Abstract
Abiotic stresses severely affect plant growth and productivity. To cope with abiotic stresses, plants have evolved tolerance mechanisms that are tightly regulated by reprogramming transcription factors (TFs). APETALA2/ethylene-responsive factor (AP2/ERF) transcription factors are known to play an important role in various abiotic stresses. However, our understanding of the molecular mechanisms remains incomplete. In this study, we identified the role of OsERF83, a member of the AP2/ERF transcription factor family, in response to drought stress. OsERF83 is a transcription factor localized to the nucleus and induced in response to various abiotic stresses, such as drought and abscisic acid (ABA). Overexpression of OsERF83 in transgenic plants (OsERF83OX) significantly increased drought tolerance, with higher photochemical efficiency in rice. OsERF83OX was also associated with growth retardation, with reduced grain yields under normal growth conditions. OsERF83 is predominantly expressed in the vascular tissue of all organs. Transcriptome analysis revealed that OsERF83 regulates drought response genes, which are related to the transporter (OsNPF8.10, OsNPF8.17, OsLH1), lignin biosynthesis (OsLAC17, OsLAC10, CAD8D), terpenoid synthesis (OsTPS33, OsTPS14, OsTPS3), cytochrome P450 family (Oscyp71Z4, CYP76M10), and abiotic stress-related genes (OsSAP, OsLEA14, PCC13-62). OsERF83 also up-regulates biotic stress-associated genes, including PATHOGENESIS-RELATED PROTEIN (PR), WALL-ASSOCIATED KINASE (WAK), CELLULOSE SYNTHASE-LIKE PROTEIN E1 (CslE1), and LYSM RECEPTOR-LIKE KINASE (RLK) genes. Our results provide new insight into the multiple roles of OsERF83 in the cross-talk between abiotic and biotic stress signaling pathways.
1. Introduction
Abiotic stresses, including drought, cold, salinity, and nutrient stress, adversely affect the cellular homeostasis of plants and ultimately impair their growth and productivity. Among these abiotic stresses, extreme heat and water deficits frequently damage plants [1]. As extreme weather disasters associated with climate change have become steadily more common, crop losses have also increased over the past several decades [2,3]. Rice (Oryza sativa. L.) productivity is severely affected by drought because rice typically requires more water than other crops [4]. It is, therefore, essential to develop rice with enhanced tolerance to drought and heat stress.
To avoid drought stress, plants have evolved a series of sophisticated strategies. Plants respond to drought stresses through stress-specific signaling pathways, leading to morphological, physiological, and biochemical changes. Under drought stresses, plants first perceive external signals through sensors, and then various transcription factors are induced by the transduction of signals [5,6]. These drought-responsive transcription factors, such as members of the AP2/ERF, MYB, bZIP, and NAC families, regulate drought-inducible genes, and consequently, plants show a tolerance to abiotic stresses [5].
The OsERF83 gene belongs to the APETALA2/ethylene-responsive factor (AP2/ERF) family, which is only present in the plant kingdom [7]. The AP2/ERF superfamily commonly possesses a highly conserved AP2 DNA-binding domain, and can be classified into four subfamilies: APETALA2 (AP2), related to abscisic acid insensitive 3/viviparous 1 (RAV), ethylene-responsive factor (ERF), and dehydration-responsive element binding protein (DREB) [8]. In the AP2/ERF superfamily, the ERF family has a single DNA-binding AP2 domain and is classified into ten subgroups (Ⅰ–X) based on putative functional motifs [7]. Many TFs belonging to the ERF family have been reported that specifically bind to the GCC box (AGCCGCC), an ethylene-responsive element (ERE) [7], to play a vital role in plant development, abiotic and biotic stresses, and hormonal signal transduction [9,10,11,12,13,14]. Recently, OsERF48 and OsERF71 were shown to be involved in drought tolerance in rice by enhancing root growth [15] and altering the root structure [16], respectively. Additionally, overexpression of OsEREBP1 confers tolerance to both biotic and abiotic stresses [17]. In Arabidopsis, AtERF019 has been reported to play an important role in plant growth and drought tolerance by delaying plant growth and senescence [18]. However, despite increasing evidence that OsERF genes enhance drought tolerance, the molecular mechanisms have not yet been entirely elucidated.
TFs belonging to group Ⅸ are associated with defense against various pathogens [7]. For example, overexpression of AtERF1 and ORA59 enhances resistance to necrotrophic pathogens in Arabidopsis. AtERF1 is a regulator of ethylene responses after pathogen attack, and ORA59 is an essential integrator of jasmonic acid (JA) [19,20]. In rice, OsERF92 negatively regulates tolerance to Magnaporthe oryzae and salt stress, which is integrated into the cross-talk between biotic and abiotic stress signaling networks [21]. OsERF83, which belongs to Group Ⅸ, is induced by drought, high salinity, low temperature, and ABA treatments [9]. A previous study demonstrated that OsERF83 is a transcription factor that positively regulates resistance to Magnaporthe oryzae and is induced by multiple phytohormone treatments, such as methyl jasmonate, ethephon, and salicylic acid. Furthermore, several PATHOGENESIS-RELATED (PR) protein genes, including PR1, PR2, PR3, PR5, and PR10, are up-regulated in OsERF83 overexpression (OsERF83OX) transgenic rice [22]. These results prompted us to study the function of OsERF83 in biotic and abiotic stresses. In this study, we found that OsERF83 confers drought tolerance and positively regulates abiotic and biotic stress-associated genes.
2. Results
2.1. OsERF83 Is a Drought-Inducible Transcription Factor
In our previous rice 3′-tiling microarray analysis, OsERF83 was induced by drought treatments [9]. Based on the results, we investigated the function of OsERF83 in response to drought. We analyzed the expression patterns using quantitative real-time PCR (qRT-PCR) (Figure 1a). We treated 2-week-old rice seedlings with various stresses, including drought, high salinity, low temperature, and ABA. The transcript level of OsERF83 was significantly induced in both leaves and roots under drought and high salinity conditions, and the degree of induction was higher in the roots than in the leaves. In contrast, the low-temperature (4 °C) and ABA (100 μM) treatments enhanced the transcript levels of OsERF83, specifically in the leaves and roots, respectively. Under drought treatments, the transcript levels of OsERF83 were increased in both the roots and leaves (Figure 1a). The regulation of OsERF83 expression by various stresses shows that it might have multiple roles in response to environmental stresses.

Figure 1.
Expression pattern of OsERF83 and subcellular localization of OsERF83 in rice protoplasts. (a) The relative expression level of OsERF83 by quantitative RT-PCR in the roots and shoots of 2-week-old seedlings treated with air-drying (drought), 400 mM NaCl (high salinity), 4 °C (cold), and 100 μM abscisic acid (ABA). OsUbi (Ubiquitin1; Os06g0681400) expression was used as an internal control for normalization. Error bars indicate the standard deviation based on three technical replicates. (h: hour) (b) The expression level of OsERF83 in various tissues and at different growth stages. (d, day; w, week; m, month; M, meiosis; BH, before heading; AH, after heading). OsUbi1 expression was used as an internal control for normalization. Error bars indicate the standard deviation based on three technical replicates. (c) Subcellular localization of the OsERF83 protein in rice protoplasts. 35S::OsERF83-GFP and 35S::OsNF-YA7-mCherry as a control vector were transiently expressed in rice leaf protoplasts. Scale bar, 10 µm.
To investigate the spatiotemporal expression patterns of OsERF83, we conducted qRT-PCR with various tissues of rice. The results revealed that OsERF83 was expressed at all developmental stages, and at particularly high levels in the roots (Figure 1b).
One study reported that OsERF83 was localized to the nucleus in onion epidermal cells [22]. To confirm the subcellular localization of OsERF83 in rice, we transformed the full-length OsERF83, fused to green fluorescent protein (GFP), into rice protoplasts (Figure S1a). We also used OsNF-YA7-mCherry as a positive control for nucleus localization [23]. Both constructs were transiently co-expressed in rice protoplasts, and the signals of both green fluorescence and mCherry fluorescence were observed in the nucleus of the rice protoplasts (Figure 1c). This indicated that the OsERF83 was localized at the nucleus. Collectively, OsERF83 is a transcription factor responsive to multiple stresses.
2.2. Overexpression of OsERF83 in Rice Confers Drought Tolerance at the Vegetative Stage
To investigate the biological role(s) of OsERF83 in rice development and the drought-stress response, we generated over 30 independent OsERF83 overexpression (OsERF83OX) transgenic rice plants using the GOS2 (rice eukaryotic translation initiation factor 1-like gene) promoter (GOS2::OsERF83), which drives expression in the whole plant body [24] (Figure S1b). Initially, we selected single-copy homozygous lines of OsERF83OX using TaqMan PCR, and then somaclonal variations were eliminated by paddy field selection for three generations. Finally, we chose three homozygous elite lines of OsERF83OX (#1, 2, 11) for further study. The transcript levels of OsERF83 were greatly enhanced in both the leaves and roots of all OsERF83OX (#1, 2, 11) plants, compared to non-transgenic (NT) plants. We checked the expression of each transgenic sister line and confirmed their overexpression (Figure S3).
We next tested the performance of OsERF83OX under drought conditions at the vegetative stage. OsERF83OX and NT plants were grown in a greenhouse for 5 weeks and then exposed to drought conditions for 2 days by withholding water. After 1 day of drought treatment, the NT plants started to display slightly rolled leaves. After 2 days of drought treatment, drought-induced visual symptoms, such as leaf rolling, wilting, and chlorosis, appeared more severely in NT plants than OsERF83OX (#1, 2, 11) plants (Figure 2a). Soil moisture consistently decreased during the drought treatment, indicating the drought stress was uniformly applied to both the NT and OsERF83OX (Figure 2b) plants. After 8 days of rewatering, most of the OsERF83OX plants had recovered and showed an 83 to 95% survival rate, while NT plants showed an approximately 20% survival rate (Figure 2c). We repeated the testing three times through T3–T4 and obtained the same results (Figure S4).
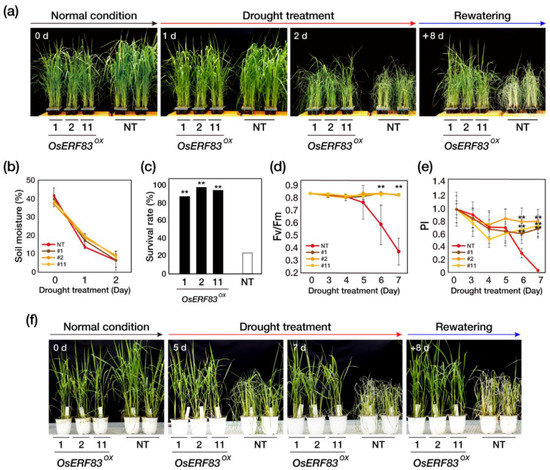
Figure 2.
Overexpression of OsERF83 in rice confers drought tolerance. (a) Drought tolerance phenotypes of OsERF83 overexpression (OsERF83OX) transgenic plants (lines 1, 2, 11). The 5-week-old plants (0 d) were exposed to drought by withholding water for 2 days and rewatering for 8 days. (b) Measurement of soil moisture content (%). Error bars represent the means ± SD (n = 10). (c) The survival rate of the transgenic plants, scored 8 days after rewatering. (d,e) Determination of the photosynthetic viability of transgenic and NT plants under drought conditions. At the indicated time point after exposure to drought stresses, the chlorophyll fluorescence (Fv/Fm) (d) and performance index (PI) (e) of overexpression plants and NT plants were measured. The data represent the mean ± SD (n = 10 points per independent line of each genotype). (f) Drought tolerance phenotypes of OsERF83OX transgenic plants (lines 1, 2, 11) in big pots. The 6-week-old plants (0 d) were exposed to drought by withholding water for 7 days and rewatering for 8 days. Asterisks (**) indicate statistically significant differences compared with non-transgenic (NT) plants according to a Student’s t-test ** p < 0.001).
To further confirm the tolerance of OsERF83OX plants to drought stress, we measured the activity of photosynthetic machinery damaged by drought stress using the JIP test. The JIP test showed the Fv/Fm (Fv: variable fluorescence; Fm: maximum fluorescence) value for the photochemical efficiency of photosystem (PS) II and the PItotal (performance index) value for the photochemical efficiency of PS I and PS II [25]. OsERF83OX and NT plants were exposed to drought conditions for 7 days after transplanting into big pots, by withholding water for 7 days (Figure 2f). The Fv/Fm values of the NT plants started to decrease at 5 days after drought treatment, whereas the Fv/Fm values of OsERF83OX plants did not decrease (Figure 2d). Additionally, the PItotal values sharply decreased in NT plants at 5 days after drought treatment, whereas the PItotal values of OsERF83OX were maintained during the drought treatment (Figure 2e). Consistent with the photochemical efficiency, after 5 days of drought treatment, the NT plants started to display drought-induced visual symptoms (Figure 2f). These results indicated that OsERF83 overexpression in rice plants protects PS I and PS II from drought stress, thereby enhancing drought tolerance; in this way, OsERF83 positively regulates drought tolerance. We concluded that overexpression of OsERF83 enhanced drought tolerance at the vegetative stage.
2.3. Overexpression of OsERF83 in Rice Leads to Growth Retardation and Affects Grain Yield under Normal Conditions
The OsERF83OX plants showed smaller plant height, including culm length and panicle length at all stages (Figure 3a,b). At the maturity stage, the average plant height of NT, OsERF83OX #1, OsERF83OX #2, and OsERF83OX #11 was 98.86 cm (100%), 75.50 cm (76.4%), 87.63 cm (87.6%), and 81.50 cm (81.5%), respectively (Figure 3b, Table S1). The yield component values of the OsERF83OX plants, such as the number of spikelets per panicle (NSP), the number of total spikelets (NTS), the number of filled grains (NFG), the grain-filling rate (FR), and total grain weight (TGW), were reduced compared to the NT plants (Figure S5, Table S1). Moreover, the OsERF83OX plants showed shorter grain width than the NT plants (Figure 3c,d). Collectively, these results indicate that overexpression of OsERF83 affects plant growth and grain yield negatively in rice.
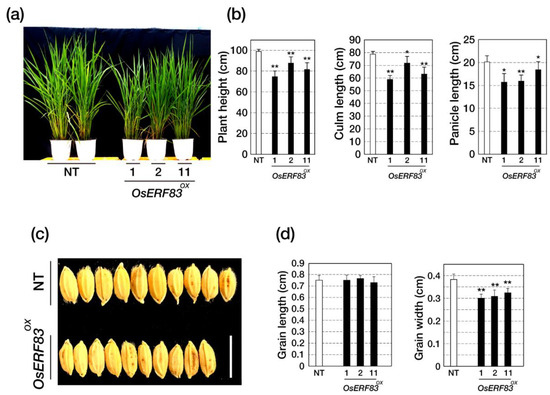
Figure 3.
Morphological analysis of OsERF83OX transgenic plants. (a) The whole plant height of 2-month-old OsERF83OX and NT plants grown in paddy fields. (b) The plant height was measured after the heading stage of the rice. A total of 18 plants for each line were measured. Data represent the mean value ± SD (n = 10). (c,d) The grain size of OsERF83OX line and NT plants. A total of 25 grains from each line were measured. Bar = 1 cm. Two asterisks (**) indicate statistically significant differences at p < 0.01, and one asterisk (*) indicates significant differences at p < 0.05 compared with NT based on the Student’s t-test. Data are shown as the mean ± SD (n = 25).
2.4. CRISPR/Cas9-Mediated Loss-of-Function Study Analysis of OsERF83
For the loss-of-function study analysis, knock-out (OsERF83KO) mutants were generated using a clustered regularly interspaced short palindromic repeats/recombinant codon-optimized Cas9 (CRISPR/Cas9) genome editing system (Figure S1c) [26], with which various types of induced mutations were found. The single-guide RNA (sgRNA) target sites were designed at the upstream region of the AP2/ERF domain for generating mutants edited in OsERF83 specific regions (Figure 4a). Four types of mutants containing 1 bp insertions were generated at the same upstream position of the PAM sequence (Figure 4a,b). Sequencing analysis revealed mutation patterns in transgenic plants, including frameshifts and premature stop codons (Figure 4c). Among 30 independent transgenic plants, 23.3% were null, and 20% were biallelic heterozygous mutant plants. A further 50% were biallelic homozygous, and we selected four independent OsERF83KO mutants for further study (Figure 4b).
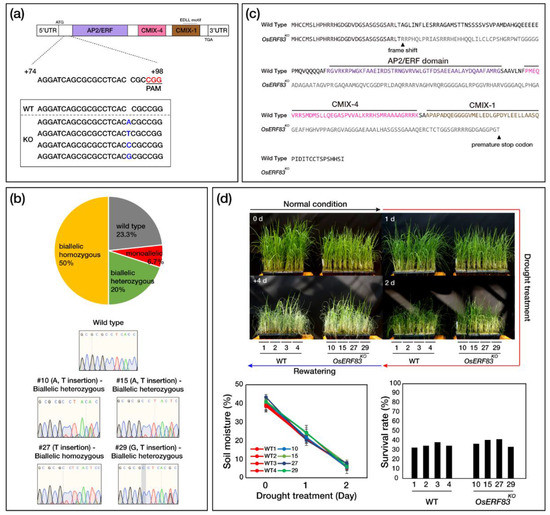
Figure 4.
Construction of knock-out (OsERF83KO) mutants and their phenotypes. (a) Schematic illustration of the target sites in the OsERF83 genomic sequence. The protospacer adjacent motif (PAM) sequence is marked in red. Mutation sequences are marked in blue. (b) Mutation pattern on OsERF83KO #10, #15, #27, and #29 plants. (c) Amino acid sequence alignment of OsERF83KO plants revealed a frameshift and premature stop codon. (d) Phenotypes of OsERF83KO (#10, #15, #27, #29) and WT plants under drought stress. All plants were grown in soil for 5 weeks under well-watered conditions, exposed to drought stress for 2 days, and then rewatered for 4 days, in the greenhouse. Measurement of the soil moisture contents (%). The data represent the mean value ± SD of five measurements performed at different locations in the soil. The survival rate of transgenic plants was scored 4 days after rewatering.
We expected that the phenotype of OsERF83KO mutants would show more sensitivity to drought stress compared with NT plants. However, OsERF83KO mutants showed similar phenotypes under drought conditions (Figure 4d). We speculated that the redundancy of other AP2/ERF transcription factor family genes caused such no significant phenotypes to occur in OsERF83KO compared to NT plants.
2.5. OsERF83 Is Expressed Predominantly in the Vascular Tissue
As shown in Figure 1b, OsERF83 was expressed ubiquitously in all the examined tissues, with high expression levels in roots. To further explore the function of OsERF83 in drought tolerance, we investigated the tissue expression pattern of OsERF83 using histochemical GUS staining with Paraplast section. We generated transgenic rice plants with a β-glucuronidase (GUS) reporter gene, driven by the native OsERF83 promoter (OsERF83::GUS) (Figure S1d). We used a coleoptile, stem, leaves, and roots of plants, as exhibited in Figure 5a–k. Interestingly, the strong GUS signals were consistently detected in vascular tissues of the coleoptile (Figure 5a), stem (Figure 5b,c), leaves (Figure 5d–g), and roots (Figure 5h–k), as shown in the longitudinal section of the roots (Figure 5i), despite the difference in developmental stage and organs. These data indicated that OsERF83 was a constitutively expressed gene and was involved in the vascular tissues.
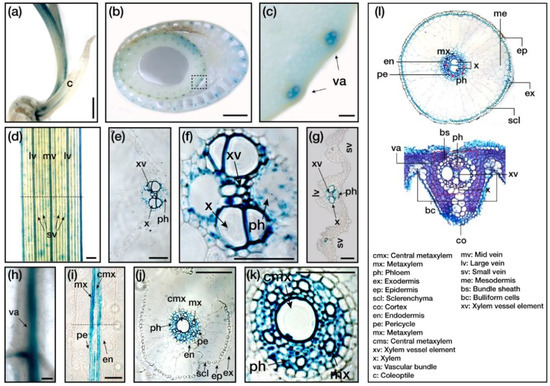
Figure 5.
Histochemical analysis of OsERF83. (a) The OsERF83 expression in different tissues of the OsERF83::GUS transgenic rice plants by GUS staining analysis, in the coleoptile of 5-day-old seedlings (a), stem cross-sections of 2-month-old plants (b,c), leaves of 2-week-old plants (d), leaf cross-sections of 2-week-old plants (e,g), the root of a 5-day-old seedling (h), longitudinal root section of 2-week-old plants (I), and root cross-sections of 2-week-old plants (j,k). Scale bars represent 1 mm in (a,b), 100 µm in (c,d) and (h), and 50 µm in (e–k). (i) Transverse section images represent structures of the roots and leaves.
To investigate possible morphological changes in the vascular tissue caused by OsERF83 expression, we observed the vascular tissue in the roots, stems, and leaves in OsERF83OX, OsERF83KO, and NT plants. However, there were no significant differences in morphology among NT, OsERF83 overexpressing, and knock-out plants (Figure S6).
2.6. Identification of Genes Involved in the OsERF83-Mediated Drought Tolerance Pathway
To perform further functional analysis of OsERF83, we generated OsERF83-6myc overexpressing transgenic (OsERF83-MYCOX) plants (Figure S1e). We confirmed protein expression levels of OsERF83 in OsERF83-MYCOX using Western blot analysis (Figure S7a,f) and transcript levels using qRT-PCR (Figure S7b). Both levels were higher in OsERF83-MYCOX than in the NT plants. OsERF83-MYCOX plants also showed drought tolerance in the vegetative stage (Figure S7c–e). To identify downstream genes regulated by OsERF83, we performed an RNA-sequencing (RNA-seq) analysis using a 3-week-old OsERF83-MYCOX. We used whole seedlings of OsERF83-MYCOX and NT, with two independent biological replicates, and calculated the reads per transcript kilobase per million fragments mapped reads (FPKM) values for each sample with count data. The heatmap indicated that the biological replicates showed a similar expression pattern (Figure 6a). A total of 33,221 differentially expressed genes (DEGs) were identified, of which 540 were up-regulated (B) and 1109 were down-regulated (A) in OsERF83 overexpressing plants (Figure 6b).
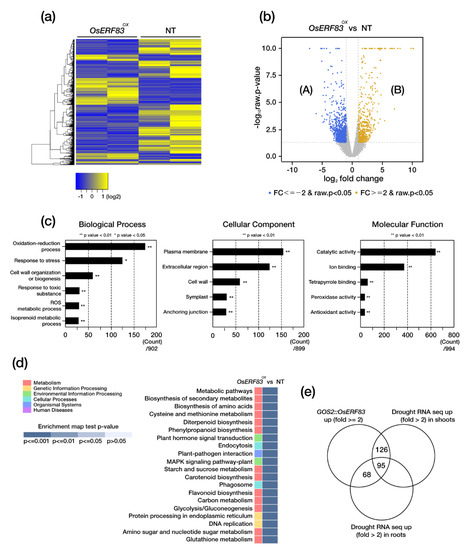
Figure 6.
RNA-seq analysis using leaves of OsERF83-MYCOX plants. (a) Heat map analysis of differentially expressed genes (DEGs) between OsERF83-MYCOX and NT plants. (b) Venn diagram of the numbers of up- and down-regulated DEGs in OsERF83-MYCOX plants compared to NT plants. (c) Gene ontology (GO) enrichment analysis of DEGs between OsERF83-MYCOX and NT plants. The most enriched GO term is shown in ‘biological process’, ‘molecular function’, and ‘cellular component’. Asterisks indicate significant enriched GO terms (** p < 0.01, * p < 0.05). (d) KEGG pathway enrichment analysis of DEGs. (e) Venn diagram of up-regulated genes amongst the DEGs identified in OsERF83-MYCOX and DEGs identified in drought-treated NT shoots and roots from public data (TENOR: http://tenor.dna.affrc.go.jp/, accessed on 30 November 2020).
To identify the putative functions of these DEGs, we conducted gene ontology analysis using gProfiler (https://biit.cs.ut.ee/gprofiler/orth). The DEGs were categorized into three categories: biological process, molecular function, and cellular component. As a result, the significantly enriched GO terms were mainly associated with the oxidation–reduction process and stress response under the biological process category, plasma membrane and the extracellular region under the cellular component category, and catalytic activity and ion binding under the molecular function category (Figure 6c). In addition, KEGG pathway analysis showed that DEGs were related to ‘diterpenoid biosynthesis’, ‘phenylpropanoid biosynthesis’, and ‘plant hormone signal transduction’ (Figure 6d). These results are consistent with our proposed role of OsERF83 in the regulation of drought stress tolerance.
Then, we identified that 289 drought-inducible genes were up-regulated in the OsERF83-MYCOX plants by filtering with a public database containing drought-treated RNA-seq data in shoots and roots (Figure 6e) [27]. These drought-inducible genes were classified into several groups: transporters, transcription factors, abiotic stress-related genes, the cytochrome P450 family, terpenoid-associated genes, disease resistance-related genes, hormone-associated genes, lignin biosynthesis-associated genes, and F-box proteins (Table 1), as well as others (Table S2). Some of the genes in each group were analyzed using qRT-PCR analysis for validation (Figure 7). The results confirmed that OsERF83-MYCOX induces a class of transporter genes (OsNPF8.10, OsNPF8.17, and OsLHT1) (Figure 7a), MYB or Myb/SANT transcription factors (Figure 7b), terpenoid-associated genes (OsTPS3 and OsCPS4) (Figure 7c), and lignin biosynthesis-associated genes (OsLAC17, OsLAC10, and CAD8D) (Figure 7d). We drew the co-expression matrix using up-regulated genes in OsERF83-MYCOX using RiceFREND to identify coregulatory networks with a high co-expression frequency (mutual rank (MR): ~20) (Figure S8) [28]. There were many drought-inducible genes in the co-expression matrix (Figure S8).

Table 1.
List of genes up-regulated (>2-fold) in OsERF83-MYCOX and drought treatment in shoots and roots.
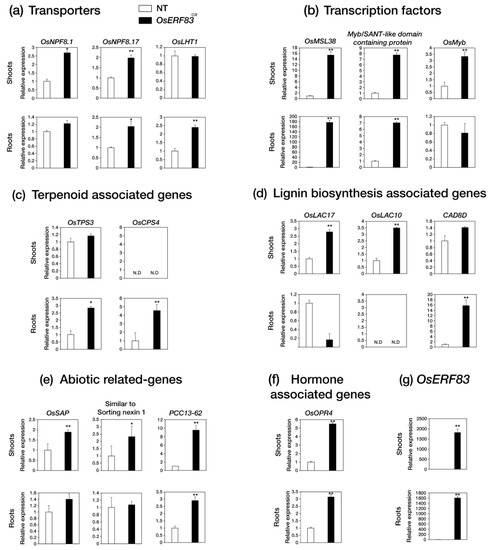
Figure 7.
Expression analysis of up-regulated genes in both OsERF83 overexpression myc-tagged transgenic (OsERF83-MYCOX) plants and drought-treated samples. (a) Relative expression levels of transporter genes (b), transcription factors (c), terpenoid-associated genes (d), lignin biosynthesis-associated genes, (e), abiotic stress-related genes. (f) and hormone associated genes, (g) qRT-PCR analysis of OsERF83 expression in 3-week-old OsERF83OX (line 1). The white bar is the expression value in NT plants, and the black bar is the expression value in OsERF83OX plants. OsUbi (Ubiquitin1; Os06g0681400) expression was used as an internal control for normalization. Error bars indicated the standard deviation based on three technical replicates. Asterisks indicate significant enriched GO terms (* p < 0.01, p < 0.05).
A previous study reported that OsERF83 interacts with the GCC box [22]. Therefore, we analyzed the presence of the GCC box within 3 kb of the 5′ upstream region of up-regulated drought-inducible genes in OsERF83-MYCOX (Table 1, Table S2). We found that most of them have the GCC box, which indicates that OsERF83 might directly regulate them.
3. Discussion
A number of studies have shown that APETALA2/ethylene-responsive factor (AP2/ERF) TFs are involved in the integration of signaling pathways in abiotic and biotic stress responses [10,11]. AP2/ERF TFs are classified into several subfamilies, including the ERF family [8]. OsERF83 is a member of group IXc, a subgroup of the ERF family reported to play an important role in abiotic stress [19,20,21]. ERF TFs have been reported that regulate stress-inducible genes by binding cis-acting elements, such as GCC box (AGCCGCC) in the promoter [29]. Previous studies have demonstrated that OsERF83 is a positive transcriptional regulator and interacts with the GCC box [22], and we analyzed the cis-element GCC box in the promoter of DEGs in our search for putative direct targets (Table 1 and Table S2).
The expression of OsERF83 was induced by ABA treatment (Figure 1a). In addition, OsERF83 was also reported to be induced by exogenous SA, JA, and ET [22]. These results indicated that OsERF83 is involved in multiple phytohormone signaling pathways during environmental stresses. It is well known that there are complex interactions between various hormones and stress signaling pathways in plants [6]. A single gene may play roles in various signaling pathways at the same time [21]. Therefore, our study suggested that OsERF83 is a positive regulator in plant responses to abiotic and biotic stresses through various hormone signaling pathways.
OsERF83OX plants showed growth retardation and low grain yields (Figure 3, Figure S5, Table S1). Generally, plants exhibiting stress tolerance have been reported to show growth retardation [30,31] because of hypersensitivity in responding to stresses, directing resources to protection from stresses rather than growing or yielding, which is known as a trade-off. Thus, genes improving stress tolerance induce growth retardation under normal growth conditions when they are constitutively overexpressed. In this study, we used the GOS2 promoter for constitutive overexpression (Figure S1b). However, if we used the stress-inducible promoter instead of the constitutive promoter, OsERF83 could be a good candidate for crop biotechnology.
The essential functions of vascular tissue are transporting water and various nutrients and supporting structures [32]. Consistent with this, there have been reports that vascular tissue development is involved in drought tolerance [33,34]. OsERF83 is predominantly expressed in vascular tissue (Figure 5). However, there were no significant differences in phenotypes of vascular tissue in this study (Figure S6). On the contrary, several transcriptome studies have shown that OsERF83 is induced under iron [35,36] and nitrate deficiency [37]. Moreover, our study revealed that various transporters were up-regulated in OsERF83-MYCOX (Table 1). Therefore, we speculated that OsERF83 might also be involved in iron and nitrate absorption.
Previously, it was reported that OsERF83 positively regulates disease resistance, and the constitutive overexpression of OsERF83 in rice enhanced resistance to Magnaporthe oryzae [22]. However, no further report on the function of OsERF83 in abiotic stress tolerance has been reported so far. In the present study, we analyzed the function of OsERF83 as a regulator of responses to drought stress. OsERF83 was strongly induced not only by drought stress, but also other abiotic stresses (Figure 1a). The OsERF83 promoter also contains several stress-related cis-elements, including ABREs, DREs regulated by ABA and DREB (Figure S9) [38]. These results support OsERF83 potentially playing an important role in abiotic stresses. OsERF83OX plants showed drought tolerance under drought treatment in the vegetative stage (Figure 2), which suggested the positive role of OsERF83 in drought tolerance. While OsERF83KO mutants showed similar phenotypes compared with non-transgenic (NT) plants under drought treatment at the vegetative stage (Figure 4), this could be explained by functional redundancy among a large number of the AP2/ERF TFs [7,12].
RNA-seq analysis identified a large number of transporter genes that were up-regulated in OsERF83 overexpression myc-tagged transgenic (OsERF83-MYCOX) plants, consistent with the vascular-specific expression of OsERF83. In a recent report, NRT1.2/NPF4.6 in Arabidopsis was found to affect ABA import activity [39]. This suggested that a group of transporter genes up-regulated in OsERF83-MYCOX (Table 1, Figure 7a), including NPFs (nitrate and peptide transporters), might affect the drought tolerance of rice through the ABA-dependent pathway. On the other hand, recent reports have shown that lignin plays an important role in protection against water losses [40,41]. A group of lignin biosynthesis-associated genes up-regulated in OsERF83-MYCOX (Table 1, Figure 7d), including OsLAC17 [42,43], OsLAC10 [44], and OsCAD8D [45], might be involved in protection against water losses and affect drought tolerance. A number of abiotic stress-related genes containing SENESCENCE-ASSOCIATED PROTEIN (OsSAP), OsLEA14/Wsi18, PCC13-62, and heat shock protein were up-regulated in OsERF83-MYCOX (Table 1, Figure 7e). OsSAP is involved in drought tolerance by virtue of its antiapoptotic activity [46]. OsLEA14/Wsi18 improves drought tolerance through higher proline and soluble sugar accumulation [47]. Desiccation-related protein (DRP)-encoding gene PCC13-62 is involved in desiccation tolerance in Linderniaceae [48]. Heat shock proteins play a role in membrane stability and regulating antioxidant enzymes and are produced under various abiotic and biotic stresses [49,50]. It has been reported that OsHSP23.7 enhances drought and salt tolerance in rice and overexpression transgenic plants show less membrane damage than NT plants [51]. It has also been shown that cytochrome P450 genes are involved in drought tolerance [52,53]. Many disease resistance-related genes, including PATHOGENESIS-RELATED PROTEIN (PR), WALL-ASSOCIATED KINASE (WAK) gene, CELLULOSE SYNTHASE-LIKE PROTEIN E1 (CslE1), LYSM RECEPTOR-LIKE KINASE (RLK), and terpenoid-associated genes, were also up-regulated (Table 1). It has been reported that many stress-responsive genes are induced by both biotic and abiotic stress treatment. PRs correlate with drought tolerance [54,55]. Cell wall-associated genes containing WAK, CslE1, and lignin biosynthesis genes are involved in both abiotic and biotic stresses [56,57]. Accordingly, we speculated that OsERF83 is involved in the core pathways in abiotic and biotic stress responses (Figure 8).
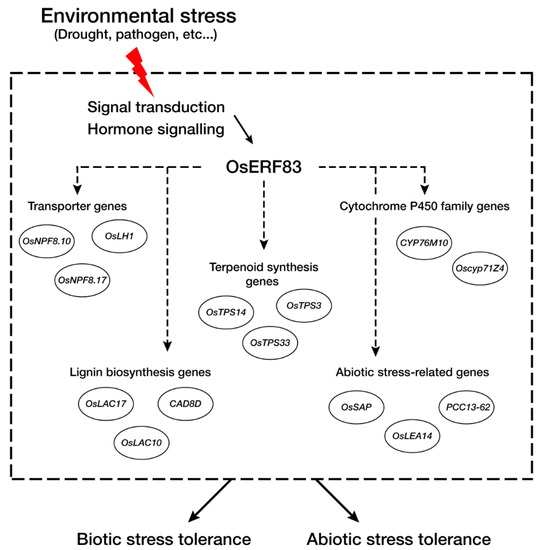
Figure 8.
Schematic representation of OsERF83-mediated stress tolerance. The rice recognizes the stress signal through a plant hormone signaling pathway, and OsERF83 is up-regulated in response to stresses. Transporter genes (OsNPF.10, OsLH1, OsNPF8.17), terpenoid synthesis genes (OsTPS14, OsTPS33, OsTPS3), cytochrome P450 genes (Oscyp71Z4, CYP76M10), lignin biosynthesis genes (OsLAC17, OsLAC10, CAD8D), and abiotic stress-related genes (OsSAP, OsLEA14, PCC13-62) were induced by OsERF83.
4. Materials and Methods
4.1. Plant Materials
To generate OsERF83 overexpressing plants (OsERF83OX), the coding sequence of OsERF83 (Os03g0860100, LOC_Os03g64260) was amplified from rice (Oryza sativa L. ssp. Japonica cv. Dongjin). The Japonica rice cultivar Dongjin (Oryza sativa. L.) was used as the non-transgenic control. The amplified OsERF83 coding sequence was cloned into rice transformation vector p700 carrying GOS2 promoter for constitutive expression using the Gateway system (Invitrogen) (Figure S2). The final constructs were introduced into Agrobacterium tumefaciens LBA4404 by triparental mating and transformed into rice (Oryza sativa cv. Dongjin). Copy numbers were determined in T0 plants through Taq-Man PCR as described by [58].
For the generation of knock-out (OsERF83KO) mutants, we used the web-based tool CRISPR RGEN Tools (http://www.rgenome.net/, accessed on 18 May 2017). The method was performed as described previously [26].
4.2. Stress Treatments
To confirm the expression levels of the OsERF83 gene under various abiotic stresses and phytohormone treatments, non-transgenic (NT) plants (Oryza sativa L. ssp. Japonica cv. Dongjin) were grown in soil for 2 weeks under standard greenhouse conditions (16 h light/8 h dark cycles at 28–30 °C). For stress treatments, the soil was moved from the roots of the seedlings, and drought stress was induced by air-drying the seedlings, while salinity stress and ABA treatment were imposed by incubating the seedlings in water containing 400 mM NaCl and 100 μM ABA, respectively at 28 °C. Low-temperature stress was induced by incubating the seedlings in water at 4 °C.
4.3. Subcellular Localization of OsERF83
To confirm the subcellular localization of OsERF83, we fused the coding regions of OsERF83 without the stop codon to the GFP. The cassette was driven by a 35S promoter and inserted into the pHBT vector (GenBank accession number EF090408) using the In-fusion system (Clonetech, CA, USA). 35S::NF-YA7-mCherry was used as the control for nuclear localization [23]. The final construct (35S::OsERF83-GFP) and the control vector (35S::NF-YA7-mCherry) were transfected into protoplasts (Oryza sativa cv. Dongjin) using a PEG-mediated protoplast transformation system [59]. The methods of rice protoplast preparation and transient gene expression were performed as described previously [60]. GFP and mCherry signals were observed 12 h after transfection using a confocal laser scanning microscope (Leica TCS SP8 STED, Wetzlar, Germany). Images were processed using Leica LAS AF Lite software. GFP was excited at 488 nm and the emitted light was detected between 512 and 560 nm. mCherry was excited at 587 nm and the emitted light was detected at 610 nm.
4.4. RNA Extraction, and Quantitative Real-Time PCR (qRT-PCR) Analysis
To investigate the spatial and temporal expression patterns of OsERF83, total RNA was isolated from the roots, stem, leaf, and flowers from different developmental stages of rice plants using a Hybrid-R RNA purification kit (GeneAll, Seoul, Korea) according to the manufacturer’s instructions. To measure the transcript levels of OsERF83, total RNA samples were extracted from the shoots and roots using a Hybrid-R RNA purification kit (GeneAll, Seoul, Korea) according to the manufacturer’s instructions. Complementary DNA (cDNA) was synthesized with Oligo-dT and random primers using RevertAid M-MuLV Reverse Transcriptase (Thermo Scientific, Massachusetts, USA). qRT-PCR was carried out using 2x qRT-PCR Pre-mix with 20x EvaGreen (SolGent, Seoul, Korea) and ROX dye (Promega, Madison, WI, USA). The amplification reactions were performed at 95 °C for 10 min, followed by 40 cycles of 95 °C for 30 s, 60 °C for 30 s, and 72 °C for 30 s in a 10 μL volume mix containing 0.5 μL EvaGreen Mix. The rice Ubiquitin1 (AK121590, Os06g0681400) transcript was used as a normalization control, and two biological and three technical replicates were analyzed for all qRT-PCRs (Table S3).
4.5. Histochemical GUS Assay
The amplified promoter region of OsERF83 was linked to the GUS reporter gene in a rice transformation vector using the Gateway system (Invitrogen, Carlsbad, CA, USA). The resulting plasmid was introduced into A. tumefaciens strain LBA 4404 by triparental mating and transformed into rice. To detect GUS staining, we used 5-day-old, 2-week-old, and 1-month-old seedlings of the OsERF83::GUS transgenic lines. The GUS staining solution contained 4 mM 5-bromo-4-chloro-3-indolyl-glucuronide cyclohexylamine salt (Gold Biotechnology, MO, USA), 0.5 mM K3Fe(CN)6, 0.5 mM C6FeK4N6, 0.1% Triton X-100, 10 mM EDTA, and 100 mM phosphate buffer. Seedlings were placed into GUS staining solution and then vacuum infiltrated for 30 min. Seedlings were then incubated at 37 °C in a dark chamber overnight. Chlorophyll was removed by washing with 70% (v/v) ethanol. We used Paraplast sections for longitudinals and cross-sections.
4.6. RNA-Sequencing Analysis
Three-week-old NT and OsERF83 overexpression myc-tagged transgenic (OsERF83-MYCOX) plants were grown under standard greenhouse conditions (16 h light/8 h dark cycles at 28–30 °C). Total RNA was extracted from 3-week-old transgenic (OsERF83-cMYCOX) and non-transgenic (NT) roots and leaves using an RNeasy plant kit (Qiagen, Hilden, German). Two biological replicates were used for RNA-seq. Library construction and next-generation sequencing (NGS) were performed by the Macrogen facility (Seoul, Korea). To make expression profiles for OsERF83-cMYCOX plants, the fragment per transcript kilobase per million fragments mapped reads (FPKM) values for each transcript were normalized with the FPKM values. The expression level of each transcript was expressed as the fragment per transcript kilobase per million fragments mapped reads (FPKM) value, which was calculated based on the number of mapped reads.
4.7. Drought Stress Treatment and Measurement of Chlorophyll Fluorescence
We evaluated the drought tolerance of transgenic, knock-out, and non-transgenic (Oryza sativa L. ssp. Japonica cv. Dongjin) plants grown in a greenhouse at the vegetative stage. Transgenic, knock-out and non-transgenic seeds were germinated on Murashige and Skoog (MS) medium (Duchefa Biochemie, Haarlem, Netherlands) with 3% sucrose in the dark for 3 days at 28 °C and transferred into light conditions for 1 day. Thirty seedlings from each transgenic, knock-out mutant, and non-transgenic plant were transplanted into ten soil pots (4 × 4 × 6 cm, three plants per pot) within a container (59 × 38.5 × 15 cm) and grown for 5 weeks in greenhouse conditions (16 h light/8 h dark cycles at 28–30 °C). Drought stress was simultaneously imposed by withholding water and rewatering. Drought-induced symptoms were monitored by imaging plants at the indicated time points using an a5000 camera (Sony, Tokyo, Japan). Soil moisture was measured at the indicated time points using an SM 150 soil moisture sensor (Delta T Devices, Cambridge, United Kingdom).
To evaluate the phenotypes of transgenic, knock-out, and non-transgenic plants exposed to drought conditions, we measured the chlorophyll fluorescence (Fv/Fm) and the performance index (PItotal). To measure Fv/Fm and PItotal, 4-week-old plants were transplanted into 15 cm diameter × 14 cm tall pots within another larger container (66 × 45.3 × 22.5 cm) and grown for 2 weeks. After each plant was subjected to drought stresses, Fv/Fm and PItotal were measured using the Handy-PEA fluorimeter (Plant Efficiency Analyzer; Hansatech Instruments, King’s Lynn, Norfolk, United Kingdom) in dark conditions to ensure sufficient dark adaptation (at least 1 h). Nine leaves of each line were measured and calculated using the Handy PEA software (version 1.31) and analyzed according to the equations of the JIP test [61].
4.8. Statistical Analysis
All data are represented as mean ± standard deviation. Each data point was compared with the control separately to determine whether they were significantly different from each other, using Student’s t-test (* p < 0.05, ** p < 0.01). Data were analyzed by Microsoft Excel software.
4.9. Accession Numbers
Genes from this article can be found in the National Center for Biotechnology Information (http://www.ncbi.nlm.nih.gov/, accessed on 19 November 2020) with the following accession numbers: OsERF83 (Os03g0860100), OsNPF8.10 (Os01g0142800), OsNPF8.17 (Os10g0112500), OsLHT1 (Os08g0127100), OsMSL38 (Os11g0282700), OsMyb (Os01g0298400), OsTPS3 (Os02g0121700), OsCPS4 (Os04g0178300), OsLAC17 (Os10g0346300), OsLAC10 (Os02g0749700), OsCAD8D (Os09g0400400), OsSAP (Os09g0425900), PCC13-62 (Os04g0404400), and OsOPR4 (Os06g0215900).
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/ijms22147656/s1.
Author Contributions
S.E.J. and S.W.B. designed the experiments; S.E.J., S.H.K. and H.-B.Y. performed the experiments; Y.S.K. and S.H.K. helped with the collection of plant materials; S.E.J. prepared the figures; S.E.J. wrote the manuscript; S.E.J. and J.S.S. edited the manuscript; S.E.J., J.S.S. and J.-K.K. review and revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was carried out with the support of “Cooperative Research Program for Agriculture Science and Technology Development (Project No. PJ01566801 to J.S.S)” Rural Development Administration, Republic of Korea.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We thank the Rural Development Administration and Kyungpook National University for providing the rice paddy fields.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zandalinas, S.I.; Mittler, R.; Balfagón, D.; Arbona, V.; Gómez-Cadenas, A. Plant adaptations to the combination of drought and high temperatures. Physiol. Plant. 2017, 162, 2–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mickelbart, M.V.; Hasegawa, P.M.; Bailey-Serres, J. Genetic mechanisms of abiotic stress tolerance that translate to crop yield stability. Nat. Rev. Genet. 2015, 16, 237–251. [Google Scholar] [CrossRef]
- Lesk, C.; Rowhani, P.; Ramankutty, N. Influence of extreme weather disasters on global crop production. Nat. Cell Biol. 2016, 529, 84–87. [Google Scholar] [CrossRef]
- Barnabás, B.; Jäger, K.; Fehér, A. The effect of drought and heat stress on reproductive processes in cereals. Plant Cell Environ. 2007, 31, 11–38. [Google Scholar] [CrossRef]
- Hirayama, T.; Shinozaki, K. Research on plant abiotic stress responses in the post-genome era: Past, present and future. Plant. J. 2010, 61, 1041–1052. [Google Scholar] [CrossRef]
- Atkinson, N.J.; Urwin, P.E. The interaction of plant biotic and abiotic stresses: From genes to the field. J. Exp. Bot. 2012, 63, 3523–3543. [Google Scholar] [CrossRef] [Green Version]
- Nakano, T.; Suzuki, K.; Fujimura, T.; Shinshi, H. Genome-Wide Analysis of the ERF Gene Family in Arabidopsis and Rice. Plant. Physiol. 2006, 140, 411–432. [Google Scholar] [CrossRef] [Green Version]
- Sakuma, Y.; Liu, Q.; Dubouzet, J.G.; Abe, H.; Shinozaki, K.; Yamaguchi-Shinozaki, K. DNA-binding specificity of the ERF/AP2 domain of Arabidopsis DREBs, transcription factors involved in dehydration- and cold-inducible gene expression. Biochem. Bioph. Res. Co. 2002, 290, 998–1009. [Google Scholar] [CrossRef]
- Oh, S.-J.; Kim, Y.S.; Kwon, C.-W.; Park, H.K.; Jeong, J.S.; Kim, J.-K. Overexpression of the transcription factor AP37 in rice improves grain yield under drought conditions. Plant. Physiol. 2009, 150, 1368–1379. [Google Scholar] [CrossRef] [Green Version]
- Mizoi, J.; Shinozaki, K.; Yamaguchi-Shinozaki, K. AP2/ERF family transcription factors in plant abiotic stress responses. Biochim. Biophys. Acta Bioenerg. 2012, 1819, 86–96. [Google Scholar] [CrossRef]
- Müller, M.; Munné-Bosch, S. Ethylene Response Factors: A Key Regulatory Hub in Hormone and Stress Signaling. Plant. Physiol. 2015, 169, 32–41. [Google Scholar] [CrossRef] [Green Version]
- Xie, Z.; Nolan, T.; Jiang, H.; Yin, Y. AP2/ERF Transcription Factor Regulatory Networks in Hormone and Abiotic Stress Responses in Arabidopsis. Front. Plant. Sci. 2019, 10, 228. [Google Scholar] [CrossRef] [Green Version]
- Cheng, M.-C.; Liao, P.-M.; Kuo, W.-W.; Lin, T.-P. The Arabidopsis ETHYLENE RESPONSE FACTOR1 Regulates Abiotic Stress-Responsive Gene Expression by Binding to Different cis-Acting Elements in Response to Different Stress Signals. Plant. Physiol. 2013, 162, 1566–1582. [Google Scholar] [CrossRef] [Green Version]
- Lorenzo, O.; Piqueras, R.; Serrano, J.J.S.; Solano, R. Ethylene Response FACTOR1 Integrates Signals from Ethylene and Jasmonate Pathways in Plant Defense. Plant. Cell 2003, 15, 165–178. [Google Scholar] [CrossRef] [Green Version]
- Jung, H.; Chung, P.J.; Park, S.-H.; Redillas, M.C.F.R.; Kim, Y.S.; Suh, J.-W.; Kim, J.-K. Overexpression of OsERF48 causes regulation of OsCML16, a calmodulin-like protein gene that enhances root growth and drought tolerance. Plant. Biotechnol. J. 2017, 15, 1295–1308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, D.K.; Jung, H.; Jang, G.; Jeong, J.S.; Kim, Y.S.; Ha, S.H.; Do Choi, Y.; Kim, J.K. Overexpression of the OsERF71 Tran-scription Factor Alters Rice Root Structure and Drought Resistance. Plant. Physiol. 2016, 172, 575–588. [Google Scholar] [CrossRef] [Green Version]
- Jisha, V.; Dampanaboina, L.; Vadassery, J.; Mithöfer, A.; Kappara, S.; Ramanan, R. Overexpression of an AP2/ERF Type Transcription Factor OsEREBP1 Confers Biotic and Abiotic Stress Tolerance in Rice. PLoS ONE 2015, 10, e0127831. [Google Scholar] [CrossRef] [PubMed]
- Scarpeci, T.E.; Frea, V.S.; Zanor, M.I.; Valle, E.M. Overexpression of AtERF019 delays plant growth and senescence, and improves drought tolerance in Arabidopsis. J. Exp. Bot. 2017, 68, 673–685. [Google Scholar] [PubMed] [Green Version]
- Berrocal-Lobo, M.; Molina, A.; Solano, R. Constitutive expression of ETHYLENE-RESPONSE-FACTOR1 in Arabidopsis con-fers resistance to several necrotrophic fungi. Plant. J. 2002, 29, 23–32. [Google Scholar] [CrossRef]
- Pré, M.; Atallah, M.; Champion, A.; De Vos, M.; Pieterse, C.; Memelink, J. The AP2/ERF Domain Transcription Factor ORA59 Integrates Jasmonic Acid and Ethylene Signals in Plant Defense. Plant. Physiol. 2008, 147, 1347–1357. [Google Scholar] [CrossRef] [Green Version]
- Liu, D.; Chen, X.; Liu, J.; Ye, J.; Guo, Z. The rice ERF transcription factor OsERF922 negatively regulates resistance to Magnaporthe oryzae and salt tolerance. J. Exp. Bot. 2012, 63, 3899–3911. [Google Scholar] [CrossRef] [Green Version]
- Tezuka, D.; Kawamata, A.; Kato, H.; Saburi, W.; Mori, H.; Imai, R. The rice ethylene response factor OsERF83 positively regulates disease resistance to Magnaporthe oryzae. Plant. Physiol. Biochem. 2019, 135, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.K.; Kim, H.I.; Jang, G.; Chung, P.J.; Jeong, J.S.; Kim, Y.S.; Bang, S.W.; Jung, H.; Choi, Y.D.; Kim, J.K. The NF-YA transcription factor OsNF-YA7 confers drought stress tolerance of rice in an abscisic acid independent manner. Plant. Sci. 2015, 241, 199–210. [Google Scholar] [CrossRef] [PubMed]
- Depater, B.S.; Vandermark, F.; Rueb, S.; Katagiri, F.; Chua, N.H.; Schilperoort, R.A.; Hensgens, L.A.M. The Promoter of the Rice Gene Gos2 Is Active in Various Different Monocot Tissues and Binds Rice Nuclear Factor Asf-1. Plant. J. 1992, 2, 837–844. [Google Scholar] [CrossRef]
- Bussotti, F.; Desotgiu, R.; Pollastrini, M.; Cascio, C. The JIP test: A tool to screen the capacity of plant adaptation to climate change. Scand. J. For. Res. 2010, 25, 43–50. [Google Scholar] [CrossRef]
- Chung, P.J.; Chung, H.; Oh, N.; Choi, J.; Bang, S.W.; Jung, S.E.; Jung, H.; Shim, J.S.; Kim, J.K. Efficiency of Recombinant CRISPR/rCas9-Mediated miRNA Gene Editing in Rice. Int. J. Mol. Sci. 2020, 21, 9606. [Google Scholar] [CrossRef] [PubMed]
- Kawahara, Y.; Oono, Y.; Wakimoto, H.; Ogata, J.; Kanamori, H.; Sasaki, H.; Mori, S.; Matsumoto, T.; Itoh, T. TENOR: Database for Comprehensive mRNA-Seq Experiments in Rice. Plant. Cell Physiol. 2016, 57, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sato, Y.; Namiki, N.; Takehisa, H.; Kamatsuki, K.; Minami, H.; Ikawa, H.; Ohyanagi, H.; Sugimoto, K.; Itoh, J.I.; Antonio, B.A.; et al. RiceFREND: A platform for retrieving coexpressed gene networks in rice. Nucl. Acids Res. 2013, 41, D1214–D1221. [Google Scholar] [CrossRef] [Green Version]
- Fujimoto, S.Y.; Ohta, M.; Usui, A.; Shinshi, H.; Ohme-Takagi, M. Arabidopsis ethylene-responsive element binding factors act as transcriptional activators or repressors of GCC box-mediated gene expression. Plant. Cell 2000, 12, 393–404. [Google Scholar]
- Kim, H.; Lee, K.; Hwang, H.; Bhatnagar, N.; Kim, D.Y.; Yoon, I.S.; Byun, M.O.; Kim, S.T.; Jung, K.H.; Kim, B.G. Overex-pression of PYL5 in rice enhances drought tolerance, inhibits growth, and modulates gene expression. J. Exp. Bot. 2014, 65, 453–464. [Google Scholar] [CrossRef] [Green Version]
- Ma, Z.; Wu, T.; Huang, K.; Jin, Y.-M.; Li, Z.; Chen, M.; Yun, S.; Zhang, H.; Yang, X.; Chen, H.; et al. A Novel AP2/ERF Transcription Factor, OsRPH1, Negatively Regulates Plant Height in Rice. Front. Plant. Sci. 2020, 11. [Google Scholar] [CrossRef]
- Lucas, W.J.; Groover, A.; Lichtenberger, R.; Furuta, K.; Yadav, S.-R.; Helariutta, Y.; He, X.-Q.; Fukuda, H.; Kang, J.; Brady, S.; et al. The Plant Vascular System: Evolution, Development and Functions. J. Integr. Plant. Biol. 2013, 55, 294–388. [Google Scholar] [CrossRef] [PubMed]
- Geng, D.; Chen, P.; Shen, X.; Zhang, Y.; Li, X.; Jiang, L.; Xie, Y.; Niu, C.; Zhang, J.; Huang, X.; et al. MdMYB88 and MdMYB124 Enhance Drought Tolerance by Modulating Root Vessels and Cell Walls in Apple. Plant. Physiol. 2018, 178, 1296–1309. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.S.; Zhang, H.; Srivastava, A.K.; Pan, Y.J.; Bai, J.J.; Fang, J.J.; Shi, H.Z.; Zhu, J.K. Knockdown of Rice MicroRNA166 Confers Drought Resistance by Causing Leaf Rolling and Altering Stem Xylem Development. Plant. Physiol. 2018, 176, 2082–2094. [Google Scholar] [CrossRef] [Green Version]
- Zheng, L.; Huang, F.; Narsai, R.; Wu, J.; Giraud, E.; He, F.; Cheng, L.; Wang, F.; Wu, P.; Whelan, J.; et al. Physiological and Transcriptome Analysis of Iron and Phosphorus Interaction in Rice Seedlings. Plant. Physiol. 2009, 151, 262–274. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.; Wang, C.; Zheng, L.; Wang, L.; Chen, Y.; Whelan, J.; Shou, H. Ethylene is involved in the regulation of iron homeostasis by regulating the expression of iron-acquisition-related genes in Oryza sativa. J. Exp. Bot. 2010, 62, 667–674. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsieh, P.-H.; Kan, C.-C.; Wu, H.-Y.; Yang, H.-C.; Hsieh, M.-H. Early molecular events associated with nitrogen deficiency in rice seedling roots. Sci. Rep. 2018, 8, 1–23. [Google Scholar] [CrossRef]
- Agarwal, P.K.; Jha, B. Transcription factors in plants and ABA dependent and independent abiotic stress signalling. Biol. Plant. 2010, 54, 201–212. [Google Scholar] [CrossRef]
- Zhang, L.; Yu, Z.; Xu, Y.; Yu, M.; Ren, Y.; Zhang, S.; Yang, G.; Huang, J.; Yan, K.; Zheng, C.; et al. Regulation of the stability and ABA import activity of NRT1.2/NPF4.6 by CEPR2-mediated phosphorylation in Arabidopsis. Mol. Plant. 2021, 14, 633–646. [Google Scholar] [CrossRef]
- Bang, S.W.; Lee, D.-K.; Jung, H.; Chung, P.J.; Kim, Y.S.; Choi, Y.D.; Suh, J.-W.; Kim, J.-K. Overexpression of OsTF1L, a rice HD-Zip transcription factor, promotes lignin biosynthesis and stomatal closure that improves drought tolerance. Plant. Biotechnol. J. 2018, 17, 118–131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tu, M.; Wang, X.; Yin, W.; Wang, Y.; Li, Y.; Zhang, G.; Li, Z.; Song, J.; Wang, X. Grapevine VlbZIP30 improves drought re-sistance by directly activating VvNAC17 and promoting lignin biosynthesis through the regulation of three peroxidase genes. Hortic. Res. 2020, 7, 150. [Google Scholar] [CrossRef]
- Sun, J.; Cui, X.; Teng, S.; Kunnong, Z.; Wang, Y.; Chen, Z.; Sun, X.; Wu, J.; Ai, P.; Quick, W.P.; et al. HD-ZIP IV gene Roc8 regulates the size of bulliform cells and lignin content in rice. Plant. Biotechnol. J. 2020, 18, 2559–2572. [Google Scholar] [CrossRef]
- Li, W.Q.; Zhang, M.J.; Gan, P.F.; Qiao, L.; Yang, S.Q.; Miao, H.; Wang, G.F.; Zhang, M.M.; Liu, W.T.; Li, H.F.; et al. CLD1/SRL1 modulates leaf rolling by affecting cell wall formation, epidermis integrity and water homeostasis in rice. Plant. J. 2017, 92, 904–923. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Q.; Luo, L.; Wang, X.; Shen, Z.; Zheng, L. Comprehensive Analysis of Rice Laccase Gene (OsLAC) Family and Ectopic Expression of OsLAC10 Enhances Tolerance to Copper Stress in Arabidopsis. Int. J. Mol. Sci. 2017, 18, 209. [Google Scholar] [CrossRef]
- Barakat, A.; Bagniewska-Zadworna, A.; Choi, A.; Plakkat, U.; DiLoreto, D.S.; Yellanki, P.; Carlson, J.E. The cinnamyl alcohol dehydrogenase gene family in Populus: Phylogeny, organization, and expression. BMC Plant. Biol. 2009, 9. [Google Scholar] [CrossRef] [Green Version]
- Ubaidillah, M.; Kim, K.-A.; Kim, Y.H.; Lee, I.-J.; Yun, B.-W.; Kim, D.H.; Loake, G. Identification of a drought-induced rice gene, OsSAP, that suppresses Bax-induced cell death in yeast. Mol. Biol. Rep. 2013, 40, 6113–6121. [Google Scholar] [CrossRef]
- Kaur, R.; Chakraborty, A.; Bhunia, R.K.; Sen, S.K.; Ghosh, A.K. Tolerance to soil water stress by Oryza sativa cv. IR20 was improved by expression of Wsi18 gene locus from Oryza nivara. Biol. Plant. 2017, 62, 129–139. [Google Scholar] [CrossRef]
- Giarola, V.; Jung, N.U.; Singh, A.; Satpathy, P.; Bartels, D. Analysis of pcC13-62 promoters predicts a link between cis-element variations and desiccation tolerance in Linderniaceae. J. Exp. Bot. 2018, 69, 3773–3784. [Google Scholar] [CrossRef]
- Driedonks, N.; Xu, J.; Peters, J.; Park, S.; Rieu, I. Multi-Level Interactions Between Heat Shock Factors, Heat Shock Proteins, and the Redox System Regulate Acclimation to Heat. Front. Plant. Sci. 2015, 6, 999. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ul Haq, S.; Khan, A.; Ali, M.; Khattak, A.M.; Gai, W.X.; Zhang, H.X.; Wei, A.M.; Gong, Z.H. Heat Shock Proteins: Dynamic Biomolecules to Counter Plant Biotic and Abiotic Stresses. Int. J. Molec. Sci. 2019, 20, 5321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zou, J.; Liu, C.; Liu, A.; Zou, D.; Chen, X. Overexpression of OsHsp17.0 and OsHsp23.7 enhances drought and salt tolerance in rice. J. Plant. Physiol. 2012, 169, 628–635. [Google Scholar] [CrossRef]
- Tamiru, M.; Undan, J.; Takagi, H.; Abe, A.; Yoshida, K.; Undan, J.Q.; Natsume, S.; Uemura, A.; Saitoh, H.; Matsumura, H.; et al. A cytochrome P450, OsDSS1, is involved in growth and drought stress responses in rice (Oryza sativa L.). Plant. Mol. Biol. 2015, 88, 85–99. [Google Scholar] [CrossRef]
- Pandian, B.A.; Sathishraj, R.; Djanaguiraman, M.; Prasad, P.V.; Jugulam, M. Role of Cytochrome P450 Enzymes in Plant Stress Response. Antioxidants 2020, 9, 454. [Google Scholar] [CrossRef]
- Wu, J.; Kim, S.G.; Kang, K.Y.; Kim, J.G.; Park, S.R.; Gupta, R.; Kim, Y.H.; Wang, Y.; Kim, S.T. Overexpression of a Path-ogenesis-Related Protein 10 Enhances Biotic and Abiotic Stress Tolerance in Rice. Plant. Pathol. J. 2016, 32, 552–562. [Google Scholar] [CrossRef] [Green Version]
- Liu, W.-X.; Zhang, F.-C.; Zhang, W.-Z.; Song, L.-F.; Wu, W.-H.; Chen, Y.-F. Arabidopsis Di19 Functions as a Transcription Factor and Modulates PR1, PR2, and PR5 Expression in Response to Drought Stress. Mol. Plant. 2013, 6, 1487–1502. [Google Scholar] [CrossRef] [Green Version]
- Le Gall, H.; Philippe, F.; Domon, J.-M.; Gillet, F.; Pelloux, J.; Rayon, C. Cell Wall Metabolism in Response to Abiotic Stress. Plants 2015, 4, 112–166. [Google Scholar] [CrossRef]
- Houston, K.; Tucker, M.; Chowdhury, J.; Shirley, N.; Little, A. The Plant Cell Wall: A Complex and Dynamic Structure As Revealed by the Responses of Genes under Stress Conditions. Front. Plant. Sci. 2016, 7, 984. [Google Scholar] [CrossRef] [Green Version]
- Bang, S.W.; Park, S.H.; Kim, Y.S.; Do Choi, Y.; Kim, J.K. The activities of four constitutively expressed promoters in sin-gle-copy transgenic rice plants for two homozygous generations. Planta 2015, 241, 1529–1541. [Google Scholar] [CrossRef]
- Zhang, Y.; Su, J.B.; Duan, S.; Ao, Y.; Dai, J.R.; Liu, J.; Wang, P.; Li, Y.G.; Liu, B.; Feng, D.R.; et al. A highly efficient rice green tissue protoplast system for transient gene expression and studying light/chloroplast-related processes. Plant. Meth. 2011. [Google Scholar] [CrossRef] [Green Version]
- Redillas, M.C.F.R.; Bang, S.W.; Lee, D.; Kim, Y.S.; Jung, H.; Chung, P.J.; Suh, J.; Kim, J. Allantoin accumulation through overexpression of ureide permease1 improves rice growth under limited nitrogen conditions. Plant. Biotechnol. J. 2018, 17, 1289–1301. [Google Scholar] [CrossRef] [Green Version]
- Redillas, M.C.F.R.; Strasser, R.J.; Jeong, J.S.; Kim, Y.S.; Kim, J.-K. The use of JIP test to evaluate drought-tolerance of transgenic rice overexpressing OsNAC10. Plant. Biotechnol. Rep. 2011, 5, 169–175. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).