Can Endothelial Glycocalyx Be a Major Morphological Substrate in Pre-Eclampsia?
Abstract
1. Background
| Function | Intact eGC | Damaged eGC |
|---|---|---|
| Regulation of mechanosensitivity of endothelial cells | Acts as a mechanotransducer, transmitting shear stress forces to endothelial cells. It accepts and dissipates the load caused by shear stress. The load is transferred to the side chains of proteoglycans, which transmit the torque to the core proteins and into the cell, activating the signal cascade reactions and the actin cytoskeleton. Stimulates the endothelial NO synthase, which regulates formation of endogenic nitric oxide synthase (eNOs)—the factor of vessels relaxation and cytoskeletal reorganization [20,21,22,23]. | Decreases mechanosensitivity of endothelial cells. Main load of fluid shear stress affects the apical membrane of endothelial cells. Blocked shear-induced NO production, disruption of vascular tone regulation, deficient vasodilatation [15,23,24]. |
| Regulation of vascular permeability | It has a structure of selective molecular sieve, with the filtration ability depending on the molecule size and charge. Facilitates permeability of low-molecular compositions. It is selectively permeable for macromolecules and performs barrier function [23,25,26]. | Removal of key structural components of eGC leads to structural damage and increase of vessel permeability for high molecular plasma proteins (albumin) and development of tissue edema and loss of barrier function [27,28]. |
| Regulation of interactions of blood cells with the vascular wall | Vascular protection via the inhibition of coagulation, leukocyte adhesion, and production and accumulation of active forms of oxygen. Outer layer of eGC bordering with blood:
| Lacking or weak vascular protection. Shedding and destruction of the outer layer leads to:
|
2. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ADMA | asymmetric dimethylarginine |
| AECA | anti-endothelial cell antibodies |
| APS | antiphospholipid antibodies |
| ASA | aspirin, acetylsalicylic acid |
| CAMs | cell adhesion molecules |
| CNS | central nervous system |
| COX | cyclooxygenase |
| CSPGs | chondroitin sulfate proteoglycans |
| DAMPs | damage-associated molecular patterns |
| DIC | disseminated intravascular coagulation |
| DSA-FACE | DNA sequencer-assisted fluorophore assisted carbohydrate electrophoresis |
| eGC | endothelial glycocalyx |
| eNOs | endothelial nitric oxide synthase |
| ec-SOD | extracellular superoxide dismutase |
| ELISA | enzyme-linked immunosorbent assay |
| EO-PE | early-onset pre-eclampsia |
| FFP | fresh frozen plasma |
| GAGs | glycosaminoglycans |
| GC | glycocalyx |
| GMB | glomerular basement membrane |
| HA | hyaruronan, hyaluronic acid |
| HELLP | Hemolysis, Elevated Liver enzymes and Low Platelet count |
| HO-1 | haem oxygenase 1 |
| HS | heparan sulfate |
| HSPGs | heparan sulfate proteoglycans |
| HUVECs | human umbilical vein endothelial cells |
| LMWH | low-molecular-weight heparin |
| LO-PE | late-onset pre-eclampsia |
| MMPs | matrix metalloproteinases |
| NO | nitric oxide |
| PE | pre-eclampsia |
| PMPs | platelet microparticles |
| qRT-PCR | quantitative real-time polymerase chain reaction |
| RBCs | red blood cells |
| SIR | systemic inflammatory response |
| S1P | sphingosine-1-phosphate |
| S1P1 | sphingosine-1-phosphate receptor 1 |
| sdc1 | syndecan 1 |
| UFH | unfractionated heparin |
References
- Valdiviezo, C.; Garovic, V.D.; Ouyang, P. Preeclampsia and hypertensive disease in pregnancy: Their contributions to cardiovascular risk. Clin. Cardiol. 2012, 35, 160–165. [Google Scholar] [CrossRef] [PubMed]
- ACOG. Practice Bulletin No. 202: Gestational Hypertension and Preeclampsia. Obstet. Gynecol. 2019, 133, e1–e25. [Google Scholar] [CrossRef] [PubMed]
- Magee, L.A.; Pels, A.; Helewa, M.; Rey, E.; Von Dadelszen, P. Diagnosis, evaluation, and management of the hypertensive disorders of pregnancy: Executive summary. J. Obstet. Gynaecol. Can. 2014, 36, 575–576. [Google Scholar] [CrossRef]
- Sheppard, S.J.; Khalil, R.A. Risk factors and mediators of the vascular dysfunction associated with hypertension in pregnancy. Cardiovasc. Hematol. Disord. Drug Targets 2010, 10, 33–52. [Google Scholar] [CrossRef]
- Van Aken, B.E.; Reitsma, P.H.; Rosendaal, F.R. Interleukin 8 and venous thrombosis: Evidence for a role of inflammation in thrombosis. Br. J. Haematol. 2002, 116, 173–177. [Google Scholar] [CrossRef]
- Laresgoiti-Servitje, E. A leading role for the immune system in the pathophysiology of preeclampsia. J. Leukoc. Biol. 2013, 94, 247–257. [Google Scholar] [CrossRef]
- Perucci, L.O.; Corrêa, M.D.; Dusse, L.M.; Gomes, K.B.; Sousa, L.P. Resolution of inflammation pathways in preeclampsia-a narrative review. Immunol. Res. 2017, 65, 774–789. [Google Scholar] [CrossRef]
- Aird, W.C. Endothelium in health and disease. Pharmacol. Rep. 2008, 60, 139–143. [Google Scholar]
- Gathiram, P.; Moodley, J. Pre-eclampsia: Its pathogenesis and pathophysiolgy. Cardiovasc. J. Afr. 2016, 27, 71–78. [Google Scholar] [CrossRef]
- Szpera-Gozdziewicz, A.; Breborowicz, G.H. Endothelial dysfunction in the pathogenesis of pre-eclampsia. Front. Biosci. 2014, 19, 734–746. [Google Scholar] [CrossRef]
- Scott, D.W.; Patel, R.P. Endothelial heterogeneity and adhesion molecules N-glycosylation: Implications in leukocyte trafficking in inflammation. Glycobiology 2013, 23, 622–633. [Google Scholar] [CrossRef]
- Pillinger, N.L.; Kam, P. Endothelial glycocalyx: Basic science and clinical implications. Anaesth. Intensive Care 2017, 45, 295–307. [Google Scholar] [CrossRef] [PubMed]
- Firth, J.A. Endothelial barriers: From hypothetical pores to membrane proteins. J. Anat. 2002, 200, 541–548. [Google Scholar] [CrossRef] [PubMed]
- Rapoport, E.M.; Andre, S.; Matveeva, V.K.; Vokhmyanina, O.A.; Gabius, H.-J.; Bovin, N.V. Localization of galectins within glycocalyx. Biochemistry 2018, 83, 727–737. [Google Scholar] [CrossRef] [PubMed]
- Tarbell, J.M.; Ebong, E.E. The endothelial glycocalyx: A mechano-sensor and -transducer. Sci. Signal. 2008, 1, pt8. [Google Scholar] [CrossRef] [PubMed]
- Tarbell, J.M.; Cancel, L.M. The glycocalyx and its significance in human medicine. J. Intern. Med. 2016, 280, 97–113. [Google Scholar] [CrossRef]
- Kundra, P.; Goswami, S. Endothelial glycocalyx: Role in body fluid homeostasis and fluid management. Indian J. Anaesth. 2019, 63, 6–14. [Google Scholar] [CrossRef]
- Ando, J.; Yamamoto, K. Flow detection and calcium signalling in vascular endothelial cells. Cardiovasc. Res. 2013, 99, 260–268. [Google Scholar] [CrossRef]
- Fabre-Gray, A.C.M.; Down, C.J.; Neal, C.R.; Foster, R.R.; Satchell, S.C.; Bills, V.L. Imaging the placental glycocalyx with transmission electron microscopy. Placenta 2018, 74, 59–61. [Google Scholar] [CrossRef] [PubMed]
- Fu, B.M.; Tarbell, J.M. Mechano-sensing and transduction by endothelial surface glycocalyx: Composition, structure, and function. Wiley Interdiscip. Rev. Syst. Biol. Med. 2013, 5, 381–390. [Google Scholar] [CrossRef]
- Tarbell, J.M.; Simon, S.I.; Curry, F.R. Mechanosensing at the vascular interface. Annu. Rev. Biomed. Eng. 2014, 16, 505–532. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Zhang, X.F.; Fu, B.M.; Tarbell, J.M. The Role of Endothelial Surface Glycocalyx in Mechanosensing and Transduction. Adv. Exp. Med. Biol. 2018, 1097, 1–27. [Google Scholar] [PubMed]
- Tarbell, J.M.; Pahakis, M.Y. Mechanotransduction and the glycocalyx. J. Intern. Med. 2006, 259, 339–350. [Google Scholar] [CrossRef] [PubMed]
- Pahakis, M.Y.; Kosky, J.R.; Dull, R.O.; Tarbell, J.M. The role of endothelial glycocalyx components in mechanotransduction of fluid shear stress. Biochem. Biophys. Res. Commun. 2007, 355, 228–233. [Google Scholar] [CrossRef] [PubMed]
- Alphonsus, C.S.; Rodseth, R.N. The endothelial glycocalyx: A review of the vascular barrier. Anaesthesia 2014, 69, 777–784. [Google Scholar] [CrossRef] [PubMed]
- Butler, M.J.; Down, C.J.; Foster, R.R.; Satchell, S.C. The pathological relevance of increased endothelial glycocalyx permeability. Am. J. Pathol. 2020, 190, 742–751. [Google Scholar] [CrossRef]
- Salmon, A.H.; Ferguson, J.K.; Burford, J.L.; Gevorgyan, H.; Nakano, D.; Harper, S.J.; Bates, D.O.; Peti-Peterdi, J. Loss of the endothelial glycocalyx links albuminuria and vascular dysfunction. J. Am. Soc. Nephrol. 2012, 23, 1339–1350. [Google Scholar] [CrossRef]
- Ushiyama, A.; Kataoka, H.; Iijima, T. Glycocalyx and its involvement in clinical pathophysiologies. J. Intensive Care 2016, 4, 59. [Google Scholar] [CrossRef]
- Lipowsky, H.H.; Gao, L.; Lescanic, A. Shedding of the endothelial glycocalyx in arterioles, capillaries, and venules and its effect on capillary hemodynamics during inflammation. Am. J. Physiol. Heart Circ. Physiol. 2011, 301, H2235–H2245. [Google Scholar] [CrossRef]
- Lipowsky, H.H. Role of the Glycocalyx as a Barrier to Leukocyte-Endothelium Adhesion. Adv. Exp. Med. Biol. 2018, 1097, 51–68. [Google Scholar]
- Lipowsky, H.H. The endothelial glycocalyx as a barrier to leukocyte adhesion and its mediation by extracellular proteases. Ann. Biomed. Eng. 2012, 40, 840–848. [Google Scholar] [CrossRef] [PubMed]
- Willhauck-Fleckenstein, M.; Moehler, T.M.; Merling, A.; Pusunc, S.; Goldschmidt, H.; Schwartz-Albiez, R. Transcriptional regulation of the vascular endothelial glycome by angiogenic and inflammatory signalling. Angiogenesis 2010, 13, 25–42. [Google Scholar] [CrossRef] [PubMed]
- Wright, R.D.; Cooper, D. Glycobiology of leukocyte trafficking in inflammation. Glycobiology 2014, 24, 1242–1251. [Google Scholar] [CrossRef] [PubMed]
- Kumagai, R.; Lu, X.; Kassab, G.S. Role of glycocalyx in flow-induced production of nitric oxide and reactive oxygen species. Free Radic. Biol. Med. 2009, 47, 600–607. [Google Scholar] [CrossRef]
- Scott, D.W.; Vallejo, M.O.; Patel, R.P. Heterogenic endothelial responses to inflammation: Role for differential N-glycosylation and vascular bed of origin. J. Am. Heart Assoc. 2013, 2, e000263. [Google Scholar] [CrossRef] [PubMed]
- Grundmann, S.; Fink, K.; Rabadzhieva, L.; Bourgeois, N.; Schwab, T.; Moser, M.; Bode, C.; Busch, H.J. Perturbation of the endothelial glycocalyx in post cardiac arrest syndrome. Resuscitation 2012, 83, 715–720. [Google Scholar] [CrossRef]
- Padberg, J.S.; Wiesinger, A.; Di Marco, G.S.; Reuter, S.; Grabner, A.; Kentrup, D.; Lukasz, A.; Oberleithner, H.; Pavenstädt, H.; Brand, M.; et al. Damage of the endothelial glycocalyx in chronic kidney disease. Atherosclerosis 2014, 234, 335–343. [Google Scholar] [CrossRef]
- Kim, Y.H.; Nijst, P.; Kiefer, K.; Tang, W.H. Endothelial Glycocalyx as Biomarker for Cardiovascular Diseases: Mechanistic and Clinical Implications. Curr. Heart Fail Rep. 2017, 14, 117–126. [Google Scholar] [CrossRef]
- Jourde-Chiche, N.; Fakhouri, F.; Dou, L.; Bellien, J.; Burtey, S.; Frimat, M.; Jarrot, P.A.; Kaplanski, G.; Le Quintrec, M.; Pernin, V.; et al. Endothelium structure and function in kidney health and disease. Nat. Rev. Nephrol. 2019, 15, 87–108. [Google Scholar] [CrossRef]
- Ligi, D.; Croce, L.; Mannello, F. Chronic Venous Disorders: The Dangerous, the Good, and the Diverse. Int. J. Mol. Sci. 2018, 19, 2544. [Google Scholar] [CrossRef]
- Uchimido, R.; Schmidt, E.P.; Shapiro, N.I. The glycocalyx: A novel diagnostic and therapeutic target in sepsis. Crit. Care 2019, 23, 16. [Google Scholar] [CrossRef]
- Yuksel, M.A.; Tuten, A.; Oncul, M.; Acikgoz, A.S.; Yuksel, I.T.; Toprak, M.S.; Ekmekci, H.; Ekmekci, O.B.; Madazli, R. Serum endocan concentration in women with pre-eclampsia. Arch. Gynecol. Obstet. 2015, 292, 69–73. [Google Scholar] [CrossRef] [PubMed]
- Chang, X.; Bian, Y.; Wu, Y.; Huang, Y.; Wang, K.; Duan, T. Endocan of the maternal placenta tissue is increased in pre-eclampsia. Int. J. Clin. Exp. Pathol. 2015, 8, 14733–14740. [Google Scholar] [PubMed]
- Khedun, S.M.; Naicker, T.; Moodley, J.; Gathiram, P. Urinary heparin sulfate proteoglycan excretion in black African women with pre-eclampsia. Acta Obstet. Gynecol. Scand. 2002, 81, 308–312. [Google Scholar] [CrossRef] [PubMed]
- Galvis-Ramírez, M.F.; Quintana-Castillo, J.C.; Bueno-Sanchez, J.C. Novel Insights into the Role of Glycans in the Pathophysiology of Glomerular Endotheliosis in Preeclampsia. Front. Physiol. 2018, 9, 1470. [Google Scholar] [CrossRef]
- Berg, S.; Engman, A.; Holmgren, S.; Lundahl, T.; Laurent, T.C. Increased plasma hyaluronan in severe pre-eclampsia and eclampsia. Scand. J. Clin. Lab. Investig. 2001, 61, 131–137. [Google Scholar]
- Marini, M.; Bonaccini, L.; Thyrion, G.D.; Vichi, D.; Parretti, E.; Sgambati, E. Distribution of sugar remains in human placentas from pregnancies complicated by hypertensive disorders. Acta Hstochem. 2011, 113, 815–825. [Google Scholar] [CrossRef]
- Hofmann-Kiefer, K.F.; Knabl, J.; Martinoff, N.; Schiessl, B.; Conzen, P.; Rehm, M.; Becker, B.F.; Chappell, D. Increased serum concentrations of circulating glycocalyx components in HELLP syndrome compared to healthy pregnancy: An observational study. Reprod. Sci. 2013, 20, 318–325. [Google Scholar] [CrossRef]
- Hofmann-Kiefer, K.F.; Chappell, D.; Knabl, J.; Frank, H.G.; Martinoff, N.; Conzen, P.; Becker, B.F.; Rehm, M. Placental syncytiotrophoblast maintains a specific type of glycocalyx at the fetomaternal border: The glycocalyx at the fetomaternal interface in healthy women and patients with HELLP syndrome. Reprod. Sci. 2013, 20, 1237–1245. [Google Scholar] [CrossRef][Green Version]
- Heyer-Chauhan, N.; Ovbude, I.J.; Hills, A.A.; Sullivan, M.H.; Hills, F.A. Placental syndecan-1 and sulphated glycosaminoglycans are decreased in preeclampsia. J. Perinat. Med. 2014, 42, 329–338. [Google Scholar] [CrossRef]
- Romão, M.; Weel, I.C.; Lifshitz, S.J.; Peraçoli, M.T. Elevated hyaluronan and extracellular matrix metalloproteinase inducer levels in women with preeclampsia. Arch. Gynecol. Obstet. 2014, 289, 575–579. [Google Scholar] [CrossRef] [PubMed]
- Alici Davutoğlu, E.; Akkaya Firat, A.; Ozel, A.; Yılmaz, N.; Uzun, I.; Temel Yuksel, I.; Madazlı, R. Evaluation of maternal serum hypoxia inducible factor-1α, progranulin and syndecan-1 levels in pregnancies with early- and late-onset preeclampsia. J. Matern. Fetal Neonatal Med. 2017, 2, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Hentschke, M.R.; Lucas, L.S.; Mistry, H.D.; Pinheiro da Costa, B.E.; Poli-de-Figueiredo, C.E. Endocan-1 concentrations in maternal and fetal plasma and placentae in pre-eclampsia in the third trimester of pregnancy. Cytokine 2015, 74, 152–156. [Google Scholar] [CrossRef] [PubMed]
- Adekola, H.; Romero, R.; Chaemsaithong, P.; Korzeniewski, S.J.; Dong, Z.; Yeo, L.; Hassan, S.S.; Chaiworapongsa, T. Endocan, a putative endothelial cell marker, is elevated in preeclampsia, decreased in acute pyelonephritis, and unchanged in other obstetrical syndromes. J. Matern. Fetal Neonatal Med. 2015, 28, 1621–1632. [Google Scholar] [CrossRef] [PubMed]
- Sukhikh, G.T.; Ziganshina, M.M.; Nizyaeva, N.V.; Kulikova, G.V.; Volkova, J.S.; Yarotskaya, E.L.; Kan, N.E.; Shchyogolev, A.I.; Tyutyunnik, V.L. Differences of glycocalyx composition in the structural elements of placenta in preeclampsia. Placenta 2016, 43, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, M.F.; Nandi, P.; Girish, G.V.; Nygard, K.; Eastabrook, G.; De Vrijer, B.; Han, V.K.; Lala, P.K. Decorin over-expression by decidual cells in preeclampsia: A potential blood biomarker. Am. J. Obstet. Gynecol. 2016, 215, e1–e361. [Google Scholar] [CrossRef]
- Cakmak, M.; Yilmaz, H.; Bağlar, E.; Darcin, T.; Inan, O.; Aktas, A.; Celik, H.T.; Ozdemir, O.; Atalay, C.R.; Akcay, A. Serum levels of endocan correlate with the presence and severity of pre-eclampsia. Clin. Exp. Hypertens. 2016, 38, 137–142. [Google Scholar] [CrossRef]
- Robajac, D.; Vanhooren, V.; Masnikosa, R.; Miković, Ž.; Mandić, V.; Libert, C.; Nedić, O. Preeclampsia transforms membrane N-glycome in human placenta. Exp. Mol. Pathol. 2016, 100, 26–30. [Google Scholar] [CrossRef]
- Gandley, R.E.; Althouse, A.; Jeyabalan, A.; Bregand-White, J.M.; McGonigal, S.; Myerski, A.C.; Gallaher, M.; Powers, R.W.; Hubel, C.A. Low Soluble Syndecan-1 Precedes Preeclampsia. PLoS ONE 2016, 11, e0157608. [Google Scholar] [CrossRef]
- Romão-Veiga, M.; Matias, M.L.; Ribeiro, V.R.; Nunes, P.R.; Borges, V.T.; Peraçoli, J.C.; Peraçoli, M.T.S. Induction of systemic inflammation by hyaluronan and hsp70 in women with pre-eclampsia. Cytokine 2018, 105, 23–31. [Google Scholar] [CrossRef]
- Weissgerber, T.L.; Garcia-Valencia, O.; Milic, N.M.; Codsi, E.; Cubro, H.; Nath, M.C.; White, W.M.; Nath, K.A.; Garovic, V.D. Early Onset Preeclampsia Is Associated With Glycocalyx Degradation and Reduced Microvascular Perfusion. J. Am. Heart Assoc. 2019, 8, e010647. [Google Scholar] [CrossRef] [PubMed]
- Kucukbas, G.N.; Sanhal, C.Y.; Uygur, D. Plasma Endocan Levels in Early and Late-Onset Preeclampsia. Fetal Pediatr. Pathol. 2019, 26, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Kornacki, J.; Wirstlein, P.; Wender-Ozegowska, E. Levels of syndecan-1 and hyaluronan in early- and late-onset preeclampsia. Pregnancy Hypertens. 2019, 18, 108–111. [Google Scholar] [CrossRef] [PubMed]
- Kuessel, L.; Husslein, H.; Montanari, E.; Kundi, M.; Himmler, G.; Binder, J.; Schiefer, J.; Zeisler, H. Dynamics of soluble syndecan-1 in maternal serum during and after pregnancies complicated by preeclampsia: A nested case control study. Clin. Chem. Lab. Med. 2019, 58, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Dane, M.J.; Van den Berg, B.M.; Avramut, M.C.; Faas, F.G.; Van der Vlag, J.; Rops, A.L.; Ravelli, R.B.; Koster, B.J.; Van Zonneveld, A.J.; Vink, H.; et al. Glomerular endothelial surface layer acts as a barrier against albumin filtration. Am. J. Pathol. 2013, 182, 1532–1540. [Google Scholar] [CrossRef]
- Wu, P.; Haththotuwa, R.; Kwok, C.S.; Babu, A.; Kotronias, R.A.; Rushton, C.; Zaman, A.; Fryer, A.A.; Kadam, U.; Chew-Graham, C.A.; et al. Preeclampsia and Future Cardiovascular Health: A Systematic Review and Meta-Analysis. Circ. Cardiovasc. Qual. Outcomes 2017, 10, e003497. [Google Scholar] [CrossRef]
- Becker, B.F.; Jacob, M.; Leipert, S.; Salmon, A.H.; Chappell, D. Degradation of the endothelial glycocalyx in clinical settings: Searching for the sheddases. Br. J. Clin. Pharmacol. 2015, 80, 389–402. [Google Scholar] [CrossRef]
- Barelli, S.; Alberio, L. The Role of Plasma Transfusion in Massive Bleeding: Protecting the Endothelial Glycocalyx? Front. Med. (Lausanne) 2018, 5, 91. [Google Scholar] [CrossRef]
- Haywood-Watson, R.J.; Holcomb, J.B.; Gonzalez, E.A.; Peng, Z.; Pati, S.; Park, P.W.; Wang, W.; Zaske, A.M.; Menge, T.; Kozar, R.A. Modulation of syndecan-1 shedding after hemorrhagic shock and resuscitation. PLoS ONE 2011, 6, e23530. [Google Scholar] [CrossRef]
- Kozar, R.A.; Peng, Z.; Zhang, R.; Holcomb, J.B.; Pati, S.; Park, P.; Ko, T.C.; Paredes, A. Plasma restoration of endothelial glycocalyx in a rodent model of hemorrhagic shock. Anesth. Analg. 2011, 112, 1289–1295. [Google Scholar] [CrossRef]
- Rath, W.; Bartz, C. Treatment of severe preeclampsia and HELLP syndrome. Zentralbl. Gynakol. 2004, 126, 293–298. [Google Scholar] [CrossRef] [PubMed]
- Martin, J.N., Jr.; Files, J.C.; Blake, P.G.; Norman, P.H.; Martin, R.W.; Hess, L.W.; Morrison, J.C.; Wiser, W.L. Plasma exchange for preeclampsia. I. Postpartum use for persistently severe preeclampsia-eclampsia with HELLP syndrome. Am. J. Obstet. Gynecol. 1990, 162, 126–137. [Google Scholar] [CrossRef]
- Jacob, M.; Paul, O.; Mehringer, L.; Chappell, D.; Rehm, M.; Welsch, U.; Kaczmarek, I.; Conzen, P.; Becker, B.F. Albumin augmentation improves condition of guinea pig hearts after 4 hr of cold ischemia. Transplantation 2009, 87, 956–965. [Google Scholar] [CrossRef] [PubMed]
- Stratta, P.; Canavese, C.; Dogliani, M.; Gurioli, L.; Porcu, M.C.; Todros, T.; Fianchino, O.; Benedetto, C.; Massobrio, M.; Balbi, L. Repeated albumin infusions do not lower blood pressure in preeclampsia. Clin. Nephrol. 1991, 36, 234–239. [Google Scholar] [PubMed]
- Palot, M.; Visseaux, H.; Pire, J.C. Indications of albumin for vascular loading during pregnancy. Ann. Fr. Anesth. Reanim. 1996, 15, 491–496. [Google Scholar] [CrossRef]
- Koseoglu, S.B.; Deveer, R.; Camuzcuoglu, A.; Kasap, B.; Camuzcuoglu, H. Massive Ascites and Pleural Effusion in Preeclampsia. J. Clin. Diagn. Res. 2017, 11, QD08–QD09. [Google Scholar] [CrossRef]
- Yini, S.; Heng, Z.; Xin, A.; Xiaochun, M. Effect of unfractionated heparin on endothelial glycocalyx in a septic shock model. Acta Anaesthesiol. Scand. 2015, 59, 160–169. [Google Scholar] [CrossRef]
- Saisto, T.; Tiitinen, A.; Ulander, V.M.; Kaaja, R. Clinical cure of severe, early onset preeclampsia with low molecular weight heparin therapy in primigravida with hyperreactio luteinalis and thrombophilia. Hum. Reprod. 2004, 19, 725–728. [Google Scholar] [CrossRef]
- Rodger, M.A.; Gris, J.C.; De Vries, J.I.P.; Martinelli, I.; Rey, É.; Schleussner, E.; Middeldorp, S.; Kaaja, R.; Langlois, N.J.; Ramsay, T.; et al. Low-molecular-weight heparin and recurrent placenta-mediated pregnancy complications: A meta-analysis of individual patient data from randomised controlled trials. Lancet 2016, 388, 2629–2641. [Google Scholar] [CrossRef]
- Raffetto, J.D.; Calanni, F.; Mattana, P.; Khalil, R.A. Sulodexide promotes arterial relaxation via endothelium-dependent nitric oxide-mediated pathway. Biochem. Pharmacol. 2019, 166, 347–356. [Google Scholar] [CrossRef]
- Scherbakov, A.Y.; Mіelikova, Т.А. Monitoring the effectiveness of the natural anticoagulant sulodexide in pregnant women with autoimmune hyperthyroidism on the background of hyperhomocysteinemia. Patologìâ 2017, 14, 57–61. [Google Scholar] [CrossRef]
- Dola, L.L.; Henyk, N.I. Optimization of management tactics of women with fetal loss syndrome against the background of thrombophilia. Pharma Innov. J. 2017, 6, 172–173. [Google Scholar]
- Popova, T.A.; Perfilova, V.N.; Zhakupova, G.A.; Verovsky, V.E.; Ostrovskij, O.V.; Tyurenkov, I.N. The effect of sulodexide on placental mitochondria function in rats with experimental preeclampsia. Biomed. KHIMIIA 2016, 62, 572–576. [Google Scholar] [CrossRef] [PubMed]
- Schött, U.; Solomon, C.; Fries, D.; Bentzer, P. The endothelial glycocalyx and its disruption, protection and regeneration: A narrative review. Scand. J. Trauma Resusc. Emerg. Med. 2016, 24, 48. [Google Scholar] [CrossRef] [PubMed]
- Kalafat, E.; Sukur, Y.E.; Abdi, A.; Thilaganathan, B.; Khalil, A. Metformin for prevention of hypertensive disorders of pregnancy in women with gestational diabetes or obesity: Systematic review and meta-analysis of randomized trials. Ultrasound Obstet. Gynecol. 2018, 52, 706–714. [Google Scholar] [CrossRef]
- Nascimento, I.B.D.; Dienstmann, G.; De Souza, M.L.R.; Fleig, R.; Hoffmann, C.B.P.C.; Silva, J.C. Evaluation of Preeclampsia Results after Use of Metformin in Gestation: Systematic Review and Meta-analysis. Rev. Bras. Ginecol. Obstet. 2018, 40, 713–721. [Google Scholar] [CrossRef] [PubMed]
- Cerny, V.; Astapenko, D.; Brettner, F.; Benes, J.; Hyspler, R.; Lehmann, C.; Zadak, Z. Targeting the endothelial glycocalyx in acute critical illness as a challenge for clinical and laboratory medicine. Crit. Rev. Clin. Lab. Sci. 2017, 54, 343–357. [Google Scholar] [CrossRef] [PubMed]
- Chang, E.Y.; Barbosa, E.; Paintlia, M.K.; Singh, A.; Singh, I. The use of N-acetylcysteine for the prevention of hypertension in the reduced uterine perfusion pressure model for preeclampsia in Sprague-Dawley rats. Am. J. Obstet. Gynecol. 2005, 193 Pt 2, 952–956. [Google Scholar] [CrossRef]
- Motawei, S.M.; Attalla, S.M.; Gouda, H.E.; Harouny, M.A.; Elmansoury, A.M. The effects of N-acetyl cysteine on oxidative stress among patients with pre-eclampsia. Int. J. Gynaecol. Obstet. 2016, 135, 226–227. [Google Scholar] [CrossRef]
- Roes, E.M.; Raijmakers, M.T.; Boo, T.M.; Zusterzeel, P.L.; Merkus, H.M.; Peters, W.H.; Steegers, E.A. Oral N-acetylcysteine administration does not stabilise the process of established severe preeclampsia. Eur. J. Obstet. Gynecol. Reprod. Biol. 2006, 127, 61–67. [Google Scholar] [CrossRef]
- Diebel, M.E.; Diebel, L.N.; Liberati, D.M. Protective effects of plasma products on the endothelial-glycocalyx barrier following trauma-hemorrhagic shock: Is sphingosine-1 phosphate responsible? J. Trauma Acute Care Surg. 2019, 87, 1061–1069. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Adamson, R.H.; Curry, F.R.; Tarbell, J.M. Sphingosine-1-phosphate protects endothelial glycocalyx by inhibiting syndecan-1 shedding. Am. J. Physiol. Heart Circ. Physiol. 2014, 306, H363–H372. [Google Scholar] [CrossRef] [PubMed]
- Mori, T.; Shinohara, K.; Wakatsuki, A.; Watanabe, K.; Fujimaki, A. Adipocytokines and endothelial function in preeclamptic women. Hypertens. Res. 2010, 33, 250–254. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Adu-Gyamfi, E.A.; Fondjo, L.A.; Owiredu, W.K.B.A.; Czika, A.; Nelson, W.; Lamptey, J.; Wang, Y.X.; Ding, Y.B. The role of adiponectin in placentation and preeclampsia. Cell Biochem. Funct. 2020, 38, 106–117. [Google Scholar] [CrossRef]
- Kerage, D.; Brindley, D.N.; Hemmings, D.G. Review: Novel insights into the regulation of vascular tone by sphingosine 1-phosphate. Placenta 2014, 35, S86–S92. [Google Scholar] [CrossRef]
- Charkiewicz, K.; Goscik, J.; Blachnio-Zabielska, A.; Raba, G.; Sakowicz, A.; Kalinka, J.; Chabowski, A.; Laudanski, P. Sphingolipids as a new factor in the pathomechanism of preeclampsia—Mass spectrometry analysis. PLoS ONE 2017, 12, e0177601. [Google Scholar] [CrossRef]
- Johnstone, E.D.; Chan, G.; Sibley, C.P.; Davidge, S.T.; Lowen, B.; Guilbert, L.J. Sphingosine-1-phosphate inhibition of placental trophoblast differentiation through a G(i)-coupled receptor response. J. Lipid Res. 2005, 46, 1833–1839. [Google Scholar] [CrossRef]
- Westwood, M.; Al-Saghir, K.; Finn-Sell, S.; Tan, C.; Cowley, E.; Berneau, S.; Adlam, D.; Johnstone, E.D. Vitamin D attenuates sphingosine-1-phosphate (S1P)-mediated inhibition of extravillous trophoblast migration. Placenta 2017, 60, 1–8. [Google Scholar] [CrossRef]
- Cao, R.N.; Tang, L.; Xia, Z.Y.; Xia, R. Endothelial glycocalyx as a potential theriapeutic target in organ injuries. Chin Med J (Engl) 2019, 132, 963–975. [Google Scholar] [CrossRef]
- Dou, H.; Song, A.; Jia, S.; Zhang, L. Heparinoids Danaparoid and Sulodexide as clinically used drugs. Prog. Mol. Biol. Transl. Sci. 2019, 163, 55–74. [Google Scholar]
- Fareed, J.; Bacher, P.; Jeske, W. Advances in Heparins and Related Research. An Epilogue. Molecules 2018, 23, 390. [Google Scholar] [CrossRef] [PubMed]
- Katsi, V.; Kanellopoulou, T.; Makris, T.; Nihoyannopoulos, P.; Nomikou, E.; Tousoulis, D. Aspirin vs Heparin for the prevention of Preeclampsia. Curr. Hypertens. Rep. 2016, 18, 57. [Google Scholar] [CrossRef] [PubMed]
- Simcox, L.E.; Ormesher, L.; Tower, C.; Greer, I.A. Thrombophilia and Pregnancy Complications. Int. J. Mol. Sci. 2015, 16, 28418–28428. [Google Scholar] [CrossRef]
- Martin, L.; Koczera, P.; Zechendorf, E.; Schuerholz, T. The Endothelial Glycocalyx: New Diagnostic and Therapeutic Approaches in Sepsis. Biomed. Res. Int. 2016, 2016, 3758278. [Google Scholar] [CrossRef] [PubMed]
- Spiess, B.D. Heparin: Effects upon the Glycocalyx and Endothelial Cells. J. Extra Corpor. Technol. 2017, 49, 192–197. [Google Scholar] [PubMed]
- Masola, V.; Zaza, G.; Onisto, M.; Lupo, A.; Gambaro, G. Glycosaminoglycans, proteoglycans and sulodexide and the endothelium: Biological roles and pharmacological effects. Int. Angiol. 2014, 33, 243–254. [Google Scholar]
- Rabelink, T.J.; De Zeeuw, D. The glycocalyx--linking albuminuria with renal and cardiovascular disease. Nat. Rev. Nephrol. 2015, 11, 667–676. [Google Scholar] [CrossRef]
- Ligi, D.; Maniscalco, R.; Mannello, F. New Frontiers for an Old Drug: What Is New on the Pleiotropic Effect of Sulodexide in Chronic Venous Disease. J. Cardiovasc. Pharmacol. 2020, 75, 208–210. [Google Scholar] [CrossRef]
- Bignamini, A.A.; Matuška, J. Sulodexide for the Symptoms and Signs of Chronic Venous Disease: A Systematic Review and Meta-analysis. Adv. Ther. 2020, 37, 1013–1033. [Google Scholar] [CrossRef]
- Targosz-Korecka, M.; Malek-Zietek, K.E.; Kloska, D.; Rajfur, Z.; Stepien, E.Ł.; Grochot-Przeczek, A.; Szymonski, M. Metformin attenuates adhesion between cancer and endothelial cells in chronic hyperglycemia by recovery of the endothelial glycocalyx barrier. Biochim. Biophys. Acta Gen. Subj. 2020, 1864, 129533. [Google Scholar] [CrossRef]
- Van Haare, J.; Kooi, M.E.; Van Teeffelen, J.W.; Vink, H.; Slenter, J.; Cobelens, H.; Strijkers, G.J.; Koehn, D.; Post, M.J.; Van Bilsen, M. Metformin and sulodexide restore cardiac microvascular perfusion capacity in diet-induced obese rats. Cardiovasc. Diabetol. 2017, 16, 47. [Google Scholar] [CrossRef] [PubMed]
- Eskens, B.J.; Zuurbier, C.J.; Van Haare, J.; Vink, H.; Van Teeffelen, J.W. Effects of two weeks of metformin treatment on whole-body glycocalyx barrier properties in db/db mice. Cardiovasc. Diabetol. 2013, 12, 175. [Google Scholar] [CrossRef] [PubMed]
- Brownfoot, F.C.; Hastie, R.; Hannan, N.J.; Cannon, P.; Tuohey, L.; Parry, L.; Senadheera, S.; Illanes, S.E.; Kaitu’u-Lino, T.J.; Tong, S. Metformin as a prevention and treatment for preeclampsia: Effects on soluble fms-like tyrosine kinase 1 and soluble endoglin secretion and endothelial dysfunction. Am. J. Obstet. Gynecol. 2016, 214, e1–e356. [Google Scholar] [CrossRef] [PubMed]
- Cui, N.; Wang, H.; Long, Y.; Su, L.; Liu, D. Dexamethasone Suppressed LPS-Induced Matrix Metalloproteinase and Its Effect on Endothelial Glycocalyx Shedding. Mediat. Inflamm. 2015, 2015, 912726. [Google Scholar] [CrossRef]
- Chappell, D.; Jacob, M.; Hofmann-Kiefer, K.; Bruegger, D.; Rehm, M.; Conzen, P.; Welsch, U.; Becker, B.F. Hydrocortisone preserves the vascular barrier by protecting the endothelial glycocalyx. Anesthesiology 2007, 107, 776–784. [Google Scholar] [CrossRef]
- Bandoli, G.; Palmsten, K.; Forbess Smith, C.J.; Chambers, C.D. A Review of Systemic Corticosteroid Use in Pregnancy and the Risk of Select Pregnancy and Birth Outcomes. Rheum. Dis. Clin. N. Am. 2017, 43, 489–502. [Google Scholar] [CrossRef]
- Nieuwdorp, M.; Van Haeften, T.W.; Gouverneur, M.C.; Mooij, H.L.; Van Lieshout, M.H.; Levi, M.; Meijers, J.C.; Holleman, F.; Hoekstra, J.B.; Vink, H.; et al. Loss of endothelial glycocalyx during acute hyperglycemia coincides with endothelial dysfunction and coagulation activation in vivo. Diabetes 2006, 55, 480–486. [Google Scholar] [CrossRef]
- Tenório, M.B.; Ferreira, R.C.; Moura, F.A.; Bueno, N.B.; Goulart, M.O.F.; Oliveira, A.C.M. Oral antioxidant therapy for prevention and treatment of preeclampsia: Meta-analysis of randomized controlled trials. Nutr. Metab. Cardiovasc. Dis. 2018, 28, 865–876. [Google Scholar] [CrossRef]
- Kobayashi, K.; Mimuro, S.; Sato, T.; Kobayashi, A.; Kawashima, S.; Makino, H.; Doi, M.; Katoh, T.; Nakajima, Y. Dexmedetomidine preserves the endothelial glycocalyx and improves survival in a rat heatstroke model. J. Anesth. 2018, 32, 880–885. [Google Scholar] [CrossRef]
- Abu-Halaweh, S.A.; Al Oweidi, A.K.; Abu-Malooh, H.; Zabalawi, M.; Alkazaleh, F.; Abu-Ali, H.; Ramsay, M.A. Intravenous dexmedetomidine infusion for labour analgesia in patient with preeclampsia. Eur. J. Anaesthesiol. 2009, 26, 86–87. [Google Scholar] [CrossRef]
- Hariharan, U. Postpartum hemorrhage and pregnancy induced hypertension during emergency lower segment cesarean section: Dexmedetomidine to our rescue. Rev. Bras. Anestesiol. 2017, 67, 538–540. [Google Scholar] [CrossRef] [PubMed]
- Eskandr, A.M.; Metwally, A.A.; Ahmed, A.A.; Elfeky, E.M.; Eldesoky, I.M.; Obada, M.A.; Abd-Elmegid, O.A. Dexmedetomidine as a part of general anaesthesia for caesarean delivery in patients with pre-eclampsia: A randomised double-blinded trial. Eur. J. Anaesthesiol. 2018, 35, 372–378. [Google Scholar] [CrossRef] [PubMed]
- El-Tahan, M.R.; El Kenany, S.; Abdelaty, E.M.; Ramzy, E.A. Comparison of the effects of low doses of dexmedetomidine and remifentanil on the maternal hemodynamic changes during caesarean delivery in patients with severe preeclampsia: A randomized trial. Minerva Anestesiol. 2018, 84, 1343–1351. [Google Scholar] [CrossRef] [PubMed]
- Poon, L.C.; Shennan, A.; Hyett, J.A.; Kapur, A.; Hadar, E.; Divakar, H.; McAuliffe, F.; Da Silva Costa, F.; Von Dadelszen, P.; McIntyre, H.D.; et al. The International Federation of Gynecology and Obstetrics (FIGO) initiative on pre-eclampsia: A pragmatic guide for first-trimester screening and prevention. Int. J. Gynaecol. Obstet. 2019, 145, 1–33. [Google Scholar] [CrossRef]
- Tan, M.Y.; Poon, L.C.; Rolnik, D.L.; Syngelaki, A.; De Paco Matallana, C.; Akolekar, R.; Cicero, S.; Janga, D.; Singh, M.; Molina, F.S.; et al. Prediction and prevention of small-for-gestational-age neonates: Evidence from SPREE and ASPRE. Ultrasound Obstet. Gynecol. 2018, 52, 52–59. [Google Scholar] [CrossRef]
- Wright, D.; Nicolaides, K.H. Aspirin delays the development of preeclampsia. Am. J. Obstet. Gynecol. 2019, 220, e1–e580. [Google Scholar] [CrossRef]
- Vane, J.R.; Bakhle, Y.S.; Botting, R.M. Cyclooxygenases 1 and 2. Annu. Rev. Pharmacol. Toxicol. 1998, 38, 97–120. [Google Scholar] [CrossRef]
- De Gaetano, G.; Donati, M.B.; Cerletti, C. Prevention of thrombosis and vascular inflammation: Benefits and limitations of selective or combined COX-1, COX-2 and 5-LOX inhibitors. Trends Pharmacol. Sci. 2003, 24, 245–252. [Google Scholar] [CrossRef]
- Altmar, R.; Luciardi, H.L.; Muntaner, J.; Herrera, R.N. The antithrombotic profile of aspirin. Aspirin resistance, or simply failure? Thromb. J. 2004, 14, 1. [Google Scholar]
- Ylikorkala, O.; Viinikka, L. The role of prostaglandins in obstetrical disorders. Baillieres Clin. Obstet. Gynaecol. 1992, 6, 809–827. [Google Scholar] [CrossRef]
- Pia, M.V. Prediction and Prevention of Pre-Eclampsia. Ph.D. Thesis, University of Helsinki, Helsinki, Finland, 2017; pp. 1–108. [Google Scholar]
- Hetzel, S.; DeMets, D.; Schneider, R.; Borzak, S.; Schneider, W.; Serebruany, V.; Schröder, H.; Hennekens, C.H. Aspirin increases nitric oxide formation in chronic stable coronary disease. J. Cardiovasc. Pharmacol. Ther. 2013, 18, 217–221. [Google Scholar] [CrossRef] [PubMed]
- Hennekens, C.H.; Schneider, W.R.; Pokov, A.; Hetzel, S.; DeMets, D.; Serebruany, V.; Schröder, H. A randomized trial of aspirin at clinically relevant doses and nitric oxide formation in humans. J. Cardiovasc. Pharmacol. Ther. 2010, 15, 344–348. [Google Scholar] [CrossRef]
- Grosser, N.; Abate, A.; Oberle, S.; Vreman, H.J.; Dennery, P.A.; Becker, J.C.; Pohle, T.; Seidman, D.S.; Schroder, H. Heme oxygenase-1 induction may explain the antioxidant profile of aspirin. Biochem. Biophys. Res. Commun. 2003, 308, 956–960. [Google Scholar] [CrossRef]
- Kim, J.; Lee, K.S.; Kim, J.H.; Lee, D.K.; Park, M.; Choi, S.; Park, W.; Kim, S.; Choi, Y.K.; Hwang, J.Y.; et al. Aspirin prevents TNF-α-induced endothelial cell dysfunction by regulating the NF-κB-dependent miR-155/eNOS pathway: Role of a miR-155/eNOS axis in preeclampsia. Free Radic. Biol. Med. 2017, 104, 185–198. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.H.; Ma, K.L.; Zhang, Y.; Hu, Z.B.; Liu, L.; Lu, J.; Chen, P.P.; Lu, C.C.; Ruan, X.Z.; Liu, B.C. Platelet microparticles contribute to aortic vascular endothelial injury in diabetes via the mTORC1 pathway. Acta Pharmacol. Sin. 2019, 40, 468–476. [Google Scholar] [CrossRef] [PubMed]
- Manicourt, D.H.; Druetz-Van Egeren, A.; Haazen, L.; Nagant de Deuxchaisnes, C. Effects of tenoxicam and aspirin on the metabolism of proteoglycans and hyaluronan in normal and osteoarthritic human articular cartilage. Br. J. Pharmacol. 1994, 113, 1113–1120. [Google Scholar] [CrossRef]
- Siddesha, J.M.; Valente, A.J.; Sakamuri, S.S.; Gardner, J.D.; Delafontaine, P.; Noda, M.; Chandrasekar, B. Acetylsalicylic acid inhibits IL-18-induced cardiac fibroblast migration through the induction of RECK. J. Cell Physiol. 2014, 229, 845–855. [Google Scholar] [CrossRef]
- Jadranko, S.; Tokmadzic, V.S.; Danijel, K.; Igor, M.; Nada, V.D.; Sanja, B.; Marijana, R.; Ana, L.B.; Gordana, L. Endothelial dysfunction mediated by interleukin-18 in patients with ischemic heart disease undergoing coronary artery bypass grafting surgery. Med. Hypotheses 2017, 104, 20–24. [Google Scholar] [CrossRef]
- Sieve, I.; Munster-Kuhnel, A.K.; Hilfiker-Kleiner, D. Regulation and function of endothelial glycocalyx layer in vascular diseases. Vascul. Pharmacol. 2018, 100, 26–33. [Google Scholar] [CrossRef]
- Ziganshina, M.M.; Pavlovich, S.V.; Bovin, N.V.; Sukhikh, G.T. Hyaluronic Acid in Vascular and Immune Homeostasis during Normal Pregnancy and Preeclampsia. Acta Nat. 2016, 8, 59–71. [Google Scholar] [CrossRef]
- Ziganshina, M.M.; Yarotskaya, E.L.; Bovin, N.V.; Sukhikh, G.T. Endothelial dysfunction as a consequence of endothelial glycocalyx damage: A role in the pathogenesis of preeclampsia. In Endothelial Dysfunction—Old Concepts and New Challenges; Lenasi, H., Ed.; IntechOpen: London, UK, 2018; pp. 113–146. [Google Scholar] [CrossRef]
- Ziganshina, M.M.; Nikolaeva, M.A.; Kan, N.E.; Vavina, O.V.; Nikolaeva, A.V.; Tyutyunnik, V.L.; Tyutyunnik, N.V.; Sukhikh, G.T.; Shilova, N.V.; Khasbiullina, N.R.; et al. Antibodies to hyaluronic acid in preeclampsia. In Glycobiology and Human Dieseases; Wiederschain, G., Ed.; CRC Press Book: Boca Raton, FL, USA, 2016; pp. 313–322. [Google Scholar]
- Nagoev, T.M.; Muminova, K.T.; Khodzhaeva, Z.S.; Kholin, A.M.; Ziganshina, M.M.; Kozlova, A.А.; Martirosyan, Y.O. Maternal hemodynamics and preeclampsia. Obstet. Gynecol. 2020. [Google Scholar] [CrossRef]
| № | Study Subject | Methods | Measurement | Findings |
|---|---|---|---|---|
| 1 | Sweden, 42 patients (11 with PE and eclampsia) | Case–control study ELISA | Plasma HA levels | Plasma HA level is increased in severe PE and eclampsia [46]. |
| 2 | South Africa, 84 patients (28 with PE) | Dimethyl-methylene blue assay | Urinary HSPGs and CSPGs levels | Urinary excretion of HSPGs was significantly increased in the PE group compared to the normotensive pregnant group and the hypertensive nonproteinuric group [44] |
| 3 | Italy, 118 patients (93 with hypertensive disorders in pregnancy, 32 with PE) | Case–control study Lectin histochemistry | Intensity of staining of carbon residues in the glycans with lectins in the placental tissue | Various alterations of the carbohydrate metabolism and GC compositions in the placentas from women with hypertensive disorders indicate correlation with the placental morpho-functional changes, characteristic for these complications, and with the degree of clinical severity [47]. |
| 4 | Germany, 55 patients (17 with HELLP-syndrome) | Observational study ELISA | GC components (sdc1, HS, and HA) were measured in serum | Increased serum levels of HS and HA were only detected in patients with HELLP. Considerable amounts of sdc1 are released into maternal blood during uncomplicated pregnancy. The HELLP syndrome is associated with an even more pronounced shedding of GC components. Maternal vasculature as well as placenta may be a possible origin of circulating GC components [48]. |
| 5 | Germany, 16 patients (8 with HELLP-syndrome) | Case–control study Immunohistochemistry Electron microscopy | Visualization and expression assessment of GC components (sdc1, HS, HA) in placenta | Large amounts of sdc1 were found, but neither HA nor HS as the major components. Intravillous fetal endothelium did not express any of the investigated GAGs. Healthy women and patients with HELLP showed no differences concerning GC composition and thickness of the syncytiotrophoblast [49]. |
| 6 | UK, 75 patients (17 with PE) | ELISA Glycosaminoglycan assay Immunohistochemistry | Concentration and expression sdc1 and sulfated GSGs in placental tissues | Decreased sGAGs and sdc1 in PE were not related to labor, gestational age, and birthweight centile [50]. |
| 7 | Brazil, 153 patients (60 with PE) | ELISA | Serum HA levels | Increased release of HA may contribute to an elevated pro-inflammatory response and tissue damage in women with PE [51]. |
| 8 | Turkey, 81 patients (49 with PE) | A cross-sectional study ELISA | Serum endocan levels | Mean endocan levels were not significantly different among groups [42]. |
| 9 | China, 22 patients (12 with PE) | Case–control study Immunohistochemistry; qRT-PCR; Western blotting; ELISA | Immunohistochemistry was used to evaluate the location of endocan. Then, the mRNA and protein levels of endocan in placenta were detected using qRT-PCR and Western blotting. Serum endocan concentration was measured by ELISA | Expression of endocan mRNA and protein were increased in the placenta tissues of PE compared with in the normal pregnancy; however, the endocan concentration of maternal serum did not differ significantly [43,52]. |
| 10 | Brazil, 117 patients (50 with PE) | Observational and case–control study MagPlex(TH)-C | Plasma endocan-1 levels | Endocan-1 is increased in women with PE. The negative correlations between endocan-1 and clinical data suggest that this molecule may also be involved in prematurity and low birth weight [53]. |
| 11 | USA, 506 patients (130 with uncomplicated pregnancy; 102 with PE; 274 with other great obstetrical syndromes) | A cross-sectional study ELISA | Plasma endocan-1 concentrations | Median maternal plasma endocan concentrations were higher in PE patients and lower in acute pyelonephritis with bacteremia than in uncomplicated pregnancy. No significant difference was observed in the median plasma endocan concentration between other great obstetrical syndromes and uncomplicated pregnancies. The difference in changes of endocan in PE and acute pyelonephritis with bacteremia may confirm that the two diseases differ in pathogenetic mechanisms, despite their associations with systemic vascular inflammation and endothelial cell activation/dysfunction [54]. |
| 12 | Russia, 23 patients (16 with moderate and severe PE) | Case–control study Lectin histochemistry | The study of carbohydrate phenotype of placenta was carried out by the lectin staining of syncytiotrophoblast membranes and the membranes of endothelial cells of terminal placental villi | The most prominent alteration of the GC composition was found in the placentas of women with severe PE. The modified glycome of syncytiotrophoblast and capillary endothelium may play an important role in pathogenesis of PE [55]. |
| 13 | Canada, 28 patients (14 with PE) | Retrospective and case–control study qRT- PCR; situ hybridization; ELISA | Decorin expression was measured at tissue and cell levels in the placenta sections. Retrospective measurements of plasma decorin levels during the second trimester were carried out. | Decorin overexpression by basal decidual cells is associated with hypoinvasive phenotype and poor endovascular differentiation of trophoblast cells in PE. Elevated plasma decorin concentration is a potential predictive biomarker for PE before the onset of clinical signs [56]. |
| 14 | Turkey, 129 patients (99 with PE) | A cross-sectional study ELISA | Serum endocan-1 concentrations | Serum endocan concentrations were significantly elevated in women with PE versus normotensive controls, and concentrations seemed to be associated with the severity of the disease [57]. |
| 15 | Serbia, 44 patients (14 with PE + IUGR) | Case–control study DSA-FACE method; electrophoresis; lectin and immunoblotting; lectin affinity chromatography | N-glycan analysis in placenta | Glycans on placental membranes were altered due to PE [58]. |
| 16 | USA, longitudinal study (n = 8); cross-sectional 3rd trimester study (34 patients, 17 with PE); case–control study (44 patients (19 with PE) | Case–control, longitudinal, and cross- sectional studies. ELISA Isolation and analysis of placental RNA Placental immunohistochemical staining and scoring | Plasma sdc1 levels and placental sdc1 expression | Soluble sdc is significantly lower before the clinical onset of PE, with reduced expression of sdc1 in the placenta after expulsion, suggesting a role of GC disturbance in PE pathophysiology [59]. |
| 17 | Turkey, 80 patients (27 with EO- PE and 27 LO- PE) | Cross-sectional study ELISA | Serum sdc1 levels | Control group presented significantly higher sdc1 levels, than EO and LO-PE [52]. |
| 18 | Brasil, 60 patients (20 with PE) | ELISA | Plasma HA levels | Significantly higher plasma levels of HA in PE than in normotensive pregnant women and non-pregnant women, suggesting involvement of HA as DAMPs in SIR [60]. |
| 19 | USA, 137 women (14 with EO-PE, 29 with LO-PE) | ELISA and noninvasive sublingual eGC measurements by sidestream dark field imaging | Plasma levels of sdc1, HA, HSPGs, perfused boundary region (width of the eGC that was permeable to RBCsreflects eGC degradation) and the percentage of vessels that were filled with RBCs ≥50% of the time (this reflects a microvascular perfusion) | In LO-PE the structural eGC changes (eGC degradation, larger perfused boundary region) was higher and percentage of vessels that were filled with RBCs was significantly lower) were accompanied by elevated plasma concentration of eGC components [61]. |
| 20 | Turkey, 78 women (25 with EO-PE and 16 with LO-PE) | ELISA | Plasma endocan levels | There was no significant difference between endocan levels in EO-PE or LO-PE compared with their corresponding control groups, nor between EO- and LO-PE groups [62]. |
| 21 | Poland, 60 women (20 with EO-PE and 20 with LO-PE) | ELISA | Serum HA and sdc1 levels | Concentration of HA was significantly higher and the level of sdc1 was significantly lower in patients with EO and LO-PE than in the control group [63]. |
| 22 | Austria, single center nested case–control study, 107 patients (95 with normal pregnancy, 12 with PE) | ELISA | Serum sdc1 levels were measured at 10 dynamic points during pregnancy | Sdc1 levels were lower in women developing PE compared to normal pregnancies, and sdc-1 might be useful to predict PE. After delivery, sdc1 levels remained higher in women with PE [64]. |
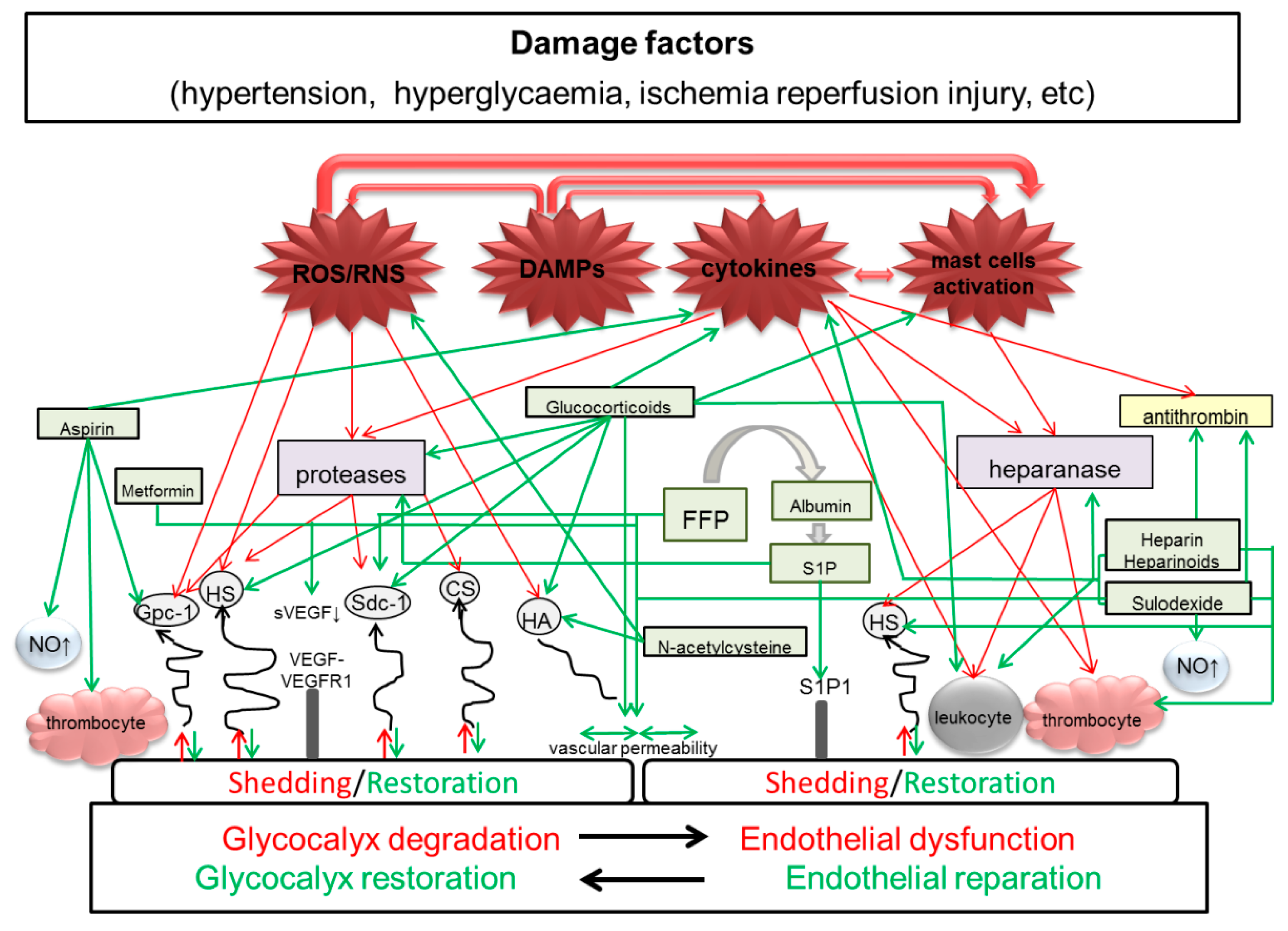
| Drugs/Molecules | Effects | Application in Pre-Eclampsia |
|---|---|---|
| Fresh frozen plasma | Improves junctional integrity of endothelial cells, partially restores eGC and preserves endothelial sdc1 [69,70] | Limited data on application in severe pre-eclampsia-eclampsia with HELLP syndrome [71,72] |
| Albumin | Reduces eGC shedding and edema formation and improves endothelial integrity [73] | No impact on blood pressure and renal function, uteroplacental, and fetoplacental resistance [74]. Used for fluid resuscitation in PE, prior to regional anesthesia for caesarean section, compensation of hemorrhagic blood loss in labor [75]. Caused positive effect in a patient with severe PE complicated with postpartum massive ascites and pleural effusion [76] |
| Heparin | Maintains the eGC thickness, inhibits neutrophil adherence and inflammation [77] | Is effective in PE and concomitant inherited or acquired thrombophilias [78]. According to meta-analysis, LMWH does not seem to reduce the risk of recurrent placenta-mediated pregnancy complications in at-risk women [79]. |
| Sulodexide | Increases GAGs synthesis [16] and promotes arterial relaxation [80] | There are few reports on effective use during pregnancy [81,82]. Decrease in the PE symptoms (lower blood pressure and less proteinuria) in experimental PE in rats [83]. |
| Hydrocortisone Methylprednisolone | Protect eGC by prevention of endothelial perturbation and glycocalyx shedding [28] | No positive effect on maternal and perinatal outcomes in PE |
| Metformin | Improves the eGC barrier properties [84] | According to several studies, metformin may prevent or treat PE [85,86] |
| N-acetylcystein | Preserves eGC [87] | Beneficial effect of the N-acetylcystein administration was noted in some clinical and experimental studies with PE [88,89,90] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ziganshina, M.M.; Yarotskaya, E.L.; Bovin, N.V.; Pavlovich, S.V.; Sukhikh, G.T. Can Endothelial Glycocalyx Be a Major Morphological Substrate in Pre-Eclampsia? Int. J. Mol. Sci. 2020, 21, 3048. https://doi.org/10.3390/ijms21093048
Ziganshina MM, Yarotskaya EL, Bovin NV, Pavlovich SV, Sukhikh GT. Can Endothelial Glycocalyx Be a Major Morphological Substrate in Pre-Eclampsia? International Journal of Molecular Sciences. 2020; 21(9):3048. https://doi.org/10.3390/ijms21093048
Chicago/Turabian StyleZiganshina, Marina M., Ekaterina L. Yarotskaya, Nicolai V. Bovin, Stanislav V. Pavlovich, and Gennady T. Sukhikh. 2020. "Can Endothelial Glycocalyx Be a Major Morphological Substrate in Pre-Eclampsia?" International Journal of Molecular Sciences 21, no. 9: 3048. https://doi.org/10.3390/ijms21093048
APA StyleZiganshina, M. M., Yarotskaya, E. L., Bovin, N. V., Pavlovich, S. V., & Sukhikh, G. T. (2020). Can Endothelial Glycocalyx Be a Major Morphological Substrate in Pre-Eclampsia? International Journal of Molecular Sciences, 21(9), 3048. https://doi.org/10.3390/ijms21093048






