Abstract
Human estrogens prescribed for hormone replacement therapy (HRT) are known to be potent carcinogens. To find safer estrogens, several chlorinated estrogens were synthesized and their carcinogenic potential were determined. A pellet containing either 2-chloro-17β-estradiol (2-ClE2) or 4-chloro-17β-estradiol (4-ClE2) was implanted subcutaneously for 52 weeks into August Copenhagen Irish (ACI) rats, a preferred animal model for human breast cancer. 17β-Estradiol (E2) frequently induced mammary tumors while both 2-ClE2 and 4-ClE2 did not. Their 17α-ethinyl forms, thought to be orally active estrogens, were also synthesized. Neither 2-chloro-17α-ethinylestradiol (2-ClEE2) nor 4-chloro-17α-ethinylestradiol (4-ClEE2) induced tumors. The less carcinogenic effects were supported by histological examination of mammary glands of ACI rats treated with the chlorinated estrogens. A chlorine atom positioned at the 2- or 4-position of E2 may prevent the metabolic activation, resulting in reducing the carcinogenicity. 2-ClE2 and 4-ClE2 administered subcutaneously and 2-ClEE2 and 4-ClEE2 given orally to ovariectomized rats all showed uterotrophic potency, albeit slightly weaker than that of E2. Our results indicate that less carcinogenic chlorinated estrogens retaining estrogenic potential could be safer alternatives to the carcinogenic estrogens now in use for HRT.
1. Introduction
Human estrogens are used in hormone replacement therapy (HRT) to alleviate menopausal symptoms and protect against osteoporosis [1,2]. Unfortunately, long-term HRT increases the incidence of breast [3,4,5,6] and endometrial cancers [7]. The risk of these cancers is correlated with the duration of HRT [4,5,6,8]. The mechanism underlying estrogen-induced carcinogenesis has not been fully explored, but both proliferative effects mediated through the estrogen receptor and/or DNA damage induced by human estrogen metabolites are significant factors in the process [9,10,11].
DNA damage has been detected in the tissues of rodents treated with human estrogens [12,13,14,15]. Human estrogens are primarily metabolized by cytochrome P450 enzymes to form 2- and 4-hydroxyestrogens (2- and 4-OHE) [16] (Figure 1). The catecholestrogens are oxidized further to 2,3-quinone and/or 3,4-quinone by P450 enzymes or peroxidase [16,17,18]. The 2,3-quinone of 2-OHE reacts with DNA to form 2-OHE-N2-dG and 2-OHE-N6-dA [19], which are potentially mutagenic [20]. The 3,4-quinone of 4-OHE reacts with dA and dG to form 4-OHE-1(α,β)-N3-dA and 4-OHE-1(α,β)-N7-dG, which are readily depurinated [19,21]. The resulting apurinic sites are mutagenic lesions [22], contributing to the initiation of cancer. During redox cycling, 2,3-quinone and/or 3,4-quinone are reduced back to their catecholestrogens. In the reduction reactions, free radicals produced cause oxidative DNA damage such as 8-oxo-7,8-dihydro-2′-deoxyguanosine [23], which has been detected in mammary DNA obtained from breast cancer patients [24]. Thus, DNA damages induced by human estrogens are capable of initiating breast and endometrial cancer.
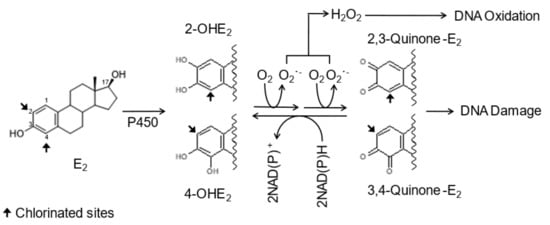
Figure 1.
Proposed oxidative mechanism of chlorinated estrogens.
The August Copenhagen Irish (ACI) rat strain has been used as a preferred animal model for studying human sporadic breast cancer. ACI rats have a very low incidence (11% over 3 years) of spontaneous mammary tumors [25,26], which is advantageous for accurately evaluating their tumorigenicity. Indeed, E2 (the structure in Figure 2) [25,26,27] and 17α-ethinylestradiol (EE2) [28] were demonstrated to induce mammary tumors. Therefore, women receiving human estrogens for HRT may have a higher risk of developing breast and reproductive cancers.
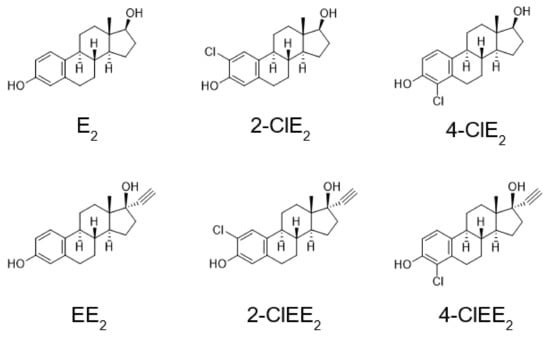
Figure 2.
Structures of E2 and EE2 and their chlorinated compounds.
In the present study, to prevent the metabolic activation of estrogens, a hydrogen atom at the 2- or 4-position of human estrogen was replaced by a chlorine atom. The tumorigenic and estrogenic potentials of the synthesized chlorinated estrogens (Figure 2), 2-chloro-17β-estradiol (2-ClE2) and 4-chloro-17β-estradiol (4-ClE2), were determined using rat models. Because E2 is inactivated when taken orally, the 17α-ethinyl formula is used for oral treatment [29,30]. The chlorinated 17α-ethinyl compounds, 2-chloro-17α-ethinylestradiol (2-ClEE2) and 4-chloro-17α-ethinylestradiol (4-ClEE2), were also synthesized and subjected to measure their tumorigenic and estrogenic potentials.
2. Results
2.1. Evidence of Mammary Tumors
Development of mammary tumors in ACI rats implanted with a pellet containing E2 or a chlorinated estrogen was monitored by palpation once a week for 52 weeks. In rats administered E2 (1.25 mg), palpable mammary tumors appeared around 38 weeks after pellet implantation; the cumulative incidence of the tumors was 80% (4/5) after 52 weeks (Figure 3 and Table 1). With 2.5 mg E2, mammary tumors appeared earlier, at 24 weeks, although the cumulative tumor incidence was 90% (9/10)—i.e., almost same as that observed with 1.25 mg E2. When 5.0 mg E2 was implanted, severe loss of body weight occurred within several weeks; therefore, the experiment with 5.0 mg E2 was terminated. Mammary tumors were confirmed by pathological examination as performed previously [27,31]. The body weight of rats treated with the following chlorinated estrogen increased as observed with the untreated rats. When dissected ACI rats treated with chlorinated estrogens at the end of experiment, no significant abnormality was observed in other organs including ovary and uterus. Among the 6 rats treated with 2.5 or 5.0 mg 2-ClE2 or 4-ClE2, no palpable mammary tumors were observed even after 52 weeks, as shown in the untreated rats (Figure 3 and Table 1). The 17α-ethinyl compounds, 2-ClEE2 and 4-ClEE2, also did not induce the tumors (Table 1). The data obtained by the 17α-ethinyl formula may support and strengthen the non-tumor evidence of 2-ClE2 and 4-ClE2.
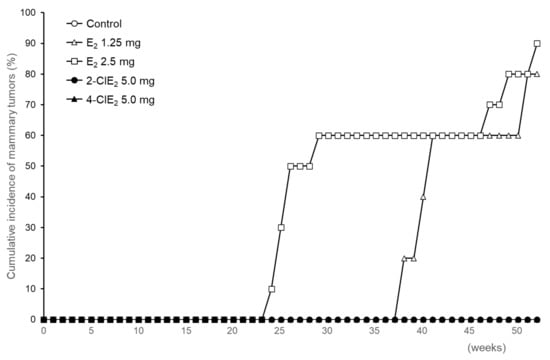
Figure 3.
Cumulative incidence of mammary tumors in chlorinated estrogen-treated rats. Development of mammary tumors in ACI rats implanted with placebo (n = 5), E2 [1.25 mg (n = 5), 2.5 mg (n = 10)], 2-ClE2 [5.0 mg (n = 6)], or 4-ClE2 [5.0 mg (n = 6)] pellets was monitored once a week for 52 weeks.

Table 1.
Incidence of mammary tumors in ACI rats implanted with a chlorinated estrogen.
2.2. Histological Examination of Mammary Glands
Mammary whole-mounts of chlorinated estrogen-treated ACI rats were subjected to histological examination. With E2 (Figure 4B), extension of the mammary glands, branching of the ducts, and the number of end buds and alveoli were all increased. Premalignant lesions such as acinar hyperplasia was also observed in whole-mount preparations. In contrast, enlargement of the mammary glands in rats treated with 2-ClE2 (Figure 4C) or 4-ClE2 (Figure 4D) was much less than that produced by E2. Mammary glands of rats treated with the 17α-ethinyl forms, 2-ClEE2 (Figure 4E) and 4-ClEE2 (Figure 4F), showed similar histological results as those treated with 2-ClE2 and 4-ClE2, respectively. No precancerous lesions were detected in rats treated with any chlorinated estrogens. Neither enlargement of the mammary glands nor premalignant lesions were detected in the vehicle-treated control ACI rats (Figure 4A).
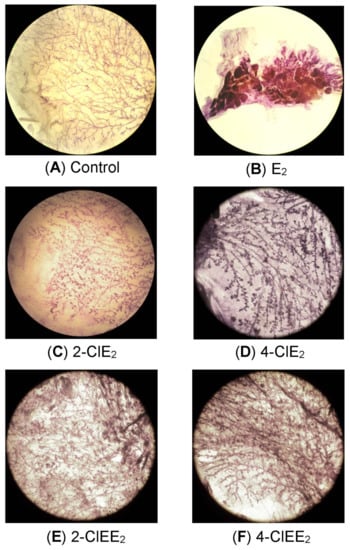
Figure 4.
Morphological examination of mammary glands of chlorinated estrogen-treated rats. Mammary glands were collected from ACI rats at the end of the experiments. Mammary tissue of ACI rats implanted with placebo (A), E2 (2.5 mg) (B), 2-ClE2 (5.0 mg) (C), 4-ClE2 (5.0 mg) (D), 2-ClEE2 (5.0 mg) (E), or 4-ClEE2 (5.0 mg) (F) and stained with hematoxylin (magnification 10×).
2.3. Uterotrophic Activity of Chlorinated Estrogens
To determine the estrogenic potential of chlorinated estrogens, 2-ClE2 or 4-ClE2 was administered subcutaneously for 3 days to OVX-rats (Figure 5A). E2 (3.0 μg), as a positive control, increased the uterine length and thickness. With 2-ClE2 or 4-ClE2 at a dose molar equivalent of E2 (3.0 μg), no increase in uterine size was observed. With a 10-times molar dose (34 μg), both 2-ClE2 and 4-ClE2 promoted uterine weight gain. The uterine weight of OVX-rats treated with E2 was 0.99 mg/g bw and that of untreated OVX-rats was 0.39 mg/g bw. Both 2-ClE2 (0.67 mg/g bw) and 4-ClE2 (0.93 mg/g bw) showed significant uterotrophic activity.
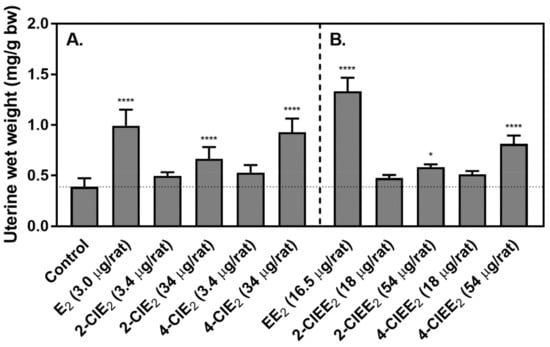
Figure 5.
Uterotrophic potential of chlorinated estrogens on OVX-rats. (A) OVX-rats (4 rats/group) were treated subcutaneously for 3 days with 2-ClE2 (3.4 or 34 μg/rat/day) or 4-ClE2 (3.4 or 34 μg/rat/day). The rats treated with E2 (3.0 μg/rat/day) were used as positive controls. The negative control rats received vehicle only. (B) OVX-rats (4 rats/group) were treated orally for 3 days with 2-ClEE2 (18 or 54 μg/rat/day) or 4-ClE2 (18 or 54 μg/rat/day). The rats treated with EE2 (16.5 μg/rat/day) were used as positive controls. On day 4, uterine wet-weight/bw ratios were calculated and compared to that obtained for OVX-rats that received vehicle, as described in Materials and Methods. Statistical analysis (one-way ANOVA with Tukey’s post hoc test) was performed for multiple comparisons to evaluate differences; *, p < 0.05 (control vs. 2-ClEE2 (54 μg/rat)); ****, p < 0.0001 (control vs. E2 (3.0 μg/rat), 2-ClE2 (34 μg/rat), 4-ClE2 (34 μg/rat), EE2 (16.5 μg/rat), or 4-ClE2 (54 μg/rat)).
Since 17α-ethinyl formula is designed for oral treatment, the uterotrophic potency of 2-ClEE2 or 4-ClEE2 was determined after given orally for 3 days (Figure 5B). At the dose molar equivalent to EE2 (16.5 μg), neither 2-ClEE2 nor 4-ClEE2 produced an increase in uterine weight. At the 3-times molar dose (54 μg), a significant increase of uterine weight was measured. The uterine weight of OVX-rats treated with EE2 was 1.33 mg/g bw. Both 2-ClEE2 (0.58 mg/g bw) and 4-ClEE2 (0.81 mg/g bw) showed significant uterotrophic activity.
3. Discussion
Trace amounts of chlorinated estrogens are produced in the environment as byproducts of reactions between estrogens and hypochlorous acid in sewage treatment plants [32,33]; their estrogenic potency was detected using an in vitro yeast two-hybrid assay with the estrogen receptor α. However, the biological properties of chlorinated estrogens in vivo are poorly understood. In the present study, the carcinogenic potential of chlorinated estrogens was determined using estrogen-sensitive ACI rats implanted with their pellets for one year. As reported previously [25,27,31], E2 (1.25 mg or 2.5 mg) was a potent inducer of mammary tumors (Figure 3 and Table 1). In contrast, 2-ClE2 and 4-ClE2 (2.5 mg and 5.0 mg) did not induce mammary tumors even after one year of treatment. The 5.0 mg of chlorinated estrogens was 4-times higher dose than 1.25 mg E2 that induced mammary tumors [27], indicating that the chlorinated estrogens appear to be less carcinogenic. The 17α-ethinyl form of E2 (EE2) was reported by another research group [28,34], to be a strong inducer of mammary tumors in ACI rats. However, both 2-ClEE2 and 4-ClEE2 did not develop mammary tumors (Table 1). The less carcinogenic potential observed for chlorinated E2 or EE2 was supported by histological examination of mammary glands of chlorinated estrogen-treated ACI rats (Figure 4).
In our recent study [27], 2-fluoro-17β-estradiol (2-FE2) did not induce mammary tumors whereas 4-fluoro-17β-estradiol (4-FE2) did. Because of the carbon–fluorine bond strength [35], fluorination inhibits metabolic hydroxylation of E2. A fluorine at the 2-position of E2 prevents 2-hydroxylation, resulting in preferential 4-hydroxylation [36]. On the other hand, a fluorine at the 4-position of E2 prevents 4-hydroxylation, resulting in preferential 2-hydroxylation. These results suggest that the development of mammary tumors might occur through the 2-hydroxylation of 4-FE2, not through the 4-hydroxylation of 2-FE2. Surprisingly, unlike 4-FE2, 4-ClE2 did not induce mammary tumors. Because a chlorine atom has greater steric hindrance and electron-withdrawing power than a fluorine atom, chlorine modification of estrogen may be more effective to diminish its carcinogenicity. The bulky chlorine atom may affect to inhibit the hydroxylation at both the 2- and 4-positions of estrogens. In addition, the resulting catecholestrogens may hardly be oxidized by the bulky chlorine atom to form their reactive quinones that can damage DNA [19,21,23].
To evaluate such evidence, the metabolites of halogenated estrogen absorbed into body should be determined. Using a radioimmunoassay (RIA), the serum E2 level in ACI rats treated with 3.0 mg E2 pellet was reported to be less than 175 pg/mL [25]. Unfortunately, the RIA used for E2 is not applicable to assay other estrogens. A newly sensitive method is required to be developed for determining such low level of halogenated estrogens and their metabolites, especially in mammary and reproductive organs.
To determine whether the chlorinated estrogens have estrogenic potency, the uterotrophic activity was measured as an indicator after treating OVX-rats subcutaneously for 3 days with 2-ClE2 or 4-ClE2. Significant uterotrophic activity was observed when treated with 34 μg of either 2-ClE2 or 4-ClE2 (Figure 5A). The body weight of OVX-rats was ~180 g; therefore, the daily dose 34 μg/rat was 189 μg/kg/day. Because the body weight of ACI rats treated for 52 weeks with a 5.0 mg chlorinated estrogen was ~80 g, the daily dose was estimated to be 172 μg/kg/day that was similar to that (189 μg/kg/day) of OVX-rats treated with 34 μg chlorinated estrogens. This indicated that the dose appearing high estrogenic potential may not always have carcinogenic potency.
E2 is generally given by parenteral treatment because if taken by mouth it is inactivated quickly. On the other hand, the 17α-ethinyl form can be absorbed more efficiently by the body and thus has a higher bioavailability after oral delivery [29,30]. In OVX-rats treated orally with either 2-ClEE2 or 4-ClEE2, both chlorinated compounds showed significant uterotrophic activity (Figure 5B). Although the chlorinated estrogens required higher doses than EE2, they provided effective estrogenic potency by oral treatment.
In our previous paper [27], both non-carcinogenic 2-FE2 and carcinogenic 4-FE2 appeared similar uterotrophic potency, indicating that estrogenic potential may not be the sole factor driving mammary tumorigenesis. 2-FE2 retaining estrogenic potential has shown to be a safer alternative for HRT. However, the difficult isolation process of 2-FE2 from 4-FE2 [37] requires the development of a specific separation method. On the other hand, both 2-ClE2 and 4-ClE2 having estrogenic potency did not present carcinogenic potency. If no separation method is established, the mixture of chlorinated estrogens may be used similarly to a combination tablet currently prescribed for HRT.
In conclusion, chlorinated estradiol derivatives retaining estrogenic potential were less mammary carcinogenic. Such chlorinated estrogens could be used as safer alternatives to carcinogenic estrogens now in use for HRT.
4. Materials and Methods
4.1. Chemicals
E2 [estra-1,3,5(10)-triene-3,17β-diol], EE2 [17α-ethinylestra-1,3,5(10)-triene-3,17β-diol] and cholesterol were purchased from Fujifilm Wako Pure Chemical Corp. (Osaka, Japan). Trichloroisocyanuric acid (TCCA) was purchased from Tokyo Chemical Industry Co., Ltd. (Tokyo, Japan).
4.2. Synthesis of Chlorinated Estrogens
2-ClE2, 4-ClE2, 2-ClEE2, and 4-ClEE2 were synthesized in good yield by a following modification of the established procedure [38]. TCCA (0.84 g) was added to an ice-cooled solution of the E2 (2.0 g) in acetonitrile (100 mL) and stirred for 1 hr. The reaction mixture was extracted with ethyl acetate. The organic layer was washed with 5% aqueous sodium hydrogen sulphite, 5% aqueous sodium carbonate and water, and evaporated. An ethanol solution (40 mL) of the residue was treated with sodium borohydride (0.67 g), and stirred at room temperature for 20 min. The reaction mixture was extracted with ethyl acetate. The organic layer was evaporated to give a residue, which was submitted to preparative HPLC equipped with an ODS column to given pure products of 2-ClE2 (0.22 g, 10%), 4-ClE2 (0.26 g, 11%), and 2,4-dichloro-17β-estradiol (2,4-diClE2; 0.22 g, 9%). Using the same procedure as described for chlorination of E2, treatment of EE2 (2.0 g) with TCCA (0.80 g) gave 2-ClEE2 (0.21 g, 9%), 4-ClEE2 (0.26 g, 12%), and 2,4-diClEE2 (0.30 g, 12%). Preparative HPLC conditions were as follows. Pump, model SP-22 (Tokyo Rikakikai, Co., LTD., Tokyo, Japan); detector, model 8011 (Tosoh Corp., Tokyo, Japan) at 270 nm; column, two 22 mm i.d. × 300 mm glass columns containing μ-Bondasphere 15 μm (Waters, Milford, MA, USA) were joined together; mobile phase, methanol—water (80:20, v/v); flow rate, 5.0 mL/min. By HPLC/UV or NMR analysis, the purity of chlorinated estrogens was determined to be >99%.
4.3. Tumorigenesis of Chlorinated Estrogens
The animal studies were approved by an ethics committee of Faculty of Pharmacy, Meijo University. All procedures with animals were conducted in compliance with the Guidelines for Proper Conduct of Animal Experiments (Science Council of Japan). Rats (ACI, 5-week-old females, Harlan) were given water and food ad libitum and kept on a 12-h light/dark cycle throughout the study. Following an established protocol for E2 [25,27,31], after one week of acclimation, the following pellet was implanted under the dorsal skin with light isoflurane anesthesia: 2-ClE2 2.5 mg or 5.0 mg (n = 6 rats), 4-ClE2 2.5 mg or 5.0 mg (n = 6), 2-ClEE2 2.5 mg or 5.0 mg (n = 5–6), or 4-ClEE2 2.5 mg or 5.0 mg (n = 5) in cholesterol (15.0 or 17.5 mg), and 20 mg cholesterol alone (n = 6) as the negative control. ACI rats implanted with E2 1.25 mg (n = 5), 2.5 mg (n = 10), or 5.0 mg (n = 5) pellet were used as the positive control. Development of mammary tumors was monitored by palpation once a week for 52 weeks. At the end of experiments, rats were euthanized by CO2 asphyxiation. Pathological determination of mammary tumors was performed following the established procedure in our laboratory [27,31].
4.4. Mammary Whole-Mount Preparation and Morphometric Analysis
Rats were euthanized under isoflurane anesthesia. Following an established protocol [27,31], the skin containing mammary glands was collected and fixed in 10% neutral-buffered formalin for at least 3 days. The mammary glands were then dissected free from the skin and processed as a whole mount. The glands were defatted in ethanol, acetone, chloroform, and ethanol again for at least 3 days in each solvent. After rehydration, the glands were stained with hematoxylin and washed with distilled water. The stained glands were cleaned up manually by viewing through a stereomicroscope, dehydrated in ethanol, cleared in xylene and mounted. Photographs were taken using a digital camera (Olympus, Tokyo, Japan) mounted on a stereomicroscope (Stemi SV11, Carl Zeiss, Jena, Germany).
4.5. Determination of Uterotrophic Potential
The uterotrophic activity of chlorinated estrogens was determined by following the protocol reported previously [27,31]. Briefly, OVX-rats (Sprague-Dawley, 6-week-old females, Japan SLC, Inc., Shizuoka, Japan; 4 rats/dose) were treated subcutaneously for 3 days with 2-ClE2 or 4-ClE2. A dose one- or ten-times molar equivalent to E2 (3.0 μg/rat/day) was used; 3.4 or 34 μg/rat/day for 2-ClE2 or 4-ClE2. The ethinyl compounds 2-ClEE2 and 4-ClEE2 were administered orally. A dose one- or three-times molar equivalent to EE2 (16.5 μg/rat/day) was used; 18 or 54 μg/rat/day for 2-ClEE2 or 4-ClEE2. The negative control rats received vehicle only. On day 4, uterine horns were dissected and trimmed of fascia and fat. The uterine weight was measured after removing the luminal fluids on filter paper. Uterine wet-weight to body-weight (bw) ratios (mg/g bw) were compared with that obtained for the OVX-rats treated subcutaneously with E2 (3.0 μg/rat/day) as a positive control. Statistical analysis (one-way ANOVA with Tukey’s post hoc test) was performed to evaluate the significance of the differences in treatment effects.
Author Contributions
Conceptualization, S.S.; Methodology, Y.O. and S.I.; Validation, Y.O., S.I. and S.S.; Formal Analysis, Y.O.; Investigation, Y.O. and S.I.; Resources, H.J. and S.I.; Data Curation, Y.O.; Writing—original draft preparation, S.S.; Writing—review and editing, Y.O., H.J. and S.I.; Visualization, Y.O. and S.S.; Project Administration, S.S.; Funding Acquisition, Y.O., H.J. and S.I. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by a grant from Takeda Science Foundation (Japan).
Institutional Review Board Statement
The studies involving animals were conducted according to the guidelines of the Declaration of Helsinki, and approved by the Ethics Committee of Faculty of Pharmacy, Meijo University (protocol #2012-P-E-24, 24 July 2012).
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We thank N. Suzuki and M. Mizukami for their technical assistance and Y. Shibutani for help in preparing the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| E2 | 17β-estradiol |
| 2-OHE | 2-hydroxyestrogen |
| 4-OHE | 4-hydroxyestrogen |
| 2-ClE2 | 2-chloro-17β-estradiol |
| 4-ClE2 | 4-chloro-17β-estradiol |
| EE2 | 17α-ethinylestradiol |
| 2-ClEE2 | 2-chloro-17α-ethinylestradiol |
| 4-ClEE2 | 4-chloro-17α-ethinylestradiol |
| 2-FE2 | 2-fluoro-17β-estradiol |
| 4-FE2 | 4-fluoro-17β-estradiol |
| ACI | August Copenhagen Irish |
| HRT | Hormone replacement therapy |
| OVX | Ovariectomized |
| bw | Body weight |
| RIA | Radioimmunoassay |
References
- Grodstein, F.; Stampfer, M.J.; Colditz, G.A.; Willett, W.C.; Manson, J.E.; Joffe, M.; Rosner, B.; Fuchs, C.; Hankinson, S.E.; Hunter, D.L.; et al. Postmenopausal hormone therapy and mortality. N. Engl. J. Med. 1997, 336, 1769–1775. [Google Scholar] [CrossRef] [PubMed]
- Bolton, J.L.; Pisha, E.; Zhang, F.; Qiu, S. Role of quinoids in estrogen carcinogenesis. Chem. Res. Toxicol. 1998, 11, 1113–1127. [Google Scholar] [CrossRef] [PubMed]
- Colditz, G.A.; Hankinson, S.E.; Hunter, D.J.; Willett, W.C.; Manson, J.E.; Stampfer, M.J.; Hennekens, C.H.; Rosner, B.; Speizer, F.E. The use of estrogens and progestins and the risk of breast cancer in postmenopausal women. N. Engl. J. Med. 1995, 332, 1589–1593. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.L.; Weiss, N.S.; Newcomb, P.; Barlow, W.; White, E. Hormone replacement therapy in relation to breast cancer. JAMA 2002, 287, 734–741. [Google Scholar] [CrossRef] [PubMed]
- Lacey, J.V., Jr.; Mink, P.J.; Lubin, J.H.; Sherman, M.E.; Troisi, R.; Hartge, P.; Schatzkin, A.; Schairer, C. Menopausal hormone replacement therapy and risk of ovarian cancer. JAMA 2002, 288, 334–341. [Google Scholar] [CrossRef] [PubMed]
- Beral, V. Million Women Study Collaborators. Breast cancer and hormone-replacement therapy in the Million Women Study. Lancet 2003, 362, 419–427. [Google Scholar] [CrossRef]
- Grady, D.; Gebretsadik, T.; Kerlikowske, K.; Emster, V.; Petitti, D. Hormone replacement therapy and endometrial cancer risk: A meta-analysis. Obstet. Gynecol. 1995, 85, 304–313. [Google Scholar] [CrossRef]
- Steinberg, K.K.; Smith, S.J.; Thacker, S.B.; Stroup, D.F. Breast cancer risk and duration of estrogen use: The role of study design in meta-analysis. Epidemiology 1994, 5, 415–421. [Google Scholar] [CrossRef]
- Liehr, J.G. Genotoxic effects of estrogens. Mutat. Res. 1990, 238, 269–276. [Google Scholar] [CrossRef]
- Fishman, J.; Osborne, M.P.; Telang, N.T. The role of estrogen in mammary carcinogenesis. Ann. N. Y. Acad. Sci. 1995, 768, 91–100. [Google Scholar] [CrossRef]
- Hernandez, L.G.; Steeg, H.; Luijten, M.; Benthem, J. Mechanisms of non-genotoxic carcinogens and importance of a weight of evidence approach. Mutat. Res. 2009, 682, 94–109. [Google Scholar] [CrossRef]
- Greenberg, E.R.; Barnes, A.B.; Resseguie, Z.; Barrett, J.A.; Burnside, S.; Lanza, L.L.; Neff, R.K.; Stevens, M.; Young, R.H.; Colton, T. Breast cancer in mothers given diethylstilbestrol in pregnancy. N. Engl. J. Med. 1984, 311, 1393–1398. [Google Scholar] [CrossRef]
- Liehr, J.G.; Avitts, T.A.; Randerath, E.; Randerath, K. Estrogen-induced endogenous DNA adduction: Possible mechanism of hormonal cancer. Proc. Nalt. Acad. Sci. USA 1986, 83, 5301–5305. [Google Scholar] [CrossRef]
- Gladek, A.; Liehr, J.G. Mechanism of genotoxicity of diethylstilbestrol in vivo. J. Biol. Chem. 1989, 264, 16847–16852. [Google Scholar] [CrossRef]
- Preston-Martin, S.; Pike, M.C.; Ross, R.K.; Jones, P.A.; Henderson, B.E. Increased cell division as a cause of human cancer. Cancer Res. 1990, 50, 7415–7421. [Google Scholar]
- Martucci, C.P.; Fishman, J. P450 enzymes of estrogen metabolism. Pharmac. Ther. 1993, 57, 237–257. [Google Scholar] [CrossRef]
- Dwivedy, I.; Devanesan, P.; Cremonesi, P.; Rogan, E.; Cavalieri, E. Synthesis and characterization of estrogen 2,3- and 3,4-quinones. Comparison of DNA adducts formed by the quinones versus horseradish peroxidase-activated catechol estrogens. Chem. Res. Toxicol. 1992, 5, 828–833. [Google Scholar] [CrossRef]
- Hayashi, N.; Hasegawa, K.; Komine, A.; Tanaka, Y.; McLachian, J.A.; Barrett, J.C.; Tsutsui, T. Estrogen-induced cell transformation and DNA adduct formation in cultured Syrian hamster embryo cells. Mol. Carcinog. 1996, 16, 149–156. [Google Scholar] [CrossRef]
- Stack, D.E.; Byun, J.; Gross, M.L.; Rogan, E.G.; Cavalieri, E.L. Molecular characteristics of catechol estrogen quinone in reactions with deoxyribonucleosides. Chem. Res. Toxicol. 1996, 9, 851–859. [Google Scholar] [CrossRef]
- Terashima, I.; Suzuki, N.; Shibutani, S. Mutagenic specificity of 2-hydroxyestrogen quinone-derived DNA adducts in mammalian cells. Biochemistry 2001, 40, 166–172. [Google Scholar] [CrossRef]
- Li, K.M.; Todorovic, R.; Devanesan, P.; Higginbotham, S.; Köfeler, H.; Ramanathan, R.; Gross, M.L.; Rogan, E.G.; Cavalieri, E.L. Metabolism and DNA binding studies of 4-hydroxyestradiol and estradiol-3,4-quinone in vitro and in female ACI rat mammary gland in vivo. Carcinogenesis 2004, 25, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Takeshita, M.; Eisenberg, W. Mechanism of mutation on DNA templates containing synthetic abasic sites: Study with a double strand vector. Nucleic Acids Res. 1994, 22, 1897–1902. [Google Scholar] [CrossRef][Green Version]
- Han, X.; Liehr, J.G. 8-Hydroxylation of guanine bases in kidney and liver DNA of hamsters treated with estradiol: Role of free radicals in estrogen-induced carcinogenesis. Cancer Res. 1994, 54, 5515–5517. [Google Scholar] [PubMed]
- Malins, D.C.; Holmes, E.H.; Polissar, N.L.; Gunselman, S.J. The etiology of breast cancer. Characteristic alteration in hydroxyl radical-induced DNA base lesions during oncogenesis with potential for evaluating incidence risk. Cancer 1993, 71, 3036–3043. [Google Scholar] [CrossRef]
- Turan, V.K.; Sanchez, R.I.; Li, J.J.; Li, S.A.; Reuhl, K.R.; Thomas, P.E.; Conney, A.H.; Gallo, M.A.; Kauffman, F.C.; Mesia-Vela, S. The effects of steroidal estrogens in ACI rat mammary carcinogenesis: 17β-estradiol, 2-hydroxyestradiol, 4-hydroxyestradiol, 16α-hydroxyestradiol, and 4-hydroxyestrone. J. Endocrinol. 2004, 183, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Shull, J.D.; Spady, T.J.; Snyder, M.C.; Johansson, S.L.; Pennington, K.L. Ovary-intact but not ovariectomized female ACI rats treated with 17β-estradiol rapidly develop mammary carcinoma. Carcinogenesis 1997, 18, 1595–1601. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Okamoto, Y.; Jinno, H.; Itoh, S.; Shibutani, S. Carcinogenic potential of fluorinated estrogens in mammary tumorigenesis. Toxicol. Lett. 2020, 318, 99–103. [Google Scholar] [CrossRef]
- Holtzman, S.; Stone, J.P.; Shellabarger, C.J. Synergism of estrogens and X-rays in mammary carcinogenesis in female ACI rats. J. Natl. Cancer Inst. 1981, 67, 455–459. [Google Scholar]
- Kuhl, H. Pharmacology of estrogens and progestogens: Influence of different routes of administration. Climacteric 2005, 8 (Suppl. 1), 3–63. [Google Scholar] [CrossRef]
- Stanczyk, F.Z.; Archer, D.F.; Bhavnani, B.R. Ethinyl estradiol and 17β-estradiol in combined oral contraceptives: Pharmacokinetics, pharmacodynamics and risk assessment. Contraception 2013, 87, 706–727. [Google Scholar] [CrossRef]
- Okamoto, Y.; Liu, X.; Suzuki, N.; Okamoto, K.; Kim, H.J.; Laxmi, Y.R.S.; Sayama, K.; Shibutani, S. Equine estrogen-induced mammary tumors in rats. Toxicol. Lett. 2010, 193, 224–228. [Google Scholar] [CrossRef]
- Hu, J.; Cheng, S.; Aizawa, T.; Terao, Y.; Kunikanw, S. Products of aqueous chlorination of 17β-estradiol and their estrogenic activities. Envirion. Sci. Technol. 2003, 37, 5665–5670. [Google Scholar] [CrossRef]
- Nakamura, H.; Shiozawa, T.; Terao, Y.; Shiraishi, F.; Fukazawa, H. By-products produced by the reaction of estrogens with hypochlorous acid and their estrogen activities. J. Heath Sci. 2006, 52, 124–131. [Google Scholar] [CrossRef][Green Version]
- Holtzman, S. Retinyl acetate inhibits estrogen-induced mammary carcinogenesis in female ACI rats. Carcinogenesis 1988, 9, 305–307. [Google Scholar] [CrossRef]
- Roberts, D.J.; Caserio, M.C. Basic Principles of Organic Chemistry; W. A. Benjamin Inc.: New York, NY, USA, 1965; Volume 77. [Google Scholar]
- Ashburn, S.P.; Han, X.; Liehr, J.G. Microsomal hydroxylation of 2- and 4-fluoroestradiol to catechol metabolites and their conversion to methyl ethers: Catechol estrogens as possible mediators of hormonal carcinogenesis. Mol. Pharmacol. 1993, 43, 534–541. [Google Scholar]
- Page, P.C.B.; Hussain, F.; Maggs, J.L.; Morgan, P.; Park, B.K. Efficient regioselective A-ring functionalization of oestrogens. Tetrahedron 1990, 46, 2059–2068. [Google Scholar] [CrossRef]
- Mukawa, F. 10β-Chloro-17β-hydroxyestra-1,4-dien-3-one and its related compounds. J. Chem. Soc. Perkin Trans. 1988, 1, 457–460. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).