The Critical Importance of Molecular Biomarkers and Imaging in the Study of Electrohypersensitivity. A Scientific Consensus International Report
Abstract
1. Introduction
2. What Is Clinical Research
3. What Is Public Health Research, and How Does It Differ from Clinical Research
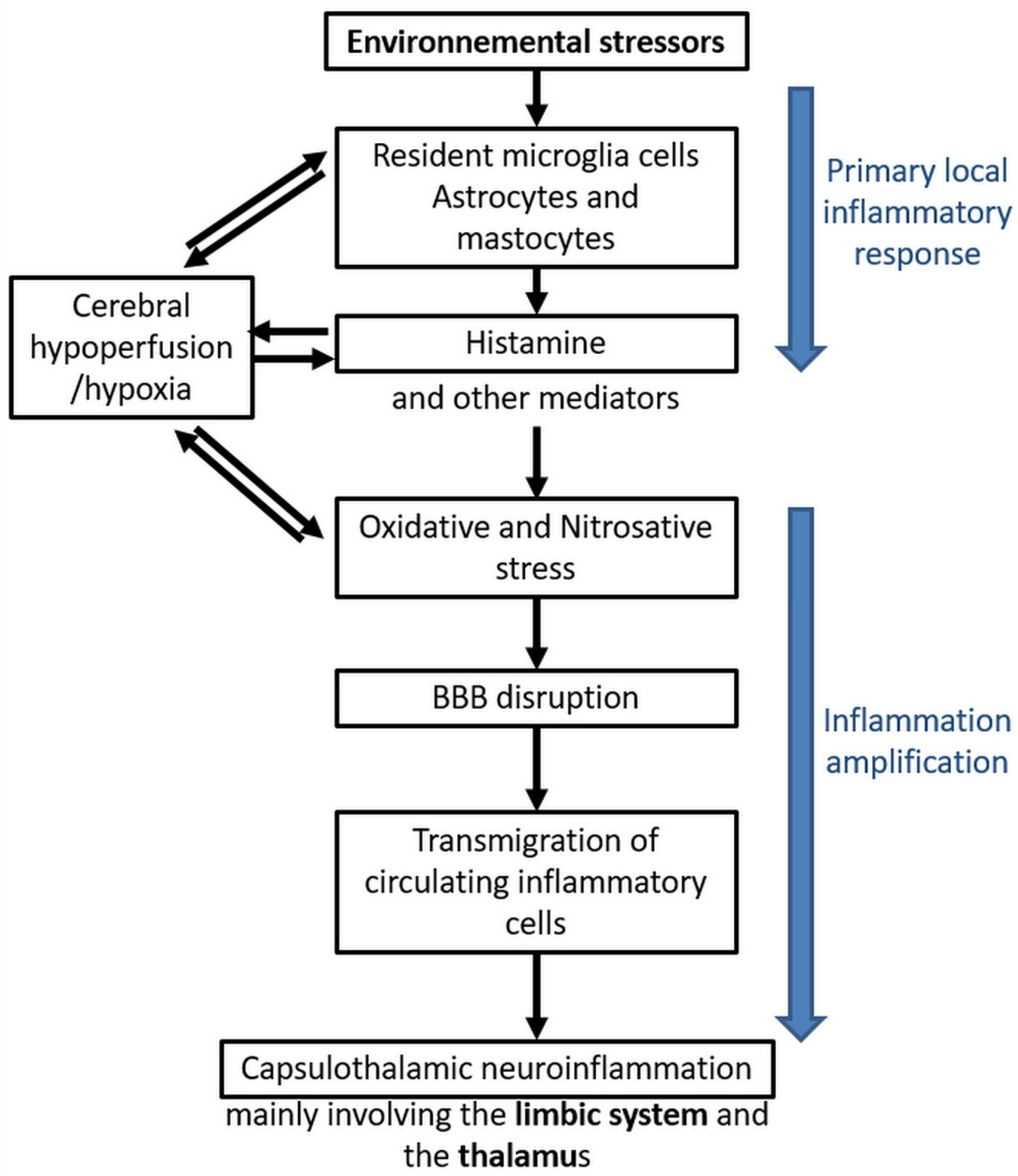
4. The Case of Electrohypersensitivity
5. Why Recent Scientifically Unfounded Claims Are Deeply Confusing
5.1. Database Selection of Patients
5.2. Biomarker Expression and EMF Exposure
5.3. “Psychological” Provocation Tests
6. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Belpomme, D.; Irigaray, P. Electrohypersensitivity as a Newly Identified and Characterized Neurologic Pathological Disorder: How to Diagnose, Treat, and Prevent It. Int. J. Mol. Sci. 2020, 21, 1915. [Google Scholar] [CrossRef]
- Leszczynski, D. EHS or not EHS: What about Are Studies by Belpomme and His Team? Available online: https://betweenrockandhardplace.wordpress.com/2020/08/22/ehs-or-not-ehs-what-about-are-studies-by-belpomme-and-his-team/ (accessed on 6 February 2021).
- Jouannna, J. Hippocratic Rationalism and the Divine. In Hippocrates; Gilman, S.L., Ed.; DeBevoise, M.B., Translator; The John Hopkins University Press: Baltimore, MD, USA; London, UK, 1999; pp. 179–181. [Google Scholar]
- Askitopoulou, H.; Stefanakis, G.; Astyrakaki, E.; Papaioannou, A.; Agouridakis, P. Emergencies and acute diseases in the collected works of Hippocrates: Observation, examination, prognosis, therapy. Eur. J. Emerg. Med. 2016, 23, 399–405. [Google Scholar] [CrossRef]
- Lüderitz, B. Hippocrates of Kos—The father of modern medicine. Clin. Res. Cardiol. Suppl. 2010, 5, 3–6. [Google Scholar] [CrossRef]
- Strimbu, K.; Tavel, J.A. What are biomarkers? Curr. Opin. HIV AIDS 2010, 5, 463–466. [Google Scholar] [CrossRef] [PubMed]
- FDA-NIH Biomarker Working Group. BEST (Biomarkers, EndpointS, and other Tools) Resource. Silver Spring (MD): Food and Drug Administration (US); Bethesda (MD): National Institutes of Health (US). 2016. Available online: www.ncbi.nlm.nih.gov/books/NBK326791/ (accessed on 16 March 2021).
- Califf, R.M. Biomarker definitions and their applications. Exp. Biol. Med. 2018, 243, 213–221. [Google Scholar] [CrossRef]
- Belpomme, D.; Campagnac, C.; Irigaray, P. Reliable disease biomarkers characterizing and identifying electrohypersensitivity and multiple chemical sensitivity as two etiopathogenic aspects of a unique pathological disorder. Rev. Environ. Health 2015, 30, 251–271, Erratum in 2016. [Google Scholar] [CrossRef] [PubMed]
- Belpomme, D.; Irigaray, P.; Sasco, A.J.; Newby, J.A.; Howard, V.; Clapp, R.; Hardell, L. The growing incidence of cancer: Role of lifestyle and screening detection. Int. J. Oncol. 2007, 30, 1037–1049. [Google Scholar] [CrossRef] [PubMed]
- Irigaray, P.; Newby, J.A.; Clapp, R.; Hardell, L.; Howard, V.; Montagnier, L.; Epstein, S.; Belpomme, D. Lifestyle-related factors and environmental agents causing cancer: An overview. Biomed. Pharmacother. 2007, 61, 640–658. [Google Scholar] [CrossRef]
- Belpomme, D.; Irigaray, P.; Hardell, L.; Clapp, R.; Montagnier, L.; Epstein, S.; Sasco, A.J. The multitude and diversity of environmental carcinogens. Environ. Res. 2007, 105, 414–429. [Google Scholar] [CrossRef]
- Irigaray, P.; Belpomme, D. Basic properties and molecular mechanisms of exogenous chemical carcinogens. Carcinogenesis 2010, 31, 135–148. [Google Scholar] [CrossRef]
- Mantzavinos, V.; Alexiou, A. Biomarkers for Alzheimer’s Disease Diagnosis. Curr. Alzheimer Res. 2017, 14, 1149–1154. [Google Scholar] [CrossRef]
- Ding, L.; Xu, Y.; Liu, S.; Bi, Y.; Xu, Y. Hemoglobin A1c and diagnosis of diabetes. J. Diabetes 2018, 10, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Body, R.; Hendry, C. Cardiac Biomarkers in Emergency Care. Cardiol. Clin. 2018, 36, 27–36. [Google Scholar] [CrossRef]
- Chen, X.-E.; Huang, S.; Kerr, D. Biomarkers in clinical medicine. In Integration of Biomarkers into Epidemiology Study Design; IARC Publication: Lyon, France, 2008; pp. 303–322. Available online: https://publications.iarc.fr/uploads/media/publication_inline/0001/02/33f111bfadb61211e9775dbf48c843c92f4220f9.pdf (accessed on 6 February 2021).
- Kuller, L.H. Epidemiology: Then and Now. Am. J. Epidemiol. 2016, 183, 372–380. [Google Scholar] [CrossRef]
- Hill, A.B. The environment and disease: Association or causation? Proc. R. Soc. Med. 1965, 58, 295–300. [Google Scholar] [CrossRef]
- Hardell, L.; Carlberg, M.; Hansson Mild, K. Re-analysis of risk for glioma in relation to mobile telephone use: Comparison with the results of the Interphone international case-control study. Int. J. Epidemiol. 2011, 40, 1126–1128. [Google Scholar] [CrossRef] [PubMed]
- Hardell, L.; Carlberg, M.; Söderqvist, F.; Mild, K.H. Pooled analysis of case-control studies on acoustic neuroma diagnosed 1997–2003 and 2007–2009 and use of mobile and cordless phones. Int. J. Oncol. 2013, 43, 1036–1044. [Google Scholar] [CrossRef]
- Kundi, M. The controversy about a possible relationship between mobile phone use and cancer. Environ. Health Perspect. 2009, 117, 316–324. [Google Scholar] [CrossRef]
- Myung, S.K.; Ju, W.; McDonnell, D.D.; Lee, Y.J.; Kazinets, G.; Cheng, C.T.; Moskowitz, J.M. Mobile phone use and risk of tumors: A meta-analysis. J. Clin. Oncol. 2009, 27, 5565–5572. [Google Scholar] [CrossRef]
- Stein, Y.; Levy-Nativ, O.; Richter, E.D. A sentinel case series of cancer patients with occupational exposures to electromagnetic non-ionizing radiation and other agents. Eur. J. Oncol. 2011, 16, 21–54. [Google Scholar] [CrossRef]
- Coureau, G.; Bouvier, G.; Lebailly, P.; Fabbro-Peray, P.; Gruber, A.; Leffondre, K.; Guillamo, J.S.; Loiseau, H.; Mathoulin-Pélissier, S.; Salamon, R.; et al. Mobile phone use and brain tumours in the CERENAT case-control study. Occup. Environ. Med. 2014, 71, 514–522. [Google Scholar] [CrossRef]
- De Vocht, F. Inferring the 1985–2014 impact of mobile phone use on selected brain cancer subtypes using Bayesian structural time series and synthetic controls. Environ. Int. 2016, 97, 100–107. [Google Scholar] [CrossRef]
- Ahlbom, A.; Day, N.; Feychting, M.; Roman, E.; Skinner, J.; Dockerty, J.; Linet, M.; McBride, M.; Michaelis, J.; Olsen, J.H.; et al. A pooled analysis of magnetic fields and childhood leukaemia. Br. J. Cancer. 2000, 83, 692–698. [Google Scholar] [CrossRef]
- Draper, G.; Vincent, T.; Kroll, M.E.; Swanson, J. Childhood cancer in relation to distance from high voltage power lines in England and Wales: A case-control study. BMJ 2005, 330, 1290. [Google Scholar] [CrossRef]
- Carles, C.; Esquirol, Y.; Turuban, M.; Piel, C.; Migault, L.; Pouchieu, C.; Bouvier, G.; Fabbro-Peray, P.; Lebailly, P.; Baldi, I. Residential proximity to power lines and risk of brain tumor in the general population. Environ. Res. 2020, 185, 109473. [Google Scholar] [CrossRef]
- IARC (International Agency for Research on Cancer). Non-ioizing radiation, Part 1: Static and extremely low-frequency (ELF) electric and magnetic fields. In IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; IARC Press: Lyon, France, 2002; Volume 80, p. 341. [Google Scholar]
- IARC (International Agency for Research on Cancer). Non-ionization radiation, Part 2: Radiofrequency electromagnetic fields. In IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; IARC Press: Lyon, France, 2013; Volume 102, p. 406. [Google Scholar]
- Choi, Y.J.; Moskowitz, J.M.; Myung, S.K.; Lee, Y.R.; Hong, Y.C. Cellular Phone Use and Risk of Tumors: Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2020, 17, 8079. [Google Scholar] [CrossRef]
- National Toxicology Program. NTP Technical Report on the Toxicology and Carcinogenesis Studies in Hsd: Sprague Dawley SD Rats Exposed to Whole-Body Radio Frequency Radiation at a Frequency (900 MHz) and Modulations (GSM and CDMA) Used by Cell Phones; NTP: Washington, DC, USA, 2018. Available online: https://ntp.niehs.nih.gov/ntp/about_ntp/trpanel/2018/march/tr595peerdraft.pdf (accessed on 16 March 2021).
- National Toxicology Program. NTP Technical Report on the Toxicology and Carcinogenesis Studies in B6C3F1/N Mice Exposed to Whole-Body Radio Frequency Radiation at a Frequency (1900 MHz) and Modulations (GSM and CDMA) Used by Cell Phones; NTP: Washington, DC, USA, 2018. Available online: https://ntp.niehs.nih.gov/ntp/about_ntp/trpanel/2018/march/tr596peerdraft.pdf (accessed on 16 March 2021).
- Falcioni, L.; Bua, L.; Tibaldi, E.; Lauriola, M.; De Angelis, L.; Gnudi, F.; Mandrioli, D.; Manservigi, M.; Manservisi, F.; Manzoli, I.; et al. Report of final results regarding brain and heart tumors in Sprague-Dawley rats exposed from prenatal life until natural death to mobile phone radiofrequency field representative of a 1.8 GHz GSM base station environmental emission. Environ. Res. 2018, 165, 496–503. [Google Scholar] [CrossRef] [PubMed]
- Soffritti, M.; Tibaldi, E.; Padovani, M.; Hoel, D.G.; Giuliani, L.; Bua, L.; Lauriola, M.; Falcioni, L.; Manservigi, M.; Manservisi, F.; et al. Synergism between sinusoidal-50 Hz magnetic field and formaldehyde in triggering carcinogenic effects in male Sprague-Dawley rats. Am. J. Ind. Med. 2016, 59, 509–521. [Google Scholar] [CrossRef] [PubMed]
- Soffritti, M.; Tibaldi, E.; Padovani, M.; Hoel, D.G.; Giuliani, L.; Bua, L.; Lauriola, M.; Falcioni, L.; Manservigi, M.; Manservisi, F.; et al. Life-span exposure to sinusoidal-50 Hz magnetic field and acute low-dose γ radiation induce carcinogenic effects in Sprague-Dawley rats. Int. J. Radiat. Biol. 2016, 92, 202–214. [Google Scholar] [CrossRef] [PubMed]
- Chou, C.K.; Guy, A.W.; Kunz, L.L.; Johnson, R.B.; Crowley, J.J.; Krupp, J.H. Long-term, low-level microwave irradiation of rats. Bioelectromagnetic 1992, 13, 469–496. [Google Scholar] [CrossRef]
- Lai, H.; Singh, N.P. Acute low-intensity microwave exposure increases DNA single-strand breaks in rat brain cells. Bioelectromagnetics 1995, 16, 207–210. [Google Scholar] [CrossRef]
- Yakymenko, I.; Tsybulin, O.; Sidorik, E.; Henshel, D.; Kyrylenko, O.; Kyrylenko, S. Oxidative mechanisms of biological activity of low-intensity radiofrequency radiation. Electromagn. Biol. Med. 2016, 35, 186–202. [Google Scholar] [CrossRef] [PubMed]
- Panagopoulos, D.J. Comparing DNA Damage Induced by Mobile Telephony and Other Types of Man-Made Electromagnetic Fields. Mutat. Res. 2019, 781, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Miller, A.B.; Morgan, L.L.; Udasin, I.; Davis, D.L. Cancer epidemiology update, following the 2011 IARC evaluation of radiofrequency electromagnetic fields (Monograph 102). Environ. Res. 2018, 167, 673–683. [Google Scholar] [CrossRef]
- Hardell, L.; Carlberg, M. Comments on the US National Toxicology Program technical reports on toxicology and carcinogenesis study in rats exposed to whole-body radiofrequency radiation at 900 MHz and in mice exposed to whole-body radiofrequency radiation at 1900 MHz. Int. J. Oncol. 2019, 54, 111–127. [Google Scholar] [CrossRef] [PubMed]
- Soffritti, M.; Giuliani, L. The carcinogenic potential of non-ionizing radiations: The cases of S-50 Hz MF and 1.8 GHz GSM radiofrequency radiation. Basic Clin. Pharmacol. Toxicol. 2019, 125, 1–12. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Framework for Developing Health-Based EMF Standards; WHO: Geneva, Switzerland, 2006; ISBN 9241594330. Available online: www.who.int/peh-emf/standards/EMF_standards_framework%5b1%5d.pdf (accessed on 6 February 2021).
- De Luca, C.; Thai, J.C.; Raskovic, D.; Cesareo, E.; Caccamo, D.; Trukhanov, A.; Korkina, L. Metabolic and genetic screening of electromagnetic hypersensitive subjects as a feasible tool for diagnostics and intervention. Mediators Inflamm. 2014, 2014, 924184. [Google Scholar] [CrossRef]
- Heuser, G.; Heuser, S.A. Functional brain MRI in patients complaining of electrohypersensitivity after long term exposure to electromagnetic fields. Rev. Environ. Health 2017, 32, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Irigaray, P.; Caccamo, D.; Belpomme, D. Oxidative stress in electrohypersensitivity self reporting patients: Results of a prospective in vivo investigation with comprehensive molecular analysis. Int. J. Mol. Med. 2018, 42, 1885–1898. [Google Scholar] [CrossRef] [PubMed]
- Irigaray, P.; Lebar, P.; Belpomme, D. How Ultrasonic Cerebral Tomosphygmography can Contribute to the Diagnosis of Electrohypersensitivity. J. Clin. Diagn. Res. 2018, 6, 143. [Google Scholar] [CrossRef]
- Greco, F. Technical Assessment of Ultrasonic Cerebral Tomosphygmography and New Scientific Evaluation of Its Clinical Interest for the Diagnosis of Electrohypersensitivity and Multiple Chemical Sensitivity. Diagnostics 2020, 10, 427. [Google Scholar] [CrossRef]
- Rea, W.J.; Pan, Y.; Fenyves, E.F.; Sujisawa, I.; Suyama, H.; Samadi, N.; Ross, G.H. Electromagnetic field sensitivity. J. Bioelectr. 1991, 10, 214–256. [Google Scholar] [CrossRef]
- WHO (World Health Organization). Electromagnetic Fields and Public Health, Electromagnetic Hypersensitivity; WHO Fact Sheet No. 296; World Health Organization: Geneva, Switzerland, 2005. [Google Scholar]
- Hansson Mild, K., Repacholi, M., van Deventer, E., Ravazzani, P., Eds.; Electromagnetic hypersensitivity. In Proceedings of the WHO International Seminar and Working Group Meeting on EMF Hypersensitivity, Prague, Czech Republic, 25–27 October 2004; World Health Organization: Geneva, Switzerland, 2006. ISBN 9241594128. [Google Scholar]
- Hocking, B. Microwave sickness: A reappraisal. Occup. Med. 2001, 51, 66–69. [Google Scholar] [CrossRef][Green Version]
- Hocking, B.; Westerman, R. Neurological changes induced by a mobile phone. Occup. Med. 2002, 52, 413–415. [Google Scholar] [CrossRef] [PubMed]
- Hocking, B.; Westerman, R. Neurological effects of radiofrequency radiation. Occup. Med. 2003, 53, 123–127. [Google Scholar] [CrossRef]
- McCarty, D.E.; Carrubba, S.; Chesson, A.L.; Frilot, C.; Gonzalez-Toledo, E.; Marino, A.A. Electromagnetic Hypersensitivity: Evidence for a Novel Neurological Syndrome. Int. J. Neurosci. 2011, 121, 670–676. [Google Scholar] [CrossRef]
- Havas, M.; Marrongelle, J.; Pollner, B.; Kelley, E.; Rees, C.; Tully, L. Provocation study using heart rate variability shows microwave radiation from 2.4 GHz cordless phone affects autonomic nervous system. In Non-Thermal Effects and Mechanisms of Interaction between Electromagnetic Fields and Living Matter; Mattioli: Fidenza, Italy, 2010; pp. 273–300. ISBN 9788862611664. [Google Scholar]
- Havas, M. Radiation from wireless technology affects the blood, the heart and the autonomic nervous system. Rev. Environ. Health 2013, 28, 75–84. [Google Scholar] [CrossRef]
- Anon. Does Short-term Exposure to Cell Phone Radiation Affect the Blood? Wise Traditions Winter. 2014. Available online: https://www.globalresearch.ca/does-short-term-exposure-tocell-phone-radiation-affect-the-blood/5429108 (accessed on 6 February 2021).
- Dodge, C.H. Clinical and hygienic aspects of exposure to electromagnetic fields. In Biological Effects and Health Implications of Microwave Radiation, Symposium Proceedings, Richmond, VA, USA, 17–19 September 1970; Cleary, S.I., Ed.; USDHEW: Boston, MA, USA, 1970; Volume 70, pp. 140–149. [Google Scholar]
- Röösli, M. Radiofrequency electromagnetic field exposure and non-specific symptoms of ill health: A systematic review. Environ. Res. 2008, 107, 277–287. [Google Scholar] [CrossRef] [PubMed]
- Johansson, A.; Nordin, S.; Heiden, M.; Sandström, M. Symptoms, personality traits, and stress in people with mobile phone-related symptoms and electromagnetic hypersensitivity. J. Psychosom. Res. 2010, 68, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Nordin, S.; Neely, G.; Olsson, D.; Sandström, M. Odor and noise intolerance in persons with self-reported electromagnetic hypersensitivity. Int. J. Environ. Res. Public Health 2014, 11, 8794–8805. [Google Scholar] [CrossRef] [PubMed]
- Medeiros, L.N.; Sanchez, T.G. Tinnitus and cell phones: The role of electromagnetic radiofrequency radiation. Braz. J. Otorhinolaryngol. 2016, 82, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Belpomme, D.; Hardell, L.; Belyaev, I.; Burgio, E.; Carpenter, D.O. Thermal and non-thermal health effects of low intensity non-ionizing radiation: An international perspective. Environ. Pollut. 2018, 242, 643–658. [Google Scholar] [CrossRef]
- Dalle-Donne, I.; Rossi, R.; Colombo, R.; Giustarini, D.; Milzani, A. Biomarkers of oxidative damage in human disease. Clin. Chem. 2006, 52, 601–623. [Google Scholar] [CrossRef]
- Stein, Y.; Udasin, I.G. Electromagnetic hypersensitivity (EHS, microwave syndrome)—Review of mechanisms. Environ. Res. 2020, 186, 109445. [Google Scholar] [CrossRef] [PubMed]
- Bartha, L.; Baumzweiger, W.; Buscher, D.S.; Callender, T.; Dahl, K.A.; Davidoff, A.; Donnay, A.; Edelson, S.B.; Elson, B.D.; Elliott, E.; et al. Multiple chemical sensitivity: A 1999 consensus. Arch. Environ. Health 1999, 54, 147–149. [Google Scholar] [CrossRef]
- Lacour, M.; Zunder, T.; Schmidtke, K.; Vaith, P.; Scheidt, C. Multiple chemical sensitivity syndrome (MCS)—Suggestions for an extension of the U.S. MCS-case definition. Int. J. Hyg. Environ. Health 2005, 208, 141–151. [Google Scholar] [CrossRef]
- Calabrò, E.; Condello, S.; Currò, M.; Ferlazzo, N.; Caccamo, D.; Magazù, S.; Ientile, R. Modulation of heat shock protein response in SH-SY5Y by mobile phone microwaves. World. J. Biol. Chem. 2012, 3, 34–40. [Google Scholar] [CrossRef]
- Calabrò, E.; Condello, S.; Currò, M.; Ferlazzo, N.; Vecchio, M.; Caccamo, D.; Magazù, S.; Ientile, R. 50 Hz electromagnetic field produced changes in FTIR spectroscopy associated with mitochondrial transmembrane potential reduction in neuronal-like SH-SY5Y cells. Oxid. Med. Cell Longev. 2013, 2013, 414393. [Google Scholar] [CrossRef]
- Calabrò, E.; Condello, S.; Currò, M.; Ferlazzo, N.; Caccamo, D.; Magazù, S.; Ientile, R. Effects of low intensity static magnetic field on FTIR spectra and ROS production in SH-SY5Y neuronal-like cells. Bioelectromagnetics 2013, 34, 618–629. [Google Scholar] [CrossRef] [PubMed]
- Kheifets, L.; Repacholi, M.; Saunders, R.; van Deventer, E. The sensitivity of children to electromagnetic fields. Pediatrics 2005, 116, e303–e313. [Google Scholar] [CrossRef]
- Herbert, M.R.; Sage, C. Autism and EMF? Plausibility of a pathophysiological link—Part, I. Pathophysiology 2013, 20, 191–209. [Google Scholar] [CrossRef]
- Herbert, M.R.; Sage, C. Autism and EMF? Plausibility of a pathophysiological link part II. Pathophysiology 2013, 20, 211–234. [Google Scholar] [CrossRef]
- Panagopoulos, D.J.; Johansson, O.; Carlo, G.L. Polarization: A Key Difference between Man-made and Natural Electromagnetic Fields, in regard to Biological Activity. Sci. Rep. 2015, 12, 14914. [Google Scholar] [CrossRef]
- Belyaev, I. Dependence of Non-Thermal Biological Effects of Microwaves on Physical and Biological Variables: Implications for Reproducibility and Safety Standards. In Non-Thermal Effects and Mechanisms of Interaction between Electromagnetic Fields and Living Matter; Mattioli: Fidenza, Italy, 2010; pp. 187–218. ISBN 9788862611664.71. [Google Scholar]
- Sage, C. The implications of non-linear biological oscillations on human electrophysiology for electrohypersensitivity (EHS) and multiple chemical sensitivity (MCS). Rev. Environ. Health 2015, 30, 293–303. [Google Scholar] [CrossRef]
- Belyaev, I. Biophysical mechanisms for nonthermal microwave effects. In Electromagnetic Fields in Biology and Medicine; Markov, M., Ed.; CRC Press: Boca Raton, FL, USA, 2015; pp. 49–68. [Google Scholar]
- Panagopoulos, D.J.; Karabarbounis, A.; Margaritis, L.H. Mechanism for action of electromagnetic fields on cells. Biochem Biophys. Res. Commun. 2002, 298, 95–102. [Google Scholar] [CrossRef]
- Pall, M.L. Electromagnetic fields act via activation of voltage-gated calcium channels to produce beneficial or adverse effects. J. Cell Mol. Med. 2013, 17, 958–965. [Google Scholar] [CrossRef]
- Pall, M.L. Electromagnetic field activation of voltage-gated calcium channels: Role in therapeutic effects. Electromagn. Biol. Med. 2014, 33, 251. [Google Scholar] [CrossRef]
- Pakhomov, A.G.; Akyel, Y.; Pakhomova, O.N.; Stuck, B.E.; Murphy, M.R. Current state and implications of research on biological effects of millimeter waves: A review of the literature. Bioelectromagnetics 1998, 19, 393–413. [Google Scholar] [CrossRef]
- Dasdag, S.; Akdag, M.Z.; Ayyildiz, O.; Demirtas, O.C.; Yayla, M.; Sert, C. Do cellular phones alter blood parameters and birth weight of rats? Electromagn. Biol. Med. 2000, 19, 107–113. [Google Scholar] [CrossRef]
- Belyaev, I.Y. Non-thermal Biological Effects of Microwaves. Microwave Rev. 2005, 11, 13–29. [Google Scholar]
- Belyaev, I.Y.; Hillert, L.; Protopopova, M.; Tamm, C.; Malmgren, L.O.; Persson, B.R.; Selivanova, G.; Harms-Ringdahl, M. 915 MHz microwaves and 50 Hz magnetic field affect chromatin conformation and 53BP1 foci in human lymphocytes from hypersensitive and healthy persons. Bioelectromagnetics 2005, 26, 173–184. [Google Scholar] [CrossRef]
- Hardell, L.; Sage, C. Biological effects from electromagnetic field exposure and public exposure standards. Biomed. Pharmacother. 2008, 62, 104–109. [Google Scholar] [CrossRef] [PubMed]
- Akdag, M.Z.; Bilgin, M.H.; Dasdag, S.; Tumer, C. Alteration of nitric oxide production in rats exposed to a prolonged, extremely low-frequency magnetic field. Electromagn. Biol. Med. 2007, 26, 99–106. [Google Scholar] [CrossRef]
- Friedman, J.; Kraus, S.; Hauptman, Y.; Schiff, Y.; Seger, R. Mechanism of short-term ERK activation by electromagnetic fields at mobile phone frequencies. Biochem. J. 2007, 405, 559–568. [Google Scholar] [CrossRef]
- Luukkonen, J.; Hakulinen, P.; Maki-Paakkanen, J.; Juutilainen, J.; Naarala, J. Enhancement of chemically induced reactive oxygen species production and DNA damage in human SH-SY5Y neuroblastoma cells by 872 MHz radiofrequency radiation. Mutat. Res. 2009, 662, 54–58. [Google Scholar] [CrossRef]
- Jorge-Mora, T.; Alvarez, F.M.; Leiro-Vidal, J.M.; Jorge-Barreiro, F.J.; Ares-Pena, F.; Lopez-Martin, M.E. Exposure to 2.45 GHz Microwave Radiation Provokes Cerebral Changes in Induction of Hsp-90 α/β Heat Shock Protein in Rat. PIER 2010, 100, 351–379. [Google Scholar] [CrossRef]
- Kim, S.J.; Jang, Y.W.; Hyung, K.E.; Lee, D.K.; Hyun, K.H.; Jeong, S.H.; Min, K.H.; Kang, W.; Jeong, J.H.; Park, S.Y.; et al. Extremely low-frequency electromagnetic field exposure enhances inflammatory response and inhibits effect of antioxidant in RAW 264.7 cells. Bioelectromagnetics 2017, 38, 374–385. [Google Scholar] [CrossRef]
- Alkis, M.E.; Akdag, M.Z.; Dasdag, S. Effects of Low-Intensity Microwave Radiation on Oxidant-Antioxidant Parameters and DNA Damage in the Liver of Rats. Bioelectromagnetics 2021, 42, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Royal Belgium Academy of Medicine. 2015, Brussels International Scientific Declaration on Electromagnetic Hypersensitivity and Multiple Chemical Sensitivity; Royal Belgium Academy of Medicine: Brussels, Belgium, 2015; Available online: Eceri-institute.org/fichiers/1441982765_Statement_EN_DEFINITIF.pdf (accessed on 6 February 2021).
- Carpenter, D.O.; Belpomme, D. Idiopathic environmental intolerance. Rev. Environ. Health 2015, 30, 257. [Google Scholar] [CrossRef] [PubMed]
- International Commission on Non-Ionizing Radiation Protection. ICNIRP statement on the ‘Guidelines for limiting exposure to time-varying electric, magnetic, and electromagnetic fields (up to 300 GHz)’. Health Phys. 2009, 97, 257–258. [Google Scholar] [CrossRef] [PubMed]
- Ribatti, D. The crucial role of mast cells in blood-brain barrier alterations. Exp. Cell Res. 2015. [Google Scholar] [CrossRef]
- Lindsberg, P.J.; Strbian, D.; Karjalainen-Lindsberg, M.L. Mast cells as early responders in the regulation of acute blood-brain barrier changes after cerebral ischemia and hemorrhage. J. Cereb. Blood Flow. Metab. 2010, 30, 689–702. [Google Scholar] [CrossRef] [PubMed]
- Nordal, R.A.; Wong, C.S. Molecular targets in radiation-induced blood-brain barrier disruption. Int. J. Radiat. Oncol. Biol. Phys. 2005, 62, 279–287. [Google Scholar] [CrossRef] [PubMed]
- Block, M.L.; Zecca, L.; Hong, J.S. Microglia-mediated neurotoxicity: Uncovering the molecular mechanisms. Nat. Rev. Neurosci. 2007, 8, 57–69. [Google Scholar] [CrossRef] [PubMed]
- De Ley, G.; Demeester, G.; Leusen, L. Cerebral histamine in hypoxia. Arch. Int. Physiol. Biochim. 1984, 94, 33–35. [Google Scholar]
- Dux, E.; Temesvári, P.; Joó, F.; Adám, G.; Clementi, F.; Dux, L.; Hideg, J.; Hossmannet, K.A. The blood-brain barrier in hypoxia: Ultrastructural aspects and adenylate cyclase activity of brain capillaries. Neuroscience 1984, 12, 951–958. [Google Scholar] [CrossRef]
- Hardebo, J.E.; Beley, A. Influence of blood pressure on bloodbrain barrier function in brain ischemia. Acta Neurol. Scand. 1984, 70, 356–359. [Google Scholar] [CrossRef] [PubMed]
- Gotoh, O.; Asano, T.; Koide, T.; Takakura, K. Ischemic brain edema following occlusion of the middle cerebral artery in the rat. I: The time courses of the brain water, sodium and potassium contents and blood-brain barrier permeability to 125I-albumin. Stroke 1985, 16, 101–109. [Google Scholar] [CrossRef]
- Hatashita, S.; Hoff, J.T. Brain edema and cerebrovascular permeability during cerebral ischemia in rats. Stroke 1990, 21, 582–588. [Google Scholar] [CrossRef] [PubMed]
- Pache, M.; Keiser, H.J.; Akhalbedashvili, N.; Lienert, C.; Dubler, B.; Kappos, L.; Flammer, J. Extraocular blood flow and endothelin-1 plasma levels in patients with multiple sclerosis. Eur. Neurol. 2003, 49, 164–168. [Google Scholar] [CrossRef]
- Davies, A.L.; Desai, R.A.; Bloomfield, P.S.; McIntosh, P.R.; Chapple, K.J.; Linington, C.; Fairless, R.; Diem, R.; Kasti, M.; Murphy, M.P.; et al. Neurological deficits caused by tissue hypoxia in neuroinflammatory disease. Ann. Neurol. 2013, 74, 815–825. [Google Scholar] [CrossRef] [PubMed]
- Hösli, L.; Hösli, E.; Schneider, U.; Wiget, W. Evidence for the existence of histamine H1- and H2-receptors on astrocytes of cultured rat central nervous system. Neurosci. Lett. 1984, 48, 287–291. [Google Scholar] [CrossRef]
- Dong, Y.; Benveniste, E.N. Immune function of astrocytes. Glia 2001, 36, 180–190. [Google Scholar] [CrossRef]
- Adachi, N. Cerebral ischemia and brain histamine. Brain Res. Rev. 2005, 50, 275–286. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Qu, C.; Lu, X.; Zhang, S. Activation of microglia by histamine and substance P. Cell Physiol. Biochem. 2014, 34, 768–780. [Google Scholar] [CrossRef] [PubMed]
- Oscar, K.J.; Hawkins, T.D. Microwave alteration of the blood-brain barrier system of rats. Brain Res. 1977, 126, 281–293. [Google Scholar] [CrossRef]
- Oscar, K.J.; Gruenau, S.P.; Folker, M.T.; Rapoport, S.I. Local cerebral blood flow after microwave exposure. Brain Res. 1981, 204, 220–225. [Google Scholar] [CrossRef]
- Albert, E.N.; Kerns, J.M. Reversible microwave effects on the blood-brain barrier. Brain Res. 1981, 230, 153–164. [Google Scholar] [CrossRef]
- Kasparová, S.; Brezová, V.; Valko, M.; Horecký, J.; Mlynárik, V.; Liptaj, T.; Vancová, O.; Ulicná, O.; Dobrota, D. Study of the oxidative stress in a rat model of chronic brain hypoperfusion. Neurochem. Int. 2005, 46, 601–611. [Google Scholar] [CrossRef] [PubMed]
- Nittby, H.; Grafström, G.; Eberhardt, J.L.; Malmgren, L.; Brun, A.; Persson, B.R.; Salford, L.G. Radiofrequency and extremely low-frequency electromagnetic field effects on the blood-brain barrier. Electromagn. Biol. Med. 2008, 27, 103–126. [Google Scholar] [CrossRef]
- Raslan, F.; Schwarz, T.; Meuth, S.G.; Austinat, M.; Bader, M.; Renné, T.; Roosen, K.; Stoll, G.; Sirén, A.L.; Kleinschnitz, C. Inhibition of bradykinin receptor B1 protects mice from focal brain injury by reducing blood-brain barrier leakage and inflammation. J. Cereb. Blood Flow. Metab. 2010, 30, 1477–1486. [Google Scholar] [CrossRef]
- Gurney, K.J.; Estrada, E.Y.; Rosenberg, G.A. Blood-brain barrier disruption by stromelysin-1 facilitates neutrophil infiltration in neuroinflammation. Neurobiol. Dis. 2006, 23, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Moretti, R.; Pansiot, J.; Bettati, D.; Strazielle, N.; Ghersi-Egea, J.F.; Damante, G.; Fleiss, B.; Titomanlio, L.; Gressens, P. Blood-brain barrier dysfunction in disorders of the developing brain. Front. Neurosci. 2015, 9, 40. [Google Scholar] [CrossRef]
- Rubin, G.J.; Das Munshi, J.; Wessely, S. Electromagnetic hypersensitivity: A systematic review of provocation studies. Psychosom. Med. 2005, 67, 224–232. [Google Scholar] [CrossRef]
- Eltiti, S.; Wallace, D.; Ridgewell, A.; Zougkou, K.; Russo, R.; Sepulveda, F.; Mirshekar-Syahkal, D.; Rasor, P.; Deeble, R.; Fox, E. Does short-term exposure to mobile phone base station signals increase symptoms in individuals who report sensitivity to electromagnetic fields? A double-blind randomized provocation study. Environ. Health Perspect. 2007, 115, 1603–1608. [Google Scholar] [CrossRef] [PubMed]
- Rubin, G.J.; Nieto-Hernandez, R.; Wessely, S. Idiopathic environmental intolerance attributed to electromagnetic fields (formerly ‘electromagnetic hypersensitivity’): An updated systematic review of provocation studies. Bioelectromagnetics 2010, 31, 1–11. [Google Scholar] [CrossRef]
- Rubin, G.J.; Hillert, L.; Nieto-Hernandez, R.; van Rongen, E.; Oftedal, G. Do people with idiopathic environmental intolerance attributed to electromagnetic fields display physiological effects when exposed to electromagnetic fields? A systematic review of provocation studies. Bioelectromagnetics 2011, 32, 593–609. [Google Scholar] [CrossRef] [PubMed]
- Baliatsas, C.; Van Kamp, I.; Lebret, E.; Rubin, G.J. Idiopathic environmental intolerance attributed to electromagnetic fields (IEI-EMF): A systematic review of identifying criteria. BMC Public Health 2012, 12, 643. [Google Scholar] [CrossRef] [PubMed]
- Eltiti, S.; Wallace, D.; Russo, R.; Fox, E. Aggregated data from two double blind base station provocation studies comparing individuals with idiopathic environmental intolerance with attribution to electromagnetic fields and controls. Bioelectromagnetics 2015, 36, 96–107. [Google Scholar] [CrossRef]
- Schmiedchen, K.; Driessen, S.; Oftedal, G. Methodological limitations in experimental studies on symptom development in individuals with idiopathic environmental intolerance attributed to electromagnetic fields (IEI-EMF)—A systematic review. Environ. Health 2019, 18, 88. [Google Scholar] [CrossRef] [PubMed]
- Röösli, M.; Mohler, E.; Frei, P. Sense and sensibility in the context of radiofrequency electromagnetic field exposure. C R Physique 2010, 11, 576–584. [Google Scholar] [CrossRef]
- Dieudonné, M. Electromagnetic hypersensitivity: A critical review of explanatory hypotheses. Environ. Health 2020, 19, 48. [Google Scholar] [CrossRef] [PubMed]
- Alasdair, P.; Peer Review and Quality of Science. Comments Posted at 07/08/2006 on Powerwatch Website. Available online: https://www.powerwatch.org.uk/columns/aphilips/index.asp (accessed on 6 February 2021).
- Wang, T. The Sick Building Syndrome: A Study of Some Contributing Factors. Ph.D. Thesis, University of Surrey, Guildford, UK, 1995. Available online: https://epubs.surrey.ac.uk/843508/ (accessed on 6 February 2021).
- Trimmel, M.; Schweiger, E. Effects of an ELF (50 Hz, 1 mT) electromagnetic field (EMF) on concentration in visual attention, perception and memory including effects of EMF sensitivity. Toxicol. Lett. 1998, 96–97, 377–382. [Google Scholar] [CrossRef]
- Arnetz, B.B.; Akerstedt, T.; Hillert, L.; Lowden, A.; Kuster, N.; Wiholm, C. The Effects of 884 MHz GSM Wireless Communication Signals on Self-reported Symptom and Sleep (EEG)—An Experimental Provocation Study. PIERS Online 2007, 3, 1148–1150. [Google Scholar] [CrossRef]
- Wiholm, C.; Lowden, A.; Kuster, N.; Hillert, L.; Arnetz, B.B.; Akerstedt, T.; Moffat, S.D. Mobile phone exposure and spatial memory. Bioelectromagnetics 2009, 30, 59–65. [Google Scholar] [CrossRef]
- Tuengler, A.; von Klitzing, L. Hypothesis on how to measure electromagnetic hypersensitivity. Electromagn. Biol. Med. 2013, 32, 281–290. [Google Scholar] [CrossRef]
- Von Klitzing, L. Artificial EMG by WLAN-Exposure. J. Biostat. Biometric App. 2021, 6, 101. [Google Scholar]
- Koppel, T.; Vilcane, I.; Ahonen, M. 50 Hz magnetic field affects heart rate variability–An experimental study. In Proceedings of the 2018 EMF-Med 1st World Conference on Biomedical Applications of Electromagnetic Fields (EMF-Med), Split, Croatia, 10–13 September 2018; IEEE: New York, NY, USA, 2018; pp. 1–2. [Google Scholar] [CrossRef]
- Kimata, H. Enhancement of allergic skin wheal responses by microwave radiation from mobile phones in patients with atopic eczema/dermatitis syndrome. Int. Arch. Allergy Immunol. 2002, 129, 348–350. [Google Scholar] [CrossRef]
- Kimata, H. Microwave radiation from cellular phones increases allergen-specific IgE production. Allergy 2005, 60, 838–839. [Google Scholar] [CrossRef] [PubMed]
- Zwamborn, A.P.M.; Vossen, S.H.J.A.; van Leersum, B.J.A.M.; Ouwens, M.A.; Mäkel, W.K. Effects of Global Communication System Radio-Frequency Fields on Well Being and Cognitive Functions of Human Subjects with and without Subjective Complaints; Netherlands Organization for Applied Scientific Research (TNO): The Hague, The Netherlands, 2003; Available online: http://www.next-up.org/pdf/tno_fel_report_03148_def.pdf (accessed on 16 March 2021).
- Braune, S.; Wrocklage, C.; Raczek, J.; Gailus, T.; Lücking, C.H. Resting blood pressure increase during exposure to a radio-frequency electromagnetic field. Lancet 1998, 351, 1857–1858. [Google Scholar] [CrossRef]
- Volkow, N.D.; Tomasi, D.; Wang, G.J.; Vaska, P.; Fowler, J.S.; Telang, F.; Alexoff, D.; Logan, J.; Wong, C. Effects of cell phone radiofrequency signal exposure on brain glucose metabolism. JAMA 2011, 305, 808–813. [Google Scholar] [CrossRef] [PubMed]
- Béres, S.; Németh, Á.; Ajtay, Z.; Kiss, I.; Németh, B.; Hejjel, L. Cellular Phone Irradiation of the Head Affects Heart Rate Variability Depending on Inspiration/Expiration Ratio. In Vivo 2018, 32, 1145–1153. [Google Scholar] [CrossRef] [PubMed]
- Bergqvist, U.; Vogel, E. Possible health implications of subjective symptoms and electromagnetic fields. In A Report Prepared by a European Group of Experts for the European Commission, DGV; Arbete Och Hälsa, 19; Swedish National Institute for Working Life: Stockholm, Sweden, 1997; Available online: http://www2.niwl.se/forlag/en/ (accessed on 23 February 2021).
- Santini, R.; Seigne, M.; Bonhomme-Faivre, L.; Bouet, S.; Defrasme, E.; Sage, M. Symptoms experienced by users of digital cellular phones: A study of a French engineering school. Electromagn. Biol. Med. 2002, 21, 81–88. [Google Scholar] [CrossRef]
- Verrender, A.; Loughran, S.P.; Anderson, V.; Hillert, L.; Rubin, G.J.; Oftedal, G.; Croft, R.J. IEI-EMF provocation case studies: A novel approach to testing sensitive individuals. Bioelectromagnetics 2018, 39, 132–143. [Google Scholar] [CrossRef]
- ANSES (French Agency for Food, Environmental and Occupational Health & Safely). Hypersensibilité Electromagnétique ou Intolérance Environnementale Idiopathique Attribuée aux Champs Electromagnétiques, Saisine n°«2011-SA-0150», Mars 2018. Available online: https://www.anses.fr/fr/system/files/AP2011SA0150Ra.pdf (accessed on 16 March 2021).
- Mortazavi, S.M.J.; Mahbudi, A.; Atefi, M.; Bagheri Sh Bahaedini, N.; Besharati, A. An old issue and a new look: Electromagnetic hypersensitivity caused by radiations emitted by GSM mobile phones. Technol. Health Care. 2011, 19, 435–443. [Google Scholar] [CrossRef]
- Parsaei, H.; Faraz, M.; Mortazavi, S.M.J. A multilayer perceptron neural network-based model for predicting subjective health symptoms in people living in the vicinity of mobile phone base stations. Ecopsychology 2017, 9, 99–105. [Google Scholar] [CrossRef]
- Dieudonné, M. Does electromagnetic hypersensitivity originate from nocebo responses? Indications from a qualitative study. Bioelectromagnetics 2016, 37, 14–24. [Google Scholar] [CrossRef]
- WHO. ICD-10 International Classification of Diseases 10th Revision The Global Standard for Diagnostic Health Information. ICD-10 Code W90 for Exposure to Other Non-Ionizing Radiation. Available online: https://icd.who.int/browse10/2010/en#/W90 (accessed on 16 March 2021).
- Belyaev, I.; Dean, A.; Eger, H.; Hubmann, G.; Jandrisovits, R.; Kern, M.; Kundi, M.; Moshammer, H.; Lercher, P.; Müller, K.; et al. EUROPAEM EMF Guideline 2016 for the prevention, diagnosis and treatment of EMF-related health problems and illnesses. Rev. Environ. Health 2016, 31, 363–397. [Google Scholar] [CrossRef] [PubMed]
- Sage, C.; Carpenter, D.O. Public health implications of wireless technologies. Pathophysiology 2009, 6, 233–246. [Google Scholar] [CrossRef]
- Bandara, P.; Carpenter, D.O. Planetary electromagnetic pollution: It is time to assess its impact. Lancet Planet Health 2018, 2, e512–e514. [Google Scholar] [CrossRef]
| 1 | Lack of precise inclusion criteria. No objective criteria based on molecular biomarkers and imaging techniques. | [62,125,127,128] |
| 2 | No clear consideration on medical anamnesis and degree of EHS severity. | [125,127] |
| 3 | No consideration for an association with MCS. | [9] |
| 4 | No consideration that EHS patients are intolerant to specific man-made waves frequencies. | [62,125,127,128] |
| 5 | Too short exposure duration. | [125,126] |
| 6 | Symptom recording made too early. | [125,127] |
| 7 | Endpoint criteria depending on subjective statements. | [62,122,123,124,125,126,127] |
| 8 | Possible EHS-associated psychological conditioning due to past suffering. | [129] |
| 9 | Possible abnormal EMF signal transmission in case of sham exposure. | [130] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Belpomme, D.; Carlo, G.L.; Irigaray, P.; Carpenter, D.O.; Hardell, L.; Kundi, M.; Belyaev, I.; Havas, M.; Adlkofer, F.; Heuser, G.; et al. The Critical Importance of Molecular Biomarkers and Imaging in the Study of Electrohypersensitivity. A Scientific Consensus International Report. Int. J. Mol. Sci. 2021, 22, 7321. https://doi.org/10.3390/ijms22147321
Belpomme D, Carlo GL, Irigaray P, Carpenter DO, Hardell L, Kundi M, Belyaev I, Havas M, Adlkofer F, Heuser G, et al. The Critical Importance of Molecular Biomarkers and Imaging in the Study of Electrohypersensitivity. A Scientific Consensus International Report. International Journal of Molecular Sciences. 2021; 22(14):7321. https://doi.org/10.3390/ijms22147321
Chicago/Turabian StyleBelpomme, Dominique, George L. Carlo, Philippe Irigaray, David O. Carpenter, Lennart Hardell, Michael Kundi, Igor Belyaev, Magda Havas, Franz Adlkofer, Gunnar Heuser, and et al. 2021. "The Critical Importance of Molecular Biomarkers and Imaging in the Study of Electrohypersensitivity. A Scientific Consensus International Report" International Journal of Molecular Sciences 22, no. 14: 7321. https://doi.org/10.3390/ijms22147321
APA StyleBelpomme, D., Carlo, G. L., Irigaray, P., Carpenter, D. O., Hardell, L., Kundi, M., Belyaev, I., Havas, M., Adlkofer, F., Heuser, G., Miller, A. B., Caccamo, D., De Luca, C., von Klitzing, L., Pall, M. L., Bandara, P., Stein, Y., Sage, C., Soffritti, M., ... Vorst, A. V. (2021). The Critical Importance of Molecular Biomarkers and Imaging in the Study of Electrohypersensitivity. A Scientific Consensus International Report. International Journal of Molecular Sciences, 22(14), 7321. https://doi.org/10.3390/ijms22147321













