MicroRNA (miRNA): A New Dimension in the Pathogenesis of Antiphospholipid Syndrome (APS)
Abstract
1. Introduction
2. Search Strategy and Inclusion Criteria
3. Origin and Function of miRNAs
4. The Role of miRNA in Immune Response
5. Innate Immune System
6. Adaptive Immune System
6.1. T-Cells
6.2. B-Cells
7. MicroRNA in Autoimmunity
8. Antiphospholipid Syndrome
9. APS: Genetic Predisposition and Family Studies
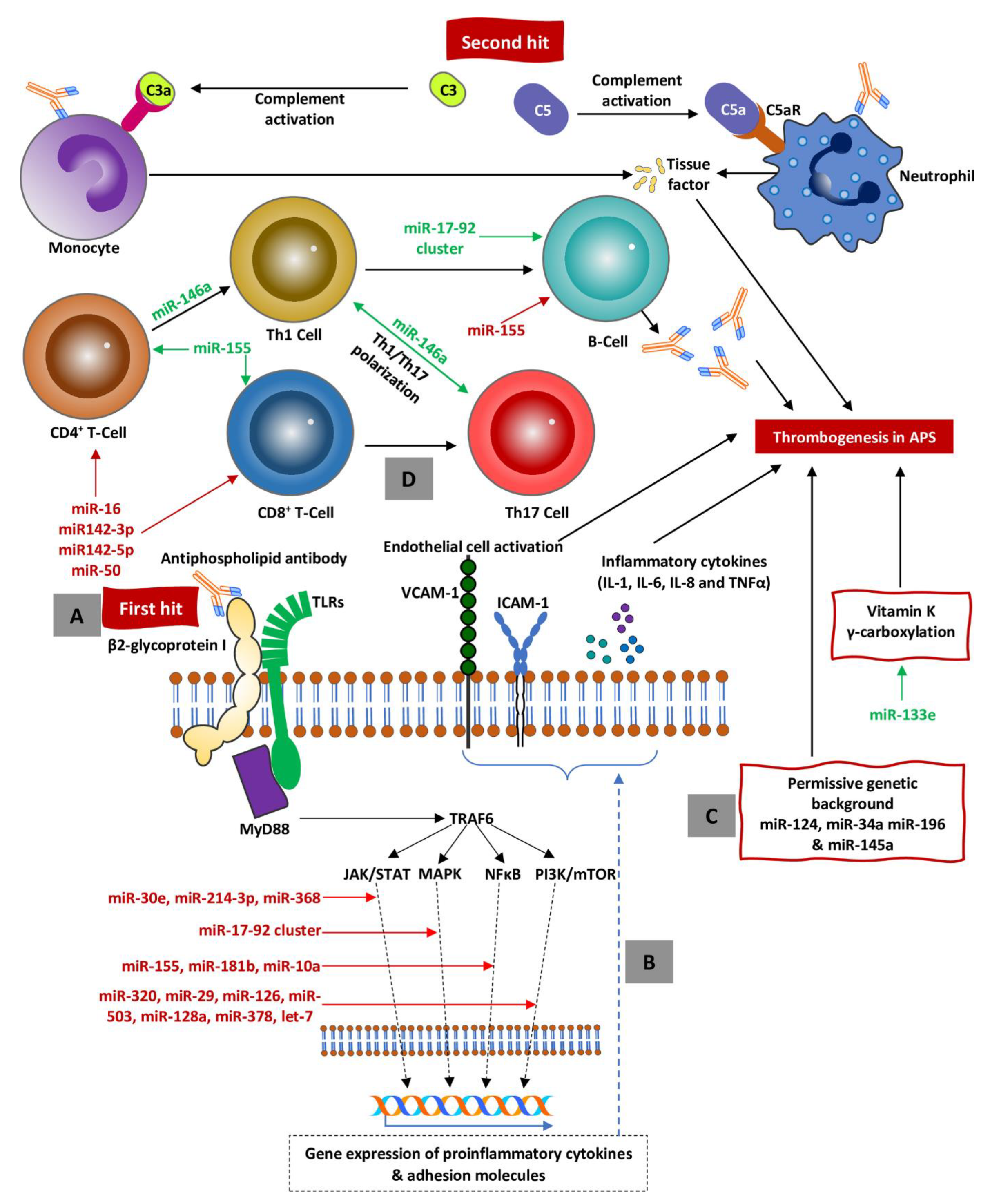
10. MicroRNA and Antiphospholipid Syndrome
11. Future Direction
12. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| miRNAs | MicroRNAs |
| mRNAs | Messenger RNAs |
| SLE | Systemic lupus erythematosus |
| RA | Rheumatoid arthritis |
| SS | Systemic sclerosis |
| MS | Multiple sclerosis |
| APS | Antiphospholipid syndrome |
| Pri-miRNA | Primary miRNA |
| dsRNA | Double-stranded RNA |
| DGCR8 | DiGeorge syndrome critical region gene 8 |
| Pre-miRNA | Precursor miRNA |
| TRBP | Trans-activator RNA binding protein |
| RISC | RNA-induced silencing complex |
| Ago | Argonaute |
| 3′-UTR | 3′-untranslated region |
| NK | Natural killer |
| PAMPs | Pathogen-associated molecular patterns |
| DAMPs | Danger-associated molecular patterns |
| TLRs | Toll-like receptors |
| NF-κB | Nuclear factor kappa-B |
| MAPK | Mitogen-activated protein kinase |
| MHC | Major histocompatibility complex |
| TCR | T-cell receptor |
| SOCS | Suppressor of cytokine signaling |
| Regulatory T | Treg |
| IFN-γ | Interferon gamma |
| BAFF | B-cell activating factor |
| aPLs | Antiphospholipid antibodies |
| LA | Lupus anticoagulants |
| aCL | Anticardiolipin |
| β2GPI | β2-glycoprotein I |
| TF | Tissue factor |
| mTOR | Mechanistic target of rapamycin |
| HLA | Human leukocyte antigen |
| TGF | Transforming growth factor |
References
- Lu, T.X.; Rothenberg, M.E. MicroRNA. J. Allergy Clin. Immunol. 2018, 141, 1202–1207. [Google Scholar] [CrossRef] [PubMed]
- Bartel, D.P. Metazoan MicroRNAs. Cell 2018, 173, 20–51. [Google Scholar] [CrossRef] [PubMed]
- Matsuyama, H.; Suzuki, H.I. Systems and Synthetic microRNA Biology: From Biogenesis to Disease Pathogenesis. Int. J. Mol. Sci. 2019, 21, 132. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.C.; Feinbaum, R.L.; Ambros, V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell 1993, 75, 843–854. [Google Scholar] [CrossRef]
- Wightman, B.; Ha, I.; Ruvkun, G. Posttranscriptional regulation of the heterochronic gene lin-14 by lin-4 mediates temporal pattern formation in C. elegans. Cell 1993, 75, 855–862. [Google Scholar] [CrossRef]
- Lim, L.P.; Lau, N.C.; Garrett-Engele, P.; Grimson, A.; Schelter, J.M.; Castle, J.; Bartel, D.P.; Linsley, P.S.; Johnson, J.M. Microarray analysis shows that some microRNAs downregulate large numbers of target mRNAs. Nature 2005, 433, 769–773. [Google Scholar] [CrossRef]
- O’connell, R.M.; Rao, D.S.; Chaudhuri, A.A.; Baltimore, D. Physiological and pathological roles for microRNAs in the immune system. Nat. Rev. Immunol. 2010, 10, 111–122. [Google Scholar] [CrossRef]
- Wang, Q.; Lin, W.; Tang, X.; Li, S.; Guo, L.; Lin, Y.; Kwok, H.F. The roles of microRNAs in regulating the expression of PD-1/PD-L1 immune checkpoint. Int. J. Mol. Sci. 2017, 18, 2540. [Google Scholar] [CrossRef]
- Ramassone, A.; Pagotto, S.; Veronese, A.; Visone, R. Epigenetics and microRNAs in cancer. Int. J. Mol. Sci. 2018, 19, 459. [Google Scholar] [CrossRef]
- Duan, W.; Zhang, W.; Jia, J.; Lu, Q.; Gershwin, M.E. Exosomal microRNA in autoimmunity. Cell Mol. Immunol. 2019, 16, 932–934. [Google Scholar] [CrossRef]
- Venkatesha, S.H.; Dudics, S.; Song, Y.; Mahurkar, A.; Moudgil, K.D. The miRNA expression profile of experimental autoimmune encephalomyelitis reveals novel potential disease biomarkers. Int. J. Mol. Sci. 2018, 19, 3990. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.-Q.; Papp, G.; Póliska, S.; Szabó, K.; Tarr, T.; Bálint, B.L.; Szodoray, P.; Zeher, M. MicroRNA expression profiles identify disease-specific alterations in systemic lupus erythematosus and primary Sjögren’s syndrome. PLoS ONE 2017, 12, e0174585. [Google Scholar] [CrossRef] [PubMed]
- Evangelatos, G.; Fragoulis, G.E.; Koulouri, V.; Lambrou, G.I. MicroRNAs in rheumatoid arthritis: From pathogenesis to clinical impact. Autoimmun. Rev. 2019, 18, 102391. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Zuo, X.X.; Li, Y.S.; Gao, S.M.; Dai, X.D.; Zhu, H.L.; Luo, H. Integration of microRNA and mRNA expression profiles in the skin of systemic sclerosis patients. Sci. Rep. 2017, 7, 42899. [Google Scholar] [CrossRef]
- Nuzziello, N.; Vilardo, L.; Pelucchi, P.; Consiglio, A.; Liuni, S.; Trojano, M.; Liguori, M. Investigating the role of MicroRNA and transcription factor co-regulatory networks in multiple sclerosis pathogenesis. Int. J. Mol. Sci. 2018, 19, 3652. [Google Scholar] [CrossRef]
- Perez-Sanchez, C.; Arias-de la Rosa, I.; Aguirre, M.A.; Luque-Tevar, M.; Ruiz-Limon, P.; Barbarroja, N.; Jimenez-Gomez, Y.; Abalos-Aguilera, M.C.; Collantes-Estevez, E.; Segui, P.; et al. Circulating microRNAs as biomarkers of disease and typification of the atherothrombotic status in antiphospholipid syndrome. Haematologica 2018, 103, 908–918. [Google Scholar] [CrossRef]
- Lagos-Quintana, M.; Rauhut, R.; Lendeckel, W.; Tuschl, T. Identification of novel genes coding for small expressed RNAs. Science 2001, 294, 853–858. [Google Scholar] [CrossRef]
- Lau, N.C.; Lim, L.P.; Weinstein, E.G.; Bartel, D.P. An abundant class of tiny RNAs with probable regulatory roles in Caenorhabditis elegans. Science 2001, 294, 858–862. [Google Scholar] [CrossRef]
- Lee, Y.; Jeon, K.; Lee, J.T.; Kim, S.; Kim, V.N. MicroRNA maturation: Stepwise processing and subcellular localization. EMBO J. 2002, 21, 4663–4670. [Google Scholar] [CrossRef]
- Lee, Y.; Kim, M.; Han, J.; Yeom, K.H.; Lee, S.; Baek, S.H.; Kim, V.N. MicroRNA genes are transcribed by RNA polymerase II. EMBO J. 2004, 23, 4051–4060. [Google Scholar] [CrossRef]
- Han, J.; Lee, Y.; Yeom, K.H.; Kim, Y.K.; Jin, H.; Kim, V.N. The Drosha-DGCR8 complex in primary microRNA processing. Genes Dev. 2004, 18, 3016–3027. [Google Scholar] [CrossRef] [PubMed]
- Shiohama, A.; Sasaki, T.; Noda, S.; Minoshima, S.; Shimizu, N. Molecular cloning and expression analysis of a novel gene DGCR8 located in the DiGeorge syndrome chromosomal region. Biochem. Biophys. Res. Commun. 2003, 304, 184–190. [Google Scholar] [CrossRef]
- Wilson, D.I.; Burn, J.; Scambler, P.; Goodship, J. DiGeorge syndrome: Part of CATCH 22. J. Med. Genet. 1993, 30, 852–856. [Google Scholar] [CrossRef] [PubMed]
- Gregory, R.I.; Yan, K.-p.; Amuthan, G.; Chendrimada, T.; Doratotaj, B.; Cooch, N.; Shiekhattar, R. The Microprocessor complex mediates the genesis of microRNAs. Nature 2004, 432, 235–240. [Google Scholar] [CrossRef]
- Pauley, K.M.; Cha, S.; Chan, E.K.L. MicroRNA in autoimmunity and autoimmune diseases. J. Autoimmun. 2009, 32, 189–194. [Google Scholar] [CrossRef]
- Iwasaki, S.; Kobayashi, M.; Yoda, M.; Sakaguchi, Y.; Katsuma, S.; Suzuki, T.; Tomari, Y. Hsc70/Hsp90 Chaperone Machinery Mediates ATP-Dependent RISC Loading of Small RNA Duplexes. Mol. Cell 2010, 39, 292–299. [Google Scholar] [CrossRef]
- Chen, J.-Q.; Papp, G.; Szodoray, P.; Zeher, M. The role of microRNAs in the pathogenesis of autoimmune diseases. Autoimmun. Rev. 2016, 15, 1171–1180. [Google Scholar] [CrossRef]
- Liu, J.; Carmell, M.A.; Rivas, F.V.; Marsden, C.G.; Thomson, J.M.; Song, J.-J.; Hammond, S.M.; Joshua-Tor, L.; Hannon, G.J. Argonaute2 is the catalytic engine of mammalian RNAi. Science 2004, 305, 1437–1441. [Google Scholar] [CrossRef]
- Wee Liang, M.; Flores-Jasso, C.F.; Salomon William, E.; Zamore Phillip, D. Argonaute Divides Its RNA Guide into Domains with Distinct Functions and RNA-Binding Properties. Cell 2012, 151, 1055–1067. [Google Scholar]
- Salomon, W.E.; Jolly, S.M.; Moore, M.J.; Zamore, P.D.; Serebrov, V. Single-molecule imaging reveals that Argonaute reshapes the binding properties of its nucleic acid guides. Cell 2015, 162, 84–95. [Google Scholar] [CrossRef]
- Bruno, I.; Wilkinson, M.F. P-bodies react to stress and nonsense. Cell 2006, 125, 1036–1038. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.P.; Massachi, I.; Manickavel, S.; Singh, S.; Rao, N.P.; Hasan, S.; Mc Curdy, D.K.; Sharma, S.; Wong, D.; Hahn, B.H. The role of miRNA in inflammation and autoimmunity. Autoimmun. Rev. 2013, 12, 1160–1165. [Google Scholar] [CrossRef] [PubMed]
- Hukowska-Szematowicz, B.; Tokarz-Deptula, B.; Deptula, W. MicroRNA (miRNA) and the immune system. Cent. Eur. J. Immunol. 2012, 37, 387–390. [Google Scholar] [CrossRef]
- Curtale, G. MiRNAs at the Crossroads between Innate Immunity and Cancer: Focus on Macrophages. Cells 2018, 7, 12. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, R.P.; Leong, J.W.; Fehniger, T.A. MicroRNA regulation of natural killer cells. Front. Immunol. 2013, 4, 44. [Google Scholar] [CrossRef] [PubMed]
- Larsen, M.T.; Hother, C.; Häger, M.; Pedersen, C.C.; Theilgaard-Mönch, K.; Borregaard, N.; Cowland, J.B. MicroRNA Profiling in Human Neutrophils during Bone Marrow Granulopoiesis and In Vivo Exudation. PLoS ONE 2013, 8, e58454. [Google Scholar] [CrossRef]
- Kumar Kingsley, S.M.; Vishnu Bhat, B. Role of MicroRNAs in the development and function of innate immune cells. Int. Rev. Immunol. 2017, 36, 154–175. [Google Scholar] [CrossRef]
- Xu, S.J.; Hu, H.T.; Li, H.L.; Chang, S. The Role of miRNAs in Immune Cell Development, Immune Cell Activation, and Tumor Immunity: With a Focus on Macrophages and Natural Killer Cells. Cells 2019, 8, 1140. [Google Scholar] [CrossRef]
- He, X.; Jing, Z.; Cheng, G. MicroRNAs: New regulators of Toll-like receptor signalling pathways. Biomed. Res. Int. 2014, 2014, 945169. [Google Scholar] [CrossRef]
- Momen-Heravi, F.; Bala, S. miRNA regulation of innate immunity. J. Leukoc. Biol. 2018, 103, 1205–1217. [Google Scholar] [CrossRef]
- Wu, H.; Neilson, J.R.; Kumar, P.; Manocha, M.; Shankar, P.; Sharp, P.A.; Manjunath, N. miRNA profiling of naive, effector and memory CD8 T cells. PLoS ONE 2007, 2, e1020. [Google Scholar] [CrossRef] [PubMed]
- Lind, E.F.; Elford, A.R.; Ohashi, P.S. Micro-RNA 155 is required for optimal CD8+ T cell responses to acute viral and intracellular bacterial challenges. J. Immunol. 2013, 190, 1210–1216. [Google Scholar] [CrossRef] [PubMed]
- Gracias, D.T.; Stelekati, E.; Hope, J.L.; Boesteanu, A.C.; Doering, T.A.; Norton, J.; Mueller, Y.M.; Fraietta, J.A.; Wherry, E.J.; Turner, M. The microRNA miR-155 controls CD8+ T cell responses by regulating interferon signaling. Nat. Immunol. 2013, 14, 593. [Google Scholar] [CrossRef] [PubMed]
- O’Connell, R.M.; Kahn, D.; Gibson, W.S.J.; Round, J.L.; Scholz, R.L.; Chaudhuri, A.A.; Kahn, M.E.; Rao, D.S.; Baltimore, D. MicroRNA-155 Promotes Autoimmune Inflammation by Enhancing Inflammatory T Cell Development. Immunity 2010, 33, 607–619. [Google Scholar] [CrossRef]
- Lu, L.-F.; Thai, T.-H.; Calado, D.P.; Chaudhry, A.; Kubo, M.; Tanaka, K.; Loeb, G.B.; Lee, H.; Yoshimura, A.; Rajewsky, K.; et al. Foxp3-Dependent MicroRNA155 Confers Competitive Fitness to Regulatory T Cells by Targeting SOCS1 Protein. Immunity 2009, 30, 80–91. [Google Scholar] [CrossRef]
- Lu, L.-F.; Boldin, M.P.; Chaudhry, A.; Lin, L.-L.; Taganov, K.D.; Hanada, T.; Yoshimura, A.; Baltimore, D.; Rudensky, A.Y. Function of miR-146a in Controlling Treg Cell-Mediated Regulation of Th1 Responses. Cell 2010, 142, 914–929. [Google Scholar] [CrossRef]
- Li, B.; Wang, X.; Choi, I.Y.; Wang, Y.-C.; Liu, S.; Pham, A.T.; Moon, H.; Smith, D.J.; Rao, D.S.; Boldin, M.P.; et al. miR-146a modulates autoreactive Th17 cell differentiation and regulates organ-specific autoimmunity. J. Clin. Investig. 2017, 127, 3702–3716. [Google Scholar] [CrossRef]
- Ventura, A.; Young, A.G.; Winslow, M.M.; Lintault, L.; Meissner, A.; Erkeland, S.J.; Newman, J.; Bronson, R.T.; Crowley, D.; Stone, J.R.; et al. Targeted deletion reveals essential and overlapping functions of the miR-17 through 92 family of miRNA clusters. Cell 2008, 132, 875–886. [Google Scholar] [CrossRef]
- Zheng, B.; Xi, Z.; Liu, R.; Yin, W.; Sui, Z.; Ren, B.; Miller, H.; Gong, Q.; Liu, C. The Function of MicroRNAs in B-Cell Development, Lymphoma, and Their Potential in Clinical Practice. Front. Immunol. 2018, 9, 936. [Google Scholar] [CrossRef]
- Lai, M.; Gonzalez-Martin, A.; Cooper, A.B.; Oda, H.; Jin, H.Y.; Shepherd, J.; He, L.; Zhu, J.; Nemazee, D.; Xiao, C. Regulation of B-cell development and tolerance by different members of the miR-17 approximately 92 family microRNAs. Nat. Commun. 2016, 7, 12207. [Google Scholar] [CrossRef]
- Xiao, C.; Calado, D.P.; Galler, G.; Thai, T.H.; Patterson, H.C.; Wang, J.; Rajewsky, N.; Bender, T.P.; Rajewsky, K. MiR-150 controls B cell differentiation by targeting the transcription factor c-Myb. Cell 2007, 131, 146–159. [Google Scholar] [CrossRef] [PubMed]
- Kong, K.Y.; Owens, K.S.; Rogers, J.H.; Mullenix, J.; Velu, C.S.; Grimes, H.L.; Dahl, R. MIR-23A microRNA cluster inhibits B-cell development. Exp. Hematol. 2010, 38, 629–640. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Yang, J.; Yuan, R.; Peng, J.; Liu, L.; Guo, X. Effects of miR-181a on the biological function of multiple myeloma. Oncol. Rep. 2019, 42, 291–300. [Google Scholar] [CrossRef] [PubMed]
- Nabhan, M.; Louka, M.L.; Khairy, E.; Tash, F.; Ali-Labib, R.; El-Habashy, S. MicroRNA-181a and its target Smad 7 as potential biomarkers for tracking child acute lymphoblastic leukemia. Gene 2017, 628, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Qi, J.; Sun, X.; Wang, W.; Wei, G.; Wu, Y.; Gao, Q.; Zheng, J. MicroRNA-181a promotes cell proliferation and inhibits apoptosis in gastric cancer by targeting RASSF1A. Oncol. Rep. 2018, 40, 1959–1970. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wu, H.; Zhao, M.; Lu, Q. Identifying the differentially expressed microRNAs in autoimmunity: A systemic review and meta-analysis. Autoimmunity 2020, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Lu, Q. Genetic and epigenetic influences on the loss of tolerance in autoimmunity. Cell Mol. Immunol. 2018, 15, 575–585. [Google Scholar] [CrossRef]
- Gonzalez-Martin, A.; Adams, B.D.; Lai, M.; Shepherd, J.; Salvador-Bernaldez, M.; Salvador, J.M.; Lu, J.; Nemazee, D.; Xiao, C. The microRNA miR-148a functions as a critical regulator of B cell tolerance and autoimmunity. Nat. Immunol. 2016, 17, 433–440. [Google Scholar] [CrossRef]
- Alsaleh, G.; François, A.; Philippe, L.; Gong, Y.-Z.; Bahram, S.; Cetin, S.; Pfeffer, S.; Gottenberg, J.-E.; Wachsmann, D.; Georgel, P.; et al. MiR-30a-3p Negatively Regulates BAFF Synthesis in Systemic Sclerosis and Rheumatoid Arthritis Fibroblasts. PLoS ONE 2014, 9, e111266. [Google Scholar] [CrossRef]
- Gumkowska-Sroka, O.; Jagoda, K.; Owczarek, A.; Helbig, G.; Giemza-Stoklosa, J.; Kotyla, P.J. Cytometric Characterization of Main Immunocompetent Cells in Patients with Systemic Sclerosis: Relationship with Disease Activity and Type of Immunosuppressive Treatment. J. Clin. Med. 2019, 8, 625. [Google Scholar] [CrossRef]
- Stypinska, B.; Wajda, A.; Walczuk, E.; Olesinska, M.; Lewandowska, A.; Walczyk, M.; Paradowska-Gorycka, A. The Serum Cell-Free microRNA Expression Profile in MCTD, SLE, SSc, and RA Patients. J. Clin. Med. 2020, 9, 161. [Google Scholar] [CrossRef] [PubMed]
- Honarpisheh, M.; Kohler, P.; von Rauchhaupt, E.; Lech, M. The Involvement of MicroRNAs in Modulation of Innate and Adaptive Immunity in Systemic Lupus Erythematosus and Lupus Nephritis. J. Immunol. Res. 2018, 2018, 4126106. [Google Scholar] [PubMed]
- Lai, N.S.; Koo, M.; Yu, C.L.; Lu, M.C. Immunopathogenesis of systemic lupus erythematosus and rheumatoid arthritis: The role of aberrant expression of non-coding RNAs in T cells. Clin. Exp. Immunol. 2017, 187, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Lam, I.K.Y.; Chow, J.X.; Lau, C.S.; Chan, V.S.F. MicroRNA-mediated immune regulation in rheumatic diseases. Cancer Lett. 2018, 431, 201–212. [Google Scholar] [CrossRef]
- Le, X.; Yu, X.; Shen, N. Novel insights of microRNAs in the development of systemic lupus erythematosus. Curr. Opin. Rheumatol. 2017, 29, 450–457. [Google Scholar] [CrossRef]
- Nalewajska, M.; Gurazda, K.; Styczynska-Kowalska, E.; Marchelek-Mysliwiec, M.; Pawlik, A.; Dziedziejko, V. The Role of MicroRNAs in Selected Forms of Glomerulonephritis. Int. J. Mol. Sci. 2019, 20, 5050. [Google Scholar] [CrossRef]
- Yan, L.; Liang, M.; Hou, X.; Zhang, Y.; Zhang, H.; Guo, Z.; Jinyu, J.; Feng, Z.; Mei, Z. The role of microRNA-16 in the pathogenesis of autoimmune diseases: A comprehensive review. Biomed. Pharmacother. 2019, 112, 108583. [Google Scholar] [CrossRef]
- Vinuesa, C.G.; Rigby, R.J.; Yu, D. Logic and extent of miRNA-mediated control of autoimmune gene expression. Int. Rev. Immunol. 2009, 28, 112–138. [Google Scholar] [CrossRef]
- Linnemann, B. Antiphospholipid syndrome—An update. Vasa 2018, 47, 451–464. [Google Scholar] [CrossRef]
- Giemza-Stoklosa, J.; Islam, M.A.; Kotyla, P.J. Hyperferritinaemia: An Iron Sword of Autoimmunity. Curr. Pharm. Des. 2019, 25, 2909–2918. [Google Scholar] [CrossRef]
- Miyakis, S.; Lockshin, M.D.; Atsumi, T.; Branch, D.W.; Brey, R.L.; Cervera, R.; Derksen, R.H.; PG, D.E.G.; Koike, T.; Meroni, P.L.; et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J. Thromb. Haemost. 2006, 4, 295–306. [Google Scholar] [CrossRef] [PubMed]
- Keeling, D.; Mackie, I.; Moore, G.W.; Greer, I.A.; Greaves, M. Guidelines on the investigation and management of antiphospholipid syndrome. Br. J. Haematol. 2012, 157, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Ugolini-Lopes, M.R.; Criado, P.R.; Parsi, K.; Kucukkaya, R.D.; Amigo, M.-C.; Tektonidou, M.G.; Andrade, D. Treatment of Non-criteria Manifestations in Antiphospholipid Syndrome. In Antiphospholipid Syndrome: Current Research Highlights and Clinical Insights; Erkan, D., Lockshin, M.D., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 247–266. [Google Scholar] [CrossRef]
- Islam, M.A.; Alam, F.; Wong, K.K. Comorbid association of antiphospholipid antibodies and migraine: A systematic review and meta-analysis. Autoimmun. Rev. 2017, 16, 512–522. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.A.; Alam, F.; Kamal, M.A.; Wong, K.K.; Sasongko, T.H.; Gan, S.H. ‘Non-Criteria’ Neurologic Manifestations of Antiphospholipid Syndrome: A Hidden Kingdom to be Discovered. CNS Neurol. Disord. Drug. Targets. 2016, 15, 1253–1265. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.A.; Alam, F.; Sasongko, T.H.; Gan, S.H. Antiphospholipid antibody-mediated thrombotic mechanisms in antiphospholipid syndrome: Towards pathophysiology-based treatment. Curr. Pharm. Des. 2016, 22, 4451–4469. [Google Scholar] [CrossRef]
- Sammaritano, L.R. Antiphospholipid syndrome. Best. Pract. Res. Clin. Rheumatol. 2019. [Google Scholar] [CrossRef] [PubMed]
- Garcia, D.; Erkan, D. Diagnosis and management of the antiphospholipid syndrome. N. Engl. J. Med. 2018, 378, 2010–2021. [Google Scholar] [CrossRef]
- Sciascia, S.; Sanna, G.; Khamashta, M.A.; Cuadrado, M.J.; Erkan, D.; Andreoli, L.; Bertolaccini, M.L. The estimated frequency of antiphospholipid antibodies in young adults with cerebrovascular events: A systematic review. Ann. Rheum. Dis. 2015, 74, 2028–2033. [Google Scholar] [CrossRef]
- Islam, M.A. Antiphospholipid antibodies and antiphospholipid syndrome in cancer: Uninvited guests in troubled times. Semin. Cancer Biol. 2019. [Google Scholar] [CrossRef]
- Islam, M.A.; Alam, S.S.; Kundu, S.; Prodhan, A.; Khandker, S.S.; Reshetnyak, T.; Kotyla, P.J.; Hassan, R.; Hossan, T. Prevalence of antiphospholipid antibodies in Behcet’s disease: A systematic review and meta-analysis. PLoS ONE 2020, 15, e0227836. [Google Scholar] [CrossRef] [PubMed]
- Yelnik, C.M.; Urbanski, G.; Drumez, E.; Sobanski, V.; Maillard, H.; Lanteri, A.; Morell-Dubois, S.; Caron, C.; Dubucquoi, S.; Launay, D.; et al. Persistent triple antiphospholipid antibody positivity as a strong risk factor of first thrombosis, in a long-term follow-up study of patients without history of thrombosis or obstetrical morbidity. Lupus 2017, 26, 163–169. [Google Scholar] [CrossRef]
- Islam, M.A.; Khandker, S.S.; Alam, F.; Kamal, M.A.; Gan, S.H. Genetic risk factors in thrombotic primary antiphospholipid syndrome: A systematic review with bioinformatic analyses. Autoimmun. Rev. 2018, 17, 226–243. [Google Scholar] [CrossRef]
- Exner, T.; Barber, S.; Kronenberg, H.; Rickard, K.A. Familial Association of the Lupus Anticoagulant. Br. J. Haematol. 1980, 45, 89–96. [Google Scholar] [CrossRef]
- Matthey, F.; Walshe, K.; Mackie, I.; Machin, S. Familial occurrence of the antiphospholipid syndrome. J. Clin. Pathol. 1989, 42, 495–497. [Google Scholar] [CrossRef]
- Jolidon, R.-M.; Knecht, H.; Humair, L.; de Torrente, A. Different clinical presentations of a lupus anticoagulant in the same family. Klin. Wochenschr. 1991, 69, 340–344. [Google Scholar] [CrossRef]
- Islam, M.A.; Wong, K.K.; Sasongko, T.H.; Gan, S.H.; Wong, J.S. Familial primary antiphospholipid syndrome: A report of co-occurrence in three Malaysian family members. Eur. J. Rheumatol. 2016, 3, 139–141. [Google Scholar] [CrossRef]
- Arnett, F.; Olsen, M.; Anderson, K.; Reveille, J. Molecular analysis of major histocompatibility complex alleles associated with the lupus anticoagulant. J. Clin. Investig. 1991, 87, 1490–1495. [Google Scholar] [CrossRef]
- Asherson, R.; Doherty, D.; Vergani, D.; Khamashta, M.; Hughes, G. Major histocompatibility complex associations with primary antiphospholipid syndrome. Arthritis. Rheum. 1992, 35, 124–125. [Google Scholar] [CrossRef]
- Caliz, R.; Atsumi, T.; Kondeatis, E.; Amengual, O.; Khamashta, M.; Vaughan, R.; Lanchbury, J.; Hughes, G. HLA class II gene polymorphisms in antiphospholipid syndrome: Haplotype analysis in 83 Caucasoid patients. Rheumatology 2001, 40, 31–36. [Google Scholar] [CrossRef]
- Granados, J.; Vargas-Alarcon, G.; Drenkard, C.; Andrade, F.; Melin-Aldana, H.; Alcocer-Varela, J.; Alarcón-Segovia, D. Relationship of anticardiolipin antibodies and antiphospholipid syndrome to HLA-DR7 in Mexican patients with systemic lupus erythematosus (SLE). Lupus 1997, 6, 57–62. [Google Scholar] [CrossRef]
- Freitas, M.V.; Da Silva, L.; Deghaide, N.H.; Donadi, E.A.; Louzada-Júnior, P. Is HLA class II susceptibility to primary antiphospholipid syndrome different from susceptibility to secondary antiphospholipid syndrome? Lupus 2004, 13, 125–131. [Google Scholar] [CrossRef]
- Al Attia, H.; Santosh, A.; Al Farhan, M. Observations on class II antigens and genetic susceptibility to primary antiphospholipid (Hughes) syndrome in Arab patients. Clin. Exp. Rheumatol. 2008, 26, 506. [Google Scholar]
- Sebastiani, G.D.; Iuliano, A.; Cantarini, L.; Galeazzi, M. Genetic aspects of the antiphospholipid syndrome: An update. Autoimmun. Rev. 2016, 15, 433–439. [Google Scholar] [CrossRef]
- Berman, H.; Ugarte-Gil, M.; Espinosa, G.; Tàssies, D.; Monteagudo, J.; Reverter, J.; Cervera, R. Can inherited thrombophilia modulate the clinical phenotype of patients with antiphospholipid syndrome. Clin. Exp. Rheumatol. 2013, 31, 926–932. [Google Scholar]
- Teruel, R.; Perez-Sanchez, C.; Corral, J.; Herranz, M.T.; Perez-Andreu, V.; Saiz, E.; Garcia-Barbera, N.; Martinez-Martinez, I.; Roldan, V.; Vicente, V.; et al. Identification of miRNAs as potential modulators of tissue factor expression in patients with systemic lupus erythematosus and antiphospholipid syndrome. J. Thromb. Haemost. 2011, 9, 1985–1992. [Google Scholar] [CrossRef]
- van den Hoogen, L.L.; Rossato, M.; Lopes, A.P.; Pandit, A.; Bekker, C.P.; Fritsch-Stork, R.D.; van Roon, J.A.; Radstake, T.R. microRNA downregulation in plasmacytoid dendritic cells in interferon-positive systemic lupus erythematosus and antiphospholipid syndrome. Rheumatology 2018, 57, 1669–1674. [Google Scholar] [CrossRef]
- Perez-Sanchez, C.; Aguirre, M.A.; Ruiz-Limon, P.; Barbarroja, N.; Jimenez-Gomez, Y.; de la Rosa, I.A.; Rodriguez-Ariza, A.; Collantes-Estevez, E.; Segui, P.; Velasco, F.; et al. Atherothrombosis-associated microRNAs in Antiphospholipid syndrome and Systemic Lupus Erythematosus patients. Sci. Rep. 2016, 6, 31375. [Google Scholar] [CrossRef]
- Zhou, H.; Wolberg, A.S.; Roubey, R.A. Characterization of monocyte tissue factor activity induced by IgG antiphospholipid antibodies and inhibition by dilazep. Blood 2004, 104, 2353–2358. [Google Scholar] [CrossRef]
- Reverter, J.-C.; Tàssies, D.; Font, J.; Monteagudo, J.; Escolar, G.S.; Ingelmo, M.; Ordinas, A. Hypercoagulable state in patients with antiphospholipid syndrome is related to high induced tissue factor expression on monocytes and to low free protein S. Arterioscler. Thromb. Vasc. Biol. 1996, 16, 1319–1326. [Google Scholar] [CrossRef]
- Kornberg, A.; Blank, M.; Kaufman, S.; Shoenfeld, Y. Induction of tissue factor-like activity in monocytes by anti-cardiolipin antibodies. J. Immunol. 1994, 153, 1328–1332. [Google Scholar]
- Dai, Y.; Huang, Y.-S.; Tang, M.; Lv, T.-Y.; Hu, C.-X.; Tan, Y.-H.; Xu, Z.-M.; Yin, Y.-B. Microarray analysis of microRNA expression in peripheral blood cells of systemic lupus erythematosus patients. Lupus 2007, 16, 939–946. [Google Scholar] [CrossRef] [PubMed]
- Perez-Andreu, V.; Teruel, R.; Corral, J.; Roldan, V.; Garcia-Barbera, N.; Salloum-Asfar, S.; Gomez-Lechon, M.J.; Bourgeois, S.; Deloukas, P.; Wadelius, M.; et al. miR-133a regulates vitamin K 2,3-epoxide reductase complex subunit 1 (VKORC1), a key protein in the vitamin K cycle. Mol. Med. 2013, 18, 1466–1472. [Google Scholar] [CrossRef] [PubMed]
- Rieder, M.J.; Reiner, A.P.; Gage, B.F.; Nickerson, D.A.; Eby, C.S.; McLeod, H.L.; Blough, D.K.; Thummel, K.E.; Veenstra, D.L.; Rettie, A.E. Effect of VKORC1 haplotypes on transcriptional regulation and warfarin dose. N. Engl. J. Med. 2005, 352, 2285–2293. [Google Scholar] [CrossRef]
- Stafford, D. The vitamin K cycle. J. Thromb. Haemost. 2005, 3, 1873–1878. [Google Scholar] [CrossRef]
- Yu, J.; Cao, X.; Zheng, Y.; Yan, L.; Wang, J. Abnormal expression of miR133a in patients with acute myocardial infarction following radical surgery for gastric cancer and the underlying mechanism. Mol. Med. Rep. 2018, 18, 5023–5029. [Google Scholar]
- Ray, M.; Gabunia, K.; Vrakas, C.N.; Herman, A.B.; Kako, F.; Kelemen, S.E.; Grisanti, L.A.; Autieri, M.V. Genetic Deletion of IL-19 (Interleukin-19) Exacerbates Atherogenesis in Il19(-/-)xLdlr(-/-) Double Knockout Mice by Dysregulation of mRNA Stability Protein HuR (Human Antigen R). Arterioscler. Thromb. Vasc. Biol. 2018, 38, 1297–1308. [Google Scholar] [CrossRef]
- Shemer, A.; Willis, R.; Gonzalez, E.B.; Romay-Penabad, Z.; Shovman, O.; Shoenfeld, Y.; Blank, M.; Amital, H. Oral administration of Domain-I of beta-2glycoprotein-I induces immunological tolerance in experimental murine antiphospholipid syndrome. J. Autoimmun. 2019, 99, 98–103. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, Q.; Song, Y.; Lai, L.; Wang, J.; Yu, H.; Cao, X.; Wang, Q. MicroRNA-98 negatively regulates IL-10 production and endotoxin tolerance in macrophages after LPS stimulation. FEBS Lett. 2011, 585, 1963–1968. [Google Scholar] [CrossRef]
- Zandman-Goddard, G.; Pierangeli, S.S.; Gertel, S.; Blank, M. Tolerogenic dendritic cells specific for beta2-glycoprotein-I Domain-I, attenuate experimental antiphospholipid syndrome. J. Autoimmun. 2014, 54, 72–80. [Google Scholar] [CrossRef]
- Nakamachi, Y.; Kawano, S.; Takenokuchi, M.; Nishimura, K.; Sakai, Y.; Chin, T.; Saura, R.; Kurosaka, M.; Kumagai, S. MicroRNA-124a is a key regulator of proliferation and monocyte chemoattractant protein 1 secretion in fibroblast-like synoviocytes from patients with rheumatoid arthritis. Arthritis. Rheum. 2009, 60, 1294–1304. [Google Scholar] [CrossRef]
- Liu, H.; Xiong, W.; Liu, F.; Lin, F.; He, J.; Liu, C.; Lin, Y.; Dong, S. MicroRNA-133b regulates the growth and migration of vascular smooth muscle cells by targeting matrix metallopeptidase 9. Pathol. Res. Pract. 2019, 215, 1083–1088. [Google Scholar] [CrossRef]
- Zheng, C.G.; Chen, B.Y.; Sun, R.H.; Mou, X.Z.; Han, F.; Li, Q.; Huang, H.J.; Liu, J.Q.; Tu, Y.X. miR-133b Downregulation Reduces Vulnerable Plaque Formation in Mice with AS through Inhibiting Macrophage Immune Responses. Mol. Ther. Nucleic. Acids. 2019, 16, 745–757. [Google Scholar] [CrossRef]
- Lv, Y.; Yi, Y.; Jia, S.; Peng, X.; Yang, H.; Guo, R. The miR-145 rs353291 C allele increases susceptibility to atherosclerosis. Front. Biosci. 2020, 25, 577–592. [Google Scholar]
- Zhang, Y.N.; Xie, B.D.; Sun, L.; Chen, W.; Jiang, S.L.; Liu, W.; Bian, F.; Tian, H.; Li, R.K. Phenotypic switching of vascular smooth muscle cells in the ‘normal region’ of aorta from atherosclerosis patients is regulated by miR-145. J. Cell Mol. Med. 2016, 20, 1049–1061. [Google Scholar] [CrossRef]
- Su, L.-C.; Xu, W.-D.; Huang, A.-F. IRAK family in inflammatory autoimmune diseases. Autoimmun. Rev. 2020, 102461. [Google Scholar] [CrossRef]
- Cheng, H.S.; Njock, M.S.; Khyzha, N.; Dang, L.T.; Fish, J.E. Noncoding RNAs regulate NF-kappaB signaling to modulate blood vessel inflammation. Front. Genet. 2014, 5, 422. [Google Scholar] [CrossRef]
- Zhou, H.; Sheng, L.; Wang, H.; Xie, H.; Mu, Y.; Wang, T.; Yan, J. Anti-β2GPI/β2GPI stimulates activation of THP-1 cells through TLR4/MD-2/MyD88 and NF-κB signaling pathways. Thromb. Res. 2013, 132, 742–749. [Google Scholar] [CrossRef]
- Xie, H.; Kong, X.; Zhou, H.; Xie, Y.; Sheng, L.; Wang, T.; Xia, L.; Yan, J. TLR4 is involved in the pathogenic effects observed in a murine model of antiphospholipid syndrome. Clin. Immunol. 2015, 160, 198–210. [Google Scholar] [CrossRef]
- Xia, L.; Xie, H.; Yu, Y.; Zhou, H.; Wang, T.; Yan, J. The Effects of NF-kappaB and c-Jun/AP-1 on the Expression of Prothrombotic and Proinflammatory Molecules Induced by Anti-beta2GPI in Mouse. PLoS ONE 2016, 11, e0147958. [Google Scholar] [CrossRef]
- Ranjbar, R.; Hesari, A.; Ghasemi, F.; Sahebkar, A. Expression of microRNAs and IRAK1 pathway genes are altered in gastric cancer patients with Helicobacter pylori infection. J. Cell Biochem. 2018, 119, 7570–7576. [Google Scholar] [CrossRef]
- Venugopal, P.; Koshy, T.; Lavu, V.; Ranga Rao, S.; Ramasamy, S.; Hariharan, S.; Venkatesan, V. Differential expression of microRNAs let-7a, miR-125b, miR-100, and miR-21 and interaction with NF-kB pathway genes in periodontitis pathogenesis. J. Cell Physiol. 2018, 233, 5877–5884. [Google Scholar] [CrossRef]
- Donners, M.M.; Beckers, L.; Lievens, D.; Munnix, I.; Heemskerk, J.; Janssen, B.J.; Wijnands, E.; Cleutjens, J.; Zernecke, A.; Weber, C.; et al. The CD40-TRAF6 axis is the key regulator of the CD40/CD40L system in neointima formation and arterial remodeling. Blood 2008, 111, 4596–4604. [Google Scholar] [CrossRef]
- Cao, Z.; Xiong, J.; Takeuchi, M.; Kurama, T.; Goeddel, D.V. TRAF6 is a signal transducer for interleukin-1. Nature 1996, 383, 443–446. [Google Scholar] [CrossRef]
- Xia, L.; Zhou, H.; Hu, L.; Xie, H.; Wang, T.; Xu, Y.; Liu, J.; Zhang, X.; Yan, J. Both NF-κB and c-Jun/AP-1 involved in anti-β2GPI/β2GPI-induced tissue factor expression in monocytes. Thromb. Haemost. 2013, 109, 643–651. [Google Scholar] [CrossRef]
- Li, Y.; Yan, L.; Zhang, W.; Hu, N.; Chen, W.; Wang, H.; Kang, M.; Ou, H. Suppression of endothelial nitric oxide synthase expression and endothelial cell proliferation by an intronic 27-ntmiRNA and it’s a novel link to AP-1. Am. J. Transl. Res. 2015, 7, 285–297. [Google Scholar]
- Bao, C.X.; Zhang, D.X.; Wang, N.N.; Zhu, X.K.; Zhao, Q.; Sun, X.L. MicroRNA-335-5p suppresses lower extremity deep venous thrombosis by targeted inhibition of PAI-1 via the TLR4 signalingpathway. J. Cell Biochem. 2018, 119, 4692–4710. [Google Scholar] [CrossRef]
- Li, N.X.; Sun, J.W.; Yu, L.M. Evaluation of the circulating MicroRNA-495 and Stat3 as prognostic and predictive biomarkers for lower extremity deep venous thrombosis. J. Cell Biochem. 2018, 119, 5262–5273. [Google Scholar] [CrossRef]
- Chen, L.J.; Yang, L.; Cheng, X.; Xue, Y.K.; Chen, L.B. Overexpression of miR-24 Is Involved in the Formation of Hypocoagulation State after Severe Trauma by Inhibiting the Synthesis of Coagulation Factor X. Dis. Markers 2017, 2017, 3649693. [Google Scholar] [CrossRef]
- Gao, J.; Ma, X.; Zhang, Y.; Guo, M.; Shi, D. The role of microRNAs in prethrombotic status associated with coronary artery disease. Thromb. Haemost. 2017, 117, 429–436. [Google Scholar] [CrossRef]
- Li, J.; Tan, M.; Xiang, Q.; Zhou, Z.; Yan, H. Thrombin-activated platelet-derived exosomes regulate endothelial cell expression of ICAM-1 via microRNA-223 during the thrombosis-inflammation response. Thromb. Res. 2017, 154, 96–105. [Google Scholar] [CrossRef]
- Sahu, A.; Jha, P.K.; Prabhakar, A.; Singh, H.D.; Gupta, N.; Chatterjee, T.; Tyagi, T.; Sharma, S.; Kumari, B.; Singh, S.; et al. MicroRNA-145 Impedes Thrombus Formation via Targeting Tissue Factor in Venous Thrombosis. EBioMedicine 2017, 26, 175–186. [Google Scholar] [CrossRef]
| miRNA | Type of Cells | Target (or Population Studied) | Activity | References |
|---|---|---|---|---|
| miR-19b | White blood cells | Tissue factor | Downregulated | [96] |
| miR-20a | ||||
| miR-296-5p | Plasma and supernatants | Tissue factor, Plasminogen activator inhibitor, Monocyte chemoattractant protein, Vascular endothelial growth factor | Upregulated | [16] |
| miR-133b | ||||
| miR-124-3p | ||||
| miR-206 | ||||
| miR-34a-5p | ||||
| miR-423-5p | ||||
| miR-122-5p | ||||
| miR-193a-5p | ||||
| miR-210-3p | ||||
| miR-192-5p | ||||
| miR-25-3p | ||||
| miR-204-5p | ||||
| miR-31-5p | ||||
| miR-205-5p | ||||
| miR-150-5p | ||||
| miR-196a-5p | ||||
| miR-885-5p | ||||
| miR-155-5p | ||||
| miR-373 | ||||
| miR-20a-5p | Downregulated | |||
| miR-30d-5p | ||||
| miR-24-3p | ||||
| miR-17-5p | ||||
| miR-30a-5p | ||||
| miR-19b-3p | ||||
| miR-191-5p | ||||
| miR-128-p | ||||
| miR-106b-5p | ||||
| miR-22-3p | ||||
| miR-26a-5p | ||||
| miR-26b-5p | ||||
| miR-376c-3p | ||||
| miR-222-3p | ||||
| miR-103a-3p | ||||
| miR-15a-5p | ||||
| miR-211-5p | ||||
| miR-145-5p | ||||
| miR-374a-5p | ||||
| miR-143-3p | ||||
| miR-125b | Dendritic cells | PAPS vs. HC | Downregulated | [97] |
| miR-127a | PAPS vs. HC | |||
| miR-150a | SLE+APS vs. HC | |||
| miR-181 a | PAPS vs. HC | |||
| miR-221a | PAPS vs. HC | |||
| miR-335 | PAPS vs. HC | |||
| miR-362 | SLE+APS vs. HC | |||
| miR-532 | SLE+APS vs. HC | |||
| miR-29a | SLE+APS vs. HC PAPS vs. HC | |||
| miR-196b | SLE+APS vs. HC | |||
| let-7g | PAPs vs. HC | |||
| miR-744 | PAPs vs. HC | |||
| miR-193b | SLE+APS vs. HC | |||
| let-7e | PAPs vs. HC | |||
| miR-30a-5p | PAPs vs. HC | |||
| miR-30d | SLE+APS vs. HC | |||
| miR-30e-3p | SLE+APS vs. HC PAPs vs. HC | |||
| mir590-3p | PAPS vs. HC | |||
| miR-126 | PAPS vs. HC | |||
| miR-1275 | SLE+APS vs. HC | |||
| miR-4443 | Neutrophils | SLE and APS vs. HC | Upregulated | [98] |
| miR-146b-5p | ||||
| miR-302d-3p | ||||
| miR-7-5p | ||||
| miR-193a-5p | ||||
| miR-320e | Downregulated | |||
| miR-346 | ||||
| miR-155-5p | ||||
| miR-22-3p | ||||
| miR-486-3p | ||||
| miR-15a-5p | ||||
| miR-144-3p | ||||
| miR-186-5p | ||||
| Let-7g-5p | ||||
| miR-151a-3p | ||||
| miR-32-5p | ||||
| miR-27b-3p | ||||
| miR-548aa | ||||
| miR-194-5p | ||||
| miR-4431 | ||||
| miR-21-5p | ||||
| miR-324-5p | ||||
| miR-374a-5p | ||||
| miR-132-3p | ||||
| miR-126-3p | ||||
| miR-450-5p | ||||
| miR-140-5p | ||||
| miR-494 | ||||
| miR-301a-3p | ||||
| miR-142-3p | ||||
| miR-92a-3p | ||||
| miR-30e-5p | ||||
| miR-590-5p | ||||
| miR-339-3p | ||||
| miR-630 | ||||
| miR-71-5p | ||||
| miR-106b-5p | ||||
| miR-1537 | ||||
| miR-197-3p | ||||
| miR-503 | ||||
| miR-582-3p | ||||
| miR-340-5p | ||||
| miR-27a-3p | ||||
| miR-26b-5p | ||||
| miR-338-3p | ||||
| miR-30b-5p | ||||
| miR-1260b | ||||
| miR-302b-3p | ||||
| miR-484 | ||||
| miR-532-5p | ||||
| miR-4454 | ||||
| miR-26a-5p | ||||
| miR-17-5p | ||||
| miR-299-3p | ||||
| miR-29b-3p | ||||
| miR-125-5p | ||||
| miR-875-5p | ||||
| miR-142-5p | ||||
| miR-363-3p |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kotyla, P.J.; Islam, M.A. MicroRNA (miRNA): A New Dimension in the Pathogenesis of Antiphospholipid Syndrome (APS). Int. J. Mol. Sci. 2020, 21, 2076. https://doi.org/10.3390/ijms21062076
Kotyla PJ, Islam MA. MicroRNA (miRNA): A New Dimension in the Pathogenesis of Antiphospholipid Syndrome (APS). International Journal of Molecular Sciences. 2020; 21(6):2076. https://doi.org/10.3390/ijms21062076
Chicago/Turabian StyleKotyla, Przemysław J., and Md Asiful Islam. 2020. "MicroRNA (miRNA): A New Dimension in the Pathogenesis of Antiphospholipid Syndrome (APS)" International Journal of Molecular Sciences 21, no. 6: 2076. https://doi.org/10.3390/ijms21062076
APA StyleKotyla, P. J., & Islam, M. A. (2020). MicroRNA (miRNA): A New Dimension in the Pathogenesis of Antiphospholipid Syndrome (APS). International Journal of Molecular Sciences, 21(6), 2076. https://doi.org/10.3390/ijms21062076






