A Comparison of Immune Responses Exerted Following Syngeneic, Allogeneic, and Xenogeneic Transplantation of Mesenchymal Stem Cells into the Mouse Brain
Abstract
1. Introduction
2. Results
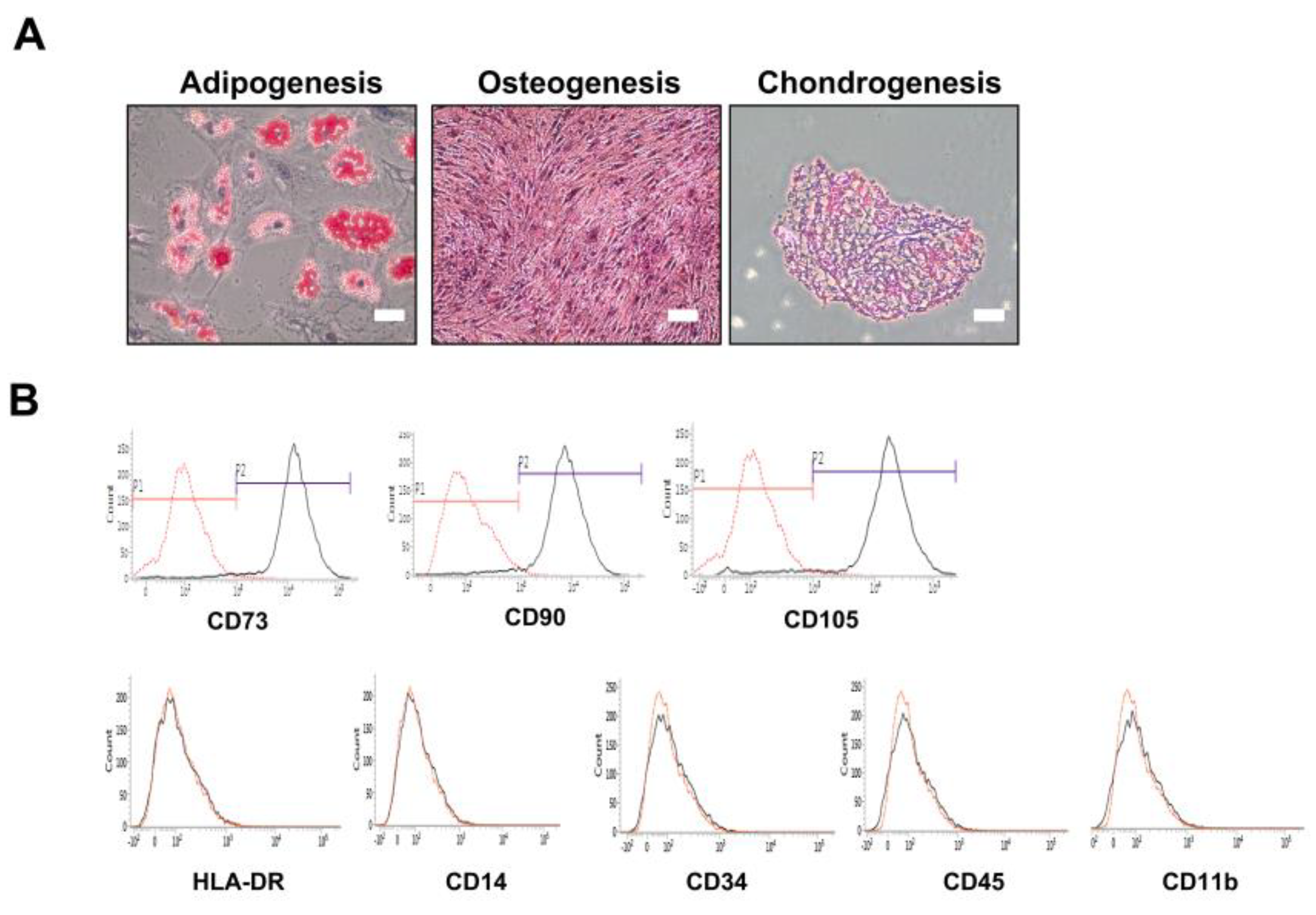
2.1. Confirmation of In Vitro Mesenchymal Trilineage Differentiation and Expression of Surface Antigens
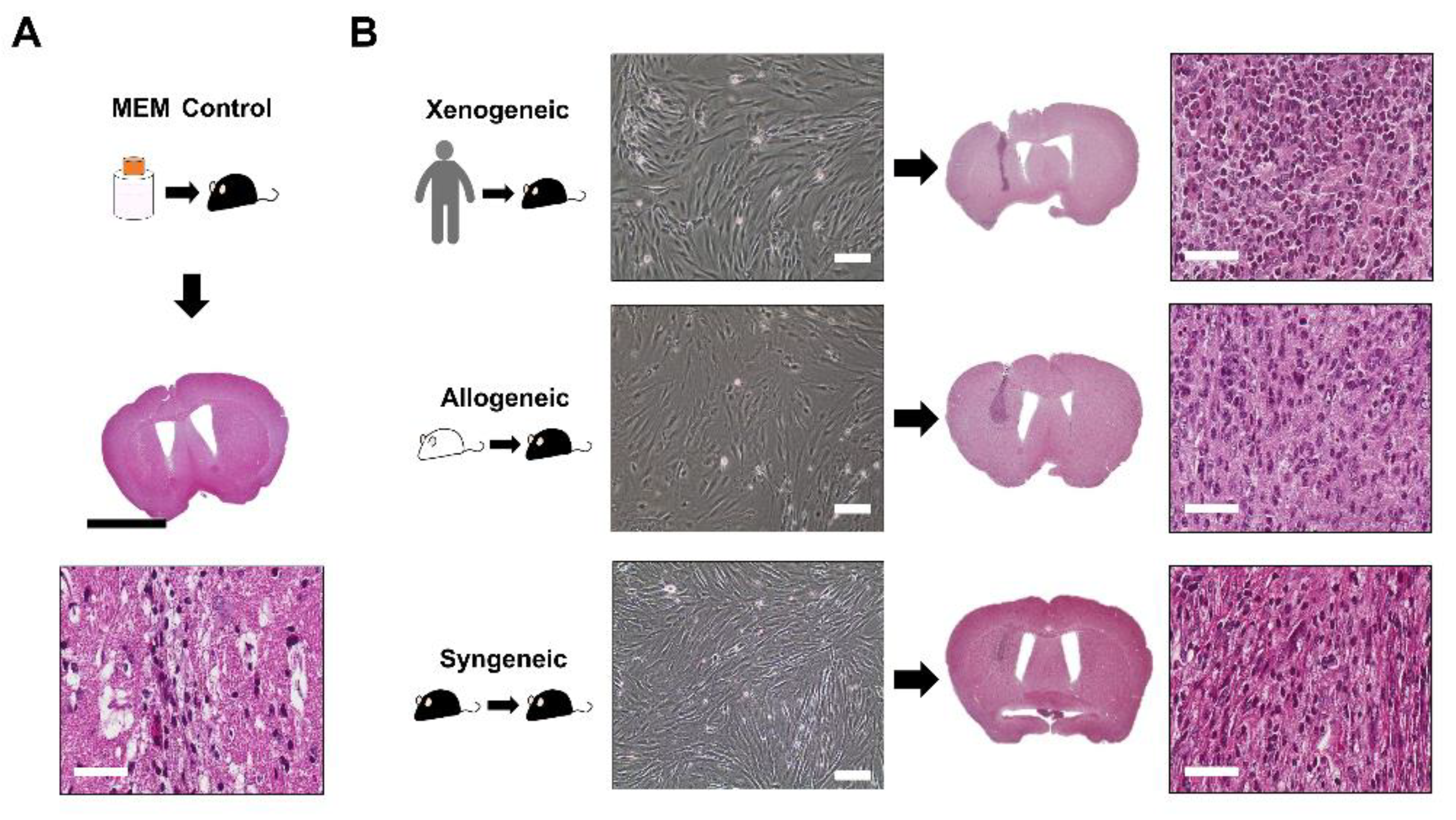
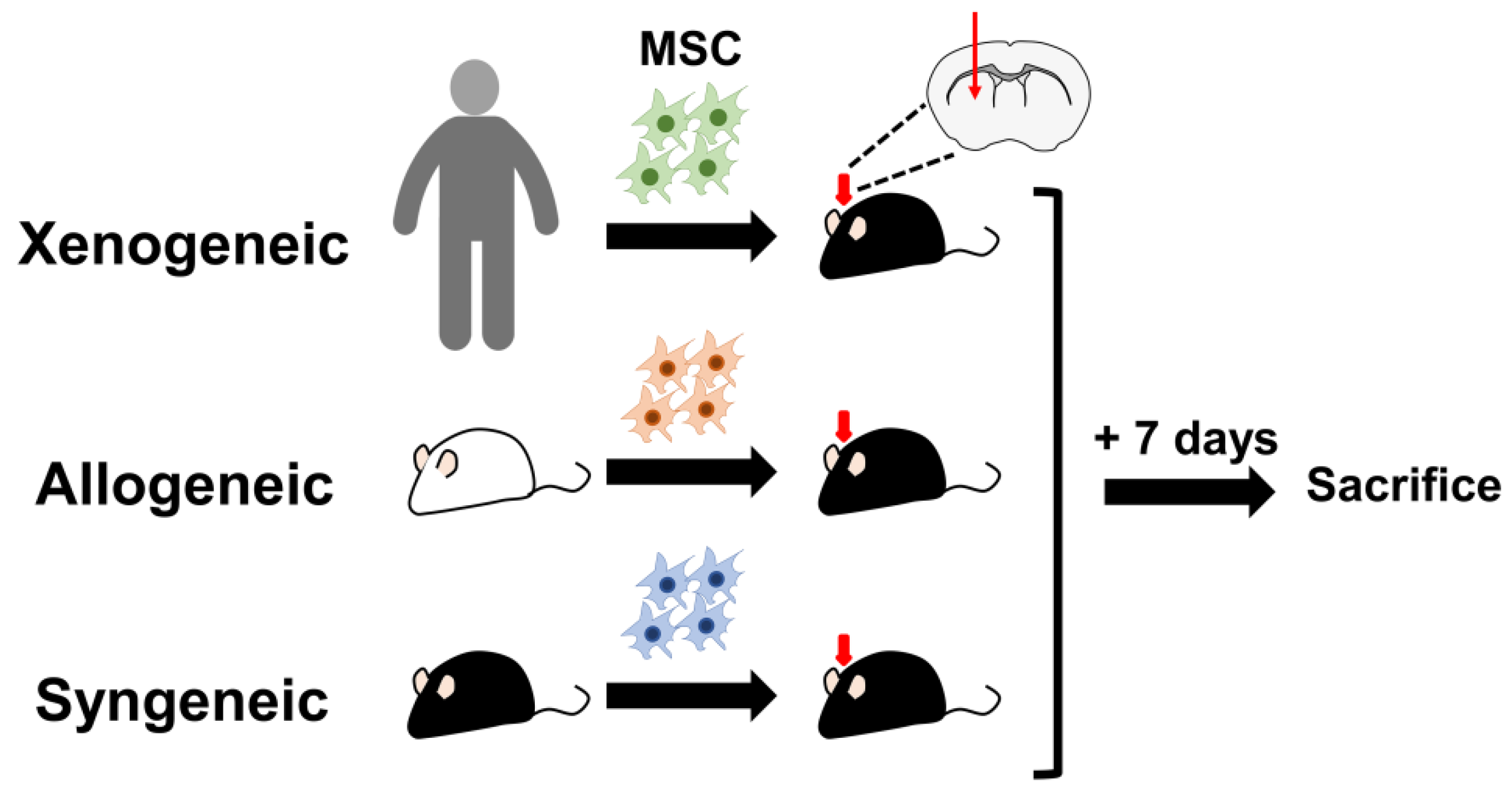
2.2. Successful Intra-Parenchymal Transplantation of Characterized MSCs
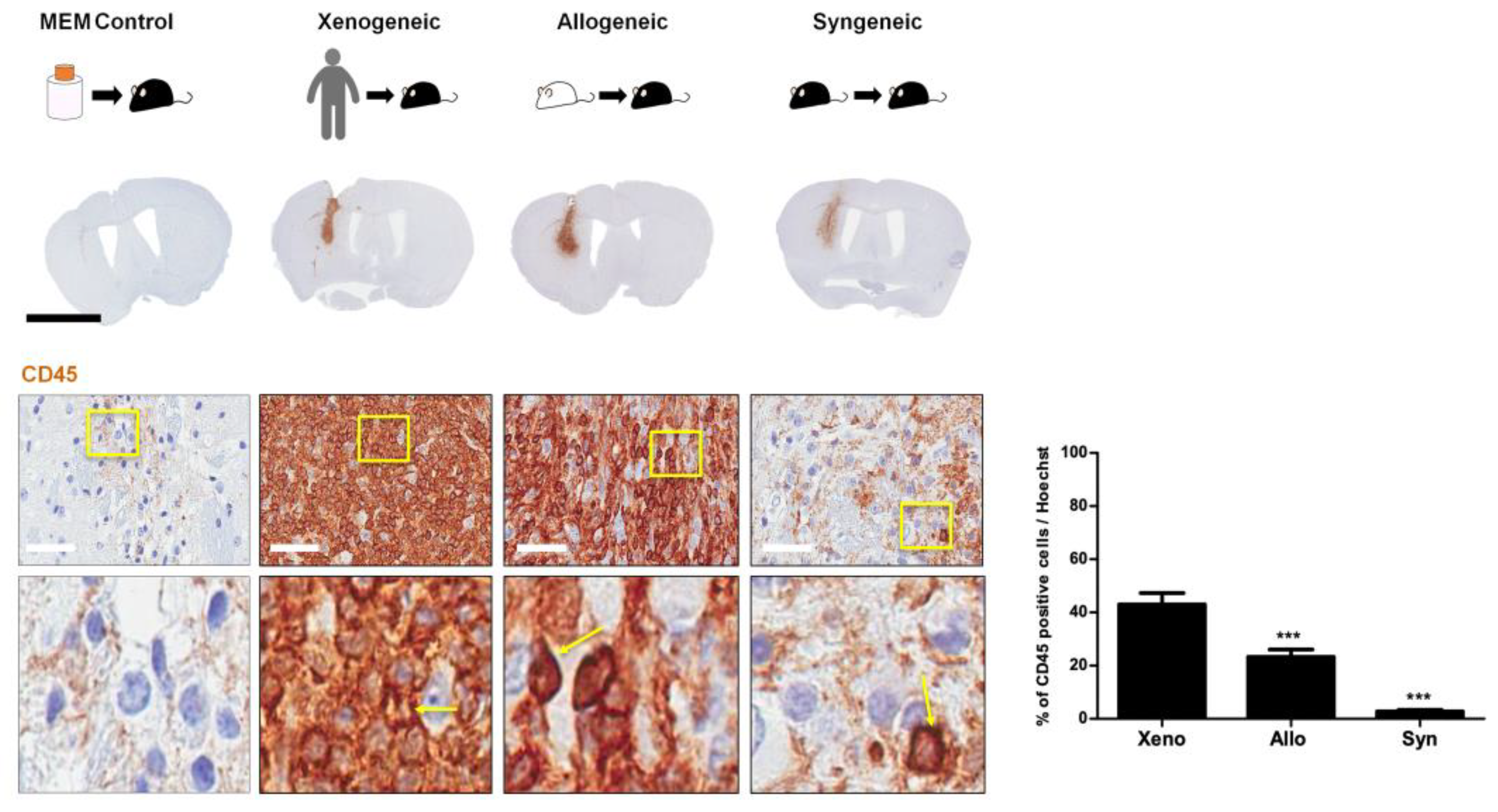
2.3. Infiltration of CD45 Positive Leukocytes Was Highest in the Xenogeneic and Lowest in the Syngeneic Group
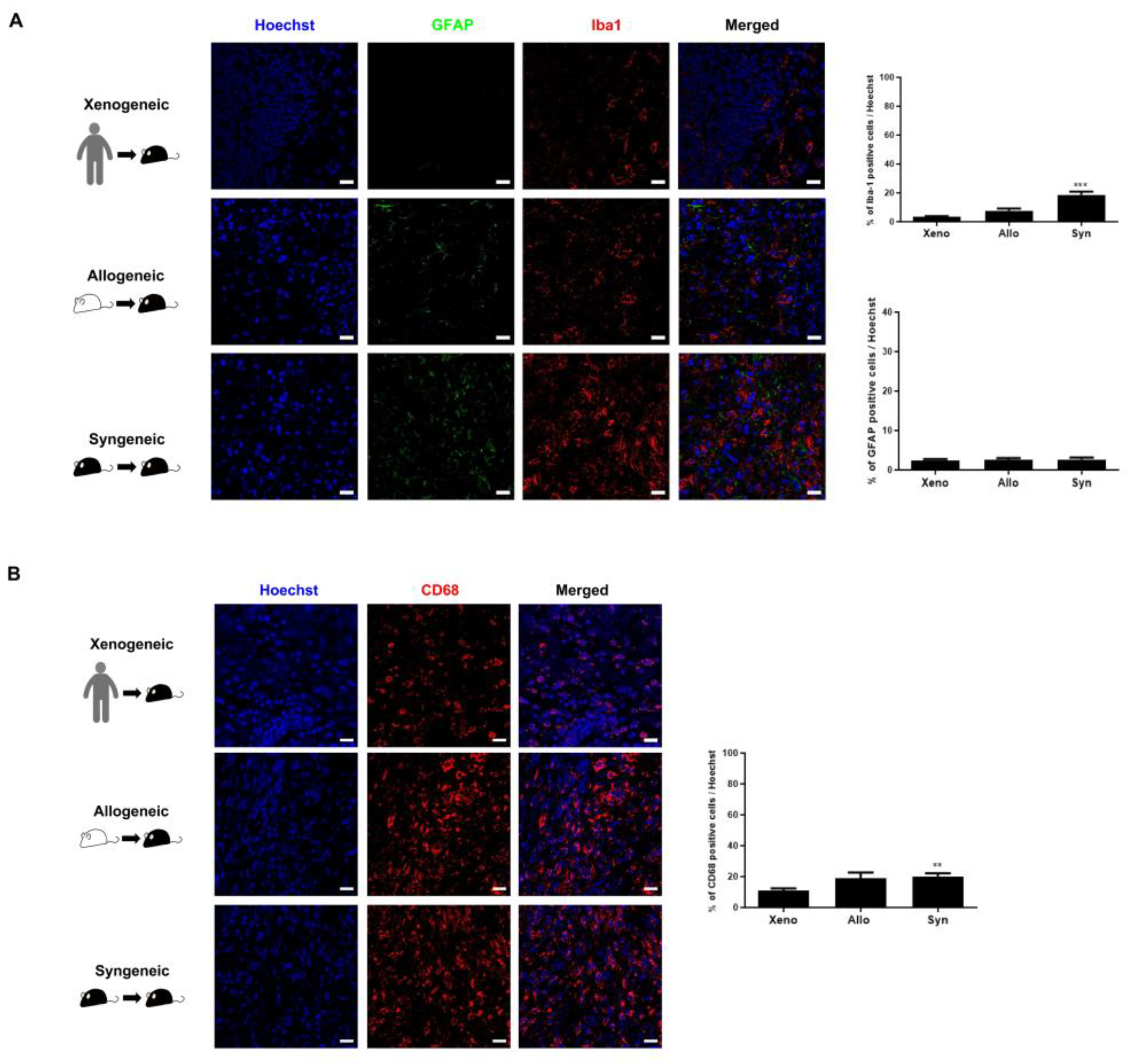
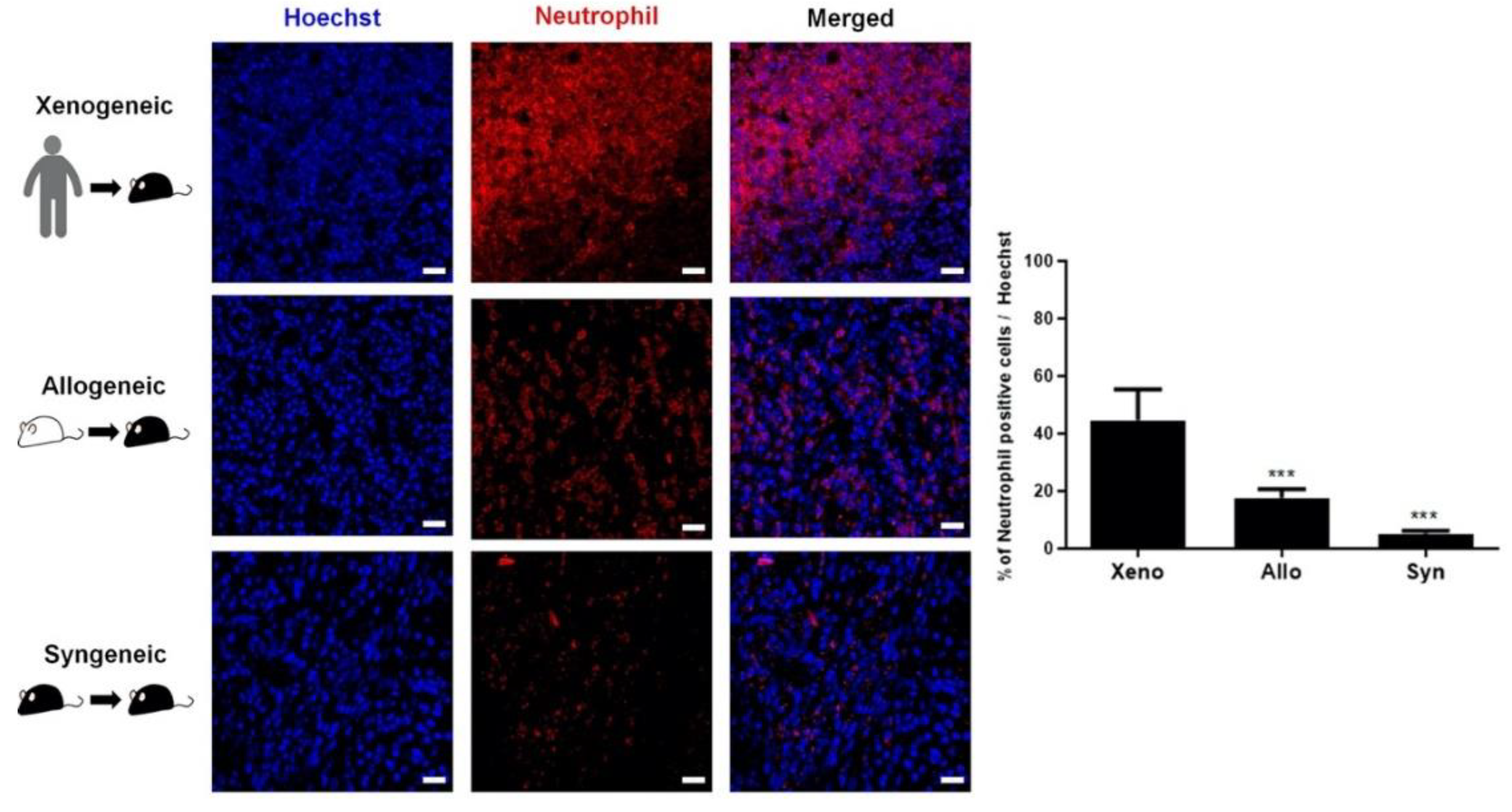
2.4. Recruitment of Other Inflammatory and Immune Cells to the Injection Site Was Identified
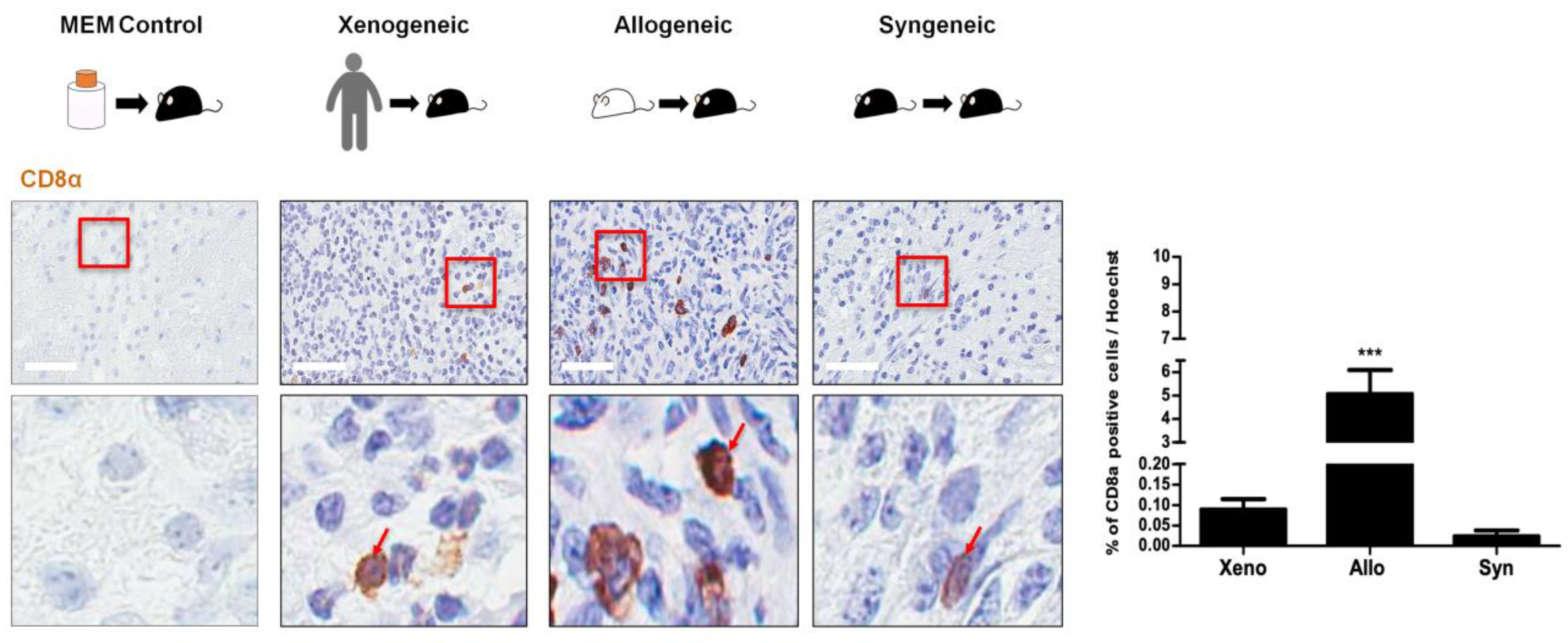
2.5. CD8 T Cell Expression Was Relatively Low for All Three Groups
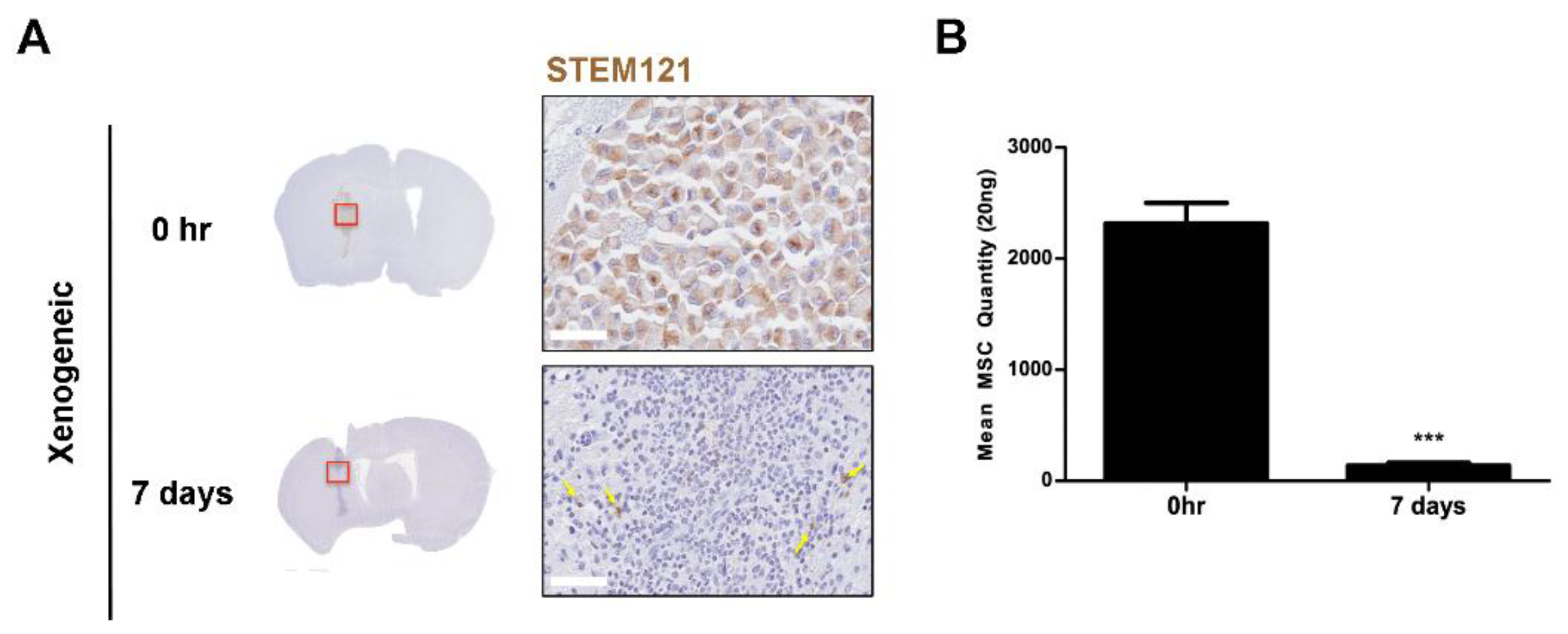
2.6. Xenogeneic MSC Persistence Was Significantly Reduced After One Week
3. Discussion
4. Materials and Methods
4.1. Ethical Statement
4.2. Culture and Preparation of Mesenchymal Stem Cells
4.3. In Vitro MSC Characterization
4.4. Experimental Animals
4.5. Transplantation of Mesenchymal Stem Cells into the Mouse Parenchyma
4.6. Histological Analysis and Quantification
4.7. Human Alu PCR Quantification of Residual Human MSCs in the Mouse Brain
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Lin, C.S.; Lin, G.; Lue, T.F. Allogeneic and xenogeneic transplantation of adipose-derived stem cells in immunocompetent recipients without immunosuppressants. Stem Cells Dev. 2012, 21, 2770–2778. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Yang, X.; Han, Z.P.; Qu, F.F.; Shao, L.; Shi, Y.F. Mesenchymal stem cells: A new trend for cell therapy. Acta Pharmacol. Sin. 2013, 34, 747–754. [Google Scholar] [CrossRef] [PubMed]
- Salem, H.K.; Thiemermann, C. Mesenchymal stromal cells: Current understanding and clinical status. Stem Cells 2010, 28, 585–596. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Seo, S.W.; Chang, J.W.; Lee, J.I.; Kim, C.H.; Chin, J.; Choi, S.J.; Kwon, H.; Yun, H.J.; Lee, J.M.; et al. Stereotactic brain injection of human umbilical cord blood mesenchymal stem cells in patients with Alzheimer’s disease dementia: A phase 1 clinical trial. Alzheimers Dement. 2015, 1, 95–102. [Google Scholar] [CrossRef]
- Lee, N.K.; Na, D.L.; Chang, J.W. Killing two birds with one stone: The multifunctional roles of mesenchymal stem cells in the treatment of neurodegenerative and muscle diseases. Histol. Histopathol. 2018, 33, 629–638. [Google Scholar]
- Eggenhofer, E.; Luk, F.; Dahlke, M.H.; Hoogduijn, M.J. The life and fate of mesenchymal stem cells. Front. Immunol. 2014, 5, 148. [Google Scholar] [CrossRef] [PubMed]
- Levy, O.; Zhao, W.; Mortensen, L.J.; Leblanc, S.; Tsang, K.; Fu, M.; Phillips, J.A.; Sagar, V.; Anandakumaran, P.; Ngai, J.; et al. mRNA-engineered mesenchymal stem cells for targeted delivery of interleukin-10 to sites of inflammation. Blood 2013, 122, e23–e32. [Google Scholar] [CrossRef]
- Mancuso, P.; Raman, S.; Glynn, A.; Barry, F.; Murphy, J.M. Mesenchymal Stem Cell Therapy for Osteoarthritis: The Critical Role of the Cell Secretome. Front. Bioeng. Biotechnol. 2019, 7, 9. [Google Scholar] [CrossRef] [PubMed]
- Fitzsimmons, R.E.B.; Mazurek, M.S.; Soos, A.; Simmons, C.A. Mesenchymal Stromal/Stem Cells in Regenerative Medicine and Tissue Engineering. Stem Cells Int. 2018, 2018, 8031718. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kim, D.; Kim, J.; Lee, D.; Jeon, H.; Kwon, S.; Kim, S.; Yoo, Y.; Lee, E.; Choi, S.J.C. Soluble intracellular adhesion molecule-1 secreted by human umbilical cord blood-derived mesenchymal stem cell reduces amyloid-β plaques. Cell Death Differ. 2012, 19, 680. [Google Scholar] [CrossRef] [PubMed]
- Atoui, R.; Chiu, R.C. Immune responses after mesenchymal stem cell implantation. Methods Mol. Biol. 2013, 1036, 107–120. [Google Scholar]
- Gao, F.; Chiu, S.; Motan, D.; Zhang, Z.; Chen, L.; Ji, H.; Tse, H.; Fu, Q.L.; Lian, Q.J.C. Mesenchymal stem cells and immunomodulation: Current status and future prospects. Cell Death Dis. 2016, 7, e2062. [Google Scholar] [CrossRef] [PubMed]
- Le Blanc, K.; Mougiakakos, D.J.N.R.I. Multipotent mesenchymal stromal cells and the innate immune system. Nat. Rev. Immunol. 2012, 12, 383. [Google Scholar] [CrossRef]
- Yanez, R.; Lamana, M.L.; García-Castro, J.; Colmenero, I.; Ramírez, M.; Bueren, J.A.J.S. Adipose tissue-derived mesenchymal stem cells have in vivo immunosuppressive properties applicable for the control of the graft-versus-host disease. Stem Cells 2006, 24, 2582–2591. [Google Scholar] [CrossRef]
- Ankrum, J.A.; Ong, J.F.; Karp, J.M. Mesenchymal stem cells: Immune evasive, not immune privileged. Nat. Biotechnol. 2014, 32, 252–260. [Google Scholar] [CrossRef] [PubMed]
- Berglund, A.K.; Fortier, L.A.; Antczak, D.F.; Schnabel, L.V. Immunoprivileged no more: Measuring the immunogenicity of allogeneic adult mesenchymal stem cells. Stem Cell Res. Ther. 2017, 8, 288. [Google Scholar] [CrossRef]
- Grinnemo, K.H.; Mansson, A.; Dellgren, G.; Klingberg, D.; Wardell, E.; Drvota, V.; Tammik, C.; Holgersson, J.; Ringden, O.; Sylven, C.; et al. Xenoreactivity and engraftment of human mesenchymal stem cells transplanted into infarcted rat myocardium. J. Thorac. Cardiovasc. Surg. 2004, 127, 1293–1300. [Google Scholar] [CrossRef]
- Jungwirth, N.; Salinas Tejedor, L.; Jin, W.; Gudi, V.; Skripuletz, T.; Stein, V.M.; Tipold, A.; Hoffmann, A.; Stangel, M.; Baumgartner, W.; et al. Mesenchymal Stem Cells Form 3D Clusters Following Intraventricular Transplantation. J. Mol. Neurosci. 2018, 65, 60–73. [Google Scholar] [CrossRef] [PubMed]
- Eliopoulos, N.; Stagg, J.; Lejeune, L.; Pommey, S.; Galipeau, J. Allogeneic marrow stromal cells are immune rejected by MHC class I- and class II-mismatched recipient mice. Blood 2005, 106, 4057–4065. [Google Scholar] [CrossRef] [PubMed]
- Zangi, L.; Margalit, R.; Reich-Zeliger, S.; Bachar-Lustig, E.; Beilhack, A.; Negrin, R.; Reisner, Y. Direct imaging of immune rejection and memory induction by allogeneic mesenchymal stromal cells. Stem Cells 2009, 27, 2865–2874. [Google Scholar] [CrossRef]
- Ryan, J.M.; Barry, F.P.; Murphy, J.M.; Mahon, B.P. Mesenchymal stem cells avoid allogeneic rejection. J. Inflamm. 2005, 2, 8. [Google Scholar] [CrossRef]
- Medawar, P.B. Immunity to homologous grafted skin; the fate of skin homografts transplanted to the brain, to subcutaneous tissue, and to the anterior chamber of the eye. Br. J. Exp. Pathol. 1948, 29, 58–69. [Google Scholar] [PubMed]
- Aron Badin, R.; Vadori, M.; Vanhove, B.; Nerriere-Daguin, V.; Naveilhan, P.; Neveu, I.; Jan, C.; Leveque, X.; Venturi, E.; Mermillod, P.; et al. Cell Therapy for Parkinson’s Disease: A Translational Approach to Assess the Role of Local and Systemic Immunosuppression. Am. J. Transplant. 2016, 16, 2016–2029. [Google Scholar] [CrossRef]
- Candolfi, M.; Curtin, J.F.; Nichols, W.S.; Muhammad, A.G.; King, G.D.; Pluhar, G.E.; McNiel, E.A.; Ohlfest, J.R.; Freese, A.B.; Moore, P.F.; et al. Intracranial glioblastoma models in preclinical neuro-oncology: Neuropathological characterization and tumor progression. J. Neurooncol. 2007, 85, 133–148. [Google Scholar] [CrossRef]
- Leveque, X.; Nerriere-Daguin, V.; Neveu, I.; Naveilhan, P. Pig neural cells derived from foetal mesencephalon as cell source for intracerebral xenotransplantation. Methods Mol. Biol. 2012, 885, 233–243. [Google Scholar]
- Irons, H.; Lind, J.G.; Wakade, C.G.; Yu, G.; Hadman, M.; Carroll, J.; Hess, D.C.; Borlongan, C.V. Intracerebral xenotransplantation of GFP mouse bone marrow stromal cells in intact and stroke rat brain: Graft survival and immunologic response. Cell Transplant. 2004, 13, 283–294. [Google Scholar] [CrossRef]
- Yang, S.; Ha, C.; Jung, M.; Jin, H.; Lee, M.; Song, H.; Choi, S.; Oh, W.; Yang, Y.J.C. Mesenchymal stem/progenitor cells developed in cultures from UC blood. Cytotherapy 2004, 6, 476–486. [Google Scholar] [CrossRef]
- Hanna, H.; Mir, L.M.; Andre, F.M.J.S. In vitro osteoblastic differentiation of mesenchymal stem cells generates cell layers with distinct properties. Stem Cell Res. Ther. 2018, 9, 203. [Google Scholar] [CrossRef]
- Kobayashi, S.D.; DeLeo, F.R. Role of neutrophils in innate immunity: A systems biology-level approach. Wiley Interdiscip. Rev. Syst. Biol. Med. 2009, 1, 309–333. [Google Scholar] [CrossRef]
- Dileepan, T.; Smith, E.D.; Knowland, D.; Hsu, M.; Platt, M.; Bittner-Eddy, P.; Cohen, B.; Southern, P.; Latimer, E.; Harley, E.; et al. Group A Streptococcus intranasal infection promotes CNS infiltration by streptococcal-specific Th17 cells. J. Clin. Investig. 2016, 126, 303–317. [Google Scholar] [CrossRef]
- Louveau, A.; Harris, T.H.; Kipnis, J. Revisiting the Mechanisms of CNS Immune Privilege. Trends Immunol. 2015, 36, 569–577. [Google Scholar] [CrossRef]
- Chan, Y.K.; Tsai, M.H.; Huang, D.C.; Zheng, Z.H.; Hung, K.D. Leukocyte nucleus segmentation and nucleus lobe counting. BMC Bioinform. 2010, 11, 558. [Google Scholar] [CrossRef]
- Le Blon, D.; Hoornaert, C.; Detrez, J.R.; Bevers, S.; Daans, J.; Goossens, H.; De Vos, W.H.; Berneman, Z.; Ponsaerts, P. Immune remodelling of stromal cell grafts in the central nervous system: Therapeutic inflammation or (harmless) side-effect? J. Tissue Eng. Regen. Med. 2017, 11, 2846–2852. [Google Scholar] [CrossRef]
- Ma, N.; Streilein, J.W. Contribution of microglia as passenger leukocytes to the fate of intraocular neuronal retinal grafts. Investig. Ophthalmol. Vis. Sci. 1998, 39, 2384–2393. [Google Scholar]
- De Witte, S.F.H.; Luk, F.; Sierra Parraga, J.M.; Gargesha, M.; Merino, A.; Korevaar, S.S.; Shankar, A.S.; O’Flynn, L.; Elliman, S.J.; Roy, D.; et al. Immunomodulation By Therapeutic Mesenchymal Stromal Cells (MSC) Is Triggered Through Phagocytosis of MSC By Monocytic Cells. Stem Cells 2018, 36, 602–615. [Google Scholar] [CrossRef]
- Ma, N.; Cheng, H.; Lu, M.; Liu, Q.; Chen, X.; Yin, G.; Zhu, H.; Zhang, L.; Meng, X.; Tang, Y.; et al. Magnetic resonance imaging with superparamagnetic iron oxide fails to track the long-term fate of mesenchymal stem cells transplanted into heart. Sci. Rep. 2015, 5, 9058. [Google Scholar] [CrossRef]
- Friedman, T.; Shimizu, A.; Smith, R.N.; Colvin, R.B.; Seebach, J.D.; Sachs, D.H.; Iacomini, J. Human CD4+ T cells mediate rejection of porcine xenografts. J. Immunol. 1999, 162, 5256–5262. [Google Scholar]
- Hoornaert, C.J.; Le Blon, D.; Quarta, A.; Daans, J.; Goossens, H.; Berneman, Z.; Ponsaerts, P. Concise Review: Innate and Adaptive Immune Recognition of Allogeneic and Xenogeneic Cell Transplants in the Central Nervous System. Stem Cells Transl. Med. 2017, 6, 1434–1441. [Google Scholar] [CrossRef]
- Machado Cde, V.; Telles, P.D.; Nascimento, I.L. Immunological characteristics of mesenchymal stem cells. Rev. Bras. Hematol. Hemoter. 2013, 35, 62–67. [Google Scholar] [CrossRef]
- Hoogduijn, M.J.; Lombardo, E. Concise Review: Mesenchymal Stromal Cells Anno 2019: Dawn of the Therapeutic Era? Stem Cells Transl. Med. 2019. [Google Scholar] [CrossRef]
- Lohan, P.; Treacy, O.; Griffin, M.D.; Ritter, T.; Ryan, A.E. Anti-Donor Immune Responses Elicited by Allogeneic Mesenchymal Stem Cells and Their Extracellular Vesicles: Are We Still Learning? Front. Immunol. 2017, 8, 1626. [Google Scholar] [CrossRef]
- Crop, M.J.; Korevaar, S.S.; De Kuiper, R.; JN, I.J.; Van Besouw, N.M.; Baan, C.C.; Weimar, W.; Hoogduijn, M.J. Human mesenchymal stem cells are susceptible to lysis by CD8(+) T cells and NK cells. Cell Transplant. 2011, 20, 1547–1559. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.H.; Park, C.G. Allogeneic ADSCs induce CD8 T cell-mediated cytotoxicity and faster cell death after exposure to xenogeneic serum or proinflammatory cytokines. Exp. Mol. Med. 2019, 51, 28. [Google Scholar] [CrossRef]
- Toma, C.; Wagner, W.R.; Bowry, S.; Schwartz, A.; Villanueva, F. Fate of culture-expanded mesenchymal stem cells in the microvasculature: In vivo observations of cell kinetics. Circ. Res. 2009, 104, 398–402. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.H.; Lee, Y.J.; Kang, J.H. Stem Cell Monitoring with a Direct or Indirect Labeling Method. Nucl. Med. Mol. Imaging 2016, 50, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Azevedo, P.O.; Sena, I.F.G.; Andreotti, J.P.; Carvalho-Tavares, J.; Alves-Filho, J.C.; Cunha, T.M.; Cunha, F.Q.; Mintz, A.; Birbrair, A. Pericytes modulate myelination in the central nervous system. J. Cell. Physiol. 2018, 233, 5523–5529. [Google Scholar] [CrossRef] [PubMed]
- Balabanov, R.; Beaumont, T.; Dore-Duffy, P. Role of central nervous system microvascular pericytes in activation of antigen-primed splenic T-lymphocytes. J. Neurosci. Res. 1999, 55, 578–587. [Google Scholar] [CrossRef]
- Asada, N.; Kunisaki, Y.; Pierce, H.; Wang, Z.; Fernandez, N.F.; Birbrair, A.; Ma’ayan, A.; Frenette, P.S. Differential cytokine contributions of perivascular haematopoietic stem cell niches. Nat. Cell Biol. 2017, 19, 214–223. [Google Scholar] [CrossRef]
- Xu, J.; Gong, T.; Heng, B.C.; Zhang, C.F. A systematic review: Differentiation of stem cells into functional pericytes. FASEB J. 2017, 31, 1775–1786. [Google Scholar] [CrossRef]
- Navarro, R.; Compte, M.; Álvarez-Vallina, L.; Sanz, L. Immune Regulation by Pericytes: Modulating Innate and Adaptive Immunity. Front. Immunol. 2016, 7, 480. [Google Scholar] [CrossRef]
- Jansen Of Lorkeers, S.J.; Eding, J.E.; Vesterinen, H.M.; van der Spoel, T.I.; Sena, E.S.; Duckers, H.J.; Doevendans, P.A.; Macleod, M.R.; Chamuleau, S.A. Similar effect of autologous and allogeneic cell therapy for ischemic heart disease: Systematic review and meta-analysis of large animal studies. Circ. Res. 2015, 116, 80–86. [Google Scholar] [CrossRef]
- Zhang, J.; Huang, X.; Wang, H.; Liu, X.; Zhang, T.; Wang, Y.; Hu, D. The challenges and promises of allogeneic mesenchymal stem cells for use as a cell-based therapy. Stem Cell Res. Ther. 2015, 6, 234. [Google Scholar] [CrossRef] [PubMed]
- Gibney, E.R.; Nolan, C.M. Epigenetics and gene expression. Heredity 2010, 105, 4–13. [Google Scholar] [CrossRef] [PubMed]
- Agodi, A.; Barchitta, M.; Maugeri, A.; Basile, G.; Zamboni, M.; Bernardini, G.; Corona, D.; Veroux, M. Unveiling the Role of DNA Methylation in Kidney Transplantation: Novel Perspectives toward Biomarker Identification. Biomed. Res. Int. 2019, 2019, 1602539. [Google Scholar] [CrossRef]
- Potaczek, D.P.; Harb, H.; Michel, S.; Alhamwe, B.A.; Renz, H.; Tost, J. Epigenetics and allergy: From basic mechanisms to clinical applications. Epigenomics 2017, 9, 539–571. [Google Scholar] [CrossRef]
- Perez-Campo, F.M.; Riancho, J.A. Epigenetic Mechanisms Regulating Mesenchymal Stem Cell Differentiation. Curr. Genom. 2015, 16, 368–383. [Google Scholar] [CrossRef][Green Version]
- Xu, L.; Liu, Y.; Sun, Y.; Wang, B.; Xiong, Y.; Lin, W.; Wei, Q.; Wang, H.; He, W.; Wang, B.; et al. Tissue source determines the differentiation potentials of mesenchymal stem cells: A comparative study of human mesenchymal stem cells from bone marrow and adipose tissue. Stem Cell Res. Ther. 2017, 8, 275. [Google Scholar] [CrossRef]
- McCaughan, J.A.; McKnight, A.J.; Courtney, A.E.; Maxwell, A.P. Epigenetics: Time to translate into transplantation. Transplantation 2012, 94, 1–7. [Google Scholar] [CrossRef]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.; Horwitz, E.J.C. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef]
- Funakoshi, K.; Bagheri, M.; Zhou, M.; Suzuki, R.; Abe, H.; Akashi, H.J.S. Highly sensitive and specific Alu-based quantification of human cells among rodent cells. Sci. Rep. 2017, 7, 13202. [Google Scholar] [CrossRef]
- Kim, H.S.; Lee, N.K.; Yoo, D.; Lee, J.; Choi, S.J.; Oh, W.; Chang, J.W.; Na, D.L.J.B. Lowering the concentration affects the migration and viability of intracerebroventricular-delivered human mesenchymal stem cells. Biochem. Biophys. Res. Commun. 2017, 493, 751–757. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hwang, J.W.; Lee, N.K.; Yang, J.H.; Son, H.J.; Bang, S.I.; Chang, J.W.; Na, D.L. A Comparison of Immune Responses Exerted Following Syngeneic, Allogeneic, and Xenogeneic Transplantation of Mesenchymal Stem Cells into the Mouse Brain. Int. J. Mol. Sci. 2020, 21, 3052. https://doi.org/10.3390/ijms21093052
Hwang JW, Lee NK, Yang JH, Son HJ, Bang SI, Chang JW, Na DL. A Comparison of Immune Responses Exerted Following Syngeneic, Allogeneic, and Xenogeneic Transplantation of Mesenchymal Stem Cells into the Mouse Brain. International Journal of Molecular Sciences. 2020; 21(9):3052. https://doi.org/10.3390/ijms21093052
Chicago/Turabian StyleHwang, Jung Won, Na Kyung Lee, Je Hoon Yang, Hyo Jin Son, Sa Ik Bang, Jong Wook Chang, and Duk L. Na. 2020. "A Comparison of Immune Responses Exerted Following Syngeneic, Allogeneic, and Xenogeneic Transplantation of Mesenchymal Stem Cells into the Mouse Brain" International Journal of Molecular Sciences 21, no. 9: 3052. https://doi.org/10.3390/ijms21093052
APA StyleHwang, J. W., Lee, N. K., Yang, J. H., Son, H. J., Bang, S. I., Chang, J. W., & Na, D. L. (2020). A Comparison of Immune Responses Exerted Following Syngeneic, Allogeneic, and Xenogeneic Transplantation of Mesenchymal Stem Cells into the Mouse Brain. International Journal of Molecular Sciences, 21(9), 3052. https://doi.org/10.3390/ijms21093052




