Abstract
The new coronavirus disease-2019 (COVID-19), which is spreading around the world and threatening people, is easily infecting a large number of people through airborne droplets; moreover, patients with hypertension, diabetes, obesity, and cardiovascular disease are more likely to experience severe conditions. Vascular endothelial dysfunction has been suggested as a common feature of high-risk patients prone to severe COVID-19, and measurement of vascular endothelial function may be recommended for predicting severe conditions in high-risk patients with COVID-19. However, fragmented vascular endothelial glycocalyx (VEGLX) is elevated in COVID-19 patients, suggesting that it may be useful as a prognostic indicator. Although the relationship between VEGLX and severe acute respiratory syndrome coronavirus 2 infections has not been well studied, some investigations into COVID-19 have clarified the relationship between VEGLX and the mechanism that leads to severe conditions. Clarifying the usefulness of VEGLX assessment as a predictive indicator of the development of severe complications is important as a strategy for confronting pandemics caused by new viruses with a high affinity for the vascular endothelium that may recur in the future.
1. Introduction
The new coronavirus disease-2019 (COVID-19) has disrupted society, accelerated economic damage, and poses a threat to the population due to insufficient information on its mode of transmission and pathogenesis. A year after the start of the COVID-19 pandemic outbreak, the identity of the causative virus, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has become more or less clear, as various studies on the disease have been reported from around the world. Findings of particular importance are: (1) the virus is highly contagious and can easily spread to a large number of people via droplets from their mouth; (2) it can be prevented by maintaining social distancing and avoiding confined spaces; and (3) although most infected people would only have a mild illness, it is not uncommon in the elderly, and in smokers. Moreover, patients with hypertension, diabetes, obesity, and cardiovascular diseases are more likely to be severely affected by COVID-19 and have a higher mortality rate. These three findings provide strategies for combating COVID-19.
Vascular endothelial damage has been identified as a common feature of high-risk patients prone to severe COVID-19 [1]. According to a report about 7 lungs obtained during autopsy from patients who died from SARS-CoV-2 infection, severe endothelial injury was found to be associated with intracellular SARS-CoV-2 virus with disrupted endothelial cell membrane [2]. The lungs from the patients with COVID-19 exhibited widespread vascular thrombosis with microangiopathy and occlusion of alveolar capillaries, and significant new vessel growth through a mechanism of intussusceptive angiogenesis [2]. On the other hand, the vascular endothelial glycocalyx (VEGLX), the extracellular matrix covering vascular endothelial cells throughout the body, is also impaired by various risk factors, and there have been numerous reports of VEGLX damage in acute respiratory distress syndrome (ARDS) and disseminated intravascular coagulation (DIC), which may be closely related to vascular endothelial damage in severe COVID-19. Recently, circulating levels of fragmented VEGLX concentrations and real-time measurements of VEGLX in sublingual capillaries have been reported in patients with COVID-19 [3].
This review discusses existing reports on COVID-19 and VEGLX damage along with their mechanisms.
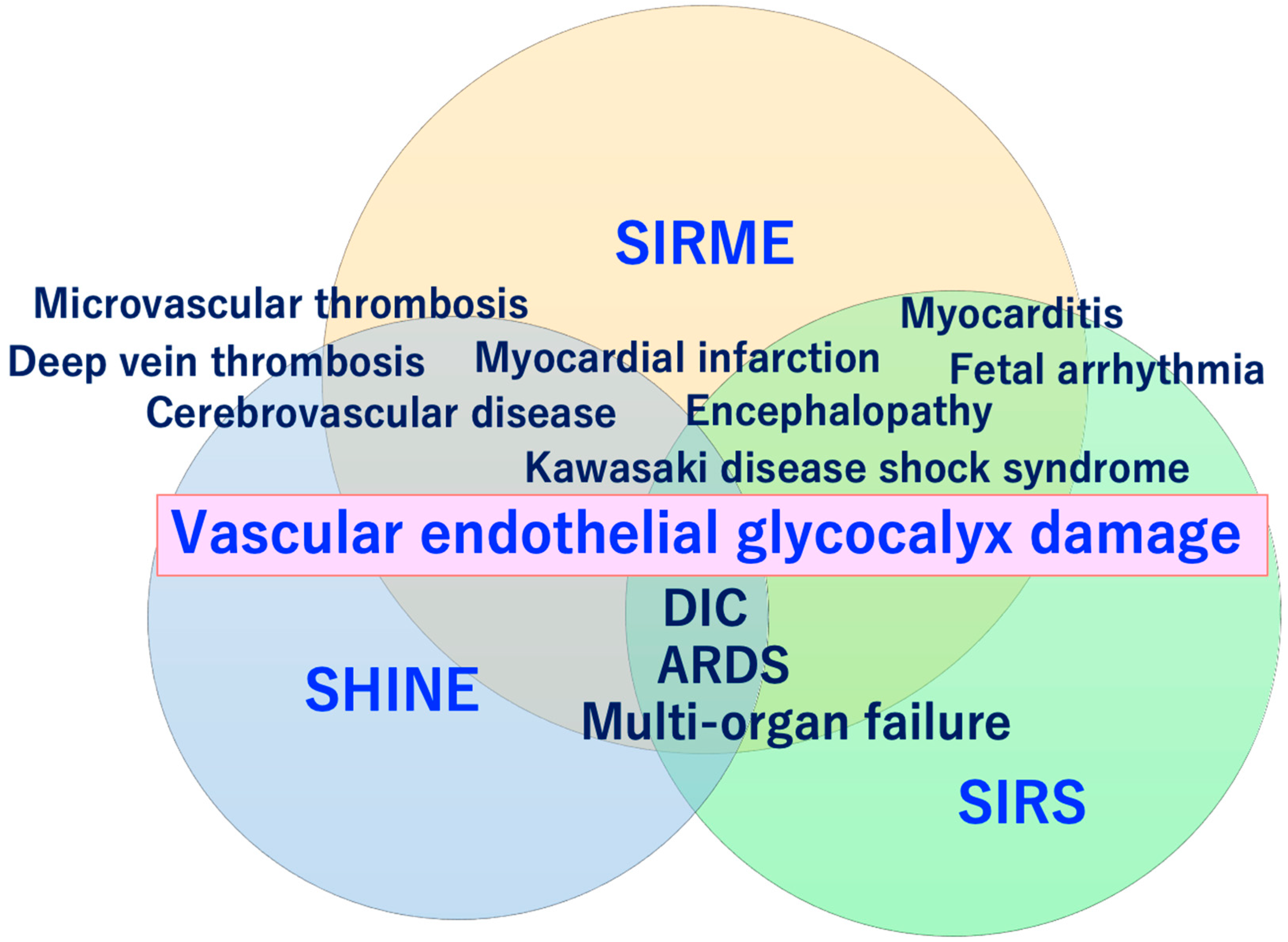
4. Systemic Inflammatory-Reactive Microvascular Endotheliopathy (SIRME)
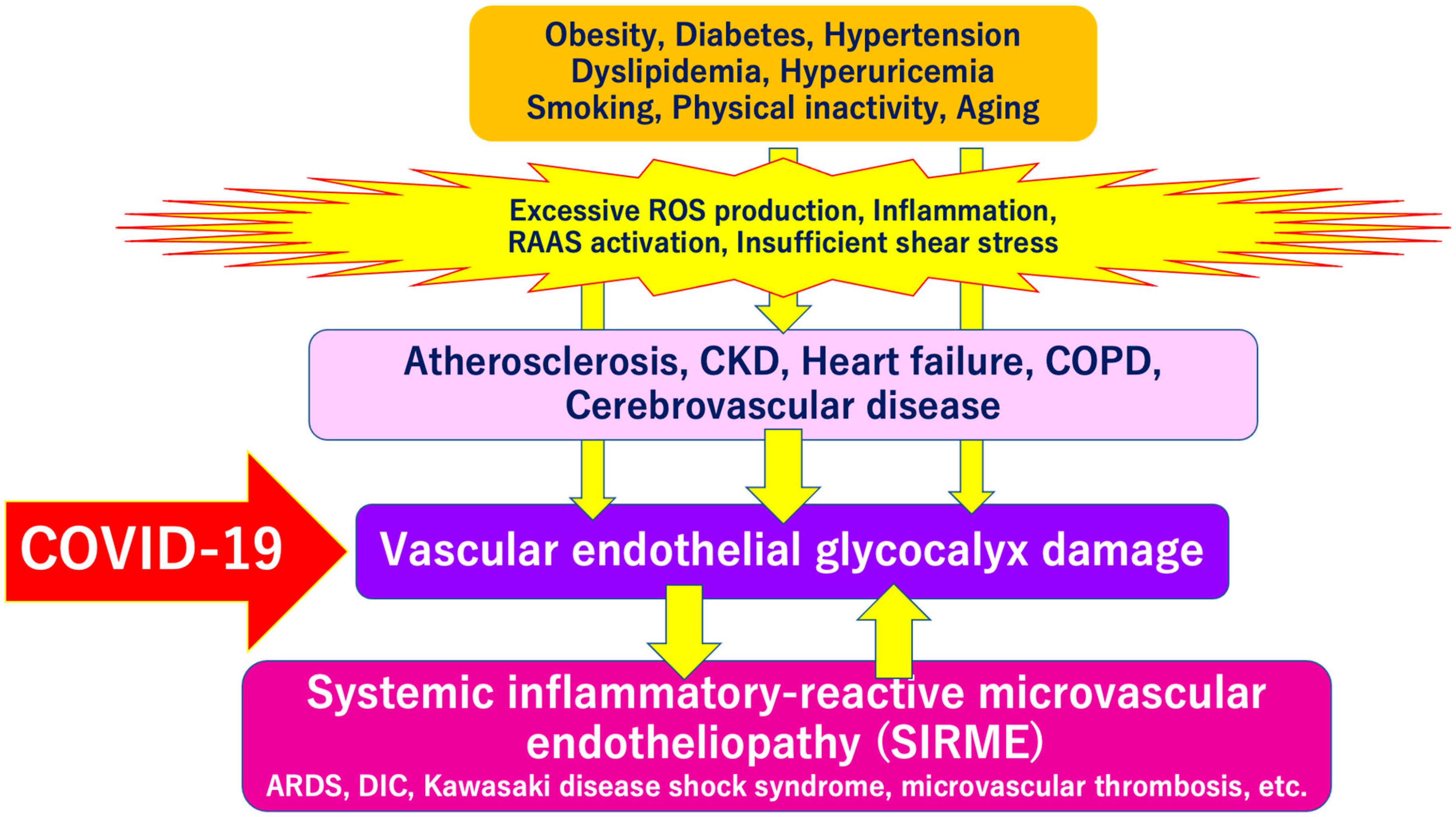
SARS-CoV-2 directly invades vascular endothelial cells and causes systemic inflammatory microvascular endothelial disorders such as leakage of plasma components from microvessels, intramicrovascular blood clotting and thrombus formation, and excessive release of inflammatory cytokines following vascular endothelial dysfunction [16]. It might play a central role in the pathogenesis of ARDS [20] and multi-organ failure [21,22,23]. Such a wide variety of serious pathologies can be explained by the concept of “systemic inflammation-reactive microvascular endotheliosis (SIRME)”, in which the VEGLX is rapidly and systemically disrupted (Figure 4).
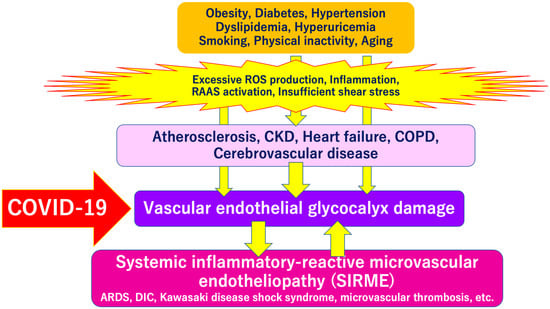
Figure 4.
Severe COVID-19 comorbidity induced by vascular endothelial glycocalyx (VEGLX) damage. The VEGLX is damaged due to various factors such as smoking, physical inactivity, hypertension, diabetes, obesity, and cardiovascular diseases. Various lethal conditions in COVID-19 (e.g., acute respiratory distress syndrome (ARDS), disseminated intravascular coagulation (DIC), Kawasaki disease shock syndrome, microvascular thrombosis, etc.) may be caused by a common mechanism, damage of VEGLX. ROS, reactive oxygen species; RAAS, renin-angiotensin-aldosterone system; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease.
On the other hand, extensive VEGLX damage has been reported to occur in patients with cardiovascular disease and its risk factors, suggesting that SARS-CoV-2 can easily penetrate endothelial cells in impaired vascular endothelial cells with a loss of barrier function, causing severe COVID-19 [24].
5. Vascular Endothelial Glycocalyx (VEGLX)
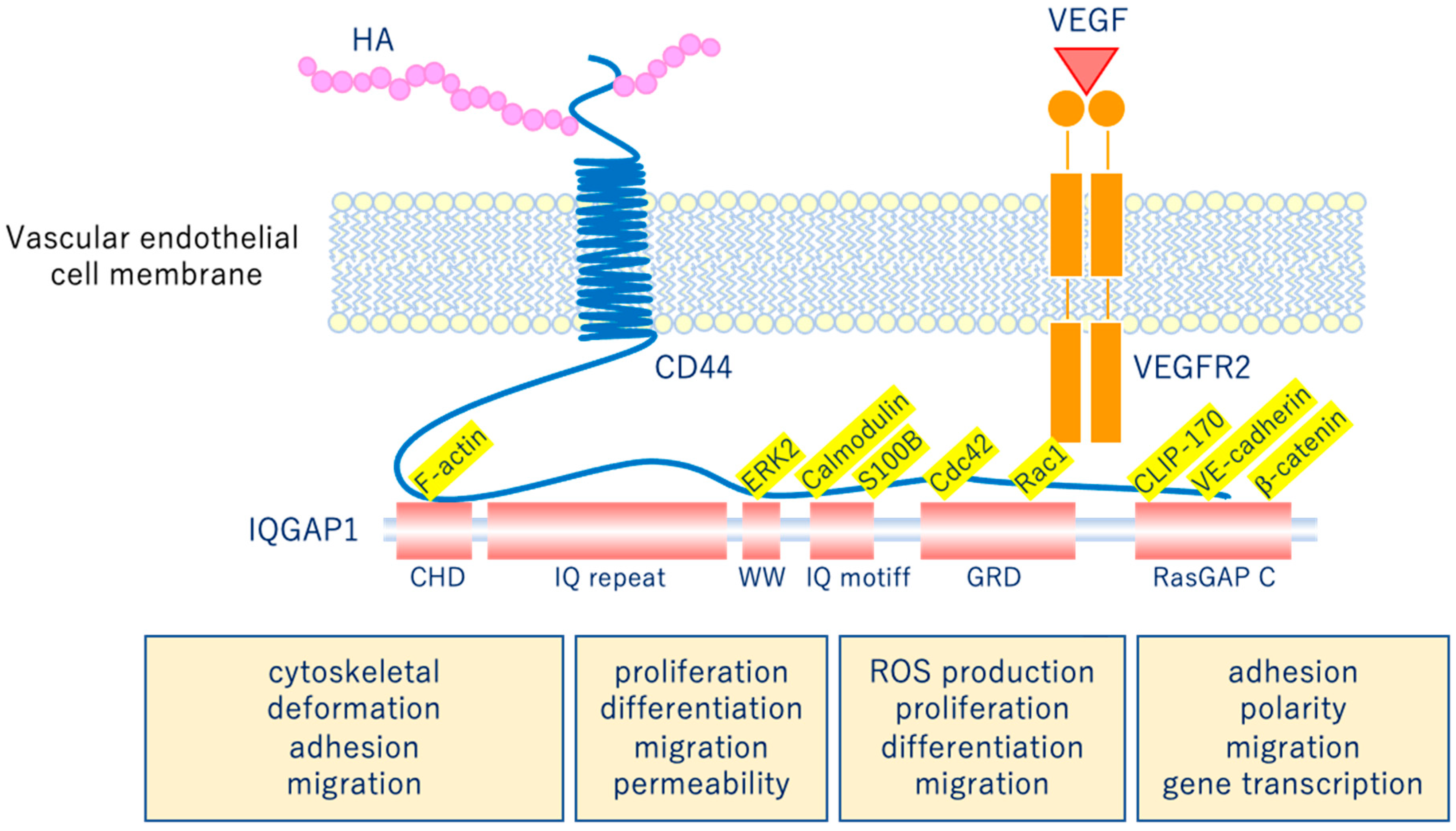
The glycocalyx is a complex gel-like layer of glycosylated lipid-protein mixtures that covers the surface of all living cells, and is known to serve as a physical protective layer as well as a buffer region between cells and the extracellular matrix to control various cellular functions [25]. Vascular endothelial cells are a monolayer of cells that comprise the innermost layer of cells in the vascular system, including arteries, veins, and capillaries, and serve a barrier function for the blood vessels surrounding all organs and in direct contact with the blood flowing through the vascular lumen. As shown in Figure 5, for example, the VEGLX plays an important role in regulating various vascular endothelial cell functions such as coagulation, inflammation, vasoconstriction and relaxation, vascular permeability, and angiogenesis [26,27].
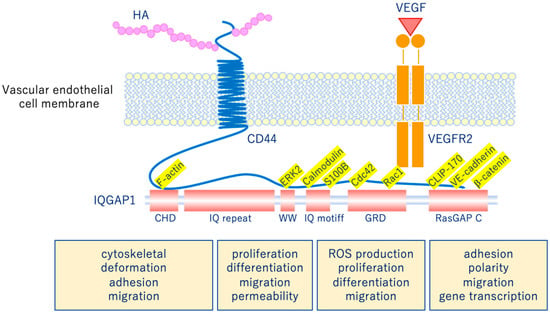
Figure 5.
Vascular endothelial glycocalyx regulates cellular functions. Hyaluronic acid (HA) activates the HA/CD44 system by binding to CD44 and regulates various intracellular signaling through the multifunctional platform IQGAP1, as well as the vascular endothelial growth factor VEGF/VEGFR2 system. VEGF, vascular endothelial growth factor; VEGFR2, VEGF receptor 2; CD44, cluster of differentiation 44; CHD, calponin homology domain; IQ repeat, IQGAP specific repeat; ERK, extracellular-signal-regulated kinase; WW, region containing two tryptophans; S100B, S100 calcium-binding protein B; IQ motif, calmodulin-binding motif; Cdc42, cell division cycle 42; Rac1, Rac family small GTPase 1; GRD, Ras GTPase-activating protein-related domain; CLIP-170, cytoplasmic linker protein 170; RasGAP C, Ras GTPase-activating protein C terminus; ROS, reactive oxygen species.
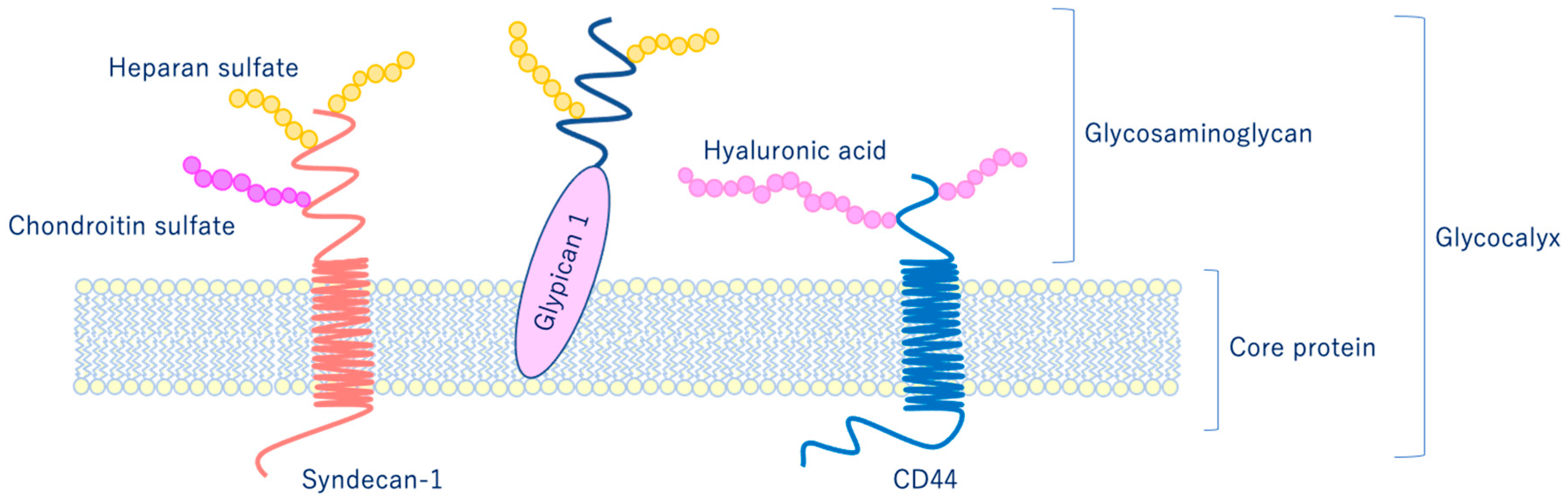
As shown in Figure 6, glycocalyx consists of sialic acid-containing glycoproteins, core proteins consisting of membrane-bound proteoglycans (syndecan, glypican, etc.), glycosaminoglycan side chains (heparan sulfate, chondroitin sulfate, etc.), and long-chain hyaluronic acid (HA) [28,29]. VEGLX is stabilized by shear stress [30] and this stabilization is crucial for nitric oxide (NO) production in vascular endothelial cells [31,32]. Although glycosaminoglycans are constantly degraded by enzymes, their levels are maintained by newly synthesized glycosaminoglycans supplied from vesicles in the golgi apparatus, regulating their homeostatic balance [33].
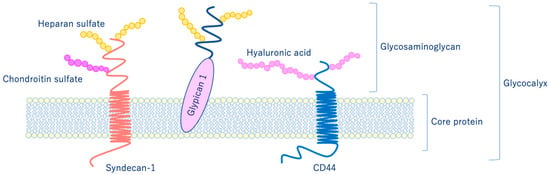
Figure 6.
Schematic representation of the vascular endothelial glycocalyx, which is present in the vascular endothelial cell membrane. The vascular endothelial glycocalyx is composed of core proteins and highly water-retaining glycosaminoglycans that bind to the cell membrane.
VEGLX protects vascular endothelial cells from the turbulence caused by blood flow and is involved in the regulatory function of the vascular permeability barrier. In addition, it plays an important role in endothelial function, especially in microvascular endothelial function, as it controls vascular reactivity and is involved in regulating the interaction between vascular endothelial cells and blood components [34]. Vascular endothelial cells in a healthy state covered by the VEGLX (Figure 7) contain a variety of cytokines, chemokines, receptors, growth factors, gap-binding proteins, extracellular superoxide dismutase (ecSOD), and endothelial nitric oxide synthase (eNOS). In addition, lipoprotein lipases are expressed that play a variety of functions required for homeostasis. Owing to its negative charge, VEGLX is also thought to be a determinant of salt sensitivity, and neutralizes cell surface charges by retaining plasma sodium in the glycocalyx layer [35].
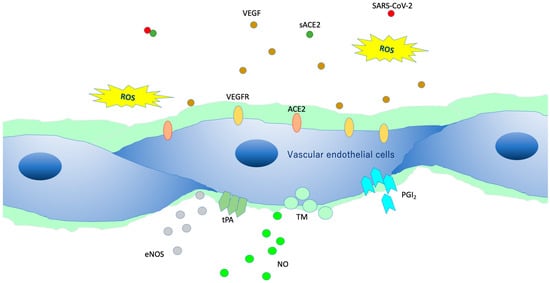
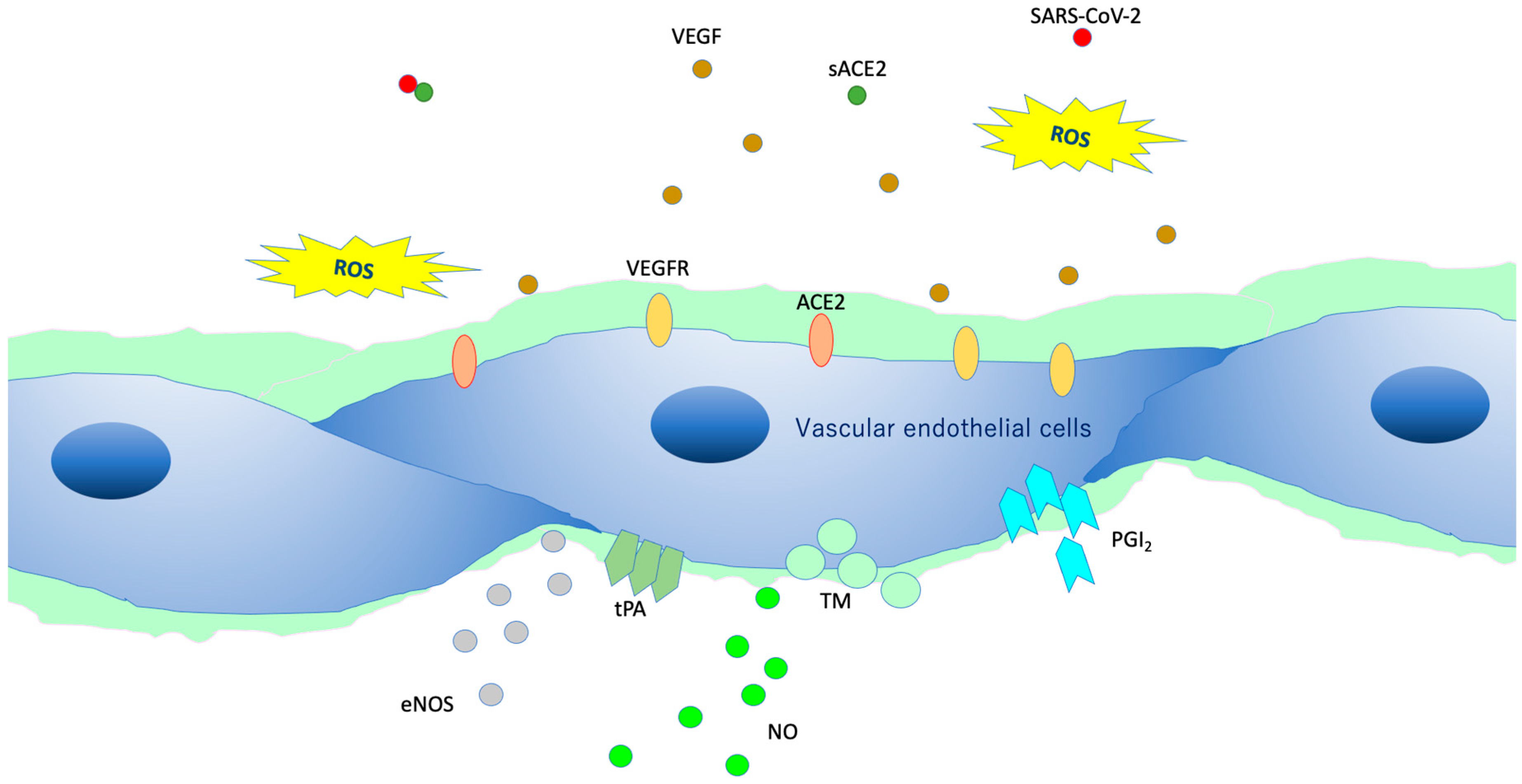
Figure 7.
Intact vascular endothelial glycocalyx (VEGLX). When vascular endothelial cells are covered enough with healthy VEGLX, even if severe acute respiratory coronavirus 2 (SARS-CoV-2) enters the body, it could be neutralized by the effects of appropriate reactive oxygen species (ROS) and soluble angiotensin-converting enzyme 2 (sACE2), and it may be possible to prevent the virus entry into the vascular endothelium. VEGF, vascular endothelial growth factor; VEGFR, VEGF receptors; NO, nitric oxide; eNOS, endothelial NO synthase; TM, thrombomodulin; tPA, tissue plasminogen activator; PGI2, prostacyclin.
6. Damage of VEGLX
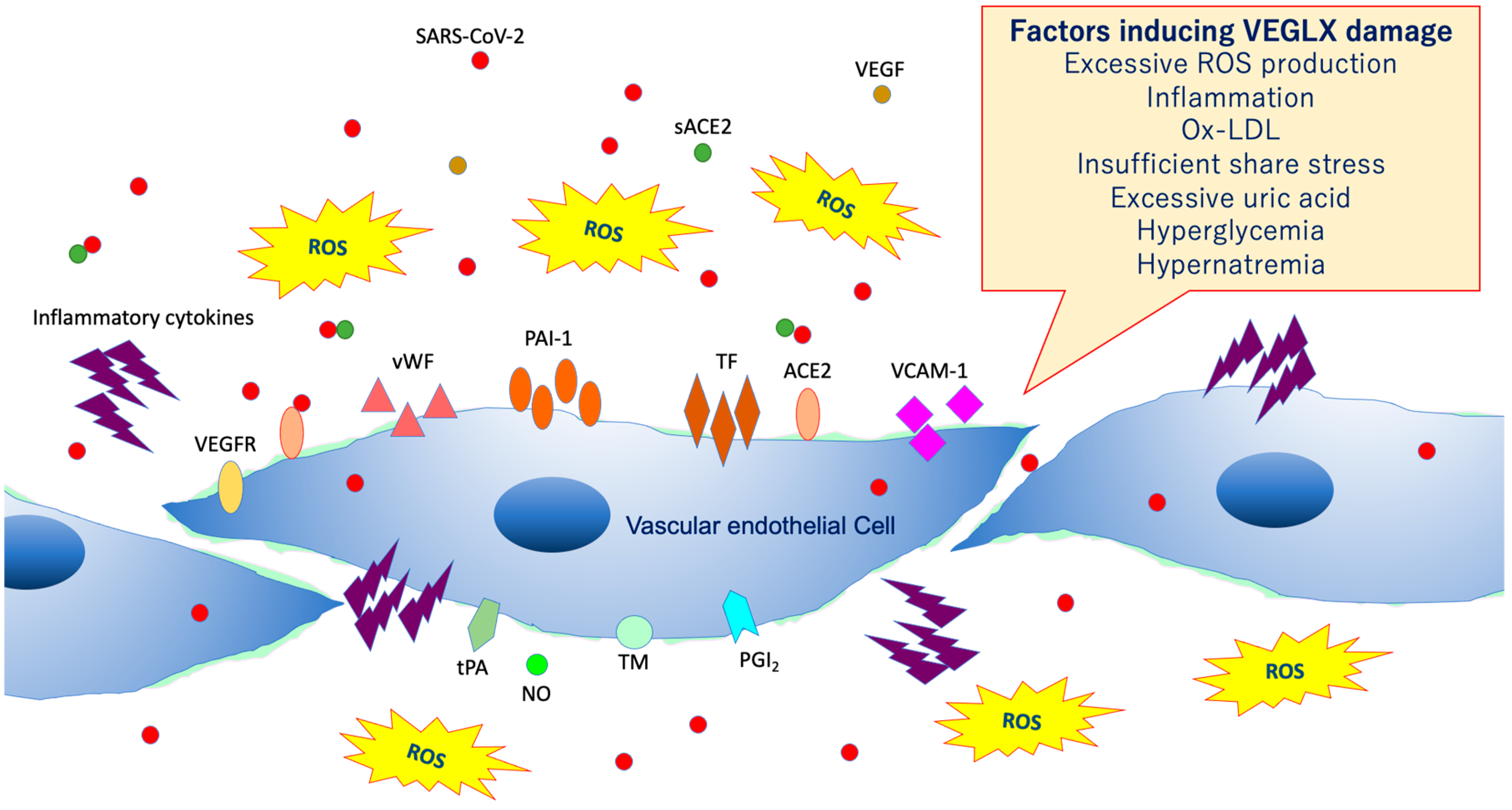
As shown in Figure 8, VEGLX shedding and degradation are known to be caused by a variety of cellular stresses [36]. Specifically, ischemia/reperfusion injury [37], endotoxin [38], inflammatory mediators [39], atrial natriuretic peptide, hypoxia, excessive reactive oxygen species (ROS), uric acid [40], hyperglycemia [41,42], hypernatremia [43], excessive fluid infusion, dehydration, decreased vascular wall shear stress, oxidized low-density lipoprotein (ox-LDL) [44], and others can cause VEGLX damage. In addition, this damage is known to be sex-specific, mostly observed in men. Systemic shedding of VEGLX has been associated with serious infections [45], Kawasaki disease [46], gestational hypertensive nephropathy (preeclampsia) [47], gestational diabetes [48], sepsis [49], acute lung injury (ALI)/ARDS [50], trauma [51], ischemic cerebral embolism [52], acute coronary syndrome [53], and shock [54]. The term shock-induced endothelial glycocalyx disturbance, called shock-induced endotheliopathy (SHINE), is an indicator of poor prognosis in fairly serious conditions, such as severe trauma, sepsis, myocardial infarction, and cardiac arrest syndrome [55]. Under these severe conditions, VEGLX is degraded via inflammatory mechanisms that promote tissue degradation, including reactive activation of metalloproteinases, heparanases, and hyaluronidases [23,56]. In patients with these acute diseases, high concentrations of fragmented VEGLX, such as syndecan-1 (soluble CD138), syndecan-4, hyaluronic acid, and heparan sulfate, can be detected in the blood. The degraded VEGLX detaches from the surface of vascular endothelial cells, thinning the glycocalyx layer, and inducing extravascular leakage in microvessels with excessive vascular permeability, contributing to further pathological deterioration by causing interstitial edema in various organs [56,57].
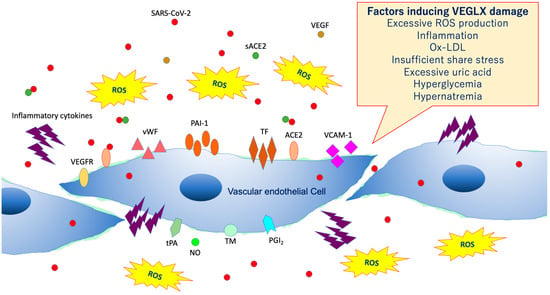
Figure 8.
Damaged vascular endothelial glycocalyx (VEGLX). VEGLX damage is associated with vascular endothelial dysfunction, which induces reduced nitric oxide (NO) bioavailability, increased excessive reactive oxygen species (ROS) production, inflammatory cytokine release, platelet adherence, coagulation, and leukocyte adhesion. SARS-CoV-2, severe acute respiratory coronavirus 2; VEGF, vascular endothelial growth factor; VEGFR, VEGF receptor; ACE2, angiotensin-converting enzyme 2; sACE2, soluble ACE2; PAI-1, plasminogen activator inhibitor-1; TF, tissue factor; vWF, von Willebrand factor; ox-LDL, oxidized low-density lipoprotein; MMPs, matrix metalloproteases; tPA, tissue plasminogen activator; PGI2, prostacyclin; TM, thrombomodulin.
7. Evaluation of VEGLX In Vivo
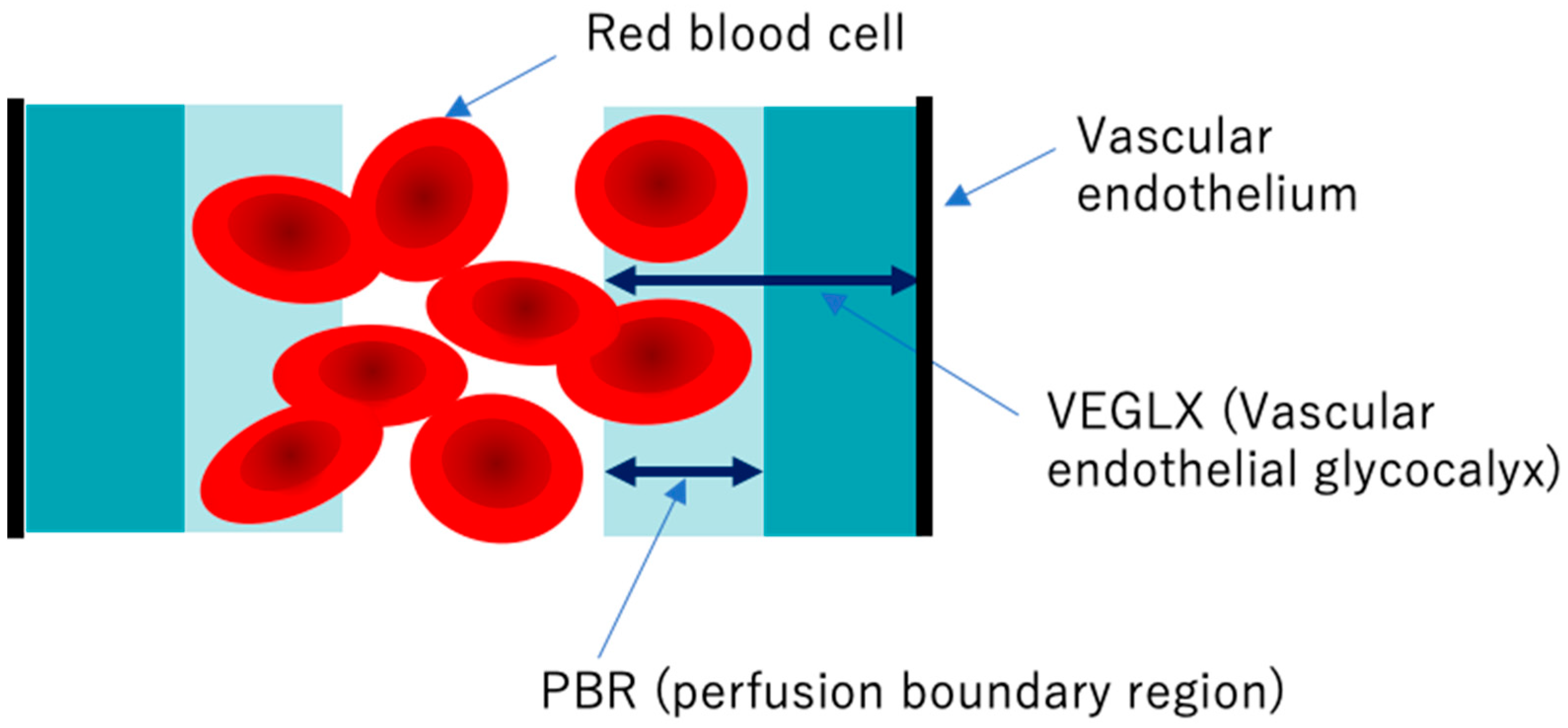
Elevated concentrations of soluble VEGLX fragments in the blood of patients with sepsis and diabetes have been previously reported and qualitatively assessed using electron micrographic imaging of the microvascular VEGLX in animal models and autopsy cases. The endothelial glycocalyx measurement device has emerged as a simple and reproducible instrument for measuring VEGLX by monitoring the sublingual microcirculation of the VEGLX vulnerable region as an indicator of the perfusion boundary region (PBR). Evaluation of the VEGLX using GlycoCheck® (Microvascular Health Solutions, UT, USA) has been used [58]. The VEGLX is composed of a tight glycocalyx layer and a coarse glycocalyx layer. The latter allows red blood cells to bounce freely within the layer, which is observed as an increased PBR (Figure 9).
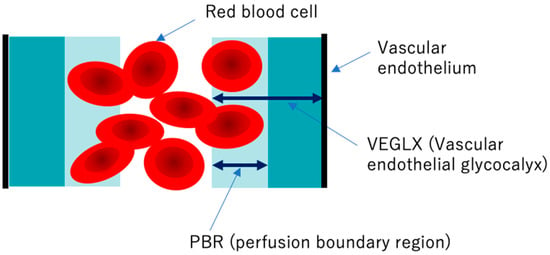
Figure 9.
Conceptual diagram of the vascular endothelial glycocalyx (VEGLX) vulnerable region (PBR). The VEGLX, which covers the surface of vascular endothelial cells, can be broadly divided into a region of dense glycans on the vascular endothelial side and a region covered by fragile glycans on the vascular lumen side. In disorders of the VEGLX, the fragile region is known to be enlarged.
To detect VEGLX damage, PBR of the sublingual arterial microvessels is measured as a noninvasive test. Higher PBR values are thought to indicate an increase in this vulnerable area of the VEGLX and represent a thinning of the tight healthy VEGLX. In addition to reports on physiological changes in the VEGLX in healthy subjects, there have been reports on PBR related to a variety of diseases. A higher PBR was observed in elderly people, women, people with low body mass index (BMI), and patients with diastolic hypertension and diabetes [59,60,61]. In the context of racial differences, one report found no obvious racial differences between 472 Chinese and 254 Flemish residents [62], while a cross-sectional report of 6169 people found racial differences in allowing for increased PBR among blacks of African descent and whites of European descent compared to Asians and Arabs [59].
In diseases associated with complications of severe coronavirus, infections include sepsis, chronic heart failure [63], cerebrovascular disease [64], microvascular angina [65], and ischemic heart disease [64], PBR has been reported to be associated with the severity of these diseases, suggesting the usefulness of VEGLX measurements [66,67].
8. Virus Infections and VEGLX
SARS-CoV-2, the virus responsible for COVID-19, is a positive-sense single-stranded RNA virus that is classified as a (+)ssRNA virus. The genome of positive-stranded RNA viruses also acts as mRNA and is translated into proteins in the host cell. These viruses are categorized into Group IV of the Baltimore Classification of Viruses, and are classified as dengue fever virus, and include SARS-CoV-1, MERS-CoV2, hepatitis C virus, yellow fever virus, Japanese encephalitis viruses, and rhinoviruses.
In case of viral infections, research on dengue fever and VEGLX has been reported. Dengue hemorrhagic fever/dengue shock syndrome (DHF/DSS) is characterized by microvascular barrier dysfunction and shock [68]. Dengue virus nonstructural protein 1 (NS1) has been identified as the only membrane-associated protein that anchors the viral replication complex to the vascular endothelial cell membrane. Increased blood levels of VEGLX components such as hyaluronic acid, heparin sulfate, claudin-5, and syndecan-1, suggesting shedding of the VEGLX and extravascular leakage of plasma components, have been associated with the development and exacerbation of severe dengue infection [69,70]. Such viral entry into and proliferation of vascular endothelial cells in severe viral infections is responsible for a variety of microvascular disorders, including ARDS and DIC [71,72].
The susceptibility of influenza A virus to host cell infection is defined by the density, glycosylation state, and length of the glycocalyx, which constitutes a protective barrier called mucin on the surface of the cell [73]. Dense mucin is the most important factor in the ability of influenza virus to penetrate the cell surface and inhibit binding to glycolipid receptors. The rate at which the virus fuses to the cell is found slower in a concentration-dependent manner with respect to mucin [73]. Several flu drugs target the interaction between the influenza virus and mucin-like proteins [73].
9. Definition of SIRME
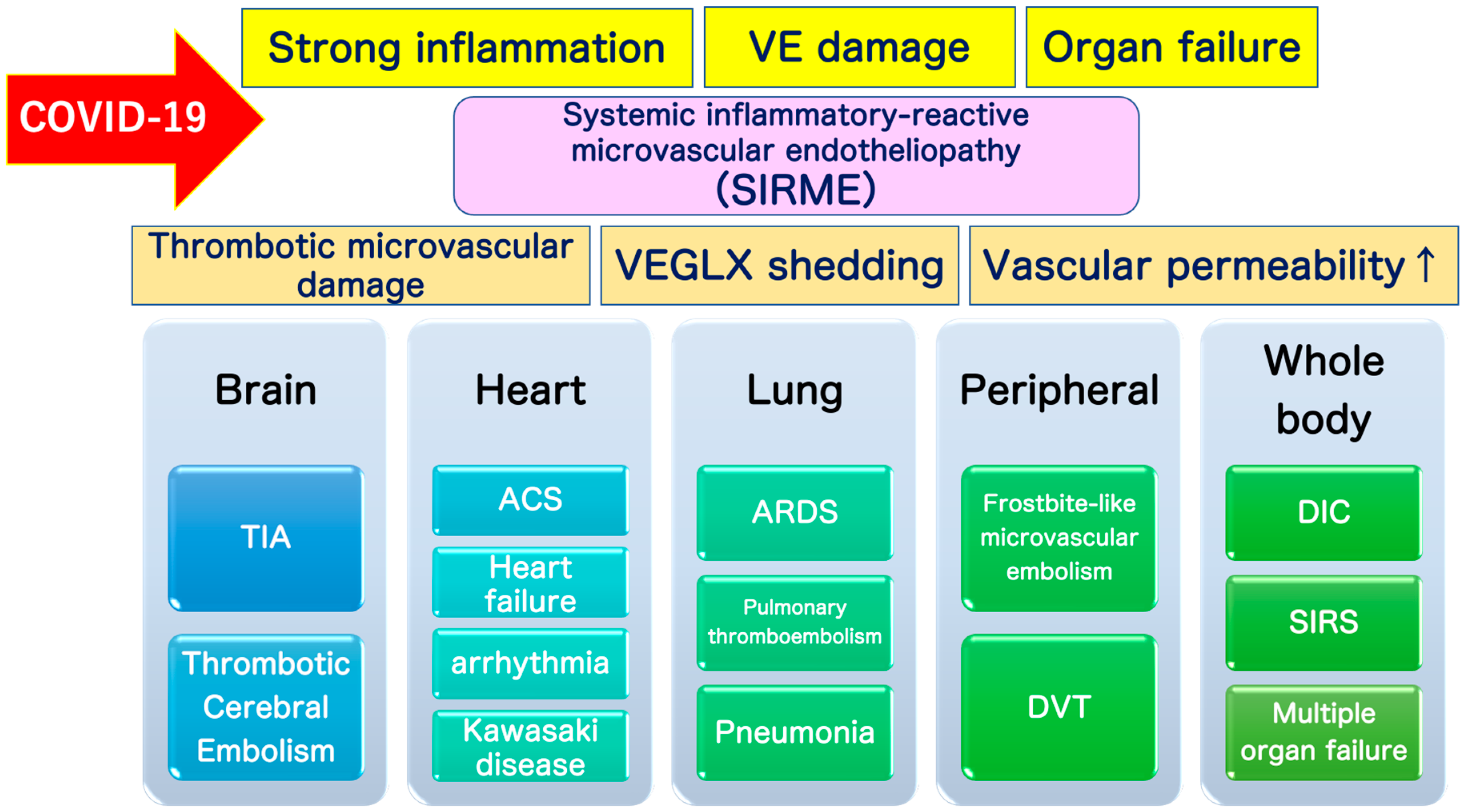
VEGLX disorders associated with sympathetic and adrenal hyperfunction, similar to shock-induced endotheliosis (SHINE) observed in extremely severe conditions such as post-cardiac arrest syndrome, also occur in COVID-19, which does not meet the criteria for systemic inflammatory response syndrome (SIRS), and in non-shock conditions. It is also a condition observed in patients with mild to moderate COVID-19. For this reason, the newly proposed systemic inflammation-reactive microvascular endotheliosis (SIRME) caused by SIRS-CoV-2 as a mechanism for the development of various complications caused by COVID-19 is shown in Figure 10.
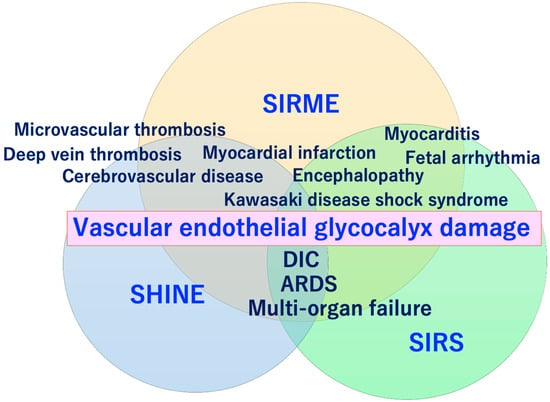
Figure 10.
Conceptual diagram showing the relationship between shock-induced endotheliopathy (SHINE)/systemic inflammatory-reactive syndrome (SIRS) and the vascular endothelial glycocalyx (VEGLX) damage that causes systemic inflammation-reactive microvascular endotheliopathy (SIRME). SIRME is caused by a systemic disorder of the VEGLX, resulting in extravascular leakage of plasma components, increased thrombogenicity, increased production of reactive oxygen species, and an excess state of inflammatory cytokines, leading to microvascular embolism, venous thrombosis, and Kawasaki disease shock syndrome. As the disease worsens, it progresses to severe SIRME, including the concept of SIRS and SHINE, leading to severe conditions such as disseminated intravascular coagulation (DIC) and acute respiratory distress syndrome (ARDS).
The VEGLX disorder as its primary mechanism, characterized by (1) thrombotic microvascular disease, (2) shedding of the VEGLX, and (3) increased vascular permeability [24]. Pathologies caused by SIRME include (1) the presence of causative inflammation (fever, high levels of C-reactive protein and proinflammatory cytokines), (2) vascular endothelial damage with strong thrombogenic tendencies (high D-dimer and FDP) and increased vascular permeability; and (3) organ damage (increased respiratory rate, high levels of lactate dehydrogenase and transaminases, and elevated myocardial deviation enzymes), and are defined as a condition in which all 3 pathologies are present at the same time (Table 1). In addition to (1) to (3), the presence of (4) abnormally high blood levels of fragmented glycocalyx, or (5) progressive multiple frosted shadows in both lungs is defined as a progressive SERMIE, which is considered a high-risk condition for progression to DIC or ARDS with poor prognosis (Figure 11).

Table 1.
SIRME and progressive SIRME.
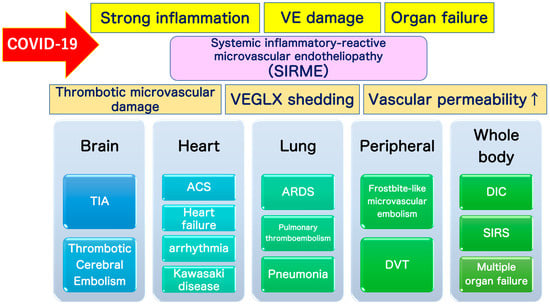
Figure 11.
Definition of systemic inflammatory-reactive microvascular endotheliopathy (SIRME). SIRME is caused by damage to the vascular endothelial glycocalyx (VEGLX), which is impaired in an inflammatory response. SIRME is characterized by (1) the presence of causative strong inflammation, (2) vascular endothelial damage with strong thrombogenic tendency and increased vascular permeability, (3) organ failure. SIRME is presumed to be one of the major mechanisms causing diverse complications of COVID-19. (↑) shows upregulation. VE, vascular endothelial; VEGLX, vascular endothelial glycocalyx; TIA, transient ischemic attack; ACS, acute coronary syndrome; ARDS, acute respiratory distress syndrome; DVT, deep vein thrombosis; DIC: disseminated intravascular coagulation; SIRS, systemic inflammatory response syndrome.
10. Severe Inflammation-Induced Vascular Endothelial Damage
Post-mortem examination of the tissues of 3 patients who died of COVID-19: a post-renal transplant patient with coronary artery disease and hypertension, an obese patient with diabetes and hypertension, and a hypertensive patient, revealed traces of viral entry into vascular endothelial cells (presence of viral bodies), aggregation of inflammatory cells, and the presence of endothelial cells [6]. In addition, apoptosis and pyotosis (inflammation-induced programmed cell death) have been observed [6]. This “COVID-19 endotheliopathy” induces systemic vascular endothelial dysfunction, especially microcirculatory dysfunction, which has been implicated in the development of various complications.
The VEGLX is responsible for maintaining vascular homeostasis [74], including regulation of vascular permeability and microvascular tonus, prevention of microvascular thrombosis, and regulation of leukocyte adhesion [71]. In case of sepsis, the VEGLX may be involved in the regulation of inflammation-related enzymes such as metalloproteinase, heparanase, and hyaluronidase. Considering the fact that microvascular endothelial damage is difficult to assess using routine imaging, delays in diagnosis and treatment due to missed early microvascular damage are likely to make it even more difficult to predict the severity of the disease in COVID-19 patients.
Systemic damage to the vascular glycocalyx, which makes up a delicate layer, causes increased transport of proteins and water out of the vessels, that is, extravascular leakage of plasma components. In sepsis, the VEGLX is impaired and its layer becomes thinner, triggering excessive permeability of microvessels and causing interstitial edema in various organs [23,56]. Systemic shedding of the VEGLX can be led by serious infections, sepsis, hemorrhagic shock, burns, traumatic brain injury [57]. It occurs rapidly in fatal medical conditions such as traumatic endotheliosis, which is a mortality-related syndrome [55]. Patients with a variety of underlying diseases have chronic systemic VEGLX disorders due to a complex mechanism, but once these patients are infected with SARS-CoV-2, COVID-19-induced SIRME occurs in the process of the development of a variety of serious complications such as ARDS [21], DIC, Kawasaki disease shock syndrome, microvascular thrombosis, and arrhythmias (Figure 11).
11. Kawasaki Disease Shock Syndrome and VEGLX
Kawasaki disease is an acute febrile systemic vasculitis occurring primarily in children younger than 5 years of age, where systemic vasculitis is especially observed in small and medium-sized arteries. Since Kawasaki disease exhibits seasonal, temporal, and regional patterns, infectious agents are thought to be the causative or precipitating factor in Kawasaki disease [75]. Serological tests have reported that HCoV-229E, a type of coronavirus, is involved in the development of Kawasaki disease [76]. The exact mechanism of Kawasaki disease development is unknown, and for coronary artery aneurysms, it is believed to be a complex interaction of genetic factors, infection, and immunity [77]. For coronary artery aneurysms, it has been reported in males, intravenous immunoglobulin (IVIG) non-responders with high neutrophil/lymphocyte ratios, cases of inotropic drug use, and cases of heart failure. Significantly more coronary artery aneurysms occur in cases with abdominal pain and neurological symptoms [78].
An increase in Kawasaki disease or Kawasaki disease-like illness has been extensively reported in countries around the world occurring at the same time as the COVID-19 outbreaks. A severe subtype of Kawasaki disease, Kawasaki disease shock syndrome, is a rare complication of Kawasaki disease and is associated with a risk of serious sequelae and death [79]. Previous reports have shown that of 187 consecutive people diagnosed with Kawasaki disease, 13 (7%) met the definition of Kawasaki disease shock syndrome. Kawasaki disease shock syndrome is characterized by more severe proinflammatory cytokine production and tends to be prone to IVIG nonresponsiveness and coronary artery abnormalities [80]. Among severe COVID-19, characteristics consistent with toxic shock syndrome with abdominal pain and gastrointestinal symptoms similar to those of Kawasaki disease shock syndrome have been identified, as an increase in the number of children with the disease has been warned.
It has been shown that in the acute phase of Kawasaki disease, circulating levels of VEGLX (syndecan-1 and hyaluronic acid) are significantly elevated, and serum hyaluronic acid is the most useful prognostic biomarker for predicting future onset or exacerbation of coronary artery lesions in Kawasaki disease [46]. Serum levels of soluble syndecan-1, one of the major core proteins expressed in the VEGLX, are thought to reflect vascular endothelial damage and inflammation in Kawasaki disease [81]. Considering the common pathogenesis of Kawasaki disease and COVID-19, VEGLX damage has been suggested to be a biomarker in Kawasaki disease, and glycocalyx-associated biomarkers will likely contribute to the early detection of severe cases of COVID-19 in children and young adults, whose incidence of the disease is rare, but are prone to severe illnesses once they develop. Research on the development of biomarkers and new therapeutic strategies for predicting the onset of severe COVID-19 and COVID-19 patients who present with Kawasaki disease shock syndrome-like symptoms is expected to be promoted.
12. Characteristics of COVID-19
The features of COVID-19 that have not been seen in previous viral infections include asymptomatic pneumonia (Figure 12 and Figure 13), ARDS [82], and DIC [83] and their rapid progression, and sudden death associated with thromboembolism [84]. In particular, thromboembolism-related complications have been reported as an important complication closely related to the severity of COVID-19 patients, with deep vein thrombosis found in as many as 58% of autopsies of deceased COVID-19 patients [85]. Patients with COVID-19 are characterized by elevated blood D-dimer and fibrinogen degradation products (FDP) levels, prolonged prothrombin time, and the development of DIC, which are all associated with severe COVID-19. It was also a poor prognostic factor for patients [4]. In addition, a number of microvascular thromboses causing characteristic frostbite-like swelling of the hands and toes have been reported in patients with COVID-19 [86].
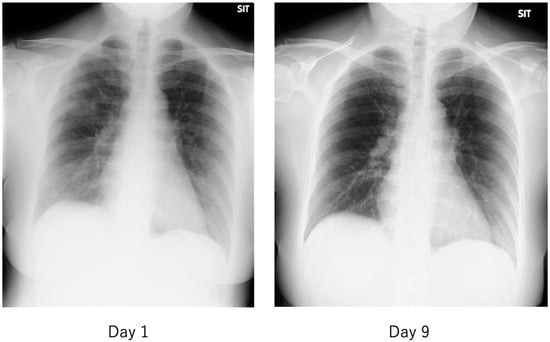
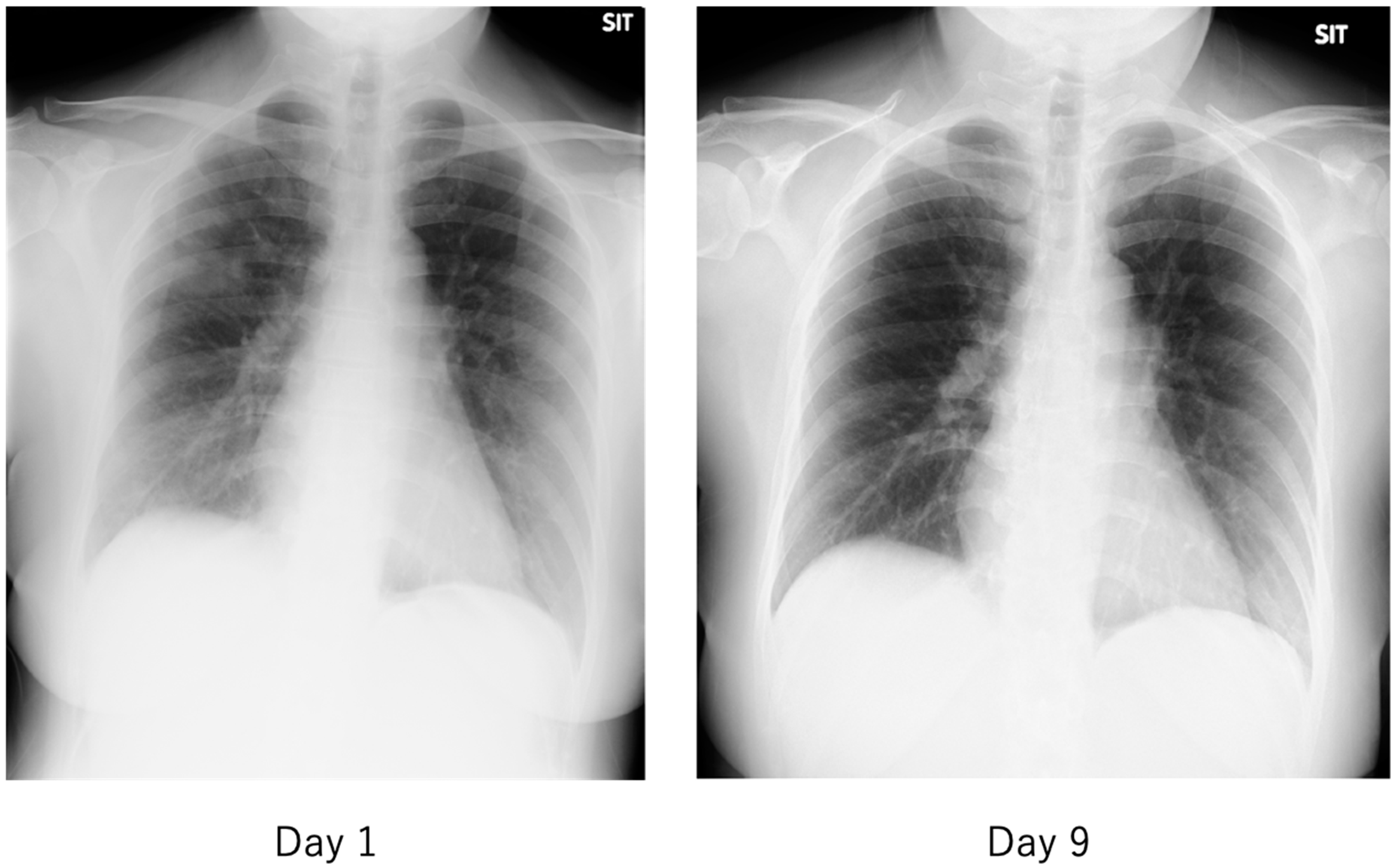
Figure 12.
Chest radiograph of a patient with mild COVID-19. The fever of 38 °C lasted only half a day, and the next day, cough and nasal discharge were noted. A polymerase chain reaction (PCR) test for SARS-CoV-2 in a nasopharyngeal swab was performed 2 days after the fever was positive, and the patient was admitted to our hospital 2 days later. On admission, the patient had a low-grade fever, cough, nasal discharge, and conjunctival hyperemia, and chest radiographs showed multiple faint frosted shadows in the bilateral lung fields (left). With follow-up observation alone, the patient’s common cold-like symptoms were mild, and the abnormal shadows on the lungs had nearly disappeared by the ninth day of the disease (right). The patient was then discharged after two PCR tests confirmed negative results.
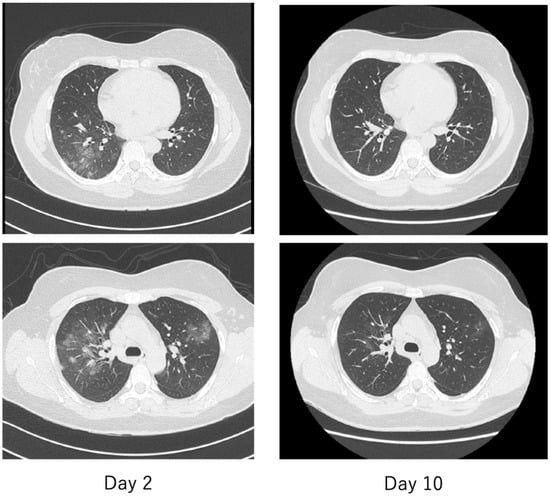
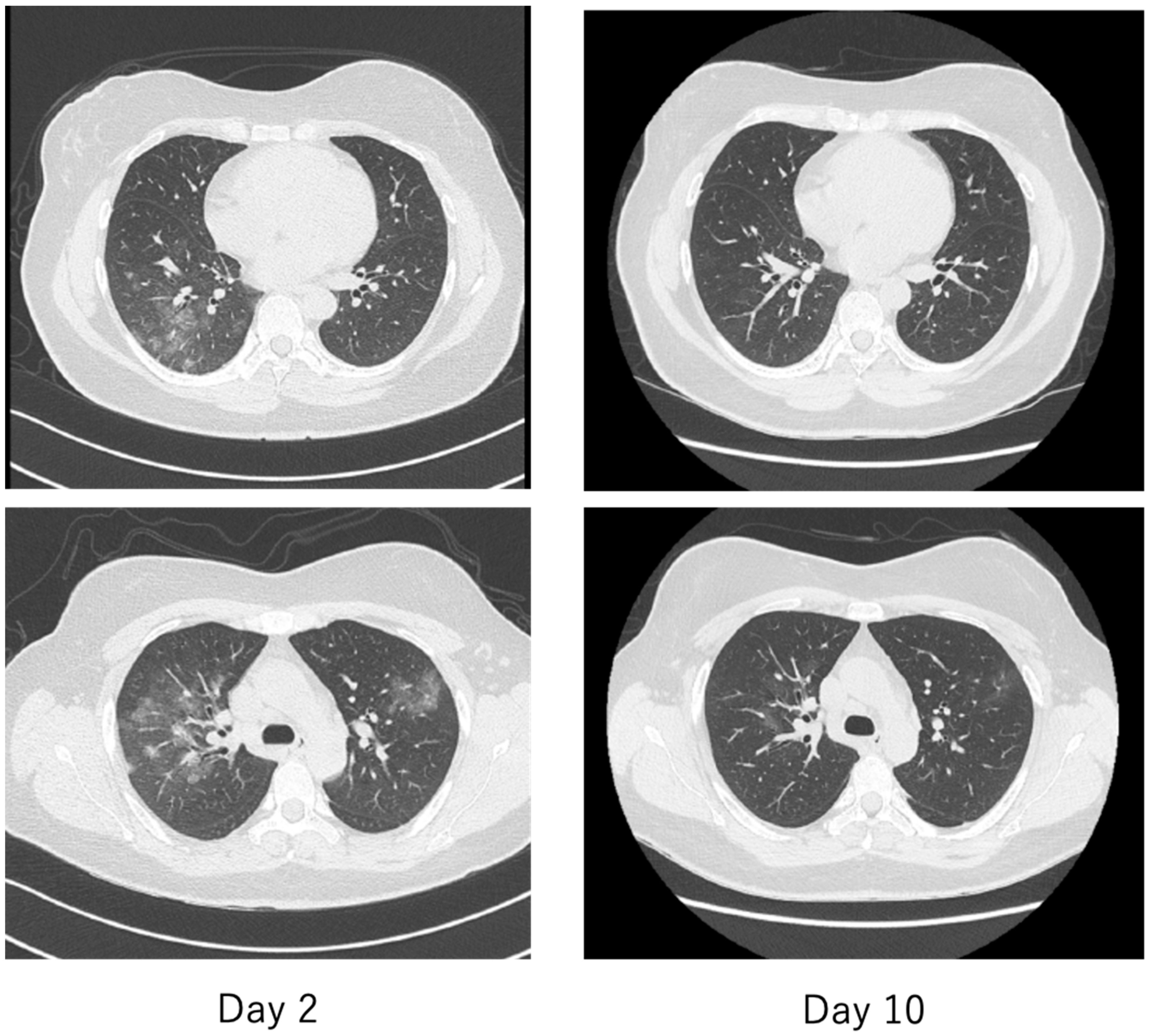
Figure 13.
Chest computed tomography (CT) of a patient with mild COVID-19, the same patient as in Figure 12. On the second day, there were multiple faint frosted shadows bilaterally (left), but by the tenth day, the abnormal shadows had almost disappeared (right).
The criteria for severity of disease in COVID-19 are generally similar to those used in previous diseases, with patients requiring oxygen and either (1) admission to an intensive care unit (ICU), (2) requiring ventilatory management, or (3) leading to death. The hallmark imaging findings of COVID-19 are asymptomatic pneumonia detected by simple chest radiography or computed tomography (CT). As shown in Figure 12 and Figure 13, the frosty shadows are multiple and bilateral, and multiple rounds. Pale frosty shadows have been observed even in patients with mild COVID-19 who have only cold symptoms (e.g., mild fever, runny nose, and cough). These characteristic abnormal chest shadows in COVID-19 patients are not necessarily an indicator of the severity of the disease, and therefore, rapidly progressing signs of deterioration can be difficult to capture, especially in poorly symptomatic cases. It is difficult to foresee severe illness or sudden death in COVID-19.
13. Literature Search Results for VEGLX in COVID-19
The keywords “glycocalyx COVID-19”, “syndecan coronavirus”, hyaluronic acid COVID-19”, “heparanase COVID-19”, and “PBR COVID-19” were searched in databases such as PubMed, the WHO Global Health research database on COVID-19, and Japan Medical Abstracts Society. The research time frame for the database was until November 2020. The language was limited to English and Japanese. The titles and abstracts of all potentially relevant articles were read to determine their relevance. Full articles were also scrutinized if the titles and abstracts were unclear. Reference lists of identified articles were screened to ensure the completeness of the search.
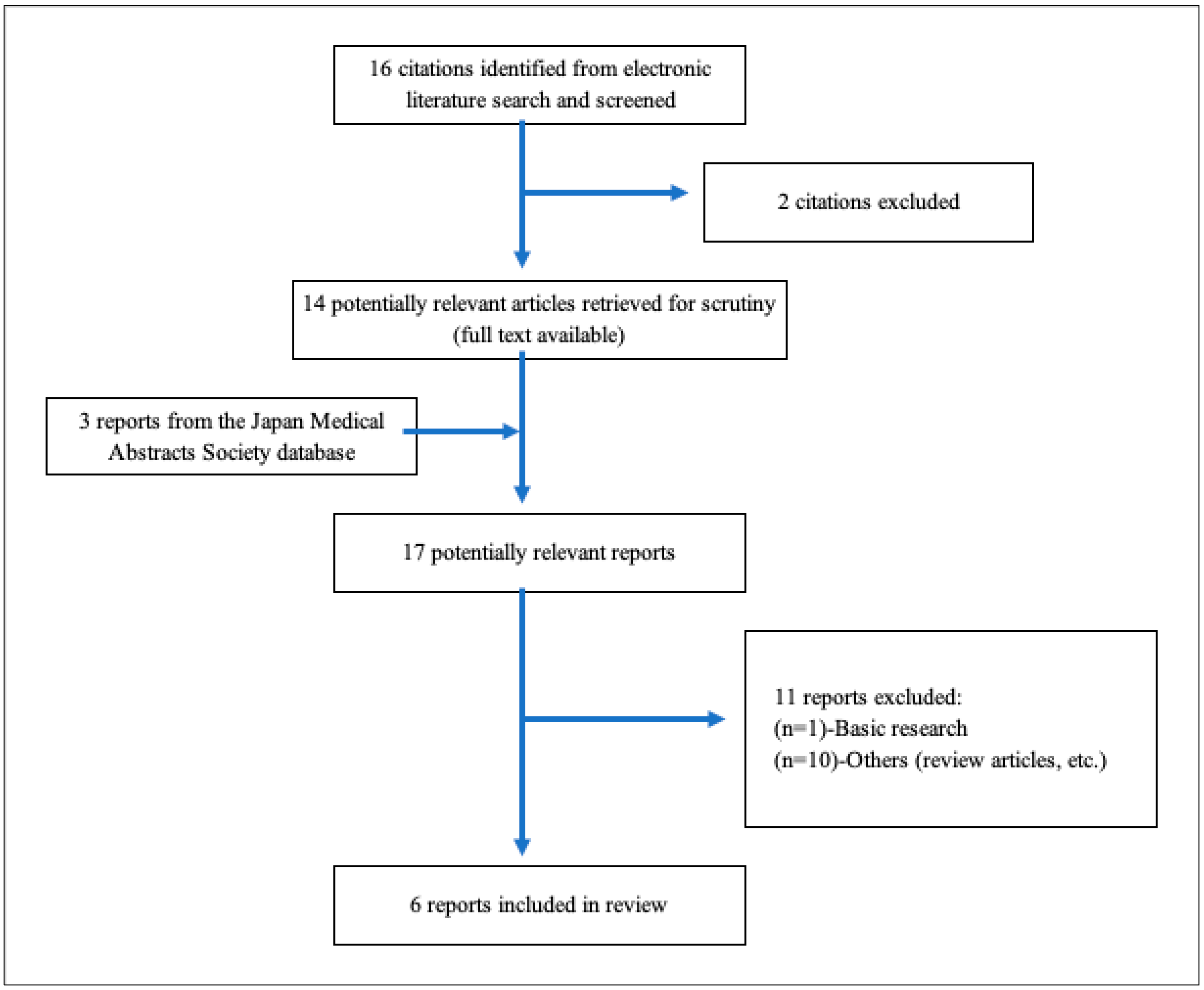
As shown in Table 2, a total of 16 studies were included for the title and abstract screening, and 2 studies were excluded due to duplicates. After a total of 11 studies were not deemed eligible for full-text review, 6 reports [3,87,88,89,90,91] were included in the review. Figure 14 presents a flowchart depicting the selection process. Table 3 summarizes the characteristics of the included studies. All studies showed that circulating levels of glycocalyx or its kinases were increased in patients with severe COVID-19. Among them, only one study also revealed that VEGLX damage detected by PBR measurement was observed in severe patients with COVID-19. At present, only a few clinical studies have been conducted on COVID-19 patients; however, its effectiveness as a biomarker for predicting the severity of the disease and as an indicator of therapeutic efficacy in patients with COVID-19 could be very promising.

Table 2.
VEGLX damage in COVID-19.
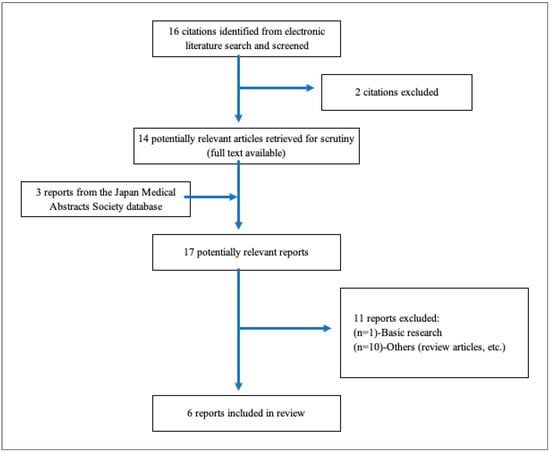
Figure 14.
A flowchart depicting the selection process. A total of 16 studies were imported for title and abstract screening after removing duplicates, and 2 studies were retained. After a total of 11 studies were deemed eligible for full-text review, 6 reports [3,87,88,89,90,91] were included in Table 3.

Table 3.
VEGLX in patients with COVID-19.
14. Future Outlook
The COVID-19 pandemic has fundamentally changed the lives of people around the world. Many cities have been sealed off, forcing people to stay in their homes, avoid contact with others, and scale back their economic activities. All non-essential activities are encouraged to stop, and even educational and work opportunities are being deprived by this epidemic. As far as we can surmise from this situation, it is getting to the point where we will never be able to return to the earlier situation again.
What we can do is to deeply understand the various aspects of the pathogenesis of viral infections, which will continue to evolve in the future, and to gather the wisdom of many to create effective countermeasures and the best way to survive together in the future. The VEGLX is a classic physical barrier common to many organisms, but this area of clinical research has not been well studied to date. It is precisely in the current challenging international climate that ideas from new areas of research must be shared to enrich our existing knowledge base.
Funding
This work was partly supported by JSPS KAKENHI grant number JP19K11371.
Acknowledgments
The author thanks all members of the COVID-19 clinical support team at Kitasato University East Hospital and Kitasato University Hospital.
Conflicts of Interest
The author declares no conflict of interest.
References
- Zhang, J.; Tecson, K.M.; McCullough, P.A. Endothelial dysfunction contributes to COVID-19-associated vascular inflammation and coagulopathy. Rev. Cardiovasc. Med. 2020, 21, 315–319. [Google Scholar] [CrossRef] [PubMed]
- Ackermann, M.; Verleden, S.E.; Kuehnel, M.; Haverich, A.; Welte, T.; Laenger, F.; Vanstapel, A.; Werlein, C.; Stark, H.; Tzankov, A.; et al. Pulmonary Vascular Endothelialitis, Thrombosis, and Angiogenesis in Covid-19. N. Engl. J. Med. 2020, 383, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Rovas, A.; Osiaevi, I.; Buscher, K.; Sackarnd, J.; Tepasse, P.R.; Fobker, M.; Kuhn, J.; Braune, S.; Gobel, U.; Tholking, G.; et al. Microvascular dysfunction in COVID-19: The MYSTIC study. Angiogenesis 2020. [Google Scholar] [CrossRef] [PubMed]
- Di Castelnuovo, A.; Bonaccio, M.; Costanzo, S.; Gialluisi, A.; Antinori, A.; Berselli, N.; Blandi, L.; Bruno, R.; Cauda, R.; Guaraldi, G.; et al. Common cardiovascular risk factors and in-hospital mortality in 3894 patients with COVID-19: Survival analysis and machine learning-based findings from the multicentre Italian CORIST Study. Nutr. Metab. Cardiovasc. Dis. 2020, 30, 1899–1913. [Google Scholar] [CrossRef]
- Yamaoka-Tojo, M. COVID-19 and Vascular Endothelium: Tips for Preventive Measures from the Perspective of Cardiovascular Preventive Medicine; Nanzando: Tokyo, Japan, 2020. [Google Scholar]
- Varga, Z.; Flammer, A.J.; Steiger, P.; Haberecker, M.; Andermatt, R.; Zinkernagel, A.S.; Mehra, M.R.; Schuepbach, R.A.; Ruschitzka, F.; Moch, H. Endothelial cell infection and endotheliitis in COVID-19. Lancet 2020, 395, 1417–1418. [Google Scholar] [CrossRef]
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Kruger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Wu, N.H.; Nitsche, A.; et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 2020, 181, 271–280.e8. [Google Scholar] [CrossRef]
- Lee, A.C.; Chakladar, J.; Li, W.T.; Chen, C.; Chang, E.Y.; Wang-Rodriguez, J.; Ongkeko, W.M. Tobacco, but Not Nicotine and Flavor-Less Electronic Cigarettes, Induces ACE2 and Immune Dysregulation. Int. J. Mol. Sci. 2020, 21, 5513. [Google Scholar] [CrossRef]
- Voinsky, I.; Baristaite, G.; Gurwitz, D. Effects of age and sex on recovery from COVID-19: Analysis of 5769 Israeli patients. J. Infect. 2020, 81, e102–e103. [Google Scholar] [CrossRef]
- Yamaoka-Tojo, M. Endothelial glycocalyx damage as a systemic inflammatory microvascular endotheliopathy in COVID-19. Biomed. J. 2020. [Google Scholar] [CrossRef]
- Danilczyk, U.; Penninger, J.M. Angiotensin-converting enzyme II in the heart and the kidney. Circ. Res. 2006, 98, 463–471. [Google Scholar] [CrossRef]
- Bunyavanich, S.; Do, A.; Vicencio, A. Nasal Gene Expression of Angiotensin-Converting Enzyme 2 in Children and Adults. JAMA 2020. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Litvinova, M.; Liang, Y.; Wang, Y.; Wang, W.; Zhao, S.; Wu, Q.; Merler, S.; Viboud, C.; Vespignani, A.; et al. Changes in contact patterns shape the dynamics of the COVID-19 outbreak in China. Science 2020, 368, 1481–1486. [Google Scholar] [CrossRef] [PubMed]
- Rabelo, L.A.; Alenina, N.; Bader, M. ACE2-angiotensin-(1-7)-Mas axis and oxidative stress in cardiovascular disease. Hypertens Res. 2011, 34, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Sahara, M.; Ikutomi, M.; Morita, T.; Minami, Y.; Nakajima, T.; Hirata, Y.; Nagai, R.; Sata, M. Deletion of angiotensin-converting enzyme 2 promotes the development of atherosclerosis and arterial neointima formation. Cardiovasc. Res. 2014, 101, 236–246. [Google Scholar] [CrossRef] [PubMed]
- Ohishi, M.; Yamamoto, K.; Rakugi, H. Angiotensin (1-7) and other angiotensin peptides. Curr. Pharm. Des. 2013, 19, 3060–3064. [Google Scholar] [CrossRef] [PubMed]
- Turner, A.J.; Hiscox, J.A.; Hooper, N.M. ACE2: From vasopeptidase to SARS virus receptor. Trends Pharmacol. Sci. 2004, 25, 291–294. [Google Scholar] [CrossRef] [PubMed]
- Ou, X.; Liu, Y.; Lei, X.; Li, P.; Mi, D.; Ren, L.; Guo, L.; Guo, R.; Chen, T.; Hu, J.; et al. Characterization of spike glycoprotein of SARS-CoV-2 on virus entry and its immune cross-reactivity with SARS-CoV. Nat. Commun. 2020, 11, 1620. [Google Scholar] [CrossRef]
- Wang, J.; Kaplan, N.; Wysocki, J.; Yang, W.; Lu, K.; Peng, H.; Batlle, D.; Lavker, R.M. The ACE2-deficient mouse: A model for a cytokine storm-driven inflammation. FASEB J. 2020. [Google Scholar] [CrossRef]
- Teuwen, L.A.; Geldhof, V.; Pasut, A.; Carmeliet, P. COVID-19: The vasculature unleashed. Nat. Rev. Immunol 2020, 20, 389–391. [Google Scholar] [CrossRef]
- Pons, S.; Fodil, S.; Azoulay, E.; Zafrani, L. The vascular endothelium: The cornerstone of organ dysfunction in severe SARS-CoV-2 infection. Crit. Care 2020, 24, 353. [Google Scholar] [CrossRef]
- Okada, H.; Yoshida, S.; Hara, A.; Ogura, S.; Tomita, H. Vascular endothelial injury exacerbates coronavirus disease 2019: The role of endothelial glycocalyx protection. Microcirculation 2020, e12654. [Google Scholar] [CrossRef] [PubMed]
- Uchimido, R.; Schmidt, E.P.; Shapiro, N.I. The glycocalyx: A novel diagnostic and therapeutic target in sepsis. Crit. Care 2019, 23, 16. [Google Scholar] [CrossRef] [PubMed]
- Yamaoka-Tojo, M. A note on systemic inflammatory-reactive microvascular endotheliopathy (SIRME): Prevention of cardiovascular disease and COVID-19. JJCDP 2020, 55, 1–14. [Google Scholar]
- Butler, P.J.; Bhatnagar, A. Mechanobiology of the abluminal glycocalyx. Biorheology 2019, 56, 101–112. [Google Scholar] [CrossRef] [PubMed]
- Betteridge, K.B.; Arkill, K.P.; Neal, C.R.; Harper, S.J.; Foster, R.R.; Satchell, S.C.; Bates, D.O.; Salmon, A.H.J. Sialic acids regulate microvessel permeability, revealed by novel in vivo studies of endothelial glycocalyx structure and function. J. Physiol. 2017, 595, 5015–5035. [Google Scholar] [CrossRef] [PubMed]
- Butler, M.J.; Ramnath, R.; Kadoya, H.; Desposito, D.; Riquier-Brison, A.; Ferguson, J.K.; Onions, K.L.; Ogier, A.S.; ElHegni, H.; Coward, R.J.; et al. Aldosterone induces albuminuria via matrix metalloproteinase-dependent damage of the endothelial glycocalyx. Kidney Int. 2019, 95, 94–107. [Google Scholar] [CrossRef]
- Curry, F.R. Microvascular solute and water transport. Microcirculation 2005, 12, 17–31. [Google Scholar] [CrossRef]
- Curry, F.E. Layer upon layer: The functional consequences of disrupting the glycocalyx-endothelial barrier in vivo and in vitro. Cardiovasc. Res. 2017, 113, 559–561. [Google Scholar] [CrossRef]
- Ueda, A.; Shimomura, M.; Ikeda, M.; Yamaguchi, R.; Tanishita, K. Effect of glycocalyx on shear-dependent albumin uptake in endothelial cells. Am. J. Physiol. Heart Circ. Physiol. 2004, 287, H2287–H2294. [Google Scholar] [CrossRef]
- Thi, M.M.; Tarbell, J.M.; Weinbaum, S.; Spray, D.C. The role of the glycocalyx in reorganization of the actin cytoskeleton under fluid shear stress: A “bumper-car” model. Proc. Natl. Acad. Sci. USA 2004, 101, 16483–16488. [Google Scholar] [CrossRef]
- Koo, A.; Dewey, C.F., Jr.; Garcia-Cardena, G. Hemodynamic shear stress characteristic of atherosclerosis-resistant regions promotes glycocalyx formation in cultured endothelial cells. Am. J. Physiol. Cell Physiol. 2013, 304, C137–C146. [Google Scholar] [CrossRef] [PubMed]
- Prydz, K. Determinants of Glycosaminoglycan (GAG) Structure. Biomolecules 2015, 5, 2003–2022. [Google Scholar] [CrossRef] [PubMed]
- Bar, A.; Targosz-Korecka, M.; Suraj, J.; Proniewski, B.; Jasztal, A.; Marczyk, B.; Sternak, M.; Przybylo, M.; Kurpinska, A.; Walczak, M.; et al. Degradation of Glycocalyx and Multiple Manifestations of Endothelial Dysfunction Coincide in the Early Phase of Endothelial Dysfunction Before Atherosclerotic Plaque Development in Apolipoprotein E/Low-Density Lipoprotein Receptor-Deficient Mice. J. Am. Heart Assoc. 2019, 8, e011171. [Google Scholar] [CrossRef] [PubMed]
- Oberleithner, H.; Wilhelmi, M. Vascular glycocalyx sodium store—Determinant of salt sensitivity? Blood Purif. 2015, 39, 7–10. [Google Scholar] [CrossRef] [PubMed]
- Brands, J.; Spaan, J.A.; Van den Berg, B.M.; Vink, H.; VanTeeffelen, J.W. Acute attenuation of glycocalyx barrier properties increases coronary blood volume independently of coronary flow reserve. Am. J. Physiol. Heart Circ. Physiol. 2010, 298, H515–H523. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rubio-Gayosso, I.; Platts, S.H.; Duling, B.R. Reactive oxygen species mediate modification of glycocalyx during ischemia-reperfusion injury. Am. J. Physiol. Heart Circ. Physiol. 2006, 290, H2247–H2256. [Google Scholar] [CrossRef]
- Nieuwdorp, M.; Meuwese, M.C.; Vink, H.; Hoekstra, J.B.; Kastelein, J.J.; Stroes, E.S. The endothelial glycocalyx: A potential barrier between health and vascular disease. Curr. Opin. Lipidol. 2005, 16, 507–511. [Google Scholar] [CrossRef]
- Nieuwdorp, M.; Meuwese, M.C.; Mooij, H.L.; van Lieshout, M.H.; Hayden, A.; Levi, M.; Meijers, J.C.; Ince, C.; Kastelein, J.J.; Vink, H.; et al. Tumor necrosis factor-alpha inhibition protects against endotoxin-induced endothelial glycocalyx perturbation. Atherosclerosis 2009, 202, 296–303. [Google Scholar] [CrossRef]
- Ko, J.; Kang, H.J.; Kim, D.A.; Kim, M.J.; Ryu, E.S.; Lee, S.; Ryu, J.H.; Roncal, C.; Johnson, R.J.; Kang, D.H. Uric acid induced the phenotype transition of vascular endothelial cells via induction of oxidative stress and glycocalyx shedding. FASEB J. 2019, 33, 13334–13345. [Google Scholar] [CrossRef]
- Zuurbier, C.J.; Demirci, C.; Koeman, A.; Vink, H.; Ince, C. Short-term hyperglycemia increases endothelial glycocalyx permeability and acutely decreases lineal density of capillaries with flowing red blood cells. J. Appl. Physiol. 2005, 99, 1471–1476. [Google Scholar] [CrossRef]
- Pahwa, R.; Nallasamy, P.; Jialal, I. Toll-like receptors 2 and 4 mediate hyperglycemia induced macrovascular aortic endothelial cell inflammation and perturbation of the endothelial glycocalyx. J. Diabetes Complicat. 2016, 30, 563–572. [Google Scholar] [CrossRef] [PubMed]
- Rorije, N.M.G.; Rademaker, E.; Schrooten, E.M.; Wouda, R.D.; Homan Van Der Heide, J.J.; Van Den Born, B.H.; Vogt, L. High-salt intake affects sublingual microcirculation and is linked to body weight change in healthy volunteers: A randomized cross-over trial. J. Hypertens. 2019, 37, 1254–1261. [Google Scholar] [CrossRef] [PubMed]
- Vink, H.; Constantinescu, A.A.; Spaan, J.A. Oxidized lipoproteins degrade the endothelial surface layer: Implications for platelet-endothelial cell adhesion. Circulation 2000, 101, 1500–1502. [Google Scholar] [CrossRef] [PubMed]
- Ostrowski, S.R.; Gaini, S.; Pedersen, C.; Johansson, P.I. Sympathoadrenal activation and endothelial damage in patients with varying degrees of acute infectious disease: An observational study. J. Crit. Care 2015, 30, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Ohnishi, Y.; Yasudo, H.; Suzuki, Y.; Furuta, T.; Matsuguma, C.; Azuma, Y.; Miyake, A.; Okada, S.; Ichihara, K.; Ohga, S.; et al. Circulating endothelial glycocalyx components as a predictive marker of coronary artery lesions in Kawasaki disease. Int. J. Cardiol. 2019, 292, 236–240. [Google Scholar] [CrossRef] [PubMed]
- Weissgerber, T.L.; Garcia-Valencia, O.; Milic, N.M.; Codsi, E.; Cubro, H.; Nath, M.C.; White, W.M.; Nath, K.A.; Garovic, V.D. Early Onset Preeclampsia Is Associated With Glycocalyx Degradation and Reduced Microvascular Perfusion. J. Am. Heart Assoc. 2019, 8, e010647. [Google Scholar] [CrossRef]
- Long, D.S.; Hou, W.; Taylor, R.S.; McCowan, L.M. Serum levels of endothelial glycocalyx constituents in women at 20 weeks’ gestation who later develop gestational diabetes mellitus compared to matched controls: A pilot study. BMJ Open 2016, 6, e011244. [Google Scholar] [CrossRef]
- Steppan, J.; Hofer, S.; Funke, B.; Brenner, T.; Henrich, M.; Martin, E.; Weitz, J.; Hofmann, U.; Weigand, M.A. Sepsis and major abdominal surgery lead to flaking of the endothelial glycocalix. J. Surg. Res. 2011, 165, 136–141. [Google Scholar] [CrossRef]
- Schmidt, E.P.; Overdier, K.H.; Sun, X.; Lin, L.; Liu, X.; Yang, Y.; Ammons, L.A.; Hiller, T.D.; Suflita, M.A.; Yu, Y.; et al. Urinary Glycosaminoglycans Predict Outcomes in Septic Shock and Acute Respiratory Distress Syndrome. Am. J. Respir. Crit. Care Med. 2016, 194, 439–449. [Google Scholar] [CrossRef]
- Chignalia, A.Z.; Yetimakman, F.; Christiaans, S.C.; Unal, S.; Bayrakci, B.; Wagener, B.M.; Russell, R.T.; Kerby, J.D.; Pittet, J.F.; Dull, R.O. The Glycocalyx and Trauma: A Review. Shock 2016, 45, 338–348. [Google Scholar] [CrossRef]
- DellaValle, B.; Hasseldam, H.; Johansen, F.F.; Iversen, H.K.; Rungby, J.; Hempel, C. Multiple Soluble Components of the Glycocalyx Are Increased in Patient Plasma After Ischemic Stroke. Stroke 2019, 50, 2948–2951. [Google Scholar] [CrossRef] [PubMed]
- Miranda, C.H.; de Carvalho Borges, M.; Schmidt, A.; Marin-Neto, J.A.; Pazin-Filho, A. Evaluation of the endothelial glycocalyx damage in patients with acute coronary syndrome. Atherosclerosis 2016, 247, 184–188. [Google Scholar] [CrossRef] [PubMed]
- Marechal, X.; Favory, R.; Joulin, O.; Montaigne, D.; Hassoun, S.; Decoster, B.; Zerimech, F.; Neviere, R. Endothelial glycocalyx damage during endotoxemia coincides with microcirculatory dysfunction and vascular oxidative stress. Shock 2008, 29, 572–576. [Google Scholar] [CrossRef] [PubMed]
- Johansson, P.I.; Stensballe, J.; Ostrowski, S.R. Shock induced endotheliopathy (SHINE) in acute critical illness—A unifying pathophysiologic mechanism. Crit. Care 2017, 21, 25. [Google Scholar] [CrossRef] [PubMed]
- Chelazzi, C.; Villa, G.; Mancinelli, P.; De Gaudio, A.R.; Adembri, C. Glycocalyx and sepsis-induced alterations in vascular permeability. Crit. Care 2015, 19, 26. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez Rodriguez, E.; Cardenas, J.C.; Cox, C.S.; Kitagawa, R.S.; Stensballe, J.; Holcomb, J.B.; Johansson, P.I.; Wade, C.E. Traumatic brain injury is associated with increased syndecan-1 shedding in severely injured patients. Scand. J. Trauma Resusc. Emerg. Med. 2018, 26, 102. [Google Scholar] [CrossRef]
- Lee, D.H.; Dane, M.J.; van den Berg, B.M.; Boels, M.G.; van Teeffelen, J.W.; de Mutsert, R.; den Heijer, M.; Rosendaal, F.R.; van der Vlag, J.; van Zonneveld, A.J.; et al. Deeper penetration of erythrocytes into the endothelial glycocalyx is associated with impaired microvascular perfusion. PLoS ONE 2014, 9, e96477. [Google Scholar] [CrossRef]
- Valerio, L.; Peters, R.J.; Zwinderman, A.H.; Pinto-Sietsma, S.J. Sublingual endothelial glycocalyx and atherosclerosis. A cross-sectional study. PLoS ONE 2019, 14, e0213097. [Google Scholar] [CrossRef]
- Broekhuizen, L.N.; Lemkes, B.A.; Mooij, H.L.; Meuwese, M.C.; Verberne, H.; Holleman, F.; Schlingemann, R.O.; Nieuwdorp, M.; Stroes, E.S.; Vink, H. Effect of sulodexide on endothelial glycocalyx and vascular permeability in patients with type 2 diabetes mellitus. Diabetologia 2010, 53, 2646–2655. [Google Scholar] [CrossRef] [PubMed]
- Groen, B.B.; Hamer, H.M.; Snijders, T.; van Kranenburg, J.; Frijns, D.; Vink, H.; van Loon, L.J. Skeletal muscle capillary density and microvascular function are compromised with aging and type 2 diabetes. J. Appl. Physiol. 2014, 116, 998–1005. [Google Scholar] [CrossRef]
- Gu, Y.M.; Wang, S.; Zhang, L.; Liu, Y.P.; Thijs, L.; Petit, T.; Zhang, Z.; Wei, F.F.; Kang, Y.Y.; Huang, Q.F.; et al. Characteristics and determinants of the sublingual microcirculation in populations of different ethnicity. Hypertension 2015, 65, 993–1001. [Google Scholar] [CrossRef] [PubMed]
- Wadowski, P.P.; Hulsmann, M.; Schorgenhofer, C.; Lang, I.M.; Wurm, R.; Gremmel, T.; Koppensteiner, R.; Steinlechner, B.; Schwameis, M.; Jilma, B. Sublingual functional capillary rarefaction in chronic heart failure. Eur. J. Clin. Invest. 2018, 48. [Google Scholar] [CrossRef] [PubMed]
- Gorshkov, A.Y.; Klimushina, M.V.; Boytsov, S.A.; Kots, A.Y.; Gumanova, N.G. Increase in perfused boundary region of endothelial glycocalyx is associated with higher prevalence of ischemic heart disease and lesions of microcirculation and vascular wall. Microcirculation 2018, 25, e12454. [Google Scholar] [CrossRef]
- Jaarsma, C.; Vink, H.; van Haare, J.; Bekkers, S.; van Rooijen, B.D.; Backes, W.H.; Wildberger, J.E.; Crijns, H.J.; van Teeffelen, J.; Schalla, S. Non-invasive assessment of microvascular dysfunction in patients with microvascular angina. Int. J. Cardiol. 2017, 248, 433–439. [Google Scholar] [CrossRef]
- Yen, W.; Cai, B.; Yang, J.; Zhang, L.; Zeng, M.; Tarbell, J.M.; Fu, B.M. Endothelial surface glycocalyx can regulate flow-induced nitric oxide production in microvessels in vivo. PLoS ONE 2015, 10, e0117133. [Google Scholar] [CrossRef]
- Ikonomidis, I.; Pavlidis, G.; Lambadiari, V.; Kousathana, F.; Varoudi, M.; Spanoudi, F.; Maratou, E.; Parissis, J.; Triantafyllidi, H.; Dimitriadis, G.; et al. Early detection of left ventricular dysfunction in first-degree relatives of diabetic patients by myocardial deformation imaging: The role of endothelial glycocalyx damage. Int. J. Cardiol. 2017, 233, 105–112. [Google Scholar] [CrossRef]
- Puerta-Guardo, H.; Glasner, D.R.; Harris, E. Dengue Virus NS1 Disrupts the Endothelial Glycocalyx, Leading to Hyperpermeability. PLoS Pathog. 2016, 12, e1005738. [Google Scholar] [CrossRef]
- Tang, T.H.; Alonso, S.; Ng, L.F.; Thein, T.L.; Pang, V.J.; Leo, Y.S.; Lye, D.C.; Yeo, T.W. Increased Serum Hyaluronic Acid and Heparan Sulfate in Dengue Fever: Association with Plasma Leakage and Disease Severity. Sci. Rep. 2017, 7, 46191. [Google Scholar] [CrossRef]
- Suwarto, S.; Sasmono, R.T.; Sinto, R.; Ibrahim, E.; Suryamin, M. Association of Endothelial Glycocalyx and Tight and Adherens Junctions With Severity of Plasma Leakage in Dengue Infection. J. Infect. Dis. 2017, 215, 992–999. [Google Scholar] [CrossRef]
- Grandoch, M.; Bollyky, P.L.; Fischer, J.W. Hyaluronan: A Master Switch Between Vascular Homeostasis and Inflammation. Circ. Res. 2018, 122, 1341–1343. [Google Scholar] [CrossRef]
- Cioffi, D.L.; Pandey, S.; Alvarez, D.F.; Cioffi, E.A. Terminal sialic acids are an important determinant of pulmonary endothelial barrier integrity. Am. J. Physiol. Lung Cell. Mol. Physiol. 2012, 302, L1067–L1077. [Google Scholar] [CrossRef] [PubMed]
- Delaveris, C.S.; Webster, E.R.; Banik, S.M.; Boxer, S.G.; Bertozzi, C.R. Membrane-tethered mucin-like polypeptides sterically inhibit binding and slow fusion kinetics of influenza A virus. Proc. Natl. Acad. Sci. USA 2020, 117, 12643–12650. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Schmidt, E.P. The endothelial glycocalyx: An important regulator of the pulmonary vascular barrier. Tissue Barriers 2013, 1. [Google Scholar] [CrossRef] [PubMed]
- Burns, J.C.; Cayan, D.R.; Tong, G.; Bainto, E.V.; Turner, C.L.; Shike, H.; Kawasaki, T.; Nakamura, Y.; Yashiro, M.; Yanagawa, H. Seasonality and temporal clustering of Kawasaki syndrome. Epidemiology 2005, 16, 220–225. [Google Scholar] [CrossRef] [PubMed]
- Shirato, K.; Imada, Y.; Kawase, M.; Nakagaki, K.; Matsuyama, S.; Taguchi, F. Possible involvement of infection with human coronavirus 229E, but not NL63, in Kawasaki disease. J. Med. Virol. 2014, 86, 2146–2153. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, S.; Agrawal, D.K. Kawasaki disease: Etiopathogenesis and novel treatment strategies. Expert Rev. Clin. Immunol. 2017, 13, 247–258. [Google Scholar] [CrossRef] [PubMed]
- Gamez-Gonzalez, L.B.; Moribe-Quintero, I.; Cisneros-Castolo, M.; Varela-Ortiz, J.; Munoz-Ramirez, M.; Garrido-Garcia, M.; Yamazaki-Nakashimada, M. Kawasaki disease shock syndrome: Unique and severe subtype of Kawasaki disease. Pediatr. Int. 2018, 60, 781–790. [Google Scholar] [CrossRef]
- Kanegaye, J.T.; Wilder, M.S.; Molkara, D.; Frazer, J.R.; Pancheri, J.; Tremoulet, A.H.; Watson, V.E.; Best, B.M.; Burns, J.C. Recognition of a Kawasaki disease shock syndrome. Pediatrics 2009, 123, e783–e789. [Google Scholar] [CrossRef]
- Li, Y.; Zheng, Q.; Zou, L.; Wu, J.; Guo, L.; Teng, L.; Zheng, R.; Jung, L.K.L.; Lu, M. Kawasaki disease shock syndrome: Clinical characteristics and possible use of IL-6, IL-10 and IFN-gamma as biomarkers for early recognition. Pediatr. Rheumatol. Online J. 2019, 17, 1. [Google Scholar] [CrossRef]
- Luo, L.; Feng, S.; Wu, Y.; Su, Y.; Jing, F.; Yi, Q. Serum Levels of Syndecan-1 in Patients With Kawasaki Disease. Pediatr. Infect. Dis. J. 2019, 38, 89–94. [Google Scholar] [CrossRef]
- Kazory, A.; Ronco, C.; McCullough, P.A. SARS-CoV-2 (COVID-19) and intravascular volume management strategies in the critically ill. Proc. Bayl. Univ. Med. Cent. 2020, 0, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Guan, W.J.; Ni, Z.Y.; Hu, Y.; Liang, W.H.; Ou, C.Q.; He, J.X.; Liu, L.; Shan, H.; Lei, C.L.; Hui, D.S.C.; et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N. Engl. J. Med. 2020, 382, 1708–1720. [Google Scholar] [CrossRef] [PubMed]
- Giannis, D.; Ziogas, I.A.; Gianni, P. Coagulation disorders in coronavirus infected patients: COVID-19, SARS-CoV-1, MERS-CoV and lessons from the past. J. Clin. Virol. 2020, 127, 104362. [Google Scholar] [CrossRef] [PubMed]
- Wichmann, D.; Sperhake, J.P.; Lutgehetmann, M.; Steurer, S.; Edler, C.; Heinemann, A.; Heinrich, F.; Mushumba, H.; Kniep, I.; Schroder, A.S.; et al. Autopsy Findings and Venous Thromboembolism in Patients With COVID-19. Ann. Intern. Med. 2020. [Google Scholar] [CrossRef]
- Piccolo, V.; Neri, I.; Filippeschi, C.; Oranges, T.; Argenziano, G.; Battarra, V.C.; Berti, S.; Manunza, F.; Fortina, A.B.; Di Lernia, V.; et al. Chilblain-like lesions during COVID-19 epidemic: A preliminary study on 63 patients. J. Eur. Acad. Dermatol. Venereol. 2020, 34, e291–e293. [Google Scholar] [CrossRef]
- Buijsers, B.; Yanginlar, C.; de Nooijer, A.; Grondman, I.; Maciej-Hulme, M.L.; Jonkman, I.; Janssen, N.A.F.; Rother, N.; de Graaf, M.; Pickkers, P.; et al. Increased Plasma Heparanase Activity in COVID-19 Patients. Front. Immunol. 2020, 11, 575047. [Google Scholar] [CrossRef]
- Fraser, D.D.; Patterson, E.K.; Slessarev, M.; Gill, S.E.; Martin, C.; Daley, M.; Miller, M.R.; Patel, M.A.; Dos Santos, C.C.; Bosma, K.J.; et al. Endothelial Injury and Glycocalyx Degradation in Critically Ill Coronavirus Disease 2019 Patients: Implications for Microvascular Platelet Aggregation. Crit. Care Explor. 2020, 2, e0194. [Google Scholar] [CrossRef]
- Stahl, K.; Gronski, P.A.; Kiyan, Y.; Seeliger, B.; Bertram, A.; Pape, T.; Welte, T.; Hoeper, M.M.; Haller, H.; David, S. Injury to the Endothelial Glycocalyx in Critically Ill Patients with COVID-19. Am. J. Respir. Crit. Care Med. 2020, 202, 1178–1181. [Google Scholar] [CrossRef]
- Hutchings, S.D.; Watchorn, J.; Trovato, F.; Napoli, S.; Mujib, S.F.; Hopkins, P.; McPhail, M. Microcirculatory, Endothelial and Inflammatory Responses in Critically Ill Patients with COVID-19 are Distinct from those Seen in Septic Shock: A Case Control Study. Shock 2020. [Google Scholar] [CrossRef]
- Ding, M.; Zhang, Q.; Li, Q.; Wu, T.; Huang, Y.Z. Correlation analysis of the severity and clinical prognosis of 32 cases of patients with COVID-19. Respir. Med. 2020, 167, 105981. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).