Regulation of the Proteolytic Activity of Cysteine Cathepsins by Oxidants
Abstract
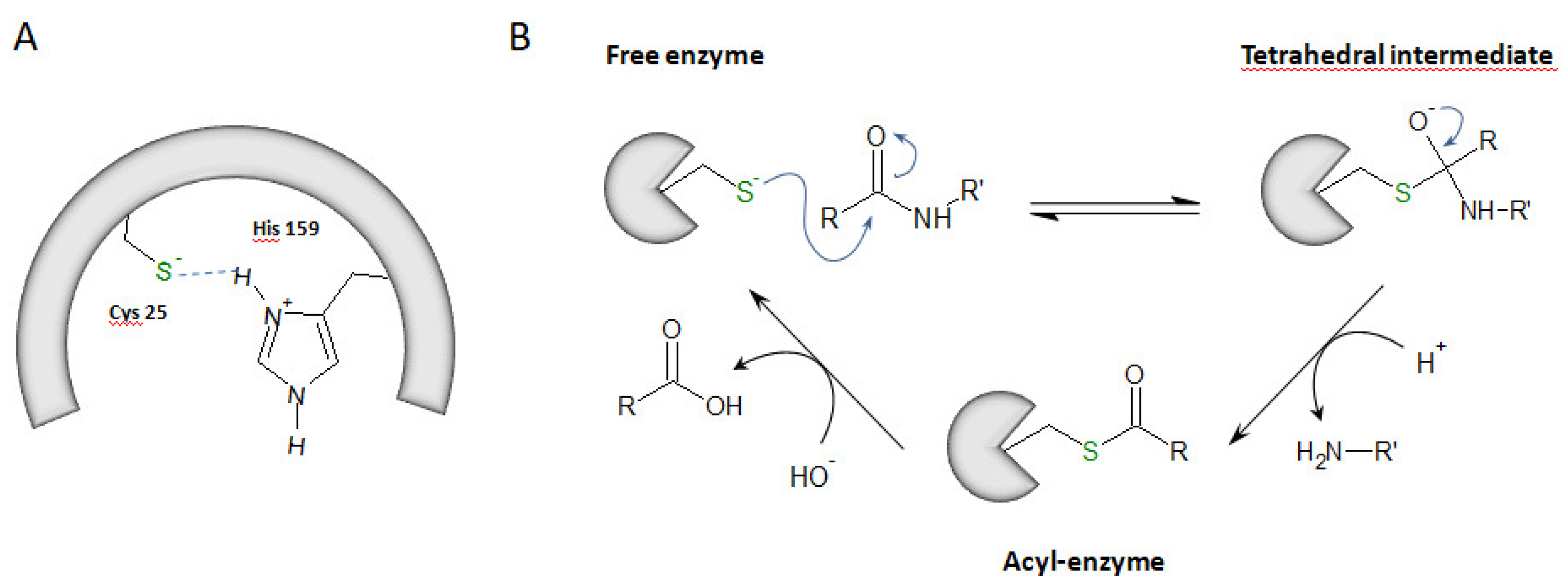
1. Cysteine Cathepsins
2. Cysteine Cathepsins in Pulmonary Diseases
3. Inactivation of Cysteine Cathepsins by Oxidants
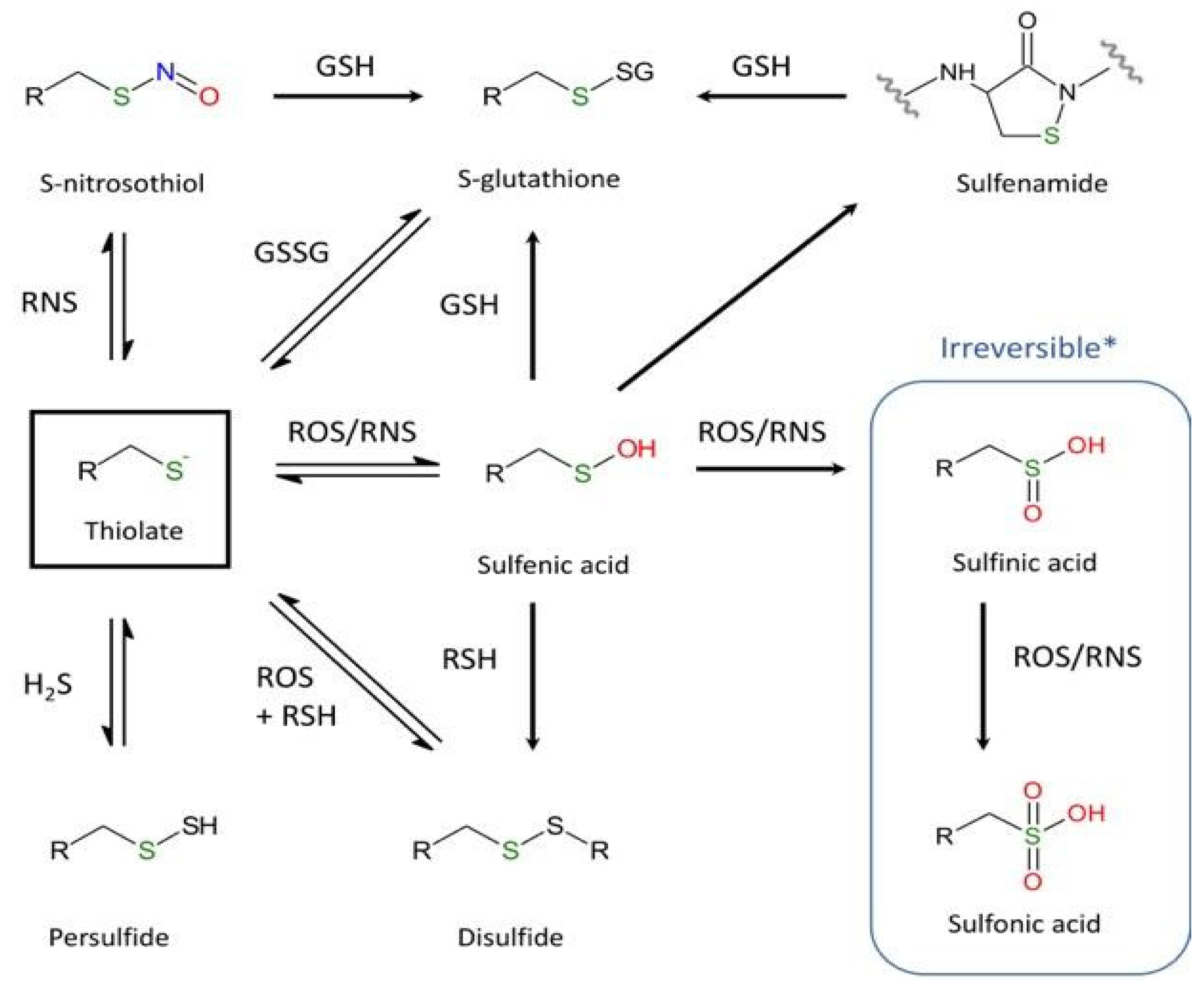
3.1. Oxidation of Thiols: A General Context
3.2. Cysteine Cathepsins and Oxidants
3.2.1. Inactivation by Reactive Nitrogen Species
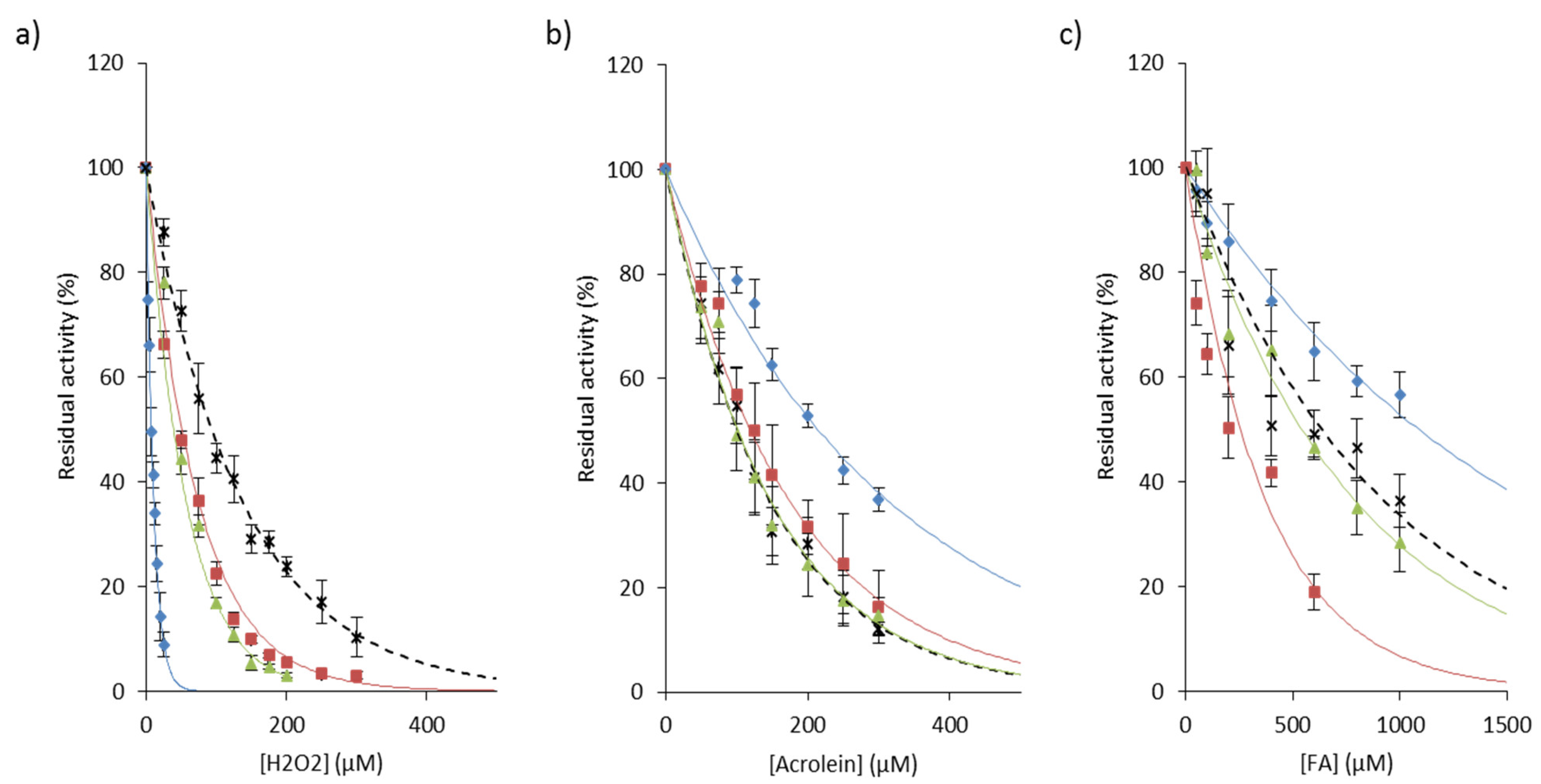
3.2.2. Inactivation by Reactive Oxygen Species
3.2.3. Redox Regulation
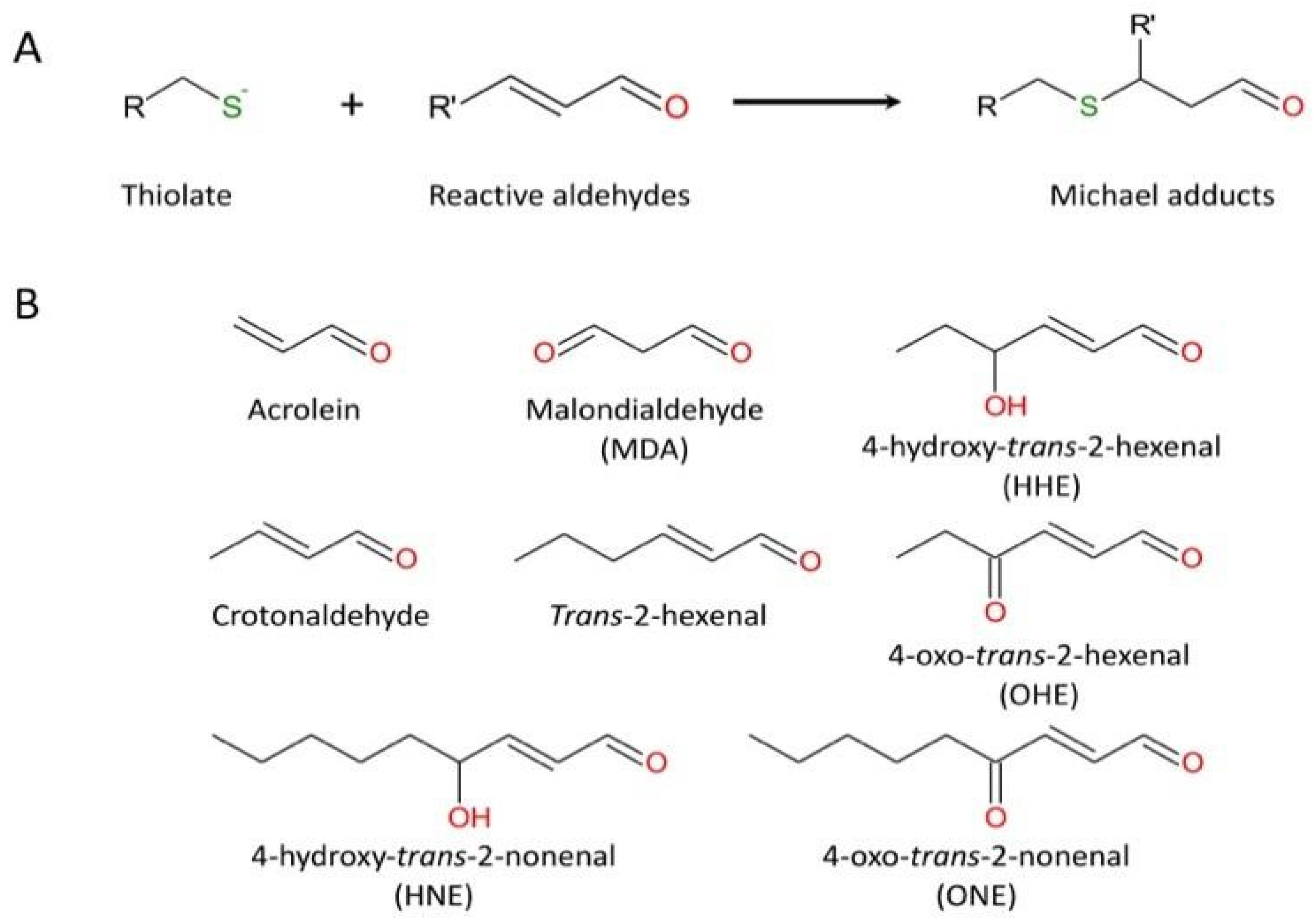
3.2.4. Carbonylation of Cysteine Cathepsins
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| C-4S | Chondroitin-4-sulfate |
| Cat | Cathepsin |
| CF | Cystic fibrosis |
| COPD | Chronic obstructive pulmonary disease |
| CSE | Cigarette smoke extract |
| ECM | Extracellular matrix |
| GAG | Glycosaminoglycan |
| GSH | Reduced glutathione |
| GSNO | S-nitrosoglutathione |
| GSSG | Oxidized glutathione |
| H2O2 | Hydrogen peroxide |
| HOCl | Hypochlorous acid |
| HNO | Nitroxyl |
| IFN | Interferon |
| IL | Interleukin |
| LDL | Low-density protein |
| MHC | Major histocompatibility complex |
| MMP | Matrix metalloproteinase |
| NO | Nitric oxide |
| ONOO− | Peroxynitrite |
| RNS | Reactive nitrogen species |
| ROS | Reactive oxygen species |
| SP | Surfactant protein |
| TGF | Transforming growth factor |
| Enzymes | Cathepsin B, (EC 3.4.22.1), cathepsin C (EC 3.4.14.1), cathepsin H (EC 3.4.22.16), cathepsin K (EC 3.4.22.38), cathepsin L (EC 3.4.22.15), cathepsin S (EC 3.4.22.27), papain (EC 3.4.22.2) |
References
- Quesada, V.; Ordóñez, G.R.; Sánchez, L.M.; Puente, X.S.; López-Otín, C. The Degradome database: Mammalian proteases and diseases of proteolysis. Nucleic Acids Res. 2009, 37, D239–D243. [Google Scholar] [CrossRef]
- López-Otín, C.; Bond, J.S. Proteases: Multifunctional enzymes in life and disease. J. Biol. Chem. 2008, 283, 30433–30437. [Google Scholar] [CrossRef] [PubMed]
- Otto, H.-H.; Schirmeister, T. Cysteine Proteases and Their Inhibitors. Chem. Rev. 1997, 97, 133–172. [Google Scholar] [CrossRef] [PubMed]
- Rawlings, N.D.; Barrett, A.J. Families of cysteine peptidases. Meth. Enzymol. 1994, 244, 461–486. [Google Scholar] [PubMed]
- Turk, V.; Stoka, V.; Vasiljeva, O.; Renko, M.; Sun, T.; Turk, B.; Turk, D. Cysteine cathepsins: From structure, function and regulation to new frontiers. Biochim. Biophys. Acta 2012, 1824, 68–88. [Google Scholar] [CrossRef]
- Lecaille, F.; Kaleta, J.; Brömme, D. Human and parasitic papain-like cysteine proteases: Their role in physiology and pathology and recent developments in inhibitor design. Chem. Rev. 2002, 102, 4459–4488. [Google Scholar] [CrossRef]
- Wilkinson, R.D.A.; Williams, R.; Scott, C.J.; Burden, R.E. Cathepsin S: Therapeutic, diagnostic, and prognostic potential. Biol. Chem. 2015, 396, 867–882. [Google Scholar] [CrossRef]
- Kasabova, M.; Saidi, A.; Naudin, C.; Sage, J.; Lecaille, F.; Lalmanach, G. Cysteine Cathepsins: Markers and Therapy Targets in Lung Disorders. Clin. Rev. Bone Miner. Metab. 2011, 9, 148–161. [Google Scholar] [CrossRef]
- Lalmanach, G.; Saidi, A.; Marchand-Adam, S.; Lecaille, F.; Kasabova, M. Cysteine cathepsins and cystatins: From ancillary tasks to prominent status in lung diseases. Biol. Chem. 2015, 396, 111–130. [Google Scholar] [CrossRef]
- Goulet, B.; Baruch, A.; Moon, N.-S.; Poirier, M.; Sansregret, L.L.; Erickson, A.; Bogyo, M.; Nepveu, A. A cathepsin L isoform that is devoid of a signal peptide localizes to the nucleus in S phase and processes the CDP/Cux transcription factor. Mol. Cell 2004, 14, 207–219. [Google Scholar] [CrossRef]
- Baici, A.; Müntener, K.; Willimann, A.; Zwicky, R. Regulation of human cathepsin B by alternative mRNA splicing: Homeostasis, fatal errors and cell death. Biol. Chem. 2006, 387, 1017–1021. [Google Scholar] [CrossRef] [PubMed]
- Brix, K.; Dunkhorst, A.; Mayer, K.; Jordans, S. Cysteine cathepsins: Cellular roadmap to different functions. Biochimie 2008, 90, 194–207. [Google Scholar] [CrossRef] [PubMed]
- Katunuma, N. Posttranslational processing and modification of cathepsins and cystatins. J. Signal Transduct. 2010, 2010, 375345. [Google Scholar] [CrossRef] [PubMed]
- Nissler, K.; Strubel, W.; Kreusch, S.; Rommerskirch, W.; Weber, E.; Wiederanders, B. The half-life of human procathepsin S. Eur. J. Biochem. 1999, 263, 717–725. [Google Scholar] [CrossRef] [PubMed]
- Vidak, E.; Javoršek, U.; Vizovišek, M.; Turk, B. Cysteine Cathepsins and their Extracellular Roles: Shaping the Microenvironment. Cells 2019, 8, 264. [Google Scholar] [CrossRef] [PubMed]
- Brömme, D.; Panwar, P.; Turan, S. Cathepsin K osteoporosis trials, pycnodysostosis and mouse deficiency models: Commonalities and differences. Expert Opin. Drug Discov. 2016, 11, 457–472. [Google Scholar] [CrossRef] [PubMed]
- Korkmaz, B.; Caughey, G.H.; Chapple, I.; Gauthier, F.; Hirschfeld, J.; Jenne, D.E.; Kettritz, R.; Lalmanach, G.; Lamort, A.-S.; Lauritzen, C.; et al. Therapeutic targeting of cathepsin C: From pathophysiology to treatment. Pharmacol. Ther. 2018, 190, 202–236. [Google Scholar] [CrossRef]
- Kramer, L.; Turk, D.; Turk, B. The Future of Cysteine Cathepsins in Disease Management. Trends Pharmacol. Sci. 2017, 38, 873–898. [Google Scholar] [CrossRef]
- Turk, B. Targeting proteases: Successes, failures and future prospects. Nat. Rev. Drug Discov. 2006, 5, 785–799. [Google Scholar] [CrossRef]
- Barrett, A.J. The cystatins: A diverse superfamily of cysteine peptidase inhibitors. Biomed. Biochim. Acta 1986, 45, 1363–1374. [Google Scholar]
- Rawlings, N.D.; Barrett, A.J.; Thomas, P.D.; Huang, X.; Bateman, A.; Finn, R.D. The MEROPS database of proteolytic enzymes, their substrates and inhibitors in 2017 and a comparison with peptidases in the PANTHER database. Nucleic Acids Res. 2018, 46, D624–D632. [Google Scholar] [CrossRef] [PubMed]
- Turk, D.; Janjić, V.; Stern, I.; Podobnik, M.; Lamba, D.; Dahl, S.W.; Lauritzen, C.; Pedersen, J.; Turk, V.; Turk, B. Structure of human dipeptidyl peptidase I (cathepsin C): Exclusion domain added to an endopeptidase framework creates the machine for activation of granular serine proteases. EMBO J. 2001, 20, 6570–6582. [Google Scholar] [CrossRef] [PubMed]
- Musil, D.; Zucic, D.; Turk, D.; Engh, R.A.; Mayr, I.; Huber, R.; Popovic, T.; Turk, V.; Towatari, T.; Katunuma, N. The refined 2.15 A X-ray crystal structure of human liver cathepsin B: The structural basis for its specificity. EMBO J. 1991, 10, 2321–2330. [Google Scholar] [CrossRef] [PubMed]
- Nägler, D.K.; Zhang, R.; Tam, W.; Sulea, T.; Purisima, E.O.; Ménard, R. Human cathepsin X: A cysteine protease with unique carboxypeptidase activity. Biochemistry 1999, 38, 12648–12654. [Google Scholar] [CrossRef]
- Storer, A.C.; Ménard, R. Catalytic mechanism in papain family of cysteine peptidases. Meth. Enzymol. 1994, 244, 486–500. [Google Scholar]
- Rzychon, M.; Chmiel, D.; Stec-Niemczyk, J. Modes of inhibition of cysteine proteases. Acta Biochim. Pol. 2004, 51, 861–873. [Google Scholar]
- Brix, K.; Scott, C.J.; Heck, M.M.S. Compartmentalization of Proteolysis. In Proteases: Structure and Function; Brix, K., Stöcker, W., Eds.; Springer: Vienna, Austria, 2013; pp. 85–125. ISBN 978-3-7091-0885-7. [Google Scholar]
- Wex, T.; Bühling, F.; Wex, H.; Günther, D.; Malfertheiner, P.; Weber, E.; Brömme, D. Human cathepsin W, a cysteine protease predominantly expressed in NK cells, is mainly localized in the endoplasmic reticulum. J. Immunol. 2001, 167, 2172–2178. [Google Scholar] [CrossRef]
- Chapman, H.A.; Munger, J.S.; Shi, G.P. The role of thiol proteases in tissue injury and remodeling. Am. J. Respir. Crit. Care Med. 1994, 150, S155–S159. [Google Scholar] [CrossRef]
- Pérez-Ramos, J.; de Lourdes Segura-Valdez, M.; Vanda, B.; Selman, M.; Pardo, A. Matrix metalloproteinases 2, 9, and 13, and tissue inhibitors of metalloproteinases 1 and 2 in experimental lung silicosis. Am. J. Respir. Crit. Care Med. 1999, 160, 1274–1282. [Google Scholar] [CrossRef]
- Scabilloni, J.F.; Wang, L.; Antonini, J.M.; Roberts, J.R.; Castranova, V.; Mercer, R.R. Matrix metalloproteinase induction in fibrosis and fibrotic nodule formation due to silica inhalation. Am. J. Physiol. Lung Cell Mol. Physiol. 2005, 288, L709–L717. [Google Scholar] [CrossRef]
- Lecaille, F.; Brömme, D.; Lalmanach, G. Biochemical properties and regulation of cathepsin K activity. Biochimie 2008, 90, 208–226. [Google Scholar] [CrossRef] [PubMed]
- Ferrall-Fairbanks, M.C.; Kieslich, C.A.; Platt, M.O. Reassessing enzyme kinetics: Considering protease-as-substrate interactions in proteolytic networks. Proc. Natl. Acad. Sci. USA 2020, 117, 3307–3318. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, Y.; Li, Z.; Greenbaum, D.; Bogyo, M.; Weber, E.; Brömme, D. Cathepsin V, a novel and potent elastolytic activity expressed in activated macrophages. J. Biol. Chem. 2004, 279, 36761–36770. [Google Scholar] [CrossRef] [PubMed]
- Bühling, F.; Kouadio, M.; Chwieralski, C.E.; Kern, U.; Hohlfeld, J.M.; Klemm, N.; Friedrichs, N.; Roth, W.; Deussing, J.M.; Peters, C.; et al. Gene targeting of the cysteine peptidase cathepsin H impairs lung surfactant in mice. PLoS ONE 2011, 6, e26247. [Google Scholar] [CrossRef] [PubMed]
- Brasch, F.; Ten Brinke, A.; Johnen, G.; Ochs, M.; Kapp, N.; Müller, K.M.; Beers, M.F.; Fehrenbach, H.; Richter, J.; Batenburg, J.J.; et al. Involvement of cathepsin H in the processing of the hydrophobic surfactant-associated protein C in type II pneumocytes. Am. J. Respir. Cell Mol. Biol. 2002, 26, 659–670. [Google Scholar] [CrossRef] [PubMed]
- Guttentag, S.; Robinson, L.; Zhang, P.; Brasch, F.; Bühling, F.; Beers, M. Cysteine protease activity is required for surfactant protein B processing and lamellar body genesis. Am. J. Respir. Cell Mol. Biol. 2003, 28, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Chapman, H.A.; Riese, R.J.; Shi, G.P. Emerging roles for cysteine proteases in human biology. Annu. Rev. Physiol. 1997, 59, 63–88. [Google Scholar] [CrossRef]
- Taggart, C.; Mall, M.A.; Lalmanach, G.; Cataldo, D.; Ludwig, A.; Janciauskiene, S.; Heath, N.; Meiners, S.; Overall, C.M.; Schultz, C.; et al. Protean proteases: At the cutting edge of lung diseases. Eur. Respir. J. 2017, 49, 1501200. [Google Scholar] [CrossRef]
- Menou, A.; Duitman, J.; Crestani, B. The impaired proteases and anti-proteases balance in Idiopathic Pulmonary Fibrosis. Matrix Biol. 2018, 68–69, 382–403. [Google Scholar] [CrossRef]
- Castranova, V.; Vallyathan, V. Silicosis and coal workers’ pneumoconiosis. Environ. Health Perspect. 2000, 108 Suppl. 4, 675–684. [Google Scholar]
- Mossman, B.T.; Churg, A. Mechanisms in the pathogenesis of asbestosis and silicosis. Am. J. Respir. Crit. Care Med. 1998, 157, 1666–1680. [Google Scholar] [CrossRef] [PubMed]
- Perdereau, C.; Godat, E.; Maurel, M.-C.; Hazouard, E.; Diot, E.; Lalmanach, G. Cysteine cathepsins in human silicotic bronchoalveolar lavage fluids. Biochim. Biophys. Acta 2006, 1762, 351–356. [Google Scholar] [CrossRef] [PubMed]
- Bühling, F.; Waldburg, N.; Reisenauer, A.; Heimburg, A.; Golpon, H.; Welte, T. Lysosomal cysteine proteases in the lung: Role in protein processing and immunoregulation. Eur. Respir. J. 2004, 23, 620–628. [Google Scholar] [CrossRef] [PubMed]
- van den Brûle, S.; Misson, P.; Bühling, F.; Lison, D.; Huaux, F. Overexpression of cathepsin K during silica-induced lung fibrosis and control by TGF-beta. Respir. Res. 2005, 6, 84. [Google Scholar] [CrossRef] [PubMed]
- Plantier, L.; Cazes, A.; Dinh-Xuan, A.-T.; Bancal, C.; Marchand-Adam, S.; Crestani, B. Physiology of the lung in idiopathic pulmonary fibrosis. Eur. Respir. Rev. 2018, 27, 170062. [Google Scholar] [CrossRef]
- Mora, A.L.; Rojas, M.; Pardo, A.; Selman, M. Emerging therapies for idiopathic pulmonary fibrosis, a progressive age-related disease. Nat. Rev. Drug Discov. 2017, 16, 755–772. [Google Scholar] [CrossRef]
- Border, W.A.; Noble, N.A. Transforming growth factor beta in tissue fibrosis. N. Engl. J. Med. 1994, 331, 1286–1292. [Google Scholar]
- Kasabova, M.; Joulin-Giet, A.; Lecaille, F.; Gilmore, B.F.; Marchand-Adam, S.; Saidi, A.; Lalmanach, G. Regulation of TGF-β1-driven differentiation of human lung fibroblasts: Emerging roles of cathepsin B and cystatin C. J. Biol. Chem. 2014, 289, 16239–16251. [Google Scholar] [CrossRef]
- Bühling, F.; Röcken, C.; Brasch, F.; Hartig, R.; Yasuda, Y.; Saftig, P.; Brömme, D.; Welte, T. Pivotal role of cathepsin K in lung fibrosis. Am. J. Pathol. 2004, 164, 2203–2216. [Google Scholar] [CrossRef]
- Zhang, D.; Leung, N.; Weber, E.; Saftig, P.; Brömme, D. The effect of cathepsin K deficiency on airway development and TGF-β1 degradation. Respir. Res. 2011, 12, 72. [Google Scholar] [CrossRef]
- Zhang, X.; Zhou, Y.; Yu, X.; Huang, Q.; Fang, W.; Li, J.; Bonventre, J.V.; Sukhova, G.K.; Libby, P.; Shi, G.-P. Differential Roles of Cysteinyl Cathepsins in TGF-β Signaling and Tissue Fibrosis. iScience 2019, 19, 607–622. [Google Scholar] [CrossRef] [PubMed]
- Vankeerberghen, A.; Cuppens, H.; Cassiman, J.-J. The cystic fibrosis transmembrane conductance regulator: An intriguing protein with pleiotropic functions. J. Cyst. Fibros. 2002, 1, 13–29. [Google Scholar] [CrossRef]
- Tate, S.; MacGregor, G.; Davis, M.; Innes, J.A.; Greening, A.P. Airways in cystic fibrosis are acidified: Detection by exhaled breath condensate. Thorax 2002, 57, 926–929. [Google Scholar] [CrossRef] [PubMed]
- Laguna, T.A.; Williams, C.B.; Nunez, M.G.; Welchlin-Bradford, C.; Moen, C.E.; Reilly, C.S.; Wendt, C.H. Biomarkers of inflammation in infants with cystic fibrosis. Respir. Res. 2018, 19, 6. [Google Scholar] [CrossRef] [PubMed]
- Martin, S.L.; Moffitt, K.L.; McDowell, A.; Greenan, C.; Bright-Thomas, R.J.; Jones, A.M.; Webb, A.K.; Elborn, J.S. Association of airway cathepsin B and S with inflammation in cystic fibrosis. Pediatr. Pulmonol. 2010, 45, 860–868. [Google Scholar] [CrossRef] [PubMed]
- Small, D.M.; Brown, R.R.; Doherty, D.F.; Abladey, A.; Zhou-Suckow, Z.; Delaney, R.J.; Kerrigan, L.; Dougan, C.M.; Borensztajn, K.S.; Holsinger, L.; et al. Targeting of cathepsin S reduces cystic fibrosis-like lung disease. Eur. Respir. J. 2019, 53, 1801523. [Google Scholar] [CrossRef] [PubMed]
- Naudin, C.; Joulin-Giet, A.; Couetdic, G.; Plésiat, P.; Szymanska, A.; Gorna, E.; Gauthier, F.; Kasprzykowski, F.; Lecaille, F.; Lalmanach, G. Human cysteine cathepsins are not reliable markers of infection by Pseudomonas aeruginosa in cystic fibrosis. PLoS ONE 2011, 6, e25577. [Google Scholar] [CrossRef]
- Andrault, P.-M.; Samsonov, S.A.; Weber, G.; Coquet, L.; Nazmi, K.; Bolscher, J.G.M.; Lalmanach, A.-C.; Jouenne, T.; Brömme, D.; Pisabarro, M.T.; et al. Antimicrobial Peptide LL-37 Is Both a Substrate of Cathepsins S and K and a Selective Inhibitor of Cathepsin L. Biochemistry 2015, 54, 2785–2798. [Google Scholar] [CrossRef]
- Lecaille, F.; Naudin, C.; Sage, J.; Joulin-Giet, A.; Courty, A.; Andrault, P.-M.; Veldhuizen, R.A.W.; Possmayer, F.; Lalmanach, G. Specific cleavage of the lung surfactant protein A by human cathepsin S may impair its antibacterial properties. Int. J. Biochem. Cell Biol. 2013, 45, 1701–1709. [Google Scholar] [CrossRef]
- Rogan, M.P.; Taggart, C.C.; Greene, C.M.; Murphy, P.G.; O’Neill, S.J.; McElvaney, N.G. Loss of microbicidal activity and increased formation of biofilm due to decreased lactoferrin activity in patients with cystic fibrosis. J. Infect. Dis. 2004, 190, 1245–1253. [Google Scholar] [CrossRef]
- Taggart, C.C.; Lowe, G.J.; Greene, C.M.; Mulgrew, A.T.; O’Neill, S.J.; Levine, R.L.; McElvaney, N.G. Cathepsin B, L, and S cleave and inactivate secretory leucoprotease inhibitor. J. Biol. Chem. 2001, 276, 33345–33352. [Google Scholar] [CrossRef] [PubMed]
- Taggart, C.C.; Greene, C.M.; Smith, S.G.; Levine, R.L.; McCray, P.B.; O’Neill, S.; McElvaney, N.G. Inactivation of human beta-defensins 2 and 3 by elastolytic cathepsins. J. Immunol. 2003, 171, 931–937. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.A.; Barrett, A.J.; Mason, R.W. Cathepsin L inactivates alpha 1-proteinase inhibitor by cleavage in the reactive site region. J. Biol. Chem. 1986, 261, 14748–14751. [Google Scholar] [PubMed]
- Hamid, Q.; Tulic, M. Immunobiology of asthma. Annu. Rev. Physiol. 2009, 71, 489–507. [Google Scholar] [CrossRef]
- Mukherjee, M.; Nair, P. Autoimmune Responses in Severe Asthma. Allergy Asthma Immunol. Res. 2018, 10, 428–447. [Google Scholar] [CrossRef]
- Fajardo, I.; Svensson, L.; Bucht, A.; Pejler, G. Increased levels of hypoxia-sensitive proteins in allergic airway inflammation. Am. J. Respir. Crit. Care Med. 2004, 170, 477–484. [Google Scholar] [CrossRef]
- Cimerman, N.; Brguljan, P.M.; Krasovec, M.; Suskovic, S.; Kos, J. Circadian and concentration profile of cathepsin S in sera from healthy subjects and asthmatic patients. Pflugers Arch. 2001, 442, R204–R206. [Google Scholar] [CrossRef]
- Honey, K.; Rudensky, A.Y. Lysosomal cysteine proteases regulate antigen presentation. Nat. Rev. Immunol. 2003, 3, 472–482. [Google Scholar] [CrossRef]
- Shi, G.P.; Bryant, R.A.; Riese, R.; Verhelst, S.; Driessen, C.; Li, Z.; Bromme, D.; Ploegh, H.L.; Chapman, H.A. Role for cathepsin F in invariant chain processing and major histocompatibility complex class II peptide loading by macrophages. J. Exp. Med. 2000, 191, 1177–1186. [Google Scholar] [CrossRef]
- Somoza, J.R.; Palmer, J.T.; Ho, J.D. The crystal structure of human cathepsin F and its implications for the development of novel immunomodulators. J. Mol. Biol. 2002, 322, 559–568. [Google Scholar] [CrossRef]
- Kharitonov, S.A.; Barnes, P.J. Exhaled markers of inflammation. Curr. Opin. Allergy Clin. Immunol. 2001, 1, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zheng, T.; Zhu, Z.; Homer, R.J.; Riese, R.J.; Chapman, H.A.; Shapiro, S.D.; Elias, J.A. Interferon gamma induction of pulmonary emphysema in the adult murine lung. J. Exp. Med. 2000, 192, 1587–1600. [Google Scholar] [CrossRef] [PubMed]
- Zheng, T.; Zhu, Z.; Wang, Z.; Homer, R.J.; Ma, B.; Riese, R.J.; Chapman, H.A.; Shapiro, S.D.; Elias, J.A. Inducible targeting of IL-13 to the adult lung causes matrix metalloproteinase- and cathepsin-dependent emphysema. J. Clin. Investig. 2000, 106, 1081–1093. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.-J.; Homer, R.J.; Gallo, A.; Lee, C.G.; Crothers, K.A.; Cho, S.J.; Rochester, C.; Cain, H.; Chupp, G.; Yoon, H.J.; et al. IL-18 is induced and IL-18 receptor alpha plays a critical role in the pathogenesis of cigarette smoke-induced pulmonary emphysema and inflammation. J. Immunol. 2007, 178, 1948–1959. [Google Scholar] [CrossRef] [PubMed]
- Andrault, P.-M.; Schamberger, A.C.; Chazeirat, T.; Sizaret, D.; Renault, J.; Staab-Weijnitz, C.A.; Hennen, E.; Petit-Courty, A.; Wartenberg, M.; Saidi, A.; et al. Cigarette smoke induces overexpression of active human cathepsin S in lungs from current smokers with or without COPD. Am. J. Physiol. Lung Cell Mol. Physiol. 2019, 317, L625–L638. [Google Scholar] [CrossRef] [PubMed]
- Wartenberg, M.; Andrault, P.-M.; Saidi, A.; Bigot, P.; Nadal-Desbarats, L.; Lecaille, F.; Lalmanach, G. Oxidation of cathepsin S by major chemicals of cigarette smoke. Free Radic. Biol. Med. 2020, 150, 53–65. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, J.A.; Gómez, I.; Chiarello, D.I.; Salsoso, R.; Klein, A.D.; Guzmán-Gutiérrez, E.; Toledo, F.; Sobrevia, L. Role of proteases in dysfunctional placental vascular remodelling in preeclampsia. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 165448. [Google Scholar] [CrossRef]
- Zhang, X.; Luo, S.; Wang, M.; Shi, G.-P. Cysteinyl cathepsins in cardiovascular diseases. Biochim. Biophys. Acta Proteins Proteom. 2020, 1868, 140360. [Google Scholar] [CrossRef]
- Blomgran, R.; Zheng, L.; Stendahl, O. Cathepsin-cleaved Bid promotes apoptosis in human neutrophils via oxidative stress-induced lysosomal membrane permeabilization. J. Leukoc. Biol. 2007, 81, 1213–1223. [Google Scholar] [CrossRef]
- Repnik, U.; Stoka, V.; Turk, V.; Turk, B. Lysosomes and lysosomal cathepsins in cell death. Biochim. Biophys. Acta 2012, 1824, 22–33. [Google Scholar] [CrossRef]
- Ni, J.; Wu, Z.; Stoka, V.; Meng, J.; Hayashi, Y.; Peters, C.; Qing, H.; Turk, V.; Nakanishi, H. Increased expression and altered subcellular distribution of cathepsin B in microglia induce cognitive impairment through oxidative stress and inflammatory response in mice. Aging Cell 2019, 18, e12856. [Google Scholar] [CrossRef] [PubMed]
- Bai, H.; Yang, B.; Yu, W.; Xiao, Y.; Yu, D.; Zhang, Q. Cathepsin B links oxidative stress to the activation of NLRP3 inflammasome. Exp. Cell Res. 2018, 362, 180–187. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, H. Microglial cathepsin B as a key driver of inflammatory brain diseases and brain aging. Neural Regen. Res. 2020, 15, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Poole, L.B. The basics of thiols and cysteines in redox biology and chemistry. Free Radic. Biol. Med. 2015, 80, 148–157. [Google Scholar] [CrossRef]
- Rullmann, J.A.; Bellido, M.N.; van Duijnen, P.T. The active site of papain. All-atom study of interactions with protein matrix and solvent. J. Mol. Biol. 1989, 206, 101–118. [Google Scholar] [CrossRef]
- Giles, N.M.; Watts, A.B.; Giles, G.I.; Fry, F.H.; Littlechild, J.A.; Jacob, C. Metal and redox modulation of cysteine protein function. Chem. Biol. 2003, 10, 677–693. [Google Scholar] [CrossRef]
- Hawkins, C.L.; Davies, M.J. Detection, identification, and quantification of oxidative protein modifications. J. Biol. Chem. 2019, 294, 19683–19708. [Google Scholar] [CrossRef]
- Reddie, K.G.; Carroll, K.S. Expanding the functional diversity of proteins through cysteine oxidation. Curr. Opin. Chem. Biol. 2008, 12, 746–754. [Google Scholar] [CrossRef]
- Rudyk, O.; Eaton, P. Biochemical methods for monitoring protein thiol redox states in biological systems. Redox Biol. 2014, 2, 803–813. [Google Scholar] [CrossRef]
- Leonard, S.E.; Carroll, K.S. Chemical “omics” approaches for understanding protein cysteine oxidation in biology. Curr. Opin. Chem. Biol. 2011, 15, 88–102. [Google Scholar] [CrossRef]
- Roos, G.; Foloppe, N.; Messens, J. Understanding the pK(a) of redox cysteines: The key role of hydrogen bonding. Antioxid. Redox Signal. 2013, 18, 94–127. [Google Scholar] [CrossRef] [PubMed]
- Moreno, M.-L.; Escobar, J.; Izquierdo-Álvarez, A.; Gil, A.; Pérez, S.; Pereda, J.; Zapico, I.; Vento, M.; Sabater, L.; Marina, A.; et al. Disulfide stress: A novel type of oxidative stress in acute pancreatitis. Free Radic. Biol. Med. 2014, 70, 265–277. [Google Scholar] [CrossRef] [PubMed]
- Biteau, B.; Labarre, J.; Toledano, M.B. ATP-dependent reduction of cysteine-sulphinic acid by S. cerevisiae sulphiredoxin. Nature 2003, 425, 980–984. [Google Scholar] [CrossRef] [PubMed]
- Woo, H.A.; Jeong, W.; Chang, T.-S.; Park, K.J.; Park, S.J.; Yang, J.S.; Rhee, S.G. Reduction of cysteine sulfinic acid by sulfiredoxin is specific to 2-cys peroxiredoxins. J. Biol. Chem. 2005, 280, 3125–3128. [Google Scholar] [CrossRef]
- Thomas, J.A.; Mallis, R.; Sies, H. Protein S -Thiolation, S-Nitrosylation, and Irreversible Sulfhydryl Oxidation: Roles in Redox Regulation. In Cellular Implications of Redox Signaling; Imperial College Press: London, UK, 2003; pp. 141–174. ISBN 978-1-86094-331-7. [Google Scholar]
- Ying, J.; Clavreul, N.; Sethuraman, M.; Adachi, T.; Cohen, R.A. Thiol oxidation in signaling and response to stress: Detection and quantification of physiological and pathophysiological thiol modifications. Free Radic. Biol. Med. 2007, 43, 1099–1108. [Google Scholar] [CrossRef]
- Hamann, M.; Zhang, T.; Hendrich, S.; Thomas, J.A. Quantitation of protein sulfinic and sulfonic acid, irreversibly oxidized protein cysteine sites in cellular proteins. Meth. Enzymol. 2002, 348, 146–156. [Google Scholar]
- Cai, J.; Bhatnagar, A.; Pierce, W.M. Protein modification by acrolein: Formation and stability of cysteine adducts. Chem. Res. Toxicol. 2009, 22, 708–716. [Google Scholar] [CrossRef]
- Jordans, S.; Jenko-Kokalj, S.; Kühl, N.M.; Tedelind, S.; Sendt, W.; Brömme, D.; Turk, D.; Brix, K. Monitoring compartment-specific substrate cleavage by cathepsins B, K, L, and S at physiological pH and redox conditions. BMC Biochem. 2009, 10, 23. [Google Scholar] [CrossRef]
- Giles, N.M.; Giles, G.I.; Jacob, C. Multiple roles of cysteine in biocatalysis. Biochem. Biophys. Res. Commun. 2003, 300, 1–4. [Google Scholar] [CrossRef]
- Lockwood, T.D. Redox control of protein degradation. Antioxid. Redox Signal. 2000, 2, 851–878. [Google Scholar] [CrossRef]
- Ascenzi, P.; Salvati, L.; Bolognesi, M.; Colasanti, M.; Polticelli, F.; Venturini, G. Inhibition of cysteine protease activity by NO-donors. Curr. Protein Pept. Sci. 2001, 2, 137–153. [Google Scholar] [CrossRef] [PubMed]
- Pillay, C.S.; Elliott, E.; Dennison, C. Endolysosomal proteolysis and its regulation. Biochem. J. 2002, 363, 417–429. [Google Scholar] [CrossRef] [PubMed]
- Wilcox, D.; Mason, R.W. Inhibition of cysteine proteinases in lysosomes and whole cells. Biochem. J. 1992, 285, 495–502. [Google Scholar] [CrossRef] [PubMed]
- Salvati, L.; Mattu, M.; Colasanti, M.; Scalone, A.; Venturini, G.; Gradoni, L.; Ascenzi, P. NO donors inhibit Leishmania infantum cysteine proteinase activity. Biochim. Biophys. Acta 2001, 1545, 357–366. [Google Scholar] [CrossRef]
- Venturini, G.; Fioravanti, E.; Colasanti, M.; Persichini, T.; Ascenzi, P. Cys25-nitrosylation inactivates papain. Biochem. Mol. Biol. Int. 1998, 46, 425–428. [Google Scholar] [CrossRef] [PubMed]
- Xian, M.; Chen, X.; Liu, Z.; Wang, K.; Wang, P.G. Inhibition of papain by S-nitrosothiols. Formation of mixed disulfides. J. Biol. Chem. 2000, 275, 20467–20473. [Google Scholar] [CrossRef]
- Giustarini, D.; Milzani, A.; Aldini, G.; Carini, M.; Rossi, R.; Dalle-Donne, I. S-nitrosation versus S-glutathionylation of protein sulfhydryl groups by S-nitrosoglutathione. Antioxid. Redox Signal. 2005, 7, 930–939. [Google Scholar] [CrossRef]
- Percival, M.D.; Ouellet, M.; Campagnolo, C.; Claveau, D.; Li, C. Inhibition of cathepsin K by nitric oxide donors: Evidence for the formation of mixed disulfides and a sulfenic acid. Biochemistry 1999, 38, 13574–13583. [Google Scholar] [CrossRef]
- Väänänen, A.J.; Kankuri, E.; Rauhala, P. Nitric oxide-related species-induced protein oxidation: Reversible, irreversible, and protective effects on enzyme function of papain. Free Radic. Biol. Med. 2005, 38, 1102–1111. [Google Scholar] [CrossRef]
- Alcock, L.J.; Perkins, M.V.; Chalker, J.M. Chemical methods for mapping cysteine oxidation. Chem. Soc. Rev. 2018, 47, 231–268. [Google Scholar] [CrossRef]
- Poole, L.B.; Zeng, B.-B.; Knaggs, S.A.; Yakubu, M.; King, S.B. Synthesis of chemical probes to map sulfenic acid modifications on proteins. Bioconjug. Chem. 2005, 16, 1624–1628. [Google Scholar] [CrossRef] [PubMed]
- Poole, L.B.; Klomsiri, C.; Knaggs, S.A.; Furdui, C.M.; Nelson, K.J.; Thomas, M.J.; Fetrow, J.S.; Daniel, L.W.; King, S.B. Fluorescent and affinity-based tools to detect cysteine sulfenic acid formation in proteins. Bioconjug. Chem. 2007, 18, 2004–2017. [Google Scholar] [CrossRef] [PubMed]
- Klomsiri, C.; Rogers, L.C.; Soito, L.; McCauley, A.K.; King, S.B.; Nelson, K.J.; Poole, L.B.; Daniel, L.W. Endosomal H2O2 production leads to localized cysteine sulfenic acid formation on proteins during lysophosphatidic acid-mediated cell signaling. Free Radic. Biol. Med. 2014, 71, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Charles, R.L.; Schröder, E.; May, G.; Free, P.; Gaffney, P.R.J.; Wait, R.; Begum, S.; Heads, R.J.; Eaton, P. Protein sulfenation as a redox sensor: Proteomics studies using a novel biotinylated dimedone analogue. Mol. Cell Proteom. 2007, 6, 1473–1484. [Google Scholar] [CrossRef]
- Reddie, K.G.; Seo, Y.H.; Muse Iii, W.B.; Leonard, S.E.; Carroll, K.S. A chemical approach for detecting sulfenic acid-modified proteins in living cells. Mol. Biosyst. 2008, 4, 521–531. [Google Scholar] [CrossRef]
- Seo, Y.H.; Carroll, K.S. Facile synthesis and biological evaluation of a cell-permeable probe to detect redox-regulated proteins. Bioorg. Med. Chem. Lett. 2009, 19, 356–359. [Google Scholar] [CrossRef]
- Leonard, S.E.; Reddie, K.G.; Carroll, K.S. Mining the thiol proteome for sulfenic acid modifications reveals new targets for oxidation in cells. ACS Chem. Biol. 2009, 4, 783–799. [Google Scholar] [CrossRef]
- Paulsen, C.E.; Truong, T.H.; Garcia, F.J.; Homann, A.; Gupta, V.; Leonard, S.E.; Carroll, K.S. Peroxide-dependent sulfenylation of the EGFR catalytic site enhances kinase activity. Nat. Chem. Biol. 2011, 8, 57–64. [Google Scholar] [CrossRef]
- Qian, J.; Klomsiri, C.; Wright, M.W.; King, S.B.; Tsang, A.W.; Poole, L.B.; Furdui, C.M. Simple synthesis of 1,3-cyclopentanedione derived probes for labeling sulfenic acid proteins. Chem. Commun. 2011, 47, 9203–9205. [Google Scholar] [CrossRef][Green Version]
- Qian, J.; Wani, R.; Klomsiri, C.; Poole, L.B.; Tsang, A.W.; Furdui, C.M. A simple and effective strategy for labeling cysteine sulfenic acid in proteins by utilization of β-ketoesters as cleavable probes. Chem. Commun. 2012, 48, 4091–4093. [Google Scholar] [CrossRef][Green Version]
- Poole, T.H.; Reisz, J.A.; Zhao, W.; Poole, L.B.; Furdui, C.M.; King, S.B. Strained Cycloalkynes as New Protein Sulfenic Acid Traps. J. Am. Chem. Soc. 2014, 136, 6167–6170. [Google Scholar] [CrossRef] [PubMed]
- Ellis, H.R.; Poole, L.B. Novel application of 7-chloro-4-nitrobenzo-2-oxa-1,3-diazole to identify cysteine sulfenic acid in the AhpC component of alkyl hydroperoxide reductase. Biochemistry 1997, 36, 15013–15018. [Google Scholar] [CrossRef] [PubMed]
- Alcock, L.J.; Oliveira, B.L.; Deery, M.J.; Pukala, T.L.; Perkins, M.V.; Bernardes, G.J.L.; Chalker, J.M. Norbornene Probes for the Detection of Cysteine Sulfenic Acid in Cells. ACS Chem. Biol. 2019, 14, 594–598. [Google Scholar] [CrossRef] [PubMed]
- Lo Conte, M.; Lin, J.; Wilson, M.A.; Carroll, K.S. A Chemical Approach for the Detection of Protein Sulfinylation. ACS Chem. Biol. 2015, 10, 1825–1830. [Google Scholar] [CrossRef]
- Hausladen, A.; Stamler, J.S. Nitrosative stress. Meth. Enzymol. 1999, 300, 389–395. [Google Scholar]
- Stamler, J.S.; Hausladen, A. Oxidative modifications in nitrosative stress. Nat. Struct. Biol. 1998, 5, 247–249. [Google Scholar] [CrossRef]
- Stamler, J.S. Redox signaling: Nitrosylation and related target interactions of nitric oxide. Cell 1994, 78, 931–936. [Google Scholar] [CrossRef]
- Stamler, J.S.; Simon, D.I.; Osborne, J.A.; Mullins, M.E.; Jaraki, O.; Michel, T.; Singel, D.J.; Loscalzo, J. S-nitrosylation of proteins with nitric oxide: Synthesis and characterization of biologically active compounds. Proc. Natl. Acad. Sci. USA 1992, 89, 444–448. [Google Scholar] [CrossRef]
- Simon, D.I.; Stamler, J.S.; Jaraki, O.; Keaney, J.F.; Osborne, J.A.; Francis, S.A.; Singel, D.J.; Loscalzo, J. Antiplatelet properties of protein S-nitrosothiols derived from nitric oxide and endothelium-derived relaxing factor. Arterioscler. Thromb. 1993, 13, 791–799. [Google Scholar] [CrossRef]
- Lemaire, G.; Guittet, O.; Vesin, M.-F.; Lepoivre, M.; Cottet, M.-H. Glutathione depletion reveals impairment of antigen processing and inhibition of cathepsin activity by nitric oxide in antigen-presenting cells. Mol. Immunol. 2009, 46, 1100–1108. [Google Scholar] [CrossRef]
- Väänänen, A.J.; Salmenperä, P.; Hukkanen, M.; Rauhala, P.; Kankuri, E. Cathepsin B is a differentiation-resistant target for nitroxyl (HNO) in THP-1 monocyte/macrophages. Free Radic. Biol. Med. 2006, 41, 120–131. [Google Scholar] [CrossRef] [PubMed]
- Väänänen, A.J.; Salmenperä, P.; Hukkanen, M.; Miranda, K.M.; Harjula, A.; Rauhala, P.; Kankuri, E. Persistent susceptibility of cathepsin B to irreversible inhibition by nitroxyl (HNO) in the presence of endogenous nitric oxide. Free Radic. Biol. Med. 2008, 45, 749–755. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.S.; Armstrong, D.A.; Gaucher, G.M. Formation and Repair of Papain Sulfenic Acid. Can. J. Biochem. 1975, 53, 298–307. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.S.; Armstrong, D.A. Glutathione mediation of papain inactivation by hydrogen peroxide and hydroxyl radicals. Radiat. Res. 1977, 69, 434–441. [Google Scholar] [CrossRef]
- Sanner, T.; Pihl, A. Studies on the active–SH group of papain and on the mechanism of papain activation by thiols. J. Biol. Chem. 1963, 238, 165–171. [Google Scholar]
- Glazer, A.N.; Smith, E.L. The sulfur distribution of papain. J. Biol. Chem. 1965, 240, 201–208. [Google Scholar]
- Lee, D.C.; Mason, C.W.; Goodman, C.B.; Holder, M.S.; Kirksey, O.W.; Womble, T.A.; Severs, W.B.; Palm, D.E. Hydrogen peroxide induces lysosomal protease alterations in PC12 cells. Neurochem. Res. 2007, 32, 1499–1510. [Google Scholar] [CrossRef]
- Weibrecht, I.; Böhmer, S.-A.; Dagnell, M.; Kappert, K.; Östman, A.; Böhmer, F.-D. Oxidation sensitivity of the catalytic cysteine of the protein-tyrosine phosphatases SHP-1 and SHP-2. Free Radic. Biol. Med. 2007, 43, 100–110. [Google Scholar] [CrossRef]
- Godat, E.; Hervé-Grépinet, V.; Veillard, F.; Lecaille, F.; Belghazi, M.; Brömme, D.; Lalmanach, G. Regulation of cathepsin K activity by hydrogen peroxide. Biol. Chem. 2008, 389, 1123–1126. [Google Scholar] [CrossRef]
- Hervé-Grépinet, V.; Veillard, F.; Godat, E.; Heuzé-Vourc’h, N.; Lecaille, F.; Lalmanach, G. Extracellular catalase activity protects cysteine cathepsins from inactivation by hydrogen peroxide. FEBS Lett. 2008, 582, 1307–1312. [Google Scholar] [CrossRef]
- Nagakannan, P.; Eftekharpour, E. Differential redox sensitivity of cathepsin B and L holds the key to autophagy-apoptosis interplay after Thioredoxin reductase inhibition in nutritionally stressed SH-SY5Y cells. Free Radic. Biol. Med. 2017, 108, 819–831. [Google Scholar] [CrossRef] [PubMed]
- Nagaoka, Y.; Otsu, K.; Okada, F.; Sato, K.; Ohba, Y.; Kotani, N.; Fujii, J. Specific inactivation of cysteine protease-type cathepsin by singlet oxygen generated from naphthalene endoperoxides. Biochem. Biophys. Res. Commun. 2005, 331, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Suto, D.; Iuchi, Y.; Ikeda, Y.; Sato, K.; Ohba, Y.; Fujii, J. Inactivation of cysteine and serine proteases by singlet oxygen. Arch. Biochem. Biophys. 2007, 461, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Davies, M.J. Protein oxidation and peroxidation. Biochem. J. 2016, 473, 805–825. [Google Scholar] [CrossRef] [PubMed]
- Headlam, H.A.; Gracanin, M.; Rodgers, K.J.; Davies, M.J. Inhibition of cathepsins and related proteases by amino acid, peptide, and protein hydroperoxides. Free Radic. Biol. Med. 2006, 40, 1539–1548. [Google Scholar] [CrossRef] [PubMed]
- Rahmanto, A.S.; Morgan, P.E.; Hawkins, C.L.; Davies, M.J. Cellular effects of peptide and protein hydroperoxides. Free Radic. Biol. Med. 2010, 48, 1071–1078. [Google Scholar] [CrossRef] [PubMed]
- Borutaite, V.; Brown, G.C. Caspases are reversibly inactivated by hydrogen peroxide. FEBS Lett. 2001, 500, 114–118. [Google Scholar] [CrossRef]
- Allan, E.R.O.; Tailor, P.; Balce, D.R.; Pirzadeh, P.; McKenna, N.T.; Renaux, B.; Warren, A.L.; Jirik, F.R.; Yates, R.M. NADPH oxidase modifies patterns of MHC class II-restricted epitopic repertoires through redox control of antigen processing. J. Immunol. 2014, 192, 4989–5001. [Google Scholar] [CrossRef]
- Rybicka, J.M.; Balce, D.R.; Khan, M.F.; Krohn, R.M.; Yates, R.M. NADPH oxidase activity controls phagosomal proteolysis in macrophages through modulation of the lumenal redox environment of phagosomes. Proc. Natl. Acad. Sci. USA 2010, 107, 10496–10501. [Google Scholar] [CrossRef]
- Rybicka, J.M.; Balce, D.R.; Chaudhuri, S.; Allan, E.R.O.; Yates, R.M. Phagosomal proteolysis in dendritic cells is modulated by NADPH oxidase in a pH-independent manner. EMBO J. 2012, 31, 932–944. [Google Scholar] [CrossRef]
- Ewanchuk, B.W.; Yates, R.M. The phagosome and redox control of antigen processing. Free Radic. Biol. Med. 2018, 125, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Balce, D.R.; Li, B.; Allan, E.R.O.; Rybicka, J.M.; Krohn, R.M.; Yates, R.M. Alternative activation of macrophages by IL-4 enhances the proteolytic capacity of their phagosomes through synergistic mechanisms. Blood 2011, 118, 4199–4208. [Google Scholar] [CrossRef] [PubMed]
- Mirković, B.; Sosič, I.; Gobec, S.; Kos, J. Redox-based inactivation of cysteine cathepsins by compounds containing the 4-aminophenol moiety. PLoS ONE 2011, 6, e27197. [Google Scholar] [CrossRef] [PubMed]
- Rahman, I.; MacNee, W. Oxidant/antioxidant imbalance in smokers and chronic obstructive pulmonary disease. Thorax 1996, 51, 348–350. [Google Scholar] [CrossRef]
- Rahman, I.; Yang, S.-R.; Biswas, S.K. Current concepts of redox signaling in the lungs. Antioxid. Redox Signal. 2006, 8, 681–689. [Google Scholar] [CrossRef]
- Morrison, D.; Rahman, I.; Lannan, S.; MacNee, W. Epithelial permeability, inflammation, and oxidant stress in the air spaces of smokers. Am. J. Respir. Crit. Care Med. 1999, 159, 473–479. [Google Scholar] [CrossRef]
- Lockwood, T.D. Cathepsin B responsiveness to glutathione and lipoic acid redox. Antioxid. Redox Signal. 2002, 4, 681–691. [Google Scholar] [CrossRef]
- Pillay, C.S.; Dennison, C. Cathepsin B stability, but not activity, is affected in cysteine: Cystine redox buffers. Biol. Chem. 2002, 383, 1199–1204. [Google Scholar] [CrossRef]
- Selent, J.; Kaleta, J.; Li, Z.; Lalmanach, G.; Brömme, D. Selective inhibition of the collagenase activity of cathepsin K. J. Biol. Chem. 2007, 282, 16492–16501. [Google Scholar] [CrossRef]
- Lecaille, F.; Chazeirat, T.; Bojarski, K.K.; Renault, J.; Saidi, A.; Prasad, V.G.N.V.; Samsonov, S.; Lalmanach, G. Rat cathepsin K: Enzymatic specificity and regulation of its collagenolytic activity. Biochim. Biophys. Acta Proteins Proteom. 2020, 1868, 140318. [Google Scholar] [CrossRef]
- Zeng, J.; Dunlop, R.A.; Rodgers, K.J.; Davies, M.J. Evidence for inactivation of cysteine proteases by reactive carbonyls via glycation of active site thiols. Biochem. J. 2006, 398, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Crabb, J.W.; O’Neil, J.; Miyagi, M.; West, K.; Hoff, H.F. Hydroxynonenal inactivates cathepsin B by forming Michael adducts with active site residues. Protein Sci. 2002, 11, 831–840. [Google Scholar] [CrossRef] [PubMed]
- O’Neil, J.; Hoppe, G.; Sayre, L.M.; Hoff, H.F. Inactivation of cathepsin B by oxidized LDL involves complex formation induced by binding of putative reactive sites exposed at low pH to thiols on the enzyme. Free Radic. Biol. Med. 1997, 23, 215–225. [Google Scholar] [CrossRef]
- Hoppe, G.; O’Neil, J.; Hoff, H.F. Inactivation of lysosomal proteases by oxidized low density lipoprotein is partially responsible for its poor degradation by mouse peritoneal macrophages. J. Clin. Investig. 1994, 94, 1506–1512. [Google Scholar] [CrossRef]
- Carr, A.C. Hypochlorous acid-modified low-density lipoprotein inactivates the lysosomal protease cathepsin B: Protection by ascorbic and lipoic acids. Redox Rep. 2001, 6, 343–349. [Google Scholar] [CrossRef]
- O’Neil, J.; Hoppe, G.; Hoff, H.F. Phospholipids in oxidized low density lipoproteins perturb the ability of macrophages to degrade internalized macromolecules and reduce intracellular cathepsin B activity. Atherosclerosis 2003, 169, 215–224. [Google Scholar] [CrossRef]
- Li, W.; Yuan, X.M.; Olsson, A.G.; Brunk, U.T. Uptake of oxidized LDL by macrophages results in partial lysosomal enzyme inactivation and relocation. Arterioscler. Thromb. Vasc. Biol. 1998, 18, 177–184. [Google Scholar] [CrossRef]
- Lougheed, M.; Zhang, H.F.; Steinbrecher, U.P. Oxidized low density lipoprotein is resistant to cathepsins and accumulates within macrophages. J. Biol. Chem. 1991, 266, 14519–14525. [Google Scholar]
- Hoff, H.F.; O’Neil, J.; Wu, Z.; Hoppe, G.; Salomon, R.L. Phospholipid hydroxyalkenals: Biological and chemical properties of specific oxidized lipids present in atherosclerotic lesions. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 275–282. [Google Scholar] [CrossRef]
- Krohne, T.U.; Kaemmerer, E.; Holz, F.G.; Kopitz, J. Lipid peroxidation products reduce lysosomal protease activities in human retinal pigment epithelial cells via two different mechanisms of action. Exp. Eye Res. 2010, 90, 261–266. [Google Scholar] [CrossRef]
- Reddy, A.T.; Lakshmi, S.P.; Muchumarri, R.R.; Reddy, R.C. Nitrated Fatty Acids Reverse Cigarette Smoke-Induced Alveolar Macrophage Activation and Inhibit Protease Activity via Electrophilic S-Alkylation. PLoS ONE 2016, 11, e0153336. [Google Scholar] [CrossRef] [PubMed]
- Thannickal, V.J.; Fanburg, B.L. Reactive oxygen species in cell signaling. Am. J. Physiol. Lung Cell Mol. Physiol. 2000, 279, L1005–L1028. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.-P.; Huang, F.L. Glutathionylation of proteins by glutathione disulfide S-oxide. Biochem. Pharmacol. 2002, 64, 1049–1056. [Google Scholar] [CrossRef]
- Poole, L.B.; Karplus, P.A.; Claiborne, A. Protein sulfenic acids in redox signaling. Annu. Rev. Pharmacol. Toxicol. 2004, 44, 325–347. [Google Scholar] [CrossRef] [PubMed]
- Saurin, A.T.; Neubert, H.; Brennan, J.P.; Eaton, P. Widespread sulfenic acid formation in tissues in response to hydrogen peroxide. Proc. Natl. Acad. Sci. USA 2004, 101, 17982–17987. [Google Scholar] [CrossRef] [PubMed]
| Target | Warhead | Tag/Detection Method | Probes (Usual Names) | Refs |
|---|---|---|---|---|
| Sulfenic acid | 1,3-cyclohexanedione (dimedone derivatives) | 7-methoxycoumarin | DCP-MCC | [113] |
| Isatoic acid | DCP-MAB | [113] | ||
| Fluorescein | DCP-FL1, DCP-FL2 | [114] | ||
| Rhodamine | DCP-Rho, DCP-Rho2 | [114,115] | ||
| Biotin | DCP-Bio1, DCP-Bio2, DCP-Bio3, Biotin dimedone | [114,115,116] | ||
| alkyne–azide “click” chemistry | DAz-1, DAz-2 | [117,118,119] | ||
| alkyne–azide “click” chemistry | DYn-1, DYn-2 | [120] | ||
| 1,3-cyclopentanedione | Biotin | BP1 | [121] | |
| Linear β-ketoester | alkyne–azide “click” chemistry | Alk-β-KE | [122] | |
| Bicyclo[6.1.0]nonyne | Biotin | BCN-Bio | [123] | |
| 7-chloro-4-nitrobenzo-2-oxa-1,3-diazole | Absorbance (347 nm) | NBD-Cl | [124] | |
| Norbornene | Biotin | Norb-Bio | [125] | |
| alkyne–azide “click” chemistry | Norb-yne | [125] | ||
| Sulfinic acid | 2-nitroso terephthalic acid | Biotin | NO-Bio | [126] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lalmanach, G.; Saidi, A.; Bigot, P.; Chazeirat, T.; Lecaille, F.; Wartenberg, M. Regulation of the Proteolytic Activity of Cysteine Cathepsins by Oxidants. Int. J. Mol. Sci. 2020, 21, 1944. https://doi.org/10.3390/ijms21061944
Lalmanach G, Saidi A, Bigot P, Chazeirat T, Lecaille F, Wartenberg M. Regulation of the Proteolytic Activity of Cysteine Cathepsins by Oxidants. International Journal of Molecular Sciences. 2020; 21(6):1944. https://doi.org/10.3390/ijms21061944
Chicago/Turabian StyleLalmanach, Gilles, Ahlame Saidi, Paul Bigot, Thibault Chazeirat, Fabien Lecaille, and Mylène Wartenberg. 2020. "Regulation of the Proteolytic Activity of Cysteine Cathepsins by Oxidants" International Journal of Molecular Sciences 21, no. 6: 1944. https://doi.org/10.3390/ijms21061944
APA StyleLalmanach, G., Saidi, A., Bigot, P., Chazeirat, T., Lecaille, F., & Wartenberg, M. (2020). Regulation of the Proteolytic Activity of Cysteine Cathepsins by Oxidants. International Journal of Molecular Sciences, 21(6), 1944. https://doi.org/10.3390/ijms21061944





