Abstract
Colorectal cancer (CRC) is one of the main causes of cancer death in the world. Post-translational modifications (PTMs) have been extensively studied in malignancies due to its relevance in tumor pathogenesis and therapy. This review is focused on the dysregulation of glycosyltransferase expression in CRC and its impact in cell function and in several biological pathways associated with CRC pathogenesis, prognosis and therapeutic approaches. Glycan structures act as interface molecules between cells and their environment and in several cases facilitate molecule function. CRC tissue shows alterations in glycan structures decorating molecules, such as annexin-1, mucins, heat shock protein 90 (Hsp90), β1 integrin, carcinoembryonic antigen (CEA), epidermal growth factor receptor (EGFR), insulin-like growth factor-binding protein 3 (IGFBP3), transforming growth factor beta (TGF-β) receptors, Fas (CD95), PD-L1, decorin, sorbin and SH3 domain-containing protein 1 (SORBS1), CD147 and glycosphingolipids. All of these are described as key molecules in oncogenesis and metastasis. Therefore, glycosylation in CRC can affect cell migration, cell–cell adhesion, actin polymerization, mitosis, cell membrane repair, apoptosis, cell differentiation, stemness regulation, intestinal mucosal barrier integrity, immune system regulation, T cell polarization and gut microbiota composition; all such functions are associated with the prognosis and evolution of the disease. According to these findings, multiple strategies have been evaluated to alter oligosaccharide processing and to modify glycoconjugate structures in order to control CRC progression and prevent metastasis. Additionally, immunotherapy approaches have contemplated the use of neo-antigens, generated by altered glycosylation, as targets for tumor-specific T cells or engineered CAR (Chimeric antigen receptors) T cells.
1. Introduction
Colorectal cancer (CRC) has been categorized worldwide as the third most common diagnosed cancer and the fourth most usual cause of cancer death [1]. The etiological heterogeneity, pathogenesis and risk evolution factors of CRC are not well defined [2,3]. Environmental, genetic, epigenetic and post-translational modifications (PTMs), as CRC risk factors, have been the focus of several research efforts, with the aim of improving not only the molecular treatments used in these patients but also biomarker profiles that could be useful for early diagnosis and a better patient follow-up during and after treatment [4].
Among the post-translational modifications differentially identified in CRC tissues, mechanisms of glycosylation have been extensively studied. Alterations of glycan biosynthesis associated with abnormal glycosylation patterns of several molecules have been related with oncogenesis, cell growth, apoptosis inhibition, increased motility, cell migration, cell adhesion, tumor cell invasiveness and metastasis. Glycans act as interface molecules between cells and microenvironment and in several cases glycan structures confer functions to the molecules. Thus, tumorigenesis and metastasis could be narrowly associated with glycan profile changes in cancer cells [5,6,7,8].
In cancer tissues, glycan expression has been found upregulated, downregulated and, interestingly, glycan structures have been shown to be truncated or modified exhibiting new structures [5,6]. Thus, the study of alterations in glycosylation profiles of molecules with a role in cancer progression and metastasis could partially explain molecular mechanisms underlying malignization and even serve as biomarkers.
Glycosylation of molecules, such as proteins or lipids, takes place in the endoplasmic reticulum and Golgi apparatus in a process catalyzed in a non-templated manner by glycosyltransferases, such as fucosyltransferases, sialyltransferases, and N-acetylglucosaminyltransferases in association with glycosidases. Proteins can be N-glycosylated and O-glycosylated and these variations determine, in part, their physical and functional properties. The considerable weight of lipid glycosylation in biology has also been demonstrated [9,10,11]. As the amount and glycosyltransferase profile could be different depending on the cell type, glycoconjugates can also be dissimilar. In fact, several biological functions are influenced by glycan biosynthetic pathways and glycosylation processes determined by the cell conditions [12]. In addition, environmental and genetic conditions have shown an impact in the glycome, thereby generating phenotypic variations [13]. In order to design new treatments aimed at specifically modifying the glycome in pathologic tissues, it is important to extend the understanding of tissue-specific glycosylation [14,15].
Downregulation or upregulation of glycosyltransferase expression, unavailability of glycosylation cofactors or substrates, changes in glycosyltransferase subcellular localization and mutations in genes encoding glycosyltransferases have been evidenced in cancer samples. These features generate aberrant glycosylation signatures that are related with tumorigenesis, metastasis and even with chemoresistance [16,17].
Accordingly, mutations in glycosyltransferases and glycosylation pathways associated genes, as well as altered gene expression coding glycosyltransferases have been described for different CRC cell lines and tumor tissues from several stages [18]. Additionally, differential glycoproteomics and glycolipid profiles have been identified and analyzed. Molecules such as annexin A1, mucins, heat shock protein 90, β1 integrin, selectin ligands, carcinoembryonic antigen (CEA), epidermal growth factor receptor (EGFR), insulin-like growth factor-binding protein 3 (IGFBP3), transforming growth factor beta (TGF-β) receptors, Fas (CD95), PD-L1, sorbin and SH3 domain-containing protein 1 (SORBS1), decorin, CD147 and glycosphingolipids show variations in their glycosylation pattern in CRC tissues when they are compared with healthy tissues. Interestingly, these changes are associated with the prognosis and evolution of the disease and response to radiation or chemotherapy agents [10,19,20]. Thus, studies aimed to identify altered glycosyltransferase gene expression together with glycosylation profile of molecules involved in CRC pathogenesis may provide a molecular basis for the identification of predictive biomarkers of treatment effectiveness and new therapeutic targets.
In this review, we intend to summarize the role of glycosyltransferases and glycosylation profile in CRC pathogenesis and discuss their therapeutic implications.
2. Glycosyltransferase Gene Expression Profile in Colorectal Cancer
The expression pattern of glycosyltransferase genes plays an important role in the biology of CRC cells [21].
Among the genes coding for glycosyltransferases, observed in CRC, 16 have been found upregulated (FUT1, FUT2, FUT3, FUT4, FUT5, FUT6, FUT8, B3GNT3, B3GNT8, B4GALNT3, C1GALT1, MGAT4B, MGAT5A, OGT, ST6GAL1 and ST6GALNAC1) and 16 have been found downregulated (FUT9, B3GNT1, B3GNT6, GALNT6, GCNT3 MGAT3, MGAT5B, ST3GAL1, ST3GAL3, ST6GALNAC2, ST6GALNAC3, ST6GALNAC6, ST8SIA1, ST8SIA3, ST8SIA4 and ST8SIA5) (Table 1) [22,23,24,25,26,27,28,29,30,31,32].
The upregulation of genes coding for beta 3 and beta 4 glycosyltransferases (B3GNT3, B3GNT8, C1GALT1 and B4GALNT3), fucosyltransferases (FUT1, FUT2, FUT3, FUT4, FUT5, FUT6 and FUT8), mannosyl-glycoprotein N-acetylglucosaminyltransferases (MGAT4B and MGAT5A), O-linked N-acetylglucosaminyltransferases (OGT) and sialyltransferases (ST6GAL1 and ST6GALNAC1) plays a crucial role in tumor cell proliferation, survival, induction of stem-like cell properties, epithelial–mesenchymal transition (EMT), metastasis and resistance to chemotherapy and radiotherapy (Table 1).
On the other hand, the downregulation of glycosyltransferases expression in CRC cells, such as beta 3-glycosyltransferases (B3GNT1 and B3GNT6), polypeptide N-acetylgalactosaminyltransferase (GALNT6), glucosaminyl (N-acetyl) transferases/xylosyltransferases (GCNT3), fucosyltransferases (FUT9), mannosyl-glycoprotein N-acetylglucosaminyltransferases (MGAT3, MGAT5b) and sialyltransferases (ST3GAL1, ST3GAL3, ST6GALNAC2, ST6GALNAC3, ST6GALNAC6, ST8SIA1, ST8SIA3, ST8SIA4 and ST8SIA5) has been also associated with tumor progression, tumor growth, cell proliferation, adhesion, migration, invasion and chemoresistance to 5-Fluorouracil (5-FU) (Table 1).
Regarding beta 3-glycosyltransferases, the role of β3GnT8 (UDP-GlcNAc:betaGalbeta-1,3-N-acetylglucosaminyltransferase 8) in CRC cell metastasis could be explained by decorating CD147 with β1,6-branched polylactosamine structures [22,27,33]. CD147, a cell surface glycoprotein also known as extracellular matrix metalloproteinase inducer (EMMPRIN) in its highly glycosylated active form, promotes matrix metalloproteinase (MMP) production in stromal and tumor cells, generates extracellular matrix degradation and thereby increases tumor cell migration and invasion [33,34]. Additionally, it has been evidenced that the overexpression of CD147 in CRC cells promotes cancer stem cell (CSC) maintenance, EMT, metastasis and low sensitivity to 5-FU through MAPK/ERK signaling pathway activation [35,36]. Furthermore, other beta-3 glycosyltransferase upregulated in CRC cells known as C1GALT1 (core 1 β1,3-galactosyltransferase) promotes cell invasion, stem-like cell phenotype and radioresistance via modifying the O-glycosylation pattern of FGFR2 (fibroblast growth factor receptor 2) and β1 integrin, respectively (Table 1) [26,37].
The upregulation of mannosyl-glycoprotein N-acetylglucosaminyltransferases, observed in CRC cells has implications in tumor progression and metastasis (Table 1). The overexpression of N-acetylglucosaminyltransferase V (MGAT5A), evidenced in CRC, promotes cancer cell migration and invasiveness through aberrant glycosylation of TIMP-1 (tissue inhibitor of metalloproteinase-1) [38]. Additionally, MGAT5A activity is involved in the preservation of angiogenesis in anti-VEGF refractory tumors through the induction of galectin 1 (Gal 1) binding to endothelial cells [39].
Alterations in the addition of fucose to precursor glycan structures promote cell proliferation, migration, adhesion to extracellular matrix, invasiveness, metastasis, maintenance of CSCs, TGF-β-induced EMT and multidrug-resistance (MDR) [40,41,42,43,44,45]. Oligosaccharides are fucosylated by FUTs in order to synthesize Lewis antigens, which are involved in cell adhesion to endothelial cells, tumor metastasis, tissue differentiation and inflammation. The overexpression of FUT3 and FUT5, evidenced in cancer cells, correlates with the increased presence of Lewis antigens on cancer cell surface that facilitates carcinoma cell–endothelial cell interactions, tumor cell rolling and metastasis [44]. Cancer cell migration and invasion are also promoted by TGF-β-induced EMT and are stimulated by fucosylation of TGF-β receptors. The overexpression of FUT3, FUT6 and FUT8 correlates with the enhanced TGF-β downstream signaling and consequently with increased cell invasiveness and metastasis [42,45]. In addition, alterations in cell surface fucosylated oligosaccharides are associated with cancer cell multidrug resistance (MDR) not only mediated by EMT but also stimulated by the positive regulation of drug efflux through ABC transporters via aberrant activation of PI3K/Akt signaling pathway. Interestingly, increased expression of FUT4, FUT6 and FUT8 has been associated with upregulation of PI3K/Akt signaling pathway and high levels of MRP1 (multidrug resistance-related protein 1) [41]. By contrast, FUT9 activity shows a dual role in CRC progression. At later stages of CRC, downregulation of FUT9 favors cancer cell proliferation and migration. On the other hand, at earlier stages of CRC development, FUT9 expression promotes CRC cell reprogramming towards a stem cell-like phenotype contributing to the expansion of CSCs or tumor-initiating cells (TICs) [46,47].
Sialylation is also a process related to CRC. Modifications in the expression of sialyltransferases are associated with cancer cell survival, proliferation, migration, invasion, metastasis, induction of stem-like cell properties, chemotherapy resistance, increased sialyl-Tn antigen expression, inflammation-driven carcinogenesis and resistance to 5-FU. In this respect, overexpression of ST6GAL1 and ST6GALNAC1 has a role in cancer cell stemness, cell survival, metastasis and chemoresistance [22,48,49,50,51]. ST6GALNAC1 induces stem-like cell properties via activation of AKT pathway, while ST6GAL1 has been evidenced to induce the expression of stem cell transcription factors Sox9 and Slug, thereby promoting cancer cell stemness. In addition, stem-like cell phenotype confers metastatic properties as well as resistance to drugs [48,50]. Furthermore, sialylation of Fas, catalyzed by ST6GAL1, inhibits Fas receptor internalization and, thus, avoids Fas-mediated apoptosis [51]. On the other hand, downregulation of ST6GALNAC2, observed in CRC tissue, stimulates metastatic processes (Table 1). ST6GALNAC2 hampers the interaction of soluble galectin-3 with the O-glycan profile on the cell surface through the modification of O-glycans. Thus, ST6GALNAC2 suppresses the retention of tumor cells at secondary sites and avoids metastasis. Consequently, downregulation of ST6GalNAc2 generates unmodified O-glycans expressed at the cell surfaces that interacts with Galectin-3 and facilitates the development of metastasis [52]. In addition, the downregulation of ST8SIA4, evidenced in CRC and other cancer cells, has been associated with increased cell proliferation, migration and invasion (Table 1) [22,53]. It has been described that ST8SIA4 gene expression is downregulated by mir-146a and miR-146b, which are overexpressed in thyroid carcinoma [53]. Similarly, epigenetic mechanisms such as DNA methylation and histone modification regulate glycosyltransferase gene expression [54]. In cancer cells, alterations of miRNAs expression profile and epigenetic mechanisms are involved in the regulation of glycosyltransferase gene expression, and glycosylation in turn participates in the epigenetic modulation of histones and transcription factors; thus, glycosylation could be considered an integral part of the epigenetic code [54].
Accordingly, changes in glycosyltransferase gene expression evidenced in carcinogenesis show consequences in glycosyltransferase joint activity and, therefore, in the cell glyco-code. Some of these alterations affect several molecular functions related with tumorigenesis, metastasis and cancer progression.

Table 1.
Upregulated and downregulated glycosyltransferase genes in CRC.
Table 1.
Upregulated and downregulated glycosyltransferase genes in CRC.
| A. Upregulated glycosyltransferase genes in CRC. Cellular effects induced by the upregulation of genes encoding glycosyltransferases are described. | |||
| Glycosyltransferases | Gene | Effects on Cancer Cells | References |
| Beta 3-glycosyltransferases | B3GNT3 | Cell migration. Cell invasion. Maintenance of CSCs. | Ashkani 2016 [22]. Barkeer 2018 [55]. |
| B3GNT8 | Cell migration. Cell invasion. Resistance to 5-FU. | Ashkani 2016 [22]. Ishida 2005 [27]. Ni 2014 [33]. Shen 2014 [56]. | |
| C1GALT1 | Induction of stem-like cell properties. Cell survival. Cell migration. Cell invasion. Radioresistance. | Hung 2014 [26]. Zhang 2018 [37]. | |
| Beta 4-glycosyltransferases | B4GALNT3 | Cell migration. Cell invasion. Maintenance of CSCs. | Che 2014 [23]. |
| Fucosyltransferases | FUT1 | Cell proliferation. Cell migration. Cell invasion. Metastasis. EMT. Maintenance of CSCs. | Ashkani 2016 [22]. Lai 2019 [43]. Petretti 2000 [57]. |
| FUT2 | Cell proliferation. Cell adhesion to extracellular matrix. Cell migration. Cell invasion. Metastasis. EMT. Maintenance of CSCs. | Ashkani 2016 [22]. Lai 2019 [43]. | |
| FUT3 | TGF-β-induced EMT. Cell adhesion to endothelium. Cell migration. | Ashkani 2016 [22]. Meng 2017 [58]. Padró 2011 [44]. Hirakawa 2014 [42]. | |
| FUT4 | MDR. | Ashkani 2016 [22]. Cheng 2013 [41]. Petretti 2000 [57]. | |
| FUT5 | Cell adhesion to endothelium. Cell migration. | Ashkani 2016 [22]. Padró 2011 [44]. | |
| FUT6 | TGF-β-induced EMT. MDR. Tumor progression. Metastasis. | Ashkani 2016 [22]. Cheng 2013 [41]. Hirakawa 2014 [42]. Sethi 2014 [31]. | |
| FUT8 | Tumor progression. Cell migration. Cell invasion. Metastasis. TGF-β-induced EMT. Tumor immune evasion. EGF-mediated cellular growth. | Sethi 2014 [31]. Tu 2017 [45]. Bastian 2021 [40]. | |
| Mannosyl-glycoprotein N-acetylglucosaminyl transferases | MGAT4B | Tumor progression. Metastasis. | Ashkani 2016 [22]. |
| MGAT5A | Tumor progression. Metastasis. Cell invasion. ↓ Anti-VEGF effectivity. Increase CCSC population. | Murata 2000 [29]. Guo 2014 [59]. Kim 2008 [38]. Croci 2014 [39]. Petretti 2000 [57]. | |
| O-linked N-acetylglucosaminyl transferases | OGT | Cell proliferation. Cell migration. Cell invasion. | Xu 2019 [32]. |
| Sialyltransferases | ST6GAL1 | Cell migration Cell invasion. Cell survival. Induction of stem-like cell properties. Chemotherapy resistance. | Ashkani 2016 [22]. Schultz 2016 [50]. Swindall 2011 [51]. Park 2012 [49]. Sethi 2014 [31]. |
| ST6GALNAC1 | ↑Sialyl-Tn expression. Maintenance of CSCs. Resistance to 5-FU. | Ashkani 2016 [22]. Marcos 2004 [60]. Ogawa 2017 [48]. | |
| B. Downregulated glycosyltransferase genes in CRC. Cellular effects induced by the downregulation of genes encoding glycosyltransferases are described. | |||
| Glycosyltransferases | Gene | Effects on Cancer cells | References |
| Beta 3-glycosyltransferases | B3GNT1 | Ashkani 2016 [22]. | |
| B3GNT6 | Cell migration. Cell invasion. Metastasis. EMT. | Iwai 2005 [28]. Gupta 2020 [61]. | |
| Polypeptide N-acetylgalactosaminyl transferases | GALNT6 | Poor differentiation. Cell migration. Cell invasion. Chemoresistance to 5-FU. ↑Tn-antigen expression. | Noda 2018 [30]. |
| Glucosaminyl (N-acetyl)transferases/ xylosyltransferases | GCNT3 | Cell proliferation. Cell adhesion. Cell migration. Cell invasion. Cell survival. Tumor growth. Chemoresistance to 5-FU. | Huang 2006 [25]. González-Vallinas 2015 [24]. Fernández 2018 [16]. |
| Fucosyltransferases | FUT9 | Cell migration. Metastasis. | Ashkani 2016 [22]. Auslander 2017 [46]. |
| Mannosyl-glycoprotein N-acetylglucosaminyl transferases | MGAT3 | Tumor progression. Metastasis. | Ashkani 2016 [22]. |
| MGAT5b | Tumor progression. Metastasis. | Ashkani 2016 [22]. | |
| Sialyltransferases | ST3GAL1 | Ashkani 2016 [22]. | |
| ST3GAL3 | Tumor progression. Metastasis. | Ashkani 2016 [22]. Sethi 2014 [31]. | |
| ST6GALNAC2 | Metastasis. | Ashkani 2016 [22]. Murugaesu 2014 [52]. Ferrer 2014 [62]. | |
| ST6GALNAC3 | Tumor progression. | Ashkani 2016 [22]. Haldrup 2018 [63]. | |
| ST6GALNAC6 | Inflammation-driven carcinogenesis. | Ashkani 2016 [22]. Huang 2020 [64]. | |
| ST8SIA1 | Ashkani 2016 [22]. | ||
| ST8SIA3 | Ashkani 2016 [22]. | ||
| ST8SIA4 | Cell proliferation. Cell migration. Cell invasion. | Ashkani 2016 [22]. Ma 2017 [53]. | |
| ST8SIA5 | Ashkani 2016 [22]. | ||
TGF-β: Transforming growth factor β. EMT: Epithelial–Mesenchymal Transition. MDR: Tumor Multidrug Resistance. CCSC: Colon Cancer Stem Cells. CSCs: Cancer Stem Cells. EGF: Epidermal Growth Factor. 5-FU: 5-fluorouracil. Tn-antigen: Cancer associated truncated glycan.
3. Glycosylated Molecules in Colorectal Cancer
Glycosylation is associated with several biological processes. Alterations in glycosyltransferase levels and glycosylation patterns have been evidenced in inflammatory conditions, tumorigenesis and metastasis [5,6,7,8,65,66]. In tumor cells, the increased dysregulation in glycosylation patterns induced, in part, by changes in cancer cell glucose metabolism favors their growth and metastasis [7,67,68].
Glycosylation of proteins generates changes on their biophysical properties, function, distribution and retention in the plasma membrane and modulates cell behavior, cellular interactions, specific ligand–receptor interactions and immune recognition [5,69,70,71]. In CRC tissues, highly glycosylated membrane proteins, such as annexin A1, have been identified (Table 2). Annexin A1 shows increased levels of GlcNAcylation in CRC compared to healthy tissues [20]. Annexin A1 is a calcium-dependent phospholipid-binding protein that interacts mainly with intracellular phospholipidic components such as phosphatidylserine and has been associated with several biological processes involved in oncogenesis, such as vesicle aggregation, membrane aggregation, exocytosis, endocytosis, actin polymerization, mitosis, cell membrane repair, apoptosis and cell differentiation. In addition, other annexin A1 functions include protection against myocardial ischemia-reperfusion injury; and the regulation of inflammatory processes, such as leukocyte migration and activation, as well as the activity of phospholipase A2 (PLA2), cyclooxygenase-2 (COX-2) and inducible nitric oxide synthase (iNOS) [72,73]. The increased GlcNAcylation described in annexin A1 from CRC tissues is defined as the addition of N-acetylglucosamine (GlcNAc) moieties to serine/threonine residues of the protein catalyzed by O-GlcNAc transferases (OGT) [74]. The importance of O-GlcNAcylation in several cellular events and even in tumorigenesis could be explained, in part, by its role in gene expression. In this regard, O-GlcNAcylation has been associated with transcription factor stability and functions. Thus, O-GlcNAcylation of transcription factors is necessary for T and B lymphocyte activation. Likewise, OGT cooperates with the regulation of epigenetic mechanisms, such as histone modification and DNA methylation that have been found dysregulated in cancer cells [74,75]. Furthermore, O-GlcNAc modifications have roles in glucose metabolism, cell differentiation, cell–cell adhesion, mitosis, Ca2+ signaling and the regulation of other ion channels among other cellular processes [76]. Thus, glycosylation of annexin A1 could have a role in CRC etiology and progression in addition to being a consequence of the carcinogenesis process.
Another protein found differentially GlcNAcylated in CRC tissues is heat shock protein 90 (HSP90β) (Table 2) [20]. HSP90β is a chaperone protein that interacts with enzymes, transcription factors, co-chaperones, structural proteins and oncoproteins among others molecules implicated in a plethora of cellular events [77]. In tumorigenesis, HSP90β has been associated with cell viability [78]; HSP90β post-translational modifications (PTMs), such as phosphorylation, acetylation and S-nitrosylation have been implicated in cancer cell immortalization via modulation of telomerase activity [79]. Additionally, it has been shown that HSP90 O-GlcNAcylation affects proteasome activity and, likewise, HSP90 glycation alters HSP90 activity and could be related with cancer progression [80].
Glycosylation of mucins has been extensively studied in cancer tissues. In this regard, MUC1 is highly glycosylated in CRC tissues and the O-glycosylation pattern of MUC2 is altered (Table 2) [19,81]. Mucin expression in intestinal epithelium is modulated by different factors, such as gut microbiota, dietary components, epigenetic mechanisms and translational processes. Since mucins are one of the most important components of the gastrointestinal associated immune system, alterations in mucin expression or in their structure and functional properties due to changes in N-glycosylation and O-glycosylation patterns among other PTMs have shown a role in immune dysregulation, loss of mucosal barrier integrity, increased risk of inflammatory or infectious diseases and changes in cell adhesive properties that alter the interaction between cells and their microenvironment [82]. In this regard, the CRC risk increases in patients affected by inflammatory bowel diseases (IBD), which are characterized by a marked dysbiosis, anomalous immunological response against gut microbiota, epigenetic dysregulation and alterations in the expression of enzymes associated with PTMs [83]. Thus, mucins’ contribution to IBD and CRC pathogenesis has been studied by several research efforts. MUC1 is one of the mucins that is more strongly implicated. Physiologically, decreased MUC1 expression in colon mucosa stimulates CD4+ T cell polarization to the Th17 phenotype and in turn Th17 cells produce cytokines that stimulate MUC1 expression, thus limiting the inflammation condition. According to these findings, alterations in MUC1 structure, such as glycosylation, can abrogate its immunoregulatory functions and ensues in chronic inflammatory conditions [84]. In addition, MUC1 contributes to metastasis generation by facilitating cell–cell and cell–extracellular matrix interactions due to its capacity to bind several molecules associated with migration and extravasation found the presence of both at the cell surface and in the cytoplasmic compartment. Thus, MUC1 can stimulate metastatic progression not only by promoting cell–cell adhesion but also by activating intracellular signaling pathways [85]. Interestingly, mucin oligosaccharides are a source of nourishment for microbiota and participates in the maintenance of colonic flora balance and distribution. Therefore, alterations in mucin glycans can generate dysbiosis and consequently abrogate gut immune balance and intestinal mucosal integrity [83]. In addition, a dense and diverse O-glycosylation pattern of mucins, such as MUC2 described as the main gel-forming mucin, is a key protective factor against colitis and CRC through the supply of health nourishment for the gut microbiota, maintenance of mucus bacterial adhesion capacity and protection from digestive proteases [19,86]. Thereby, mucin glycosylation patterns are critical not only for mucin structure but also for its stability, functionality and protection. Therefore, aberrant mucin glycosylation has strong implications in IBD and CRC pathogenesis and progression.
Adhesion molecules, such as selectin, integrin and their respective ligands, have an essential role in tumor cell endothelial adhesion, migration and organ invasion [87]. Changes in the expression level and glycosylation pattern of adhesion molecules have been related with tumor progression, poor prognosis and metastasis. In this regard, the hypersialylation of β1 integrin evidenced in CRC cells (Table 1) facilitates β1 integrin binding to its ligands, collagen I and laminin and interestingly promotes intracytoplasmic association of β1 integrin with talin, stimulating cell adhesion, motility and migration [88]. Studies focused on selectins have been performed with respect to the ability of circulating tumor cells to spread to distant organs and lymph nodes. In CRC, the overexpression of E-selectin ligands, specifically the sialyl LewisX (sLeX) determinants that mediate cell rolling, have been described. Thus, metastasis is closely associated with an altered glycosylation profile [89].
Regarding the immune response, alterations of the glycosylation profile of mucins or other molecules affect tumor cells interactions with human lectin receptors on antigen presenting cells (APCs), such as MGL (macrophage galactose-type lectin), DC-SIGN (dendritic cell-specific intercellular adhesion molecule-3-grabbing non-integrin) and galectin-3. In CRC tissues, aberrant glycosylation of MUC1 and other CRC antigens, such as carcinoembryonic antigen (CEA), can alter the mammalian lectin receptor interactions with tumor cells and could participate in immune modulation and immune evasion (Table 2) [81]. In addition, glycosylation pattern of T cells and their ligands, determine thymus homing of T cell precursors, T cell migration, endothelial adhesion and T cell interaction with MHCII on APC. Additionally, N-glycosylation of T cell receptor (TCR) and T cell co-receptor regulates T cell activation through stabilization of immunological synapses and inhibition of proteases degradation. Moreover, CD4+ T cell polarization is modulated, in part, by the expression and function of glycosyltransferases [90].
Additionally, in CRC, CEA shows high levels of Lewis antigens such as Lewis X and Lewis Y. These carbohydrates mediate the binding of CEA with DC-SIGN which is expressed mainly on immature dendritic cells. The interaction of tumor cells with immature dendritic cells through DC-SIGN could be related with immune tolerance due to the fact that immature dendritic cells are not effective in priming naive T cells. Thus, recruitment of immature dendritic cells by DC-SIGN through Lewis antigens decorating CEA can arrest dendritic cell differentiation towards a mature phenotype, thereby contributing to tumor cell immune evasion (Table 2) [81,91].
Aberrant glycosylation of the epidermal growth factor receptor (EGFR) has been widely studied in CRC cells (Table 2). N-linked glycosylation of EGFR is required for its trafficking to the plasma membrane and it is necessary for its role in tumor growth [92]. In addition, the EGFR glycosylation pattern in CRC cells is modified by β1,4-N-acetylgalactosaminyltransferase III (B4GALNT3) that has been found overexpressed in advanced CRC stages and has been related with poor prognosis (Table 1). PTMs catalyzed by B4GALNT3 confer stability to EGFR and avoids its degradation, contributes with EGFR signaling pathways that mediate the maintenance of stemness and consequently generates cancer cell growth, survival and attenuated differentiation (Table 2) [23].
Furthermore, insulin-like growth factor-binding protein 3 (IGFBP3) has shown an altered glycosylation pattern in serum and tumor cell membrane samples isolated from CRC patients. In CRC, IGFBP3 has been found decorated with high amounts of α2,6 Sialic acid and low levels of Fuc and GlcNAc moieties [93]. The main function of IGFBP3 is to protect and transport the insulin-like growth factors (IGFs), in circulation, thereby regulating IGFs activity and availability. IGFs play a role in cell proliferation, survival, differentiation and migration. In addition, IGFBP3 has shown biological associations with cell growth inhibitors and molecules involved in tumorigenesis such as retinoic acid, retinoid X receptor (RXR), retinoic acid receptor (RAR), nuclear factor kappa B (NF-ĸB), transforming growth factor beta (TGF-β), tumor necrosis factor-alpha (TNFα), glucose-regulated protein 78 (GRP78), butyrate (histone deacetylase inhibitor), dietary supplements with anti-inflammatory and anti-cancer properties, caveolin-1 and interestingly with the tumor suppressor gene p53 [94,95,96,97,98]. In addition, the glycosylation pattern of IGFBP3 is crucial for IGFBP3 affinity, its selective binding with its ligands and for IGFBP3 stability, half-life, function and activity. Thus, low expression of IGFBP3 has been related with cancer development and resistance to radiotherapy and chemotherapy and interestingly alterations in the glycosylation pattern of IGFBP3 can modify several molecular events and intracellular pathways related with tumorigenesis [93,94,95,99].
In CRC tissues, altered glycosylation patterns of glycosphingolipids have been described (Table 1) [10]. Glycosphingolipids are plasma membrane molecules that consist of glycan structures linked to sphingosine and closely related lipids. They are implicated in several events related with membrane integrity and cellular interactions. In cancer cells, they are involved in cell proliferation, cell migration, chemoresistance, EMT, metastasis, reduced apoptosis and poor cell differentiation [9,10]. Interestingly, CRC tissues have shown an increased fucosylation and sialylation accompanied by low acetylation and sulfation of glycosphingolipid glycans (Table 2) [10,100]. Thus, PTMs affecting glycosphingolipid glycan profile in tumor tissues could be implicated in CRC progression and metastasis.

Table 2.
Summary of molecules identified to be glycosylated in colorectal cancer samples. Glycosylation and associated biological effects on cancer cells have been collected.
Table 2.
Summary of molecules identified to be glycosylated in colorectal cancer samples. Glycosylation and associated biological effects on cancer cells have been collected.
| Target Molecules | Glycosylation | Effects on Cancer Cells | References |
|---|---|---|---|
| Annexin A1 | GlcNAcylation | Mitosis. Apoptosis. Cell differentiation. | Li et al. [20]. Yang et al. [74]. |
| HSP90 | GlcNAcylation | Cell viability. Cancer progression. | Li et al. [20]. Zou et al. [78]. Overath et al. [80]. |
| Carcinoembryonic antigen (CEA) | ↑ Fucose ↑ Mannose ↑ Thomsen–Friedenreich antigen ↓ N-acetylgalactosamine ↓ N-acetylglucosamine ↓ Galactose | Immune tolerance. CRC tumorigenesis. CRC progression. | Zhao et al. [101]. van Gisbergen et al. [91]. |
| IGFBP3 | ↑Sialylation (α2,3) ↓Fucosylation ↓GlcNAcylation | Cell proliferation. Cell survival. Cell differentiation. Cell migration. | Zámorová et al. [93]. |
| Decorin | O-glycosylation. | CRC development and progression. Metastasis. Cell-cell adhesion. Cell migration. | Wei et al. [102]. |
| SORBS1 | O-glycosylation. | CRC development and progression. Metastasis. Cell-cell adhesion. | Wei et al. [102]. |
| EGFR | Sialylation (Loss of α2,6 sialylation) Modification of N- with LacdiNAc structures. | Cell proliferation. Tumor growth. Cancer cell survival. Attenuated cancer cell differentiation. Chemoresistance. | Li et al. [92]. Che et al. [23]. Park et al. [49]. |
| TGF-β receptors | Fucosylation | EMT. Metastasis. | Hirakawa et al. [42]. |
| MucinsMUC1 MUC2 | O-glycosylation | Alteration of the interactions of mammalian lectin receptors with tumor cells. Immune dysregulation. Loss of mucosal barrier integrity. Gut dysbiosis. | Pothuraju et al. [82]. Peixoto et al. [69]. Brockhausen et al. [103]. Venkitachalam et al. [17]. Kawashima et al. [19]. Arike et al. [86]. |
| Podoplanin | O-glycosylation | Cell migration. Cell invasion. | Liu et al. [104]. |
| β1 integrin | α2-6 sialylation | Cell adhesion. Cell motility. Cell migration. Tumor cell survival. | Seales et al. [88]. Zhuo et al. [105]. |
| Fas (CD95) | α2-6 sialylation | Anti-apoptotic effect. | Swindall et al. [51]. |
| PD-L1 | N-glycosylation | Immune response evasion. | Ruan et al. [106]. |
| CD147 | Modification with Beta1,6-branched polylactosamine structures. | Cell migration. Cell invasion. Metastasis. | Ni et al. [33]. |
| Glycosphingolipids | ↑ Fucosylation ↑ Sialylation ↓ Glycans acetylation ↓ Glycans sulfation | Cell proliferation. Cell migration. Chemoresistance. EMT. Metastasis. Reduced apoptosis. Poor cell differentiation. | Holst et al. [10]. Misonou et al. [100]. Cumin et al. [9]. Gb3: Distler et al. [107]. Gb4: Park et al. [108]. GCS: Haynes et al. [109]. NEU3: Yamaguchi et al. [110]. GD1a and GM1: Kwak et al. [111]. α-GalCer: Yoshioka et al. [112]. GM3: Chung et al. [113]. |
EMT: Epithelial–Mesenchymal Transition.
4. Implications of Glycosylation and Glycosyltransferases in Colorectal Cancer Therapy
As previously mentioned, CRC is considered one of the most lethal cancer with a high global prevalence [114]. Current treatments include surgical interventions in order to remove primary tumors and to avoid metastasis either before or after chemotherapy or radiotherapy [115,116,117,118]. Despite these treatments, metastasis is a common occurrence in most cases because of the late diagnoses and the problems in surgical elimination [2,119,120,121]. For these reasons, chemotherapy is a good option to reduce death related to CRC.
Chemotherapy agents are varied and there are regimens including only one agent or a combination of effective drugs that affect one or several targets [121]. Indeed, the most frequently used treatment in present times is a combination of 5-fluorouracil (5-FU)/leucovorin (LV, folinic acid) with oxalipatin or irinitecan (camptothecin-11, CPT-11). Other agents include monoclonal antibodies such as cetuximab or bevacizumab targeting EGFR or the vascular endothelial growth factor (VEGF), respectively [122].
Modulation of glycosyltransferase expression by drugs has been studied for CRC. The expression of GCNT3 gene, which encodes the enzyme mucin-type core 2 1,6-N-acetylglucosaminyltransferase (C2GnT-M), has been found downregulated in bad prognosis CRC tissues (Table 1). In this regard, Gonzalez-Vallinas et al. have shown that the expression of GCNT3 is induced by chemotherapeutic drugs, such as pyrimidine analogue 5-FU, proteasome inhibitor bortezomib or the mitotic inhibitor paclitaxel and these effects correlate with suppression of cell viability in cancer cells [24].
Regarding tumor cell epitopes, it has been described that the altered glycosylation generates neo-antigens that become targets for tumor-specific T cells [90]. Thereby, CRC associated glycosylation can be involved in tumor immune evasion and immune regulation. Based on these findings, immunotherapy challenges, such as those that include the design and generation of engineered CAR (Chimeric antigen receptors) T cells using differentially glycosylated molecules as targets, have ensued (Figure 1) [123]. In addition, innovative immunotherapies focused on enhancing T cell activity through modification of T cell N-glycosylation could avoid the differentiation of naive CD4+ T cells into regulatory or exhaustion phenotypes that favor tumor immune evasion and it is usually observed in precancerous conditions and in tumor microenvironment (Figure 1) [124,125,126,127,128].
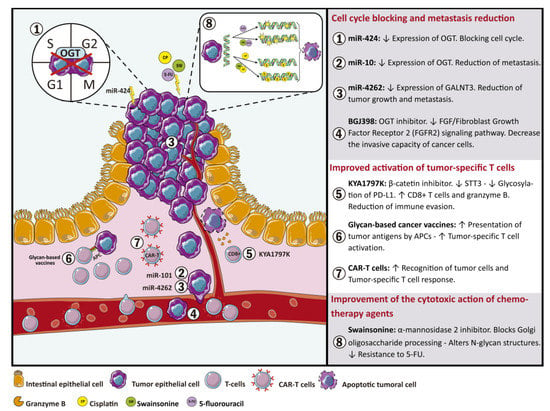
Figure 1.
Glycan-based therapeutic approaches for colorectal cancer. Details of therapy strategies based on modifications of glycan and glycosyltransferase expression includes: (1–4) Glycosyltransferases as a target for therapeutic miRNAs and glycosyltransferase inhibitors in order to reduce metastasis and block cell cycle. (5–7) Strategies based on glycan profile modifications to stimulate the immune system. (8) Implementation of modified glycan structures to increase cytotoxic action of chemotherapy agents.
According to CRC, O-GlcNAcylation has been shown to be elevated in CRC due to the Warburg effect and it has been recognized as an axis for the development and progression of metastasis [129]. CRC cells replace their aerobic glucose metabolism, even in the presence of relatively normal levels of oxygen, by anaerobic glucose metabolism which leads to a significant increase in glucose absorption in a process known as the Warburg effect [130]. Thus, the Warburg effect provide cancer cells with an incredible adaptive advantage compared to normal cells since glucose and glutamine are basic for cellular growth [131,132]. This process is also associated with a high risk of metastasis in CRC patients who have an excessive caloric intake [133] or diabetes comorbidity [134]. In this regard, various miRNAs have been shown to regulate the expression of O-GlcNAcylation transferases (OGT) [135,136]. Among these, miR-424 downregulates the expression of OGT and other glycosyltransferases, such as MGAT4A, with a resultant arrest of the cell cycle (Figure 1) [137]. In addition, OGT and histone methyltransferase (EZH2) are regulated by miR-101. The evidenced decrease in miR-101 in CRC tissues induces an increase in O-GlcNAcylation of Ser75 that stabilizes EZH2. OGT and EZH2 activity produces a decrease of miR-101 expression, thereby generating a negative feedback loop that promotes metastasis (Figure 1) [138]. Thus, OGT and O-GlcNAcylation patterns are interesting therapeutic targets for metastatic CRC [139].
As previously stated, alterations in glycosylation patterns, mediated by the modified glycosyltransferase expression, are involved in CRC malignant transformation [11,140]. Therefore, glycoconjugates and the molecules involved in their synthesis are interesting targets for new therapeutic approaches. In this context, Jian-Jun Qu et al. evaluated the effect of miR-4262 in CRC cell lines and human tissues and observed a significant decrease of miR-4262 levels when compared with control samples. Additionally, upregulation of miR-4262 expression decreased GALNT4 expression. GALNT4 catalyzes the O-glycosylation of threonine residues 44 and 57 in P-selectin glycoprotein ligand-1 (PSGL-1). Thr57 glycosylation is necessary for PSGL-1/P-selectin interaction, which stimulates tumor growth, tumor cell propagation into the bloodstream and metastasis. Accordingly, the administration of miR-4262 could reduce tumor growth and metastasis through the downregulation of GALNT4 expression (Figure 1) [141,142,143,144].
It has been shown that BGJ398, an OGT inhibitor, can significantly inhibit the activity of core 1 O-Glycan T-Synthase (C1GALT1) that catalyzes the transfer of Gal from UDP-Gal to GalNAc-alpha-1-Ser/Thr of cell surface proteins, such as the fibroblast growth factor receptor 2 (FGFR2), resulting in a significant decrease in the invasive capacity of CRC cells (Figure 1). These data suggest that FGF/FGFR2 signaling pathway is part of the phenotypic changes modulated by C1GALT1 and could be a therapy for CRC (Figure 1) [26].
Regarding tumor immune evasion, it has been described that KYA1797K, a β-catenin inhibitor, stimulates the immune response through the regulation of glycosyltransferase expression. The β-catenin and dolichyl-diphosphooligosaccharide-protein glycosyltransferase subunit STT3 (STT3) are overexpressed in CRC. The expression of STT3 is upregulated by β-catenin. Furthermore, upregulation of both proteins correlates with CRC’s worsened prognosis. In this regard, KYA1797K mediates suppression of β-catenin expression with the subsequent downregulation of STT3. STT3 downregulation decreases the stability and the immunosuppressive activity of programmed death receptor 1 (PD-L1) through the inhibition of PD-L1 glycosylation and consequently increases the population of CD8+ T cells and the expression of granzyme B. Therefore, the inhibition of PD-L1 glycosylation reduces the immune evasion through blocking Wnt/β-catenin/STT3 signaling pathway in CRC cells (Figure 1) [106].
The use of glycans as target molecules for immunotherapy has been extensively studied in cancer cells. Glycan-based cancer vaccines are used to decorate tumors with glycan structures, such as Lewis antigens, in order to enhance the presentation of tumor antigens by APCs, including dendritic cells internalization and antigen presentation, thereby improving tumor-specific T cell activation (Figure 1) [90]. Additionally, therapeutic approaches using engineered CAR-T cells with specificity for glycan epitopes differentially expressed in cancer cells have also been described (Figure 1) [123].
Drug resistance has been associated with aberrant glycosylation profiles. Hamaguchi et al. used glycosylation inhibitors in order to study the relationship between N-glycosylation and drug resistance. Specifically, they observed an effect of swainsonine, an α-mannosidase 2 inhibitor which blocks Golgi oligosaccharide processing, generating altered N-glycan structures that affect 5-FU mechanisms of resistance in CRC cell lines [145]. In the same way, other authors have shown that swainsonine administration improves tumor cell sensitivity towards the apoptotic effect of cisplatin, which results in avoiding drug resistance. These data suggest that inhibition of N-glycosylation could facilitate the cytotoxic action of chemotherapy agents [146].
All these considerations highlight aberrant glycosylation as a crucial issue in CRC treatment and point out some potential targets to improve and design specific therapies.
5. Conclusions
Glycosylation is a regulatory mechanism implicated in a number of physiological and pathological processes. The mechanisms of glycosylation are related with several biological events such as membrane integrity, cellular interactions and cell proliferation. In cancer cells, glycosylation has been related to cell migration, chemoresistance, epithelial-to-mesenchymal transition, metastasis, reduced apoptosis, tumor growth and poor cell differentiation. In CRC, alterations in glycoprotein profiles, such as increased sialylation or branched glycan structures and overexpression of fucosylation, have been extensively found. Thereby, characterization and analysis of glycoconjugates have great potential to improve early diagnosis and therapy designs based on new therapeutic targets.
Author Contributions
Conceptualization, C.F.-P., N.G.-D., E.N.Q., I.S.-G. and R.N.Q.; resources, L.G.E., L.A.I., R.F.-C., G.A.M. and F.G.-C.; writing—preparation of the original draft, C.F.-P., N.G.-D., E.N.Q., I.S.-G. and R.N.Q.; writing—proofreading and editing, L.G.E., L.A.I., R.F.-C., G.A.M. and F.G.-C. All authors have read and agreed to the published version of the manuscript.
Funding
We declare that the funds or sources of support received in this specific internal report study were from the Simón Bolívar University, Barranquilla, Colombia and by the Integrated Territorial Investment (ITI), Junta de Andalucía, Spain (PI-0030-2017). The external funding was from the Ministry of Science, Technology and Innovation of Colombia, subsidy 125380763038, 125380763188 and SGR code BPIN 2020000100144. We clarified that the funder had no role in the design of the study, in the collection and analysis of data, in the decision to publish or in the preparation of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data sharing is not applicable to this article.
Acknowledgments
We thank the Simón Bolívar University, Ministry of Science, Technology and Innovation of Colombia and Integrated Territorial Investment (ITI), Junta de Andalucía for facilitating the resources for article publication. Images in figure were obtained and modified from SMART: Service Medical ART (http://smart.servier.com accessed on 29 May 2021).
Conflicts of Interest
The authors declare that they have no competing interests.
References
- Araghi, M.; Soerjomataram, I.; Jenkins, M.; Brierley, J.; Morris, E.; Bray, F.; Arnold, M. Global trends in colorectal cancer mortality: Projections to the year 2035. Int. J. Cancer 2019, 144, 2992–3000. [Google Scholar] [CrossRef]
- Keum, N.; Giovannucci, E. Global burden of colorectal cancer: Emerging trends, risk factors and prevention strategies. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 713–732. [Google Scholar] [CrossRef]
- Rawla, P.; Sunkara, T.; Barsouk, A. Epidemiology of colorectal cancer: Incidence, mortality, survival, and risk factors. Prz. Gastroenterol. 2019, 14, 89–103. [Google Scholar] [CrossRef] [PubMed]
- De Freitas, J.C.M., Jr.; Morgado-Díaz, J.A. The role of N-glycans in colorectal cancer progression: Potential biomarkers and therapeutic applications. Oncotarget 2016, 7, 19395–19413. [Google Scholar] [CrossRef] [PubMed]
- Hoja-Łukowicz, D.; Link-Lenczowski, P.; Carpentieri, A.; Amoresano, A.; Pocheć, E.; Artemenko, K.A.; Bergquist, J.; Lityńska, A. L1CAM from human melanoma carries a novel type of N-glycan with Galβ1-4Galβ1- motif. Involvement of N-linked glycans in migratory and invasive behaviour of melanoma cells. Glycoconj. J. 2013, 30, 205–225. [Google Scholar] [CrossRef] [PubMed]
- Julien, S.; Ivetic, A.; Grigoriadis, A.; QiZe, D.; Burford, B.; Sproviero, D.; Picco, G.; Gillett, C.; Papp, S.L.; Schaffer, L.; et al. Selectin ligand sialyl-Lewis x antigen drives metastasis of hormone-dependent breast cancers. Cancer Res. 2011, 71, 7683–7693. [Google Scholar] [CrossRef]
- Kim, Y.-S.; Ahn, Y.H.; Song, K.J.; Kang, J.G.; Lee, J.H.; Jeon, S.K.; Kim, H.-C.; Yoo, J.S.; Ko, J.-H. Overexpression and β-1,6-N-acetylglucosaminylation-initiated aberrant glycosylation of TIMP-1: A “double whammy” strategy in colon cancer progression. J. Biol. Chem. 2012, 287, 32467–32478. [Google Scholar] [CrossRef]
- Wei, T.; Liu, Q.; He, F.; Zhu, W.; Hu, L.; Guo, L.; Zhang, J. The role of N-acetylglucosaminyltransferases V in the malignancy of human hepatocellular carcinoma. Exp. Mol. Pathol. 2012, 93, 8–17. [Google Scholar] [CrossRef]
- Cumin, C.; Huang, Y.L.; Everest-Dass, A.; Jacob, F. Deciphering the Importance of Glycosphingolipids on Cellular and Molecular Mechanisms Associated with Epithelial-to-Mesenchymal Transition in Cancer. Biomolecules 2021, 11, 62. [Google Scholar] [CrossRef]
- Holst, S.; Stavenhagen, K.; Balog, C.I.; Koeleman, C.A.; McDonnell, L.M.; Mayboroda, O.A.; Verhoeven, A.; Mesker, W.E.; Tollenaar, R.A.; Deelder, A.M.; et al. Investigations on aberrant glycosylation of glycosphingolipids in colorectal cancer tissues using liquid chromatography and matrix-assisted laser desorption time-of-flight mass spectrometry (MALDI-TOF-MS). Mol. Cell. Proteom. 2013, 12, 3081–3093. [Google Scholar] [CrossRef]
- Holst, S.; Wuhrer, M.; Rombouts, Y. Glycosylation characteristics of colorectal cancer. Adv. Cancer Res. 2015, 126, 203–256. [Google Scholar]
- Varki, A. Biological roles of glycans. Glycobiology 2017, 27, 3–49. [Google Scholar] [CrossRef]
- Marth, J.D.; Grewal, P.K. Mammalian glycosylation in immunity. Nat. Rev. Immunol. 2008, 8, 874–887. [Google Scholar] [CrossRef] [PubMed]
- Reily, C.; Stewart, T.J.; Renfrow, M.B.; Novak, J. Glycosylation in health and disease. Nat. Rev. Nephrol. 2019, 15, 346–366. [Google Scholar] [CrossRef]
- Stanley, P. Golgi glycosylation. Cold Spring Harb. Perspect. Biol. 2011, 3, a005199. [Google Scholar] [CrossRef] [PubMed]
- Fernández, L.P.; Sánchez-Martínez, R.; Vargas, T.; Herranz, J.; Martín-Hernández, R.; Mendiola, M.; Hardisson, D.; Reglero, G.; Feliu, J.; Redondo, A.; et al. The role of glycosyltransferase enzyme GCNT3 in colon and ovarian cancer prognosis and chemoresistance. Sci. Rep. 2018, 8, 8485. [Google Scholar] [CrossRef]
- Venkitachalam, S.; Guda, K. Altered glycosyltransferases in colorectal cancer. Expert Rev. Gastroenterol. Hepatol. 2017, 11, 5–7. [Google Scholar] [CrossRef]
- Venkitachalam, S.; Revoredo, L.; Varadan, V.; Fecteau, R.E.; Ravi, L.; Lutterbaugh, J.; Markowitz, S.D.; Willis, J.E.; Gerken, T.A.; Guda, K. Biochemical and functional characterization of glycosylation-associated mutational landscapes in colon cancer. Sci. Rep. 2016, 6, 23642. [Google Scholar] [CrossRef] [PubMed]
- Kawashima, H. Roles of the gel-forming MUC2 mucin and its O-glycosylation in the protection against colitis and colorectal cancer. Biol. Pharm. Bull. 2012, 35, 1637–1641. [Google Scholar] [CrossRef]
- Li, Y.; Wen, T.; Zhu, M.; Li, L.; Wei, J.; Wu, X.; Guo, M.; Liu, S.; Zhao, H.; Xia, S.; et al. Glycoproteomic analysis of tissues from patients with colon cancer using lectin microarrays and nanoLC-MS/MS. Mol. Biosyst. 2013, 9, 1877–1887. [Google Scholar] [CrossRef]
- Dall’olio, F. Protein glycosylation in cancer biology: An overview. Clin. Mol. Pathol. 1996, 49, M126–M135. [Google Scholar] [CrossRef] [PubMed]
- Ashkani, J.; Naidoo, K.J. Glycosyltransferase Gene Expression Profiles Classify Cancer Types and Propose Prognostic Subtypes. Sci. Rep. 2016, 6, 26451. [Google Scholar] [CrossRef] [PubMed]
- Che, M.I.; Huang, J.; Hung, J.S.; Lin, Y.C.; Huang, M.J.; Lai, H.S.; Hsu, W.M.; Liang, J.T.; Huang, M.C. β1, 4-N-acetylgalactosaminyltransferase III modulates cancer stemness through EGFR signaling pathway in colon cancer cells. Oncotarget 2014, 5, 3673–3684. [Google Scholar] [CrossRef] [PubMed]
- González-Vallinas, M.; Vargas, T.; Moreno-Rubio, J.; Molina, S.; Herranz, J.; Cejas, P.; Burgos, E.; Aguayo, C.; Custodio, A.; Reglero, G.; et al. Clinical relevance of the differential expression of the glycosyltransferase gene GCNT3 in colon cancer. Eur. J. Cancer 2015, 51, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.C.; Chen, H.Y.; Huang, H.C.; Huang, J.; Liang, J.T.; Shen, T.L.; Lin, N.Y.; Ho, C.C.; Cho, I.M.; Hsu, S.M. C2GnT-M is downregulated in colorectal cancer and its re-expression causes growth inhibition of colon cancer cells. Oncogene 2006, 25, 3267–3276. [Google Scholar] [CrossRef]
- Hung, J.S.; Huang, J.; Lin, Y.C.; Huang, M.J.; Lee, P.H.; Lai, H.S.; Liang, J.T.; Huang, M.C. C1GALT1 overexpression promotes the invasive behavior of colon cancer cells through modifying O-glycosylation of FGFR2. Oncotarget 2014, 5, 2096–2106. [Google Scholar] [CrossRef]
- Ishida, H.; Togayachi, A.; Sakai, T.; Iwai, T.; Hiruma, T.; Sato, T.; Okubo, R.; Inaba, N.; Kudo, T.; Gotoh, M.; et al. A novel beta1,3-N-acetylglucosaminyltransferase (beta3Gn-T8), which synthesizes poly-N-acetyllactosamine, is dramatically upregulated in colon cancer. FEBS Lett. 2005, 579, 71–78. [Google Scholar] [CrossRef]
- Iwai, T.; Kudo, T.; Kawamoto, R.; Kubota, T.; Togayachi, A.; Hiruma, T.; Okada, T.; Kawamoto, T.; Morozumi, K.; Narimatsu, H. Core 3 synthase is down-regulated in colon carcinoma and profoundly suppresses the metastatic potential of carcinoma cells. Proc. Natl. Acad. Sci. USA 2005, 102, 4572–4577. [Google Scholar] [CrossRef]
- Murata, K.; Miyoshi, E.; Kameyama, M.; Ishikawa, O.; Kabuto, T.; Sasaki, Y.; Hiratsuka, M.; Ohigashi, H.; Ishiguro, S.; Ito, S.; et al. Expression of N-acetylglucosaminyltransferase V in colorectal cancer correlates with metastasis and poor prognosis. Clin. Cancer Res. 2000, 6, 1772–1777. [Google Scholar] [PubMed]
- Noda, M.; Okayama, H.; Tachibana, K.; Sakamoto, W.; Saito, K.; Thar Min, A.K.; Ashizawa, M.; Nakajima, T.; Aoto, K.; Momma, T.; et al. Glycosyltransferase Gene Expression Identifies a Poor Prognostic Colorectal Cancer Subtype Associated with Mismatch Repair Deficiency and Incomplete Glycan Synthesis. Clin. Cancer Res. 2018, 24, 4468–4481. [Google Scholar] [CrossRef] [PubMed]
- Sethi, M.K.; Thaysen-Andersen, M.; Smith, J.T.; Baker, M.S.; Packer, N.H.; Hancock, W.S.; Fanayan, S. Comparative N-glycan profiling of colorectal cancer cell lines reveals unique bisecting GlcNAc and α-2,3-linked sialic acid determinants are associated with membrane proteins of the more metastatic/aggressive cell lines. J. Proteome Res. 2014, 13, 277–288. [Google Scholar] [CrossRef]
- Xu, D.; Wang, W.; Bian, T.; Yang, W.; Shao, M.; Yang, H. Increased expression of O-GlcNAc transferase (OGT) is a biomarker for poor prognosis and allows tumorigenesis and invasion in colon cancer. Int. J. Clin. Exp. Pathol. 2019, 12, 1305–1314. [Google Scholar]
- Ni, J.; Jiang, Z.; Shen, L.; Gao, L.; Yu, M.; Xu, X.; Zou, S.; Hua, D.; Wu, S. beta3GnT8 regulates the metastatic potential of colorectal carcinoma cells by altering the glycosylation of CD147. Oncol. Rep. 2014, 31, 1795–1801. [Google Scholar] [CrossRef][Green Version]
- Sun, J.; Hemler, M.E. Regulation of MMP-1 and MMP-2 production through CD147/extracellular matrix metalloproteinase inducer interactions. Cancer Res. 2001, 61, 2276–2281. [Google Scholar]
- Very, N.; Lefebvre, T.; El Yazidi-Belkoura, I. Drug resistance related to aberrant glycosylation in colorectal cancer. Oncotarget 2017, 9, 1380–1402. [Google Scholar] [CrossRef]
- Xu, T.; Zhou, M.; Peng, L.; Kong, S.; Miao, R.; Shi, Y.; Sheng, H.; Li, L. Upregulation of CD147 promotes cell invasion, epithelial-to-mesenchymal transition and activates MAPK/ERK signaling pathway in colorectal cancer. Int. J. Clin. Exp. Pathol. 2014, 7, 7432–7441. [Google Scholar] [PubMed]
- Zhang, C.; Deng, X.; Qiu, L.; Peng, F.; Geng, S.; Shen, L.; Luo, Z. Knockdown of C1GalT1 inhibits radioresistance of human esophageal cancer cells through modifying β1-integrin glycosylation. J. Cancer 2018, 9, 2666–2677. [Google Scholar] [CrossRef]
- Kim, Y.S.; Hwang, S.Y.; Kang, H.Y.; Sohn, H.; Oh, S.; Kim, J.Y.; Yoo, J.S.; Kim, Y.H.; Kim, C.H.; Jeon, J.H.; et al. Functional proteomics study reveals that N-Acetylglucosaminyltransferase V reinforces the invasive/metastatic potential of colon cancer through aberrant glycosylation on tissue inhibitor of metalloproteinase-1. Mol. Cell. Proteom. 2008, 7, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Croci, D.O.; Cerliani, J.P.; Dalotto-Moreno, T.; Mendez-Huergo, S.P.; Mascanfroni, I.D.; Dergan-Dylon, S.; Toscano, M.A.; Caramelo, J.J.; Garcia-Vallejo, J.J.; Ouyang, J.; et al. Glycosylation-dependent lectin-receptor interactions preserve angiogenesis in anti-VEGF refractory tumors. Cell 2014, 156, 744–758. [Google Scholar] [CrossRef] [PubMed]
- Bastian, K.; Scott, E.; Elliott, D.J.; Munkley, J. FUT8 Alpha-(1,6)-Fucosyltransferase in Cancer. Int. J. Mol. Sci. 2021, 22, 455. [Google Scholar] [CrossRef]
- Cheng, L.; Luo, S.; Jin, C.; Ma, H.; Zhou, H.; Jia, L. FUT family mediates the multidrug resistance of human hepatocellular carcinoma via the PI3K/Akt signaling pathway. Cell Death Dis. 2013, 4, e923. [Google Scholar] [CrossRef]
- Hirakawa, M.; Takimoto, R.; Tamura, F.; Yoshida, M.; Ono, M.; Murase, K.; Sato, Y.; Osuga, T.; Sato, T.; Iyama, S.; et al. Fucosylated TGF-β receptors transduces a signal for epithelial-mesenchymal transition in colorectal cancer cells. Br. J. Cancer 2014, 110, 156–163. [Google Scholar] [CrossRef]
- Lai, T.-Y.; Chen, I.J.; Lin, R.-J.; Liao, G.-S.; Yeo, H.-L.; Ho, C.-L.; Wu, J.-C.; Chang, N.-C.; Lee, A.C.-L.; Yu, A.L. Fucosyltransferase 1 and 2 play pivotal roles in breast cancer cells. Cell Death Discov. 2019, 5, 74. [Google Scholar] [CrossRef] [PubMed]
- Padró, M.; Cobler, L.; Garrido, M.; de Bolós, C. Down-regulation of FUT3 and FUT5 by shRNA alters Lewis antigens expression and reduces the adhesion capacities of gastric cancer cells. Biochim. Biophys. Acta 2011, 1810, 1141–1149. [Google Scholar] [CrossRef]
- Tu, C.-F.; Wu, M.-Y.; Lin, Y.-C.; Kannagi, R.; Yang, R.-B. FUT8 promotes breast cancer cell invasiveness by remodeling TGF-β receptor core fucosylation. Breast Cancer Res. 2017, 19, 111. [Google Scholar] [CrossRef]
- Auslander, N.; Cunningham, C.E.; Toosi, B.M.; McEwen, E.J.; Yizhak, K.; Vizeacoumar, F.S.; Parameswaran, S.; Gonen, N.; Freywald, T.; Bhanumathy, K.K.; et al. An integrated computational and experimental study uncovers FUT9 as a metabolic driver of colorectal cancer. Mol. Syst. Biol. 2017, 13, 956. [Google Scholar] [CrossRef] [PubMed]
- Blanas, A.; Zaal, A.; van der Haar Àvila, I.; Kempers, M.; Kruijssen, L.; de Kok, M.; Popovic, M.A.; van der Horst, J.C.; van Vliet, S.J. FUT9-Driven Programming of Colon Cancer Cells towards a Stem Cell-Like State. Cancers 2020, 12, 2580. [Google Scholar] [CrossRef]
- Ogawa, T.; Hirohashi, Y.; Murai, A.; Nishidate, T.; Okita, K.; Wang, L.; Ikehara, Y.; Satoyoshi, T.; Usui, A.; Kubo, T.; et al. ST6GALNAC1 plays important roles in enhancing cancer stem phenotypes of colorectal cancer via the Akt pathway. Oncotarget 2017, 8, 112550–112564. [Google Scholar] [CrossRef] [PubMed]
- Park, J.J.; Yi, J.Y.; Jin, Y.B.; Lee, Y.J.; Lee, J.S.; Lee, Y.S.; Ko, Y.G.; Lee, M. Sialylation of epidermal growth factor receptor regulates receptor activity and chemosensitivity to gefitinib in colon cancer cells. Biochem. Pharmacol. 2012, 83, 849–857. [Google Scholar] [CrossRef]
- Schultz, M.J.; Holdbrooks, A.T.; Chakraborty, A.; Grizzle, W.E.; Landen, C.N.; Buchsbaum, D.J.; Conner, M.G.; Arend, R.C.; Yoon, K.J.; Klug, C.A.; et al. The Tumor-Associated Glycosyltransferase ST6Gal-I Regulates Stem Cell Transcription Factors and Confers a Cancer Stem Cell Phenotype. Cancer Res. 2016, 76, 3978–3988. [Google Scholar] [CrossRef]
- Swindall, A.F.; Bellis, S.L. Sialylation of the Fas death receptor by ST6Gal-I provides protection against Fas-mediated apoptosis in colon carcinoma cells. J. Biol. Chem. 2011, 286, 22982–22990. [Google Scholar] [CrossRef]
- Murugaesu, N.; Iravani, M.; van Weverwijk, A.; Ivetic, A.; Johnson, D.A.; Antonopoulos, A.; Fearns, A.; Jamal-Hanjani, M.; Sims, D.; Fenwick, K.; et al. An in vivo functional screen identifies ST6GalNAc2 sialyltransferase as a breast cancer metastasis suppressor. Cancer Discov. 2014, 4, 304–317. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Zhao, X.; Liang, L.; Wang, G.; Li, Y.; Miao, X.; Zhao, Y. miR-146a and miR-146b promote proliferation, migration and invasion of follicular thyroid carcinoma via inhibition of ST8SIA4. Oncotarget 2017, 8, 28028–28041. [Google Scholar] [CrossRef] [PubMed]
- Dall’Olio, F.; Trinchera, M. Epigenetic Bases of Aberrant Glycosylation in Cancer. Int. J. Mol. Sci. 2017, 18, 998. [Google Scholar] [CrossRef]
- Barkeer, S.; Chugh, S.; Karmakar, S.; Kaushik, G.; Rauth, S.; Rachagani, S.; Batra, S.K.; Ponnusamy, M.P. Novel role of O-glycosyltransferases GALNT3 and B3GNT3 in the self-renewal of pancreatic cancer stem cells. BMC Cancer 2018, 18, 1157. [Google Scholar] [CrossRef]
- Shen, L.; Yu, M.; Xu, X.; Gao, L.; Ni, J.; Luo, Z.; Wu, S. Knockdown of β3GnT8 reverses 5-fluorouracil resistance in human colorectal cancer cells via inhibition the biosynthesis of polylactosamine-type N-glycans. Int. J. Oncol. 2014, 45, 2560–2568. [Google Scholar] [CrossRef]
- Petretti, T.; Kemmner, W.; Schulze, B.; Schlag, P.M. Altered mRNA expression of glycosyltransferases in human colorectal carcinomas and liver metastases. Gut 2000, 46, 359. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.; Xu, L.; Yang, Y.; Zhou, L.; Chang, Y.; Shi, T.; Tan, C.; An, H.; Zhu, Y.; Xu, J. High expression of FUT3 is linked to poor prognosis in clear cell renal cell carcinoma. Oncotarget 2017, 8, 61036–61047. [Google Scholar] [CrossRef]
- Guo, H.; Nagy, T.; Pierce, M. Post-translational glycoprotein modifications regulate colon cancer stem cells and colon adenoma progression in Apc(min/+) mice through altered Wnt receptor signaling. J. Biol. Chem. 2014, 289, 31534–31549. [Google Scholar] [CrossRef]
- Marcos, N.T.; Pinho, S.; Grandela, C.; Cruz, A.; Samyn-Petit, B.; Harduin-Lepers, A.; Almeida, R.; Silva, F.; Morais, V.; Costa, J.; et al. Role of the human ST6GalNAc-I and ST6GalNAc-II in the synthesis of the cancer-associated sialyl-Tn antigen. Cancer Res. 2004, 64, 7050–7057. [Google Scholar] [CrossRef]
- Gupta, R.; Leon, F.; Rauth, S.; Batra, S.K.; Ponnusamy, M.P. A Systematic Review on the Implications of O-linked Glycan Branching and Truncating Enzymes on Cancer Progression and Metastasis. Cells 2020, 9, 446. [Google Scholar] [CrossRef]
- Ferrer, C.M.; Reginato, M.J. Sticking to sugars at the metastatic site: Sialyltransferase ST6GalNAc2 acts as a breast cancer metastasis suppressor. Cancer Discov. 2014, 4, 275–277. [Google Scholar] [CrossRef][Green Version]
- Haldrup, C.; Pedersen, A.L.; Øgaard, N.; Strand, S.H.; Høyer, S.; Borre, M.; Ørntoft, T.F.; Sørensen, K.D. Biomarker potential of ST6GALNAC3 and ZNF660 promoter hypermethylation in prostate cancer tissue and liquid biopsies. Mol. Oncol. 2018, 12, 545–560. [Google Scholar] [CrossRef]
- Huang, H.C.; Cai, B.H.; Suen, C.S.; Lee, H.Y.; Hwang, M.J.; Liu, F.T.; Kannagi, R. BGN/TLR4/NF-B Mediates Epigenetic Silencing of Immunosuppressive Siglec Ligands in Colon Cancer Cells. Cells 2020, 9, 397. [Google Scholar] [CrossRef]
- Dube, D.H.; Bertozzi, C.R. Glycans in cancer and inflammation—Potential for therapeutics and diagnostics. Nat. Rev. Drug Discov. 2005, 4, 477–488. [Google Scholar] [CrossRef]
- Orntoft, T.F.; Vestergaard, E.M. Clinical aspects of altered glycosylation of glycoproteins in cancer. Electrophoresis 1999, 20, 362–371. [Google Scholar] [CrossRef]
- Christiansen, M.N.; Chik, J.; Lee, L.; Anugraham, M.; Abrahams, J.L.; Packer, N.H. Cell surface protein glycosylation in cancer. Proteomics 2014, 14, 525–546. [Google Scholar] [CrossRef]
- Jóźwiak, P.; Forma, E.; Bryś, M.; Krześlak, A. O-GlcNAcylation and Metabolic Reprograming in Cancer. Front. Endocrinol. 2014, 5, 145. [Google Scholar]
- Peixoto, A.; Relvas-Santos, M.; Azevedo, R.; Santos, L.L.; Ferreira, J.A. Protein Glycosylation and Tumor Microenvironment Alterations Driving Cancer Hallmarks. Front. Oncol. 2019, 9, 380. [Google Scholar] [CrossRef]
- Shental-Bechor, D.; Levy, Y. Effect of glycosylation on protein folding: A close look at thermodynamic stabilization. Proc. Natl. Acad. Sci. USA 2008, 105, 8256–8261. [Google Scholar] [CrossRef]
- Vagin, O.; Kraut, J.A.; Sachs, G. Role of N-glycosylation in trafficking of apical membrane proteins in epithelia. Am. J. Physiol. Ren. Physiol. 2009, 296, F459–F469. [Google Scholar] [CrossRef]
- Moraes, L.A.; Ampomah, P.B.; Lim, L.H.K. Annexin A1 in inflammation and breast cancer: A new axis in the tumor microenvironment. Cell Adhes. Migr. 2018, 12, 417–423. [Google Scholar] [CrossRef]
- Lizarbe, M.A.; Barrasa, J.I.; Olmo, N.; Gavilanes, F.; Turnay, J. Annexin-Phospholipid Interactions. Functional Implications. Int. J. Mol. Sci. 2013, 14, 2652–2683. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Qian, K. Protein O-GlcNAcylation: Emerging mechanisms and functions. Nat. Rev. Mol. Cell Biol. 2017, 18, 452–465. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.P.; Zhang, K.; Wu, J.; Yang, X. O-GlcNAc signaling in cancer metabolism and epigenetics. Cancer Lett. 2015, 356, 244–250. [Google Scholar] [CrossRef]
- Fisi, V.; Miseta, A.; Nagy, T.A.-O. The Role of Stress-Induced O-GlcNAc Protein Modification in the Regulation of Membrane Transport. Oxidative Med. Cell. Longev. 2017, 2017, 1308692. [Google Scholar] [CrossRef] [PubMed]
- Zuehlke, A.D.; Beebe, K.; Neckers, L.; Prince, T. Regulation and function of the human HSP90AA1 gene. Gene 2015, 570, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Zou, M.; Bhatia, A.; Dong, H.; Jayaprakash, P.; Guo, J.; Sahu, D.; Hou, Y.; Tsen, F.; Tong, C.; O’Brien, K.; et al. Evolutionarily conserved dual lysine motif determines the non-chaperone function of secreted Hsp90alpha in tumour progression. Oncogene 2017, 36, 2160–2171. [Google Scholar] [CrossRef]
- Trepel, J.; Mollapour, M.; Giaccone, G.; Neckers, L. Targeting the dynamic HSP90 complex in cancer. Nat. Rev. Cancer 2010, 10, 537–549. [Google Scholar] [CrossRef] [PubMed]
- Overath, T.; Kuckelkorn, U.; Henklein, P.; Strehl, B.; Bonar, D.; Kloss, A.; Siele, D.; Kloetzel, P.M.; Janek, K. Mapping of O-GlcNAc sites of 20 S proteasome subunits and Hsp90 by a novel biotin-cystamine tag. Mol. Cell Proteom. 2012, 11, 467–477. [Google Scholar] [CrossRef] [PubMed]
- Saeland, E.; Belo, A.I.; Mongera, S.; van Die, I.; Meijer, G.A.; van Kooyk, Y. Differential glycosylation of MUC1 and CEACAM5 between normal mucosa and tumour tissue of colon cancer patients. Int. J. Cancer 2012, 131, 117–128. [Google Scholar] [CrossRef]
- Pothuraju, R.; Krishn, S.R.; Gautam, S.K.; Pai, P.; Ganguly, K.; Chaudhary, S.; Rachagani, S.; Kaur, S.; Batra, S.K. Mechanistic and Functional Shades of Mucins and Associated Glycans in Colon Cancer. Cancers (Basel) 2020, 12, 649. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Ponce, C.; Navarro Quiroz, R.; Díaz Perez, A.; Aroca Martinez, G.; Cadena Bonfanti, A.; Acosta Hoyos, A.; Gómez Escorcia, L.; Hernández Agudelo, S.; Orozco Sánchez, C.; Villarreal Camacho, J.; et al. MicroRNAs overexpressed in Crohn’s disease and their interactions with mechanisms of epigenetic regulation explain novel aspects of Crohn’s disease pathogenesis. Clin. Epigenet. 2021, 13, 39. [Google Scholar] [CrossRef]
- Nishida, A.; Lau, C.W.; Zhang, M.; Andoh, A.; Shi, H.N.; Mizoguchi, E.; Mizoguchi, A. The membrane-bound mucin Muc1 regulates T helper 17-cell responses and colitis in mice. Gastroenterology 2012, 142, 865–874.e2. [Google Scholar] [CrossRef]
- Agrawal, B.; Gupta, N.; Konowalchuk, J.D. MUC1 Mucin: A Putative Regulatory (Checkpoint) Molecule of T Cells. Front. Immunol. 2018, 9, 2391. [Google Scholar] [CrossRef]
- Arike, L.; Hansson, G.C. The Densely O-Glycosylated MUC2 Mucin Protects the Intestine and Provides Food for the Commensal Bacteria. J. Mol. Biol. 2016, 428, 3221–3229. [Google Scholar] [CrossRef]
- Läubli, H.; Borsig, L. Altered Cell Adhesion and Glycosylation Promote Cancer Immune Suppression and Metastasis. Front. Immunol. 2019, 10, 2120. [Google Scholar] [CrossRef]
- Seales, E.C.; Jurado, G.A.; Brunson, B.A.; Wakefield, J.K.; Frost, A.R.; Bellis, S.L. Hypersialylation of beta1 integrins, observed in colon adenocarcinoma, may contribute to cancer progression by up-regulating cell motility. Cancer Res. 2005, 65, 4645–4652. [Google Scholar] [CrossRef]
- Deschepper, F.M.; Zoppi, R.; Pirro, M.; Hensbergen, P.J.; Dall’Olio, F.; Kotsias, M.; Gardner, R.A.; Spencer, D.I.R.; Videira, P.A. L1CAM as an E-selectin Ligand in Colon Cancer. Int. J. Mol. Sci. 2020, 21, 8286. [Google Scholar] [CrossRef]
- De Bousser, E.; Meuris, L.; Callewaert, N.; Festjens, N. Human T cell glycosylation and implications on immune therapy for cancer. Hum. Vaccines Immunother. 2020, 16, 2374–2388. [Google Scholar] [CrossRef]
- Van Gisbergen, K.P.; Aarnoudse, C.A.; Meijer, G.A.; Geijtenbeek, T.B.; van Kooyk, Y. Dendritic cells recognize tumor-specific glycosylation of carcinoembryonic antigen on colorectal cancer cells through dendritic cell-specific intercellular adhesion molecule-3-grabbing nonintegrin. Cancer Res. 2005, 65, 5935–5944. [Google Scholar] [CrossRef]
- Li, H.; Al-Japairai, K.; Tao, Y.; Xiang, Z. RPN2 promotes colorectal cancer cell proliferation through modulating the glycosylation status of EGFR. Oncotarget 2017, 8, 72633–72651. [Google Scholar] [CrossRef] [PubMed]
- Zámorová, M.; Holazová, A.; Miljuš, G.; Robajac, D.; Šunderić, M.; Malenković, V.; Đukanović, B.; Gemeiner, P.; Katrlík, J.; Nedić, O. Analysis of changes in the glycan composition of serum, cytosol and membrane glycoprotein biomarkers of colorectal cancer using a lectin-based protein microarray. Anal. Methods 2017, 9, 2660–2666. [Google Scholar] [CrossRef]
- Baxter, R.C. Insulin-like growth factor binding protein-3 (IGFBP-3): Novel ligands mediate unexpected functions. J. Cell Commun. Signal. 2013, 7, 179–189. [Google Scholar] [CrossRef]
- Cai, Q.; Dozmorov, M.; Oh, Y. IGFBP-3/IGFBP-3 Receptor System as an Anti-Tumor and Anti-Metastatic Signaling in Cancer. Cells 2020, 9, 1261. [Google Scholar] [CrossRef]
- Grkovic, S.; O’Reilly, V.C.; Han, S.; Hong, M.; Baxter, R.C.; Firth, S.M. IGFBP-3 binds GRP78, stimulates autophagy and promotes the survival of breast cancer cells exposed to adverse microenvironments. Oncogene 2013, 32, 2412–2420. [Google Scholar] [CrossRef] [PubMed]
- Walker, G.E.; Wilson, E.M.; Powell, D.; Oh, Y. Butyrate, a histone deacetylase inhibitor, activates the human IGF binding protein-3 promoter in breast cancer cells: Molecular mechanism involves an Sp1/Sp3 multiprotein complex. Endocrinology 2001, 142, 3817–3827. [Google Scholar] [CrossRef]
- Williams, A.C.; Smartt, H.; AM, H.Z.; Macfarlane, M.; Paraskeva, C.; Collard, T.J. Insulin-like growth factor binding protein 3 (IGFBP-3) potentiates TRAIL-induced apoptosis of human colorectal carcinoma cells through inhibition of NF-kappaB. Cell Death Differ. 2007, 14, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Baricević, I.; Masnikosa, R.; Lagundzin, D.; Golubović, V.; Nedić, O. Alterations of insulin-like growth factor binding protein 3 (IGFBP-3) glycosylation in patients with breast tumours. Clin. Biochem. 2010, 43, 725–731. [Google Scholar] [CrossRef]
- Misonou, Y.; Shida, K.; Korekane, H.; Seki, Y.; Noura, S.; Ohue, M.; Miyamoto, Y. Comprehensive Clinico-Glycomic Study of 16 Colorectal Cancer Specimens: Elucidation of Aberrant Glycosylation and Its Mechanistic Causes in Colorectal Cancer Cells. J. Proteome Res. 2009, 8, 2990–3005. [Google Scholar] [CrossRef]
- Zhao, Q.; Zhan, T.; Deng, Z.; Li, Q.; Liu, Y.; Yang, S.; Ji, D.; Li, Y. Glycan analysis of colorectal cancer samples reveals stage-dependent changes in CEA glycosylation patterns. Clin. Proteom. 2018, 15, 9. [Google Scholar] [CrossRef]
- Wei, H.; Cheng, Z.; Ouyang, C.; Zhang, Y.; Hu, Y.; Chen, S.; Wang, C.; Lu, F.; Zhang, J.; Wang, Y.; et al. Glycoprotein screening in colorectal cancer based on differentially expressed Tn antigen. Oncol. Rep. 2016, 36, 1313–1324. [Google Scholar] [CrossRef] [PubMed]
- Brockhausen, I. Mucin-type O-glycans in human colon and breast cancer: Glycodynamics and functions. EMBO Rep. 2006, 7, 599–604. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Cui, Y.; Yang, F.; Xu, Z.; Da, L.T.; Zhang, Y. Inhibition of polypeptide N-acetyl-α-galactosaminyltransferases is an underlying mechanism of dietary polyphenols preventing colorectal tumorigenesis. Bioorg. Med. Chem. 2019, 27, 3372–3382. [Google Scholar] [CrossRef] [PubMed]
- Zhuo, Y.; Chammas, R.; Bellis, S.L. Sialylation of beta1 integrins blocks cell adhesion to galectin-3 and protects cells against galectin-3-induced apoptosis. J. Biol. Chem. 2008, 283, 22177–22185. [Google Scholar] [CrossRef]
- Ruan, Z.; Liang, M.; Lai, M.; Shang, L.; Deng, X.; Su, X. KYA1797K down-regulates PD-L1 in colon cancer stem cells to block immune evasion by suppressing the β-catenin/STT3 signaling pathway. Int. Immunopharmacol. 2020, 78, 106003. [Google Scholar] [CrossRef]
- Distler, U.; Souady, J.; Hülsewig, M.; Drmić-Hofman, I.; Haier, J.; Friedrich, A.W.; Karch, H.; Senninger, N.; Dreisewerd, K.; Berkenkamp, S.; et al. Shiga toxin receptor Gb3Cer/CD77: Tumor-association and promising therapeutic target in pancreas and colon cancer. PLoS ONE 2009, 4, e6813. [Google Scholar] [CrossRef]
- Park, S.Y.; Kwak, C.Y.; Shayman, J.A.; Kim, J.H. Globoside promotes activation of ERK by interaction with the epidermal growth factor receptor. Biochim. Biophys. Acta 2012, 1820, 1141–1148. [Google Scholar] [CrossRef]
- Haynes, T.A.; Filippov, V.; Filippova, M.; Yang, J.; Zhang, K.; Duerksen-Hughes, P.J. DNA damage induces down-regulation of UDP-glucose ceramide glucosyltransferase, increases ceramide levels and triggers apoptosis in p53-deficient cancer cells. Biochim. Biophys. Acta 2012, 1821, 943–953. [Google Scholar] [CrossRef]
- Yamaguchi, K.; Shiozaki, K.; Moriya, S.; Koseki, K.; Wada, T.; Tateno, H.; Sato, I.; Asano, M.; Iwakura, Y.; Miyagi, T. Reduced susceptibility to colitis-associated colon carcinogenesis in mice lacking plasma membrane-associated sialidase. PLoS ONE 2012, 7, e41132. [Google Scholar] [CrossRef]
- Kwak, D.H.; Ryu, J.-S.; Kim, C.-H.; Ko, K.; Ma, J.Y.; Hwang, K.-A.; Choo, Y.-K. Relationship between ganglioside expression and anti-cancer effects of the monoclonal antibody against epithelial cell adhesion molecule in colon cancer. Exp. Mol. Med. 2011, 43, 693–701. [Google Scholar] [CrossRef]
- Yoshioka, K.; Ueno, Y.; Tanaka, S.; Nagai, K.; Onitake, T.; Hanaoka, R.; Watanabe, H.; Chayama, K. Role of natural killer T cells in the mouse colitis-associated colon cancer model. Scand. J. Immunol. 2012, 75, 16–26. [Google Scholar] [CrossRef]
- Chung, T.-W.; Choi, H.-J.; Kim, S.-J.; Kwak, C.-H.; Song, K.-H.; Jin, U.-H.; Chang, Y.-C.; Chang, H.W.; Lee, Y.-C.; Ha, K.-T.; et al. The ganglioside GM3 is associated with cisplatin-induced apoptosis in human colon cancer cells. PLoS ONE 2014, 9, e92786. [Google Scholar] [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Brown, K.G.M.; Solomon, M.J.; Mahon, K.; O’Shannassy, S. Management of colorectal cancer. BMJ 2019, 366, l4561. [Google Scholar] [CrossRef]
- Kuipers, E.J.; Grady, W.M.; Lieberman, D.; Seufferlein, T.; Sung, J.J.; Boelens, P.G.; van de Velde, C.J.; Watanabe, T. Colorectal cancer. Nat. Rev. Dis. Primers 2015, 1, 15065. [Google Scholar] [CrossRef]
- Messersmith, W.A. NCCN Guidelines Updates: Management of Metastatic Colorectal Cancer. J. Natl. Compr. Cancer Netw. 2019, 17, 599–601. [Google Scholar]
- Van Cutsem, E.; Cervantes, A.; Nordlinger, B.; Arnold, D.; Group, E.G.W. Metastatic colorectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2014, 25 (Suppl. S3), iii1–iii9. [Google Scholar] [CrossRef]
- Sanchez-Gundin, J.; Fernandez-Carballido, A.M.; Martinez-Valdivieso, L.; Barreda-Hernandez, D.; Torres-Suarez, A.I. New Trends in the Therapeutic Approach to Metastatic Colorectal Cancer. Int. J. Med. Sci. 2018, 15, 659–665. [Google Scholar] [CrossRef]
- Van der Stok, E.P.; Spaander, M.C.W.; Grunhagen, D.J.; Verhoef, C.; Kuipers, E.J. Surveillance after curative treatment for colorectal cancer. Nat. Rev. Clin. Oncol. 2017, 14, 297–315. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.H.; Chen, Y.X.; Fang, J.Y. Comprehensive review of targeted therapy for colorectal cancer. Signal. Transduct. Target. Ther. 2020, 5, 22. [Google Scholar] [CrossRef] [PubMed]
- Yaffee, P.; Osipov, A.; Tan, C.; Tuli, R.; Hendifar, A. Review of systemic therapies for locally advanced and metastatic rectal cancer. J. Gastrointest. Oncol. 2015, 6, 185–200. [Google Scholar] [PubMed]
- Steentoft, C.; Migliorini, D.; King, T.R.; Mandel, U.; June, C.H.; Posey, A.D., Jr. Glycan-directed CAR-T cells. Glycobiology 2018, 28, 656–669. [Google Scholar] [CrossRef]
- Dominguez-Villar, M.; Fernandez-Ponce, C.; Munoz-Suano, A.; Gomez, E.; Rodríguez-Iglesias, M.; Garcia-Cozar, F. Up-regulation of FOXP3 and induction of suppressive function in CD4+ Jurkat T-cells expressing hepatitis C virus core protein. Clin. Sci. 2012, 123, 15–27. [Google Scholar] [CrossRef]
- Fernandez-Ponce, C.; Dominguez-Villar, M.; Aguado, E.; Garcia-Cozar, F. CD4+ primary T cells expressing HCV-core protein upregulate Foxp3 and IL-10, suppressing CD4 and CD8 T cells. PLoS ONE 2014, 9, e85191. [Google Scholar] [CrossRef]
- Fernández-Ponce, C.; Dominguez-Villar, M.; Muñoz-Miranda, J.P.; Arbulo-Echevarria, M.M.; Litrán, R.; Aguado, E.; García-Cozar, F. Immune modulation by the hepatitis C virus core protein. J. Viral Hepat. 2017, 24, 350–356. [Google Scholar] [CrossRef]
- Fernández-Ponce, C.; Durán-Ruiz, M.C.; Narbona-Sánchez, I.; Muñoz-Miranda, J.P.; Arbulo-Echevarria, M.M.; Serna-Sanz, A.; Baumann, C.; Litrán, R.; Aguado, E.; Bloch, W.; et al. Ultrastructural Localization and Molecular Associations of HCV Capsid Protein in Jurkat T Cells. Front. Microbiol. 2018, 8, 2595. [Google Scholar] [CrossRef]
- Sasawatari, S.; Okamoto, Y.; Kumanogoh, A.; Toyofuku, T. Blockade of N-Glycosylation Promotes Antitumor Immune Response of T Cells. J. Immunol. 2020, 204, 1373–1385. [Google Scholar] [CrossRef]
- Steenackers, A.; Olivier-Van Stichelen, S.; Baldini, S.F.; Dehennaut, V.; Toillon, R.A.; Le Bourhis, X.; El Yazidi-Belkoura, I.; Lefebvre, T. Silencing the Nucleocytoplasmic O-GlcNAc Transferase Reduces Proliferation, Adhesion, and Migration of Cancer and Fetal Human Colon Cell Lines. Front. Endocrinol. 2016, 7, 46. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Warburg, O. On the origin of cancer cells. Science 1956, 123, 309–314. [Google Scholar] [CrossRef]
- Warburg, O. On respiratory impairment in cancer cells. Science 1956, 124, 269–270. [Google Scholar]
- Siegel, R.; Desantis, C.; Jemal, A. Colorectal cancer statistics, 2014. CA Cancer J. Clin. 2014, 64, 104–117. [Google Scholar] [CrossRef]
- Giovannucci, E.; Michaud, D. The role of obesity and related metabolic disturbances in cancers of the colon, prostate, and pancreas. Gastroenterology 2007, 132, 2208–2225. [Google Scholar] [CrossRef]
- Babae, N.; Bourajjaj, M.; Liu, Y.; Van Beijnum, J.R.; Cerisoli, F.; Scaria, P.V.; Verheul, M.; Van Berkel, M.P.; Pieters, E.H.; Van Haastert, R.J.; et al. Systemic miRNA-7 delivery inhibits tumor angiogenesis and growth in murine xenograft glioblastoma. Oncotarget 2014, 5, 6687–6700. [Google Scholar] [CrossRef] [PubMed]
- Luo, P.; He, T.; Jiang, R.; Li, G. MicroRNA-423-5p targets O-GlcNAc transferase to induce apoptosis in cardiomyocytes. Mol. Med. Rep. 2015, 12, 1163–1168. [Google Scholar] [CrossRef]
- Vaiana, C.A.; Kurcon, T.; Mahal, L.K. MicroRNA-424 Predicts a Role for β-1,4 Branched Glycosylation in Cell Cycle Progression. J. Biol. Chem. 2016, 291, 1529–1537. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Xu, B.; Li, X.; Shang, Y.; Chu, Y.; Wang, W.; Chen, D.; Wu, N.; Hu, S.; Zhang, S.; et al. O-GlcNAcylation promotes colorectal cancer metastasis via the miR-101-O-GlcNAc/EZH2 regulatory feedback circuit. Oncogene 2019, 38, 301–316. [Google Scholar] [CrossRef]
- Guo, H.; Zhang, B.; Nairn, A.V.; Nagy, T.; Moremen, K.W.; Buckhaults, P.; Pierce, M. O-Linked N-Acetylglucosamine (O-GlcNAc) Expression Levels Epigenetically Regulate Colon Cancer Tumorigenesis by Affecting the Cancer Stem Cell Compartment via Modulating Expression of Transcriptional Factor MYBL1. J. Biol. Chem. 2017, 292, 4123–4137. [Google Scholar] [CrossRef]
- Holm, M.; Nummela, P.; Heiskanen, A.; Satomaa, T.; Kaprio, T.; Mustonen, H.; Ristimäki, A.; Haglund, C. N-glycomic profiling of colorectal cancer according to tumor stage and location. PLoS ONE 2020, 15, e0234989. [Google Scholar] [CrossRef]
- Bennett, E.P.; Hassan, H.; Mandel, U.; Mirgorodskaya, E.; Roepstorff, P.; Burchell, J.; Taylor-Papadimitriou, J.; Hollingsworth, M.A.; Merkx, G.; van Kessel, A.G.; et al. Cloning of a Human UDP-N-Acetyl-α-d-Galactosamine:PolypeptideN-Acetylgalactosaminyltransferase That Complements Other GalNAc-Transferases in Complete O-Glycosylation of the MUC1 Tandem Repeat. J. Biol. Chem. 1998, 273, 30472–30481. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Short, H.J.; Qian, K.X.; Elhammer, A.P.; Geng, J.G. Characterization of glycoprotein ligands for P-selectin on a human small cell lung cancer cell line NCI-H345. Biochem. Biophys. Res. Commun. 2001, 288, 637–644. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Ramachandran, V.; Kang, J.; Kishimoto, T.K.; Cummings, R.D.; McEver, R.P. Identification of N-terminal residues on P-selectin glycoprotein ligand-1 required for binding to P-selectin. J. Biol. Chem. 1998, 273, 7078–7087. [Google Scholar] [CrossRef] [PubMed]
- Qu, J.J.; Qu, X.Y.; Zhou, D.Z. miR-4262 inhibits colon cancer cell proliferation via targeting of GALNT4. Mol. Med. Rep. 2017, 16, 3731–3736. [Google Scholar] [CrossRef] [PubMed]
- Hamaguchi, J.; Nakagawa, H.; Takahashi, M.; Kudo, T.; Kamiyama, N.; Sun, B.; Oshima, T.; Sato, Y.; Deguchi, K.; Todo, S.; et al. Swainsonine reduces 5-fluorouracil tolerance in the multistage resistance of colorectal cancer cell lines. Mol. Cancer 2007, 6, 58. [Google Scholar] [CrossRef] [PubMed]
- Santos, F.M.; Latorre, A.O.; Hueza, I.M.; Sanches, D.S.; Lippi, L.L.; Gardner, D.R.; Spinosa, H.S. Increased antitumor efficacy by the combined administration of swainsonine and cisplatin in vivo. Phytomedicine 2011, 18, 1096–1101. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).