Acellular Dermal Matrix Used in Diabetic Foot Ulcers: Clinical Outcomes Supported by Biochemical and Histological Analyses
Abstract
1. Introduction
2. Methods and Materials
2.1. Patients, Surgical Methods, and Clinical Outcomes
2.2. Quantitative Real Time PCR (qRT-PCR) on Tissue Biopsies
2.3. Western Blotting
2.4. Sample Preparation for Histology
2.5. Statistical Analyses
2.6. Endpoints
3. Results
3.1. Surgical Method and Clinical Evaluation
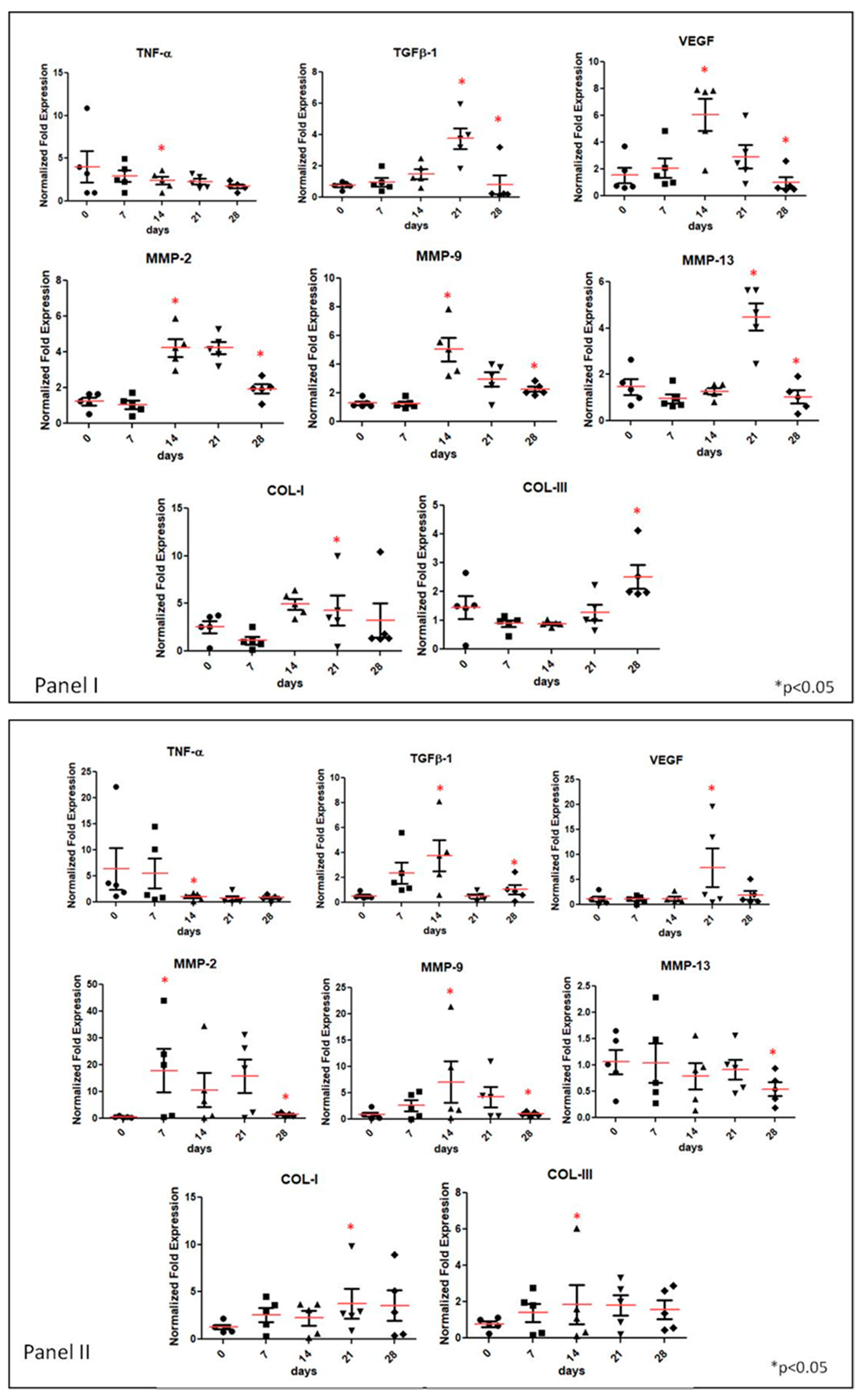
3.2. Gene Expression Analyses of Specific Tissue Recovery by Using qRT-PCR
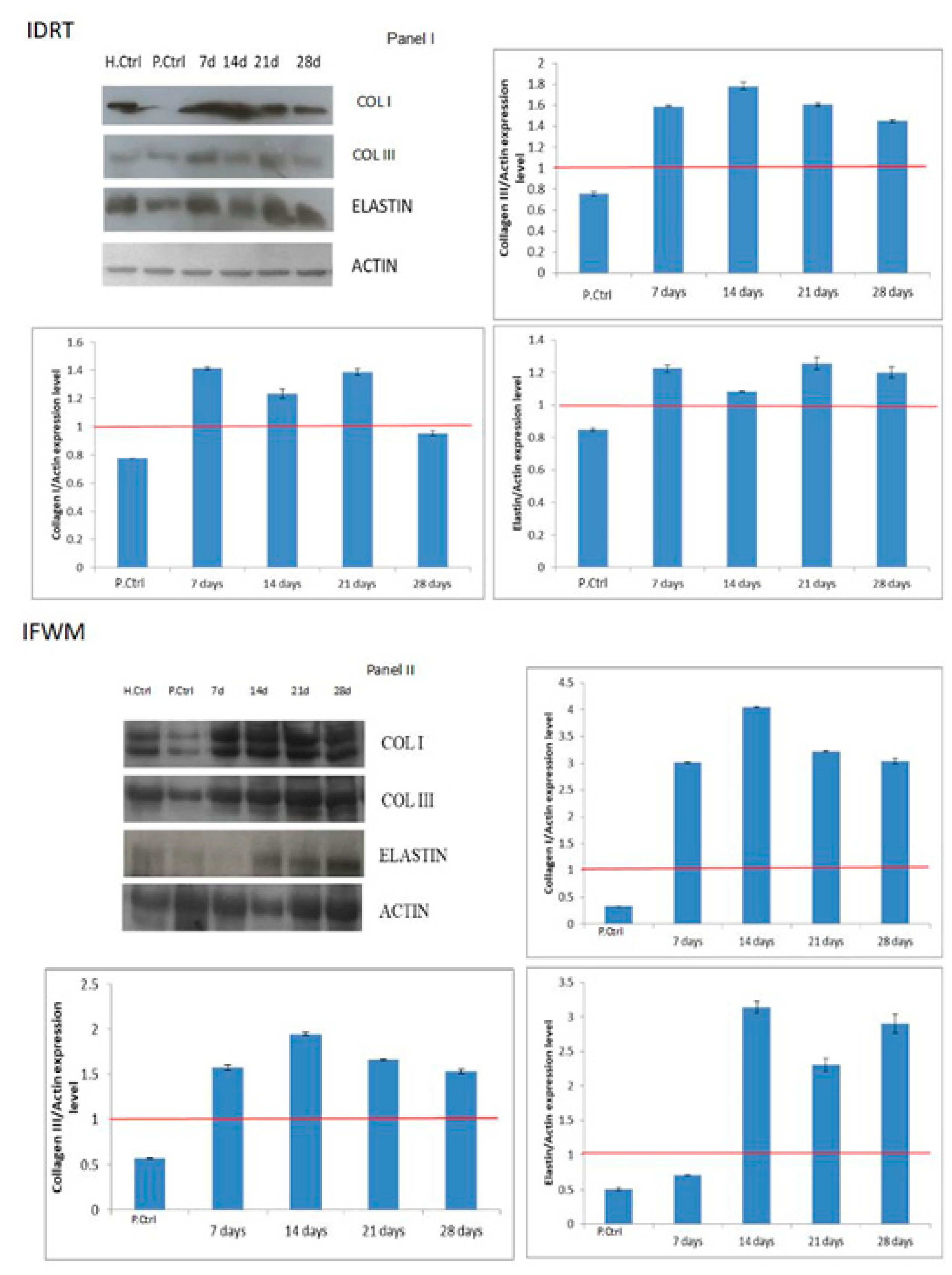
3.3. Western Blotting
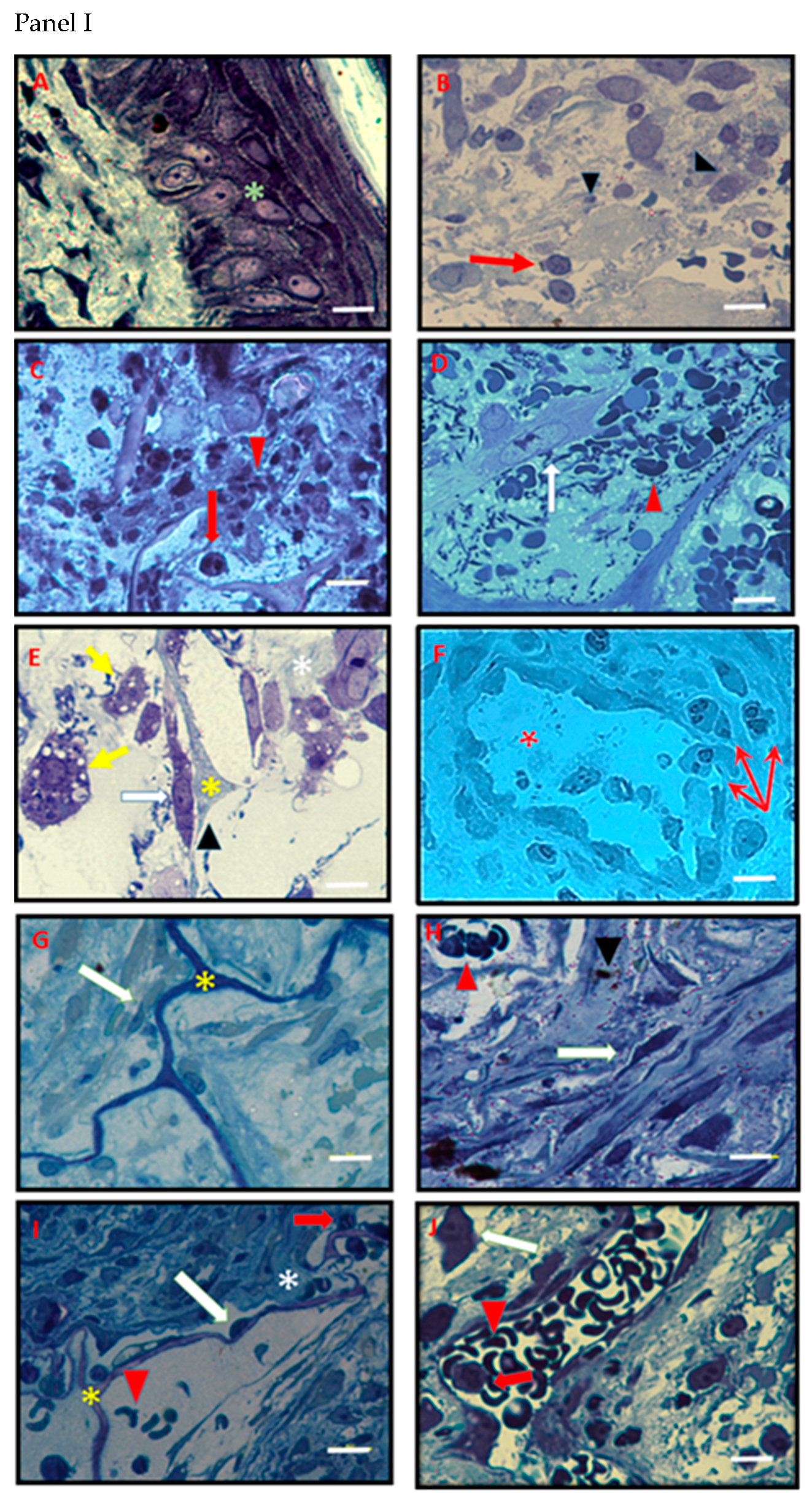
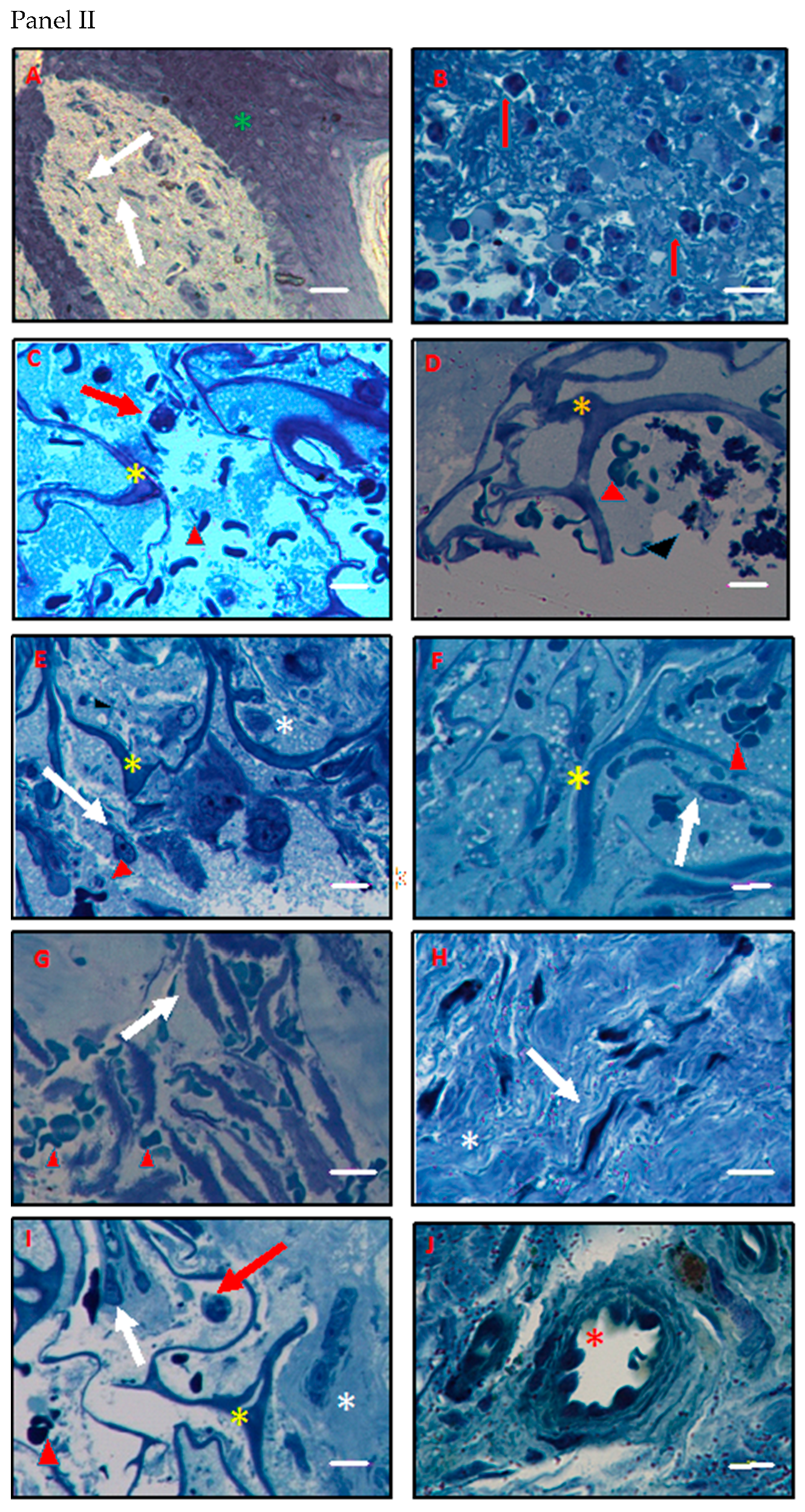
3.4. Histology and Ultrastructural Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shi, C.; Wang, C.; Liu, H.; Li, Q.; Li, R.; Zhang, Y.; Liu, Y.; Shao, Y.; Wang, J. Selection of Appropriate Wound Dressing for Various Wounds. Front. Bioeng. Biotechnol. 2020, 8, 182. [Google Scholar] [CrossRef] [PubMed]
- Harper, D.; Young, A.; McNaught, C.E. The physiology of wound healing. Surgery 2014, 32, 9. [Google Scholar] [CrossRef]
- Kiritsi, D.; Nyström, A. The role of TGFβ in wound healing pathologies. Mech. Ageing Dev. 2018, 172, 51–58. [Google Scholar] [CrossRef]
- Barrientos, S.; Stojadinovic, O.; Golinko, M.S.; Brem, H.; Tomic-Canic, M. PERSPECTIVE ARTICLE: Growth factors and cytokines in wound healing. Wound Repair Regen. 2008, 16, 585–601. [Google Scholar] [CrossRef] [PubMed]
- Bao, P.; Kodra, A.; Tomic-Canic, M.; Golinko, M.S.; Ehrlich, H.P.; Brem, H. The Role of Vascular Endothelial Growth Factor in Wound Healing. J. Surg. Res. 2009, 153, 347–358. [Google Scholar] [CrossRef] [PubMed]
- Tardáguila-García, A.; García-Morales, E.; García-Alamino, J.M.; Álvaro-Afonso, F.J.; Molines-Barroso, R.J.; Lázaro-Martínez, J.L. Metalloproteinases in chronic and acute wounds: A systematic review and meta-analysis. Wound Repair Regen. 2019, 27, 415–420. [Google Scholar] [CrossRef]
- Naoum, J.J.; Arbid, E.J. Endovascular Techniques in Limb Salvage: Infrapopliteal Angioplasty. Methodist DeBakey Cardiovasc. J. 2013, 9, 103–107. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Armstrong, D.G.; Lavery, L.A. Evidence-based options for off-loading diabetic wounds. Clin. Podiatr. Med. Surg. 1998, 15, 95–104. [Google Scholar]
- Lipsky, B.A.; Berendt, A.R.; Cornia, P.B.; Pile, J.C.; Peters, E.; Armstrong, D.G.; Deery, H.G.; Embil, J.M.; Joseph, W.S.; Karchmer, A.W.; et al. 2012 Infectious Diseases Society of America Clinical Practice Guideline for the Diagnosis and Treatment of Diabetic Foot Infections. Clin. Infect. Dis. 2012, 54, e132–e173. [Google Scholar] [CrossRef]
- Canonico, S.; Campitiello, F.; Della, C.A.; Fattopace, A. The use of a dermal substitute and thin skin grafts in the cure of “complex” leg ulcers. Dermatol. Surg 2009, 35, 195–200. [Google Scholar] [CrossRef]
- Gottlieb, M.E.; Furman, J. Successful management and surgical closure of chronic and pathological wounds using Integra. J. Burns Wounds 2004, 3, 4–60. [Google Scholar]
- Campitiello, E.; Della Corte, A.; Fattopace, A.; D’Acunzi, D.; Canonico, S. The use of artificial dermis in the treatment of chronic and acute wounds: Regeneration of dermis and wound healing. Acta Biomed. Atenei Parm. 2005, 76 (Suppl. 1), 69–71. [Google Scholar]
- Campitiello, F.; Mancone, M.; Della Corte, A.; Guerniero, R.; Canonico, S. To evaluate the efficacy of an acellular Flowable matrix in comparison with a wet dressing for the treatment of patients with diabetic foot ulcers: A randomized clinical trial. Updat. Surgery 2017, 69, 523–529. [Google Scholar] [CrossRef]
- Campitiello, F.; Mancone, M.; Della Corte, A.; Guerniero, R.; Canonico, S. A Short Commentary about Efficacy of a Flowable Matrix in the Treatment of Diabetic Foot Ulcers. Clin. Res. Foot Ankle 2017, 5, 241. [Google Scholar] [CrossRef]
- Campitiello, F.; Mancone, M.; Della Corte, A.; Guerniero, R.; Canonico, S. Acellular Flowable Matrix in the Treatment of Tunneled or Cavity Ulcers in Diabetic Feet: A Preliminary Report. Adv. Ski. Wound Care 2018, 31, 270–275. [Google Scholar] [CrossRef]
- Sorrentino, V.P.; Della Corte, A.; Campitiello, F.; Freda, F.; Petronella, P.; Canonico, S. The use of a dermal substitute and thin skin graft in the cure of lower limbs wounds from vasculitis: Observational study. BMC Geriatr. 2010, 10, 1–2. [Google Scholar] [CrossRef]
- Driver, V.R.; Lavery, L.A.; Reyzelman, A.M.; Dutra, T.G.; Dove, C.R.; Kotsis, S.V.; Kim, H.M.; Chung, K.C. A clinical trial of Integra Template for diabetic foot ulcer treatment. Wound Repair Regen. 2015, 23, 891–900. [Google Scholar] [CrossRef] [PubMed]
- Nicoli, F.; Rampinelli, I.; Godwin, Y. The application of Integra in a challenging context. Scars Burns Heal. 2016, 2. [Google Scholar] [CrossRef]
- Feldman, E.L.; Stevens, M.J. Clinical Testing in Diabetic Peripheral Neuropathy. Can. J. Neurol. Sci. J. Can. Sci. Neurol. 1994, 21, S3–S7. [Google Scholar] [CrossRef] [PubMed]
- Norgren, L.; Hiatt, W.; Dormandy, J.; Nehler, M.; Harris, K.; Fowkes, F. Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II). J. Vasc. Surg. 2007, 45, S5–S67. [Google Scholar] [CrossRef] [PubMed]
- Stellavato, A.; Corsuto, L.; D’Agostino, A.; La Gatta, A.; Diana, P.; Bernini, P.; De Rosa, M.; Schiraldi, C. Hyaluronan Hybrid Cooperative Complexes as a Novel Frontier for Cellular Bioprocesses Re-Activation. PLoS ONE 2016, 11, e0163510. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, D.G.; Boulton, A.J.M.; Bus, S.A. Diabetic Foot Ulcers and Their Recurrence. N. Engl. J. Med. 2017, 376, 2367–2375. [Google Scholar] [CrossRef] [PubMed]
- Rice, J.B.; Desai, U.; Cummings, A.K.G.; Birnbaum, H.G.; Skornicki, M.; Parsons, N.B. Burden of Diabetic Foot Ulcers for Medicare and Private Insurers. Diabetes Care 2014, 37, 651–658. [Google Scholar] [CrossRef]
- Hobizal, K.B.; Wukich, D.K. Diabetic foot infections: Current concept review. Diabetes Foot Ankle 2012, 3, 18409. [Google Scholar] [CrossRef]
- Yannas, I.V.; Lee, E.; Orgill, D.P.; Skrabut, E.M.; Murphy, G.F. Synthesis and characterization of a model extracellular matrix that induces partial regeneration of adult mammalian skin. Proc. Natl. Acad. Sci. USA 1989, 86, 933–937. [Google Scholar] [CrossRef] [PubMed]
- Soller, E.C.; Tzeranis, D.; Miu, K.; So, P.T.; Yannas, I.V. Common features of optimal collagen scaffolds that disrupt wound contraction and enhance regeneration both in peripheral nerves and in skin. Biomaterials 2012, 33, 4783–4791. [Google Scholar] [CrossRef] [PubMed]
- Tzeranis, D.S.; Soller, E.C.; Buydash, M.C.; So, P.T.; Yannas, I.V. In situ quantification of the surface chemistry of biomaterials. Ann. Biomed. Eng. 2014, submitted. [Google Scholar]
- Conese, M.; Annacontini, L.; Carbone, A.; Beccia, E.; Cecchino, L.R.; Parisi, D.; Di Gioia, S.; Lembo, F.; Angiolillo, A.; Mastrangelo, F.; et al. The Role of Adipose-Derived Stem Cells, Dermal Regenerative Templates, and Platelet-Rich Plasma in Tissue Engineering-Based Treatments of Chronic Skin Wounds. Stem Cells Int. 2020, 2020, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Yannas, I.V.; Burke, J.F.; Huang, C.; Gordon, P.L. Suppression of in vivo degradability and of immunogenicity of collagen by reaction with glycosaminoglycans. Polym. Prepr. 1975, 16, 209–214. [Google Scholar]
- Yannas, I.V.; Tzeranis, D.; So, P.T. Surface biology of collagen scaffold explains blocking of wound contraction and regeneration of skin and peripheral nerves. Biomed. Mater. 2015, 11, 014106. [Google Scholar] [CrossRef]
- Katz, I.A.; Harlan, A.; Miranda-Palma, B.; Prieto-Sanchez, L.; Armstrong, D.G.; Bowker, J.H.; Mizel, M.S.; Boulton, A.J. A Randomized Trial of Two Irremovable Off-Loading Devices in the Management of Plantar Neuropathic Diabetic Foot Ulcers. Diabetes Care 2005, 28, 555–559. [Google Scholar] [CrossRef]
- Veves, A.; Falanga, V.; Armstrong, D.G.; Sabolinski, M.L. Graftskin, a Human Skin Equivalent, Is Effective in the Management of Noninfected Neuropathic Diabetic Foot Ulcers: A prospective randomized multicenter clinical trial. Diabetes Care 2001, 24, 290–295. [Google Scholar] [CrossRef] [PubMed]
- Margolis, D.J.; Allen-Taylor, L.; Hoffstad, O.; Berlin, J.A. Diabetic neuropathic foot ulcers: Predicting which ones will not heal. Am. J. Med. 2003, 115, 627–631. [Google Scholar] [CrossRef] [PubMed]
- Vas, P.; Rayman, G.; Dhatariya, K.; Driver, V.; Hartemann, A.; Londahl, M.; Piaggesi, A.; Apelqvist, J.; Attinger, C.; Game, F. Effectiveness of interventions to enhance healing of chronic foot ulcers in diabetes: A systematic review. Diabetes/Metab. Res. Rev. 2020, 36, e3284. [Google Scholar] [CrossRef]
- Rosa, S.D.; Rosa, M.F.; Fonseca, M.A.; Luz, G.V.; Avila, C.F.; Domínguez, A.G.; Dantas, A.G.; Richter, V.B. Evidence in Practice of Tissue Healing with Latex Biomembrane: Integrative Review. J. Diabetes Res. 2019, 2019, 7457295. [Google Scholar] [CrossRef]
- Gu, X.-Y.; Shen, S.-E.; Huang, C.-F.; Liu, Y.-N.; Chen, Y.-C.; Luo, L.; Zeng, Y.; Wang, A.-P. Effect of activated autologous monocytes/macrophages on wound healing in a rodent model of experimental diabetes. Diabetes Res. Clin. Pract. 2013, 102, 53–59. [Google Scholar] [CrossRef]
- Stellavato, A.; Tirino, V.; De Novellis, F.; Della Vecchia, A.; Cinquegrani, F.; De Rosa, M.; Papaccio, G.; Schiraldi, C. Biotechnological Chondroitin a Novel Glycosamminoglycan With Remarkable Biological Function on Human Primary Chondrocytes. J. Cell. Biochem. 2016, 117, 2158–2169. [Google Scholar] [CrossRef]
- Russo, R.; Vassallo, V.; Stellavato, A.; Valletta, M.; Cimini, D.; Pedone, P.V.; Schiraldi, C.; Chambery, A. Differential Secretome Profiling of Human Osteoarthritic Synoviocytes Treated with Biotechnological Unsulfated and Marine Sulfated Chondroitins. Int. J. Mol. Sci. 2020, 21, 3746. [Google Scholar] [CrossRef]
- Corsuto, L.; Rother, S.; Koehler, L.; Bedini, E.; Moeller, S.; Schnabelrauch, M.; Hintze, V.; Schiraldi, C.; Scharnweber, D. Sulfation degree not origin of chondroitin sulfate derivatives modulates keratinocyte response. Carbohydr. Polym. 2018, 191, 53–64. [Google Scholar] [CrossRef] [PubMed]
- Xue, M.; Jackson, C.J. Extracellular Matrix Reorganization during Wound Healing and Its Impact on Abnormal Scarring. Adv. Wound Care 2015, 4, 3. [Google Scholar] [CrossRef]
- Yannas, I.V.; Tzeranis, D.S.; Peter, T.C.S. Regeneration of injured skin and peripheral nerves requires control of wound contraction, not scar formation. Wound Repair Regen. 2017, 25, 177–191. [Google Scholar] [CrossRef] [PubMed]
- Hirche, C.; Senghaas, A.; Fischer, S.; Hollenbeck, S.T.; Kremer, T.; Kneser, U. Novel use of a Flowable collagen glycosaminoglycan matrix (Integra™ Flowable Wound Matrix) combined with percutaneous cannula scartissue release in treatment of post burn malfunction of the hand: A preliminary 6 month follow-up. Burns 2016, 42, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Palao, R.; Gómez, P.; Huguet, P. Burned breast reconstructive surgery with Integra dermal regeneration template. Br. J. Plast. Surg. 2003, 56, 252–259. [Google Scholar] [CrossRef]

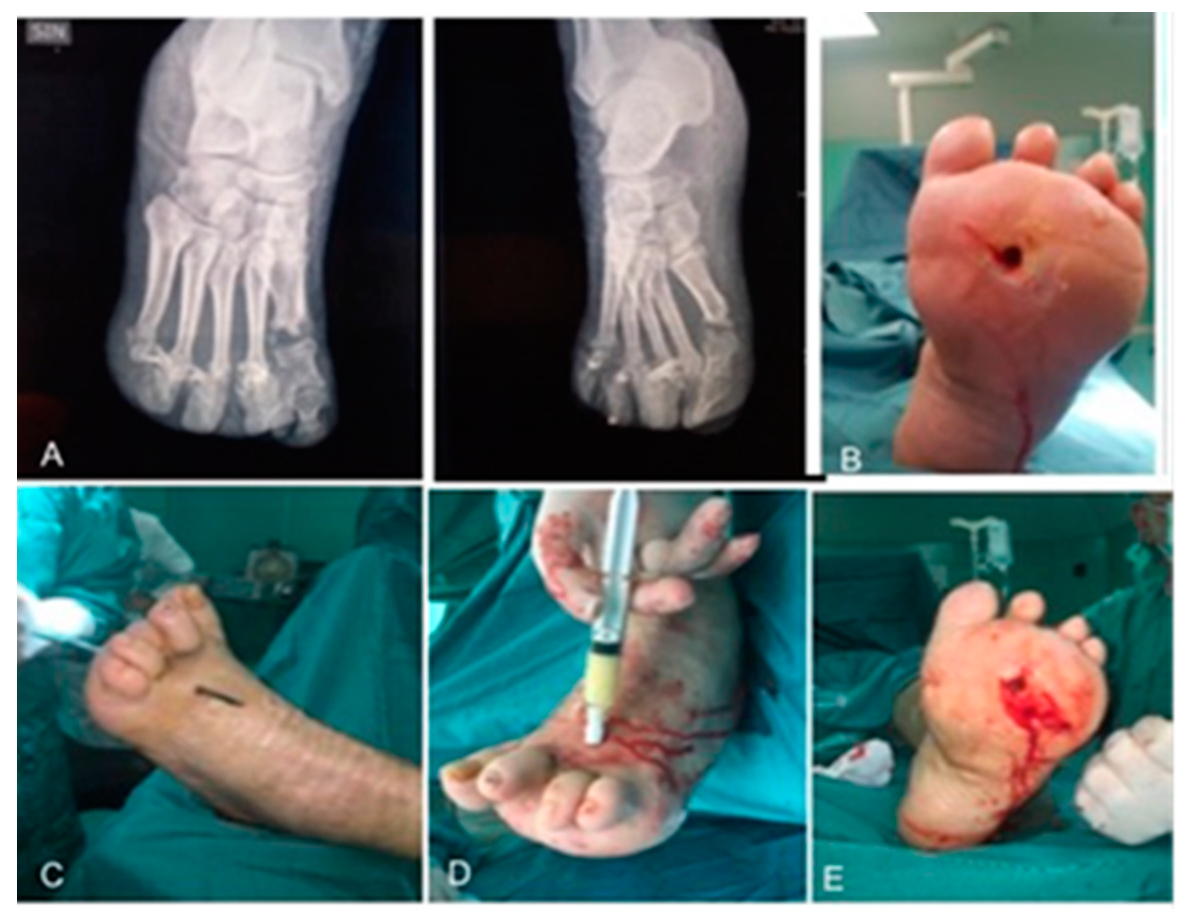
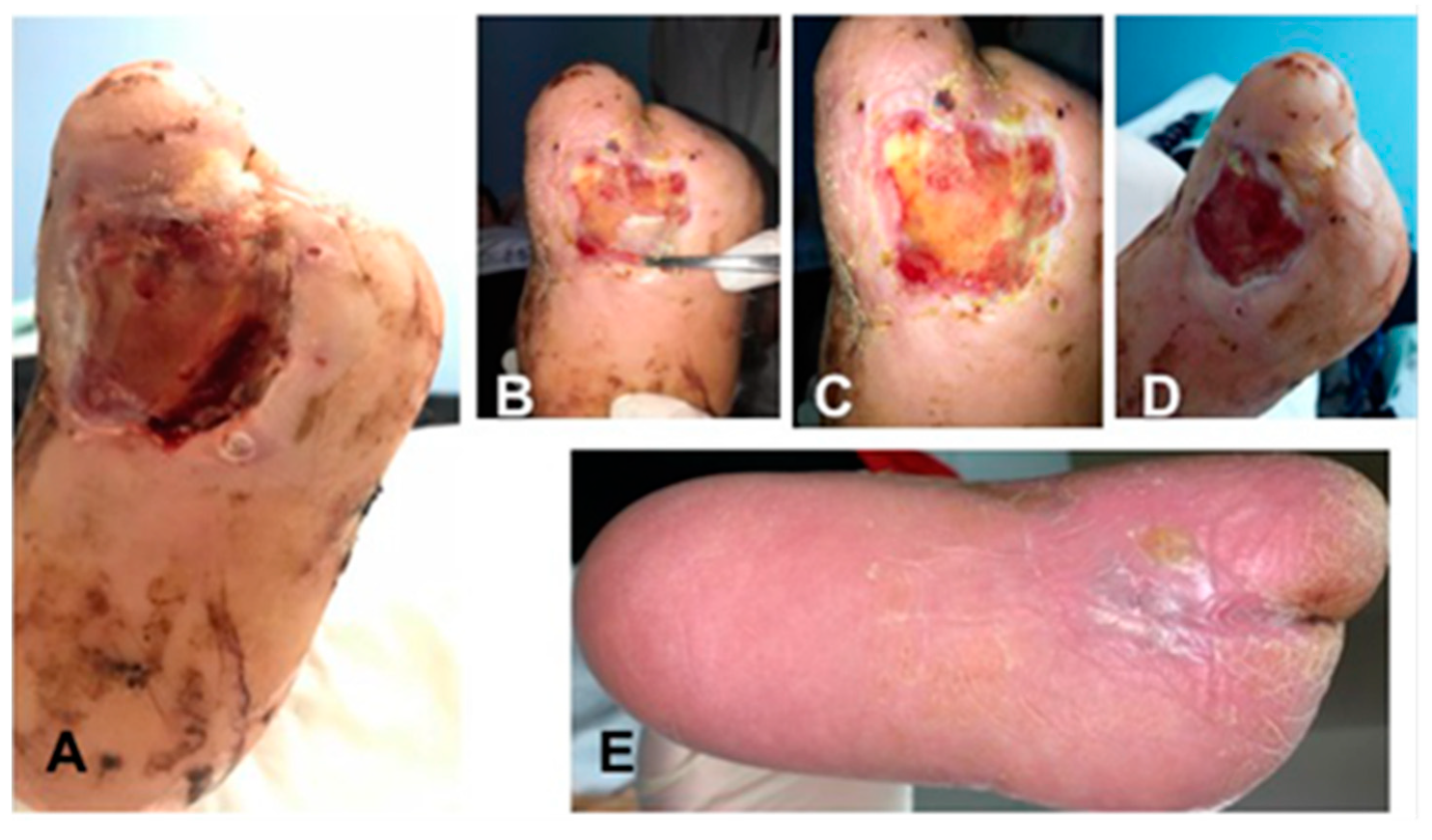

| Procedures | T0 (Enrollment) | T1 (1 Week) | T2 (2 Weeks) | T3 (3 Weeks) | T4 (4 Weeks) |
|---|---|---|---|---|---|
| Informed consent | X | ||||
| Clinical Examination | X (before debridement) | X | X | X | X |
| Laboratory Blood Tests | X (before debridement) | X | X | ||
| Wound Biopsy | X (before debridement) | X | X | X | X |
| Complications | X | X | X | X |
| Gene Name (Symbol) | PCR Primer Sequence 5′ → 3′ | Amplicon Length (bp) |
|---|---|---|
| Ipoxantina-guanina fosforibosiltransferasi (HPRT) | CATCCTGCACCACCAACTG CACAGTCTTCTGAGTGGCAG | 117 |
| Transforming growth factor, beta 1 (TGF-â1) | CTCGCAGCTGGAGGACTCC CTCGTCCAGGATGGCGTAG | 103 |
| Tumor necrosis factor alpha (TNF-á) | CGAGTGACAAGCCTGTAGC GGTGTGGGTGAGGAGCACAT | 102 |
| Matrix metallopeptidase 2 (MMP-2) | GCCGCCTTTAACTGGAGCAA TTCCAGGCATCTGCGATGAG | 106 |
| Matrix metallopeptidase 9 (MMP-9) | GCGCCACCACAGCCAACTATG TggATGCCgTCTATgTCgTCTTTA | 104 |
| Matrix metallopeptidase 13 (MMP-13) | TCCCTGAAGGGAAGGAGC CTCGTCCAGGATGGCGTAG | 105 |
| Vascular endothelial growth factor (VEGF) | AAGCTTGTGCGATGTTACCC CCAGGAGAGAATGTGGCAGT | 110 |
| Type I collagen (COL-1A1) | CAGCCGCTTCACCTACAGC TTTTGTATTCAATCACTGTCTTGCC | 132 |
| Type III collagen (COL-3A1) | TGGTCCCCAAGGTGTCAAAG GGGGGTCCTGGGTTACCATTA | 125 |
| IDRT | IFWM | |
|---|---|---|
| Age (Years) | 61.20 ± 5. 26 | 55.00 ± 3.74 |
| Gender (Male/Female) | ||
| Females Males | 1 (20%) 4 (80%) | 2 (40%) 3 (60%) |
| Body Mass Index (kg/m2) | 27.75 ± 3.70 | 29.06 ± 6.21 |
| Ankle Brachial Index | 0.94 ± 0.05 | 1 ± 0.12 |
| Osteomyelitis Charcot Foot | 4 (80%) 3 (60%) | 3 (60%) 3 (60%) |
| Depth (cm) Length (cm) Width (cm) | ------ 11. 40 ± 3.91 6.40 ± 2.41 | 8.40 ± 2.30 2.80 ± 1.92 2.00 ± 0.71 |
| Ulcer Location | ||
| Forefoot Forefoot-Midfoot | 2 (40%) 3 (60%) | 4 (80%) 1 (20%) |
| Mean Duration of Ulcer (Months) | 15.00 ± 5.20 | 7.00 ± 9.72 |
| Surgical Debridement Surgical Debridement + Minor Amputation STSG | 3 (60%) 1 (20%) 2 (40%) | 4 (80%) 1 (20%) ------ |
| T0 | T2 | T4 | ||
|---|---|---|---|---|
| IDRT | Glycaemia (mg/dL) | 185.80 ± 100.47 | 137.20 ± 28.98 | 132.40 ± 21.98 |
| WBC (U/µL) | 8582.00 ± 1975.02 | 7700.00 ± 1396.42 | 7286.00 ± 1163.61 | |
| HbA1c (%) | 7.38 ± 1.12 | 6.74 ± 0.88 | 6.38± 0.99 | |
| ESR (mm/h) | 49.20 ± 23.57 | 36.00 ± 16.75 | 35.40 ± 22.58 | |
| PCR (mg/L) | 15.94 ± 24.79 | 6.70 ± 7.71 | 3.29 ± 3.83 | |
| IFWM | Glycaemia (mg/dL) | 161.00 ± 31.10 | 133.80 ± 31.10 | 134.80 ± 21.32 |
| WBC (U/µL) | 9298.40 ± 1989.11 | 8558.20 ± 1547,96 | 7996.00 ± 1547.96 | |
| HbA1c (%) | 7.86 ± 1.74 | 7.78 ± 1.73 | 7.26± 1.57 | |
| ESR (mm/h) | 52.80 ± 27.95 | 46.20 ± 24.09 | 41.40 ± 23.60 | |
| PCR (mg/L) | 7.54 ± 5.15 | 4.98 ± 3.99 | 2.97 ± 2.86 |
| IDRT | IFWM | |
|---|---|---|
| Time to Healing (Days) | 56.00 ± 5.948 | 34.00 ± 8.94 |
| Rate Wound Healing at 16 Weeks | 100% | 100% |
| Interval between Surgery and the Deambulation (Days) | 92.00 ± 40.87 | 58.00 ± 8.37 |
| Duration of Hospital Stay (Days) | 14.60 ± 3.97 | 12.80 ± 3.27 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Campitiello, F.; Mancone, M.; Cammarota, M.; D’Agostino, A.; Ricci, G.; Stellavato, A.; Della Corte, A.; Pirozzi, A.V.A.; Scialla, G.; Schiraldi, C.; et al. Acellular Dermal Matrix Used in Diabetic Foot Ulcers: Clinical Outcomes Supported by Biochemical and Histological Analyses. Int. J. Mol. Sci. 2021, 22, 7085. https://doi.org/10.3390/ijms22137085
Campitiello F, Mancone M, Cammarota M, D’Agostino A, Ricci G, Stellavato A, Della Corte A, Pirozzi AVA, Scialla G, Schiraldi C, et al. Acellular Dermal Matrix Used in Diabetic Foot Ulcers: Clinical Outcomes Supported by Biochemical and Histological Analyses. International Journal of Molecular Sciences. 2021; 22(13):7085. https://doi.org/10.3390/ijms22137085
Chicago/Turabian StyleCampitiello, Ferdinando, Manfredi Mancone, Marcella Cammarota, Antonella D’Agostino, Giulia Ricci, Antonietta Stellavato, Angela Della Corte, Anna Virginia Adriana Pirozzi, Gianluca Scialla, Chiara Schiraldi, and et al. 2021. "Acellular Dermal Matrix Used in Diabetic Foot Ulcers: Clinical Outcomes Supported by Biochemical and Histological Analyses" International Journal of Molecular Sciences 22, no. 13: 7085. https://doi.org/10.3390/ijms22137085
APA StyleCampitiello, F., Mancone, M., Cammarota, M., D’Agostino, A., Ricci, G., Stellavato, A., Della Corte, A., Pirozzi, A. V. A., Scialla, G., Schiraldi, C., & Canonico, S. (2021). Acellular Dermal Matrix Used in Diabetic Foot Ulcers: Clinical Outcomes Supported by Biochemical and Histological Analyses. International Journal of Molecular Sciences, 22(13), 7085. https://doi.org/10.3390/ijms22137085









