google Scholar] [CrossRef]
- Wu, S.; Zhu, H.; Liu, J.; Yang, Q.; Shao, X.; Bi, F.; Hu, C.; Huo, H.; Chen, K.; Yi, G. Establishment of a PEG-Mediated Protoplast Transformation System Based on DNA and CRISPR/Cas9 Ribonucleoprotein Complexes for Banana. BMC Plant Biol. 2020, 20, 425. [Google Scholar] [CrossRef]
- Lin, C.-S.; Hsu, C.-T.; Yang, L.-H.; Lee, L.-Y.; Fu, J.-Y.; Cheng, Q.-W.; Wu, F.-H.; Hsiao, H.C.-W.; Zhang, Y.; Zhang, R.; et al. Application of Protoplast Technology to CRISPR/Cas9 Mutagenesis: From Single-Cell Mutation Detection to Mutant Plant Regeneration. Plant Biotechnol. J. 2018, 16, 1295–1310. [Google Scholar] [CrossRef]
- Weiss, T.; Wang, C.; Kang, X.; Zhao, H.; Elena Gamo, M.; Starker, C.G.; Crisp, P.A.; Zhou, P.; Springer, N.M.; Voytas, D.F.; et al. Optimization of Multiplexed CRISPR/Cas9 System for Highly Efficient Genome Editing in Setaria viridis. Plant J. 2020, 104, 828–838. [Google Scholar] [CrossRef] [PubMed]
- Mamidi, S.; Healey, A.; Huang, P.; Grimwood, J.; Jenkins, J.; Barry, K.; Sreedasyam, A.; Shu, S.; Lovell, J.T.; Feldman, M.; et al. A Genome Resource for Green Millet Setaria viridis Enables Discovery of Agronomically Valuable Loci. Nat. Biotechnol. 2020, 38, 1203–1210. [Google Scholar] [CrossRef]
- Curtin, S.J.; Xiong, Y.; Michno, J.-M.; Campbell, B.W.; Stec, A.O.; Čermák, T.; Starker, C.; Voytas, D.F.; Eamens, A.L.; Stupar, R.M. CRISPR/Cas9 and TALENs Generate Heritable Mutations for Genes Involved in Small RNA Processing of Glycine max and Medicago truncatula. Plant Biotechnol. J. 2018, 16, 1125–1137. [Google Scholar] [CrossRef]
- Meng, Y.; Hou, Y.; Wang, H.; Ji, R.; Liu, B.; Wen, J.; Niu, L.; Lin, H. Targeted Mutagenesis by CRISPR/Cas9 System in the Model Legume Medicago truncatula. Plant Cell Rep. 2017, 36, 371–374. [Google Scholar] [CrossRef]
- Che, P.; Chang, S.; Simon, M.K.; Zhang, Z.; Shaharyar, A.; Ourada, J.; O’Neill, D.; Torres-Mendoza, M.; Guo, Y.; Marasigan, K.M.; et al. Developing a Rapid and Highly Efficient Cowpea Regeneration, Transformation and Genome Editing System Using Embryonic Axis Explants. Plant J. 2021, 106, 817–830. [Google Scholar] [CrossRef] [PubMed]
- Badhan, S.; Ball, A.S.; Mantri, N. First Report of CRISPR/Cas9 Mediated DNA-Free Editing of 4CL and RVE7 Genes in Chickpea Protoplasts. Int. J. Mol. Sci. 2021, 22, 396. [Google Scholar] [CrossRef]
- Mao, Y.; Botella, J.; Liu, Y.; Zhu, J.-K. Gene Editing in Plants: Progress and Challenges. Natl. Sci. Rev. 2019, 6, 421–437. [Google Scholar] [CrossRef]
- Jansing, J.; Schiermeyer, A.; Schillberg, S.; Fischer, R.; Bortesi, L. Genome Editing in Agriculture: Technical and Practical Considerations. Int. J. Mol. Sci. 2019, 20, 2888. [Google Scholar] [CrossRef]
- Yang, B. Grand Challenges in Genome Editing in Plants. Front. Genome Ed. 2020, 2, 2. [Google Scholar] [CrossRef]
- Kumar, A.; Kumar, S. ; Komal; Ramchiary, N.; Singh, P. Role of Traditional Ethnobotanical Knowledge and Indigenous Communities in Achieving Sustainable Development Goals. 2021; 13. [Google Scholar] [CrossRef]
- Evans, P.; Quinoa Boom Offers Hard Lesson in Food Economics. CBC News, 13 January 2013. Available online: https://www.cbc.ca/news/business/quinoa-boom-offers-hard-lesson-in-food-economics-1.1358699 (accessed on 7 June 2021).
- FAO. The Impact of the Quinoa Boom on Bolivian Family Farmers. Available online: http://www.fao.org/resources/infographics/infographics-details/en/c/225070/ (accessed on 7 July 2021).
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).