The Gut-Muscle Axis in Older Subjects with Low Muscle Mass and Performance: A Proof of Concept Study Exploring Fecal Microbiota Composition and Function with Shotgun Metagenomics Sequencing
Abstract
1. Introduction
2. Results
2.1. Clinical and Nutritional Characteristics of Participants
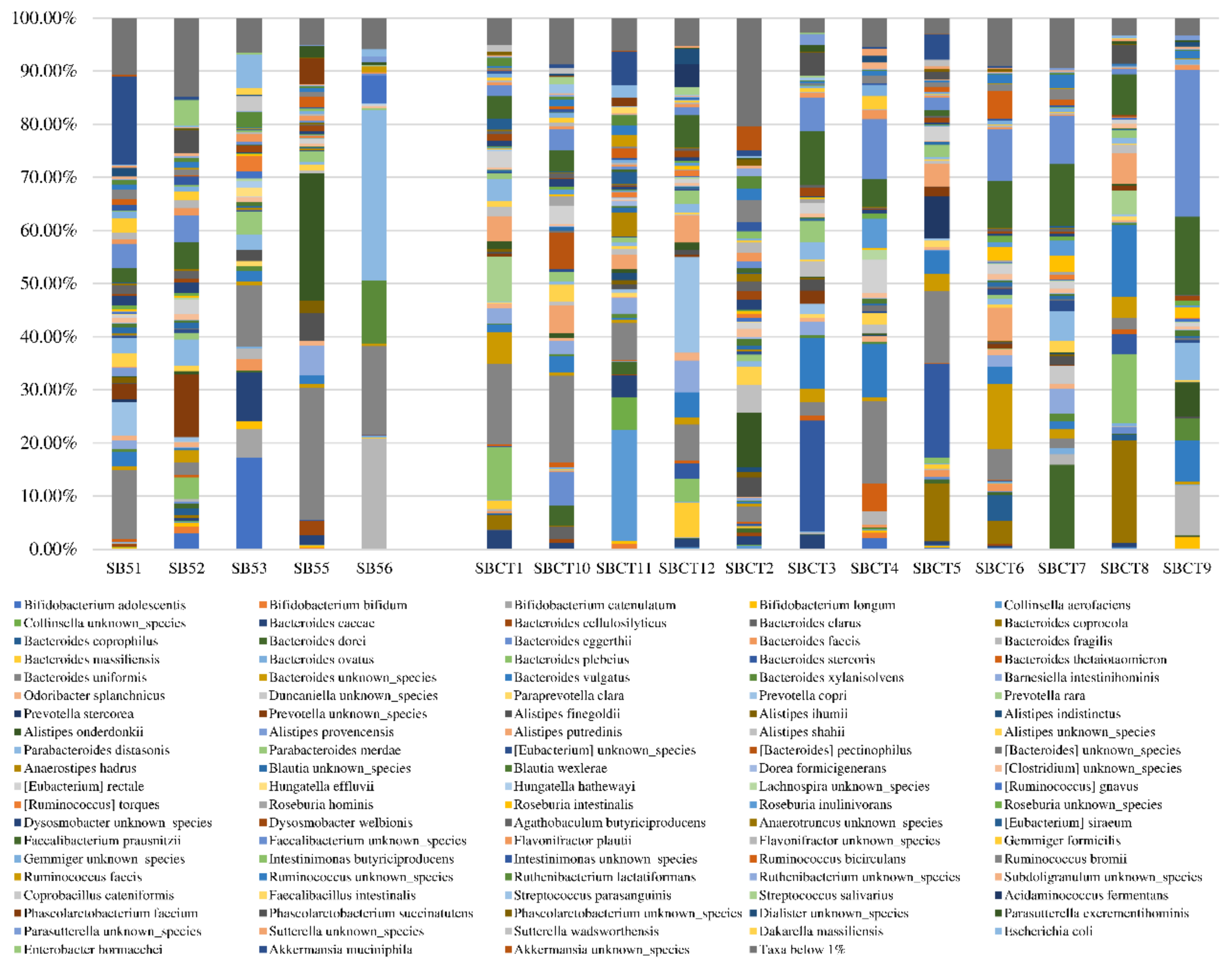
2.2. Composition of the Fecal Microbiota
2.3. Function of the Fecal Microbiota
3. Discussion
4. Materials and Methods
4.1. Study Design, Participants, and Setting
4.2. Study Procedures
4.3. Microbiota Analyses
4.4. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Data Accessibility
Abbreviations
| BMI | Body Mass Index |
| IQR | Interquartile Range |
| PCoA | Principal Coordinate Analysis |
| PF&S | Physical Frailty and Sarcopenia |
| SMI | Skeletal Muscle Index |
| SMM | Skeletal Muscle Mass |
| SPPB | Short Physical Performance Battery |
References
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Writing Group for the European Working Group on Sarcopenia in Older People 2 (EWGSOP2); Extended Group for EWGSOP2. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef] [PubMed]
- Picca, A.; Calvani, R.; Cesari, M.; Landi, F.; Bernabei, R.; Coelho-Júnior, H.J.; Marzetti, E. Biomarkers of physical frailty and sarcopenia: Coming up to the place? Int. J. Mol. Sci. 2020, 21, 5635. [Google Scholar] [CrossRef] [PubMed]
- Martone, A.M.; Marzetti, E.; Calvani, R.; Picca, A.; Tosato, M.; Santoro, L.; Di Giorgio, A.; Nesci, A.; Sisto, A.; Santoliquido, A.; et al. Exercise and protein intake: A synergistic approach against sarcopenia. Biomed. Res. Int. 2017, 2017, 2672435. [Google Scholar] [CrossRef] [PubMed]
- Calvani, R.; Picca, A.; Marini, F.; Biancolillo, A.; Cesari, M.; Pesce, V.; Lezza, A.M.S.; Bossola, M.; Leeuwenburgh, C.; Bernabei, R.; et al. The “BIOmarkers associated with Sarcopenia and PHysical frailty in EldeRly pErsons” (BIOSPHERE) study: Rationale, design and methods. Eur. J. Intern. Med. 2018, 56, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Clegg, A.; Young, J.; Iliffe, S.; Olde Rikkert, M.; Rockwood, K. Frailty in older people. Lancet 2013, 381, 752–762. [Google Scholar] [CrossRef]
- Landi, F.; Calvani, R.; Cesari, M.; Tosato, M.; Martone, A.M.; Bernabei, R.; Onder, G.; Marzetti, E. Sarcopenia as the biological substrate of physical frailty. Clin. Geriatr. Med. 2015, 31, 367–374. [Google Scholar] [CrossRef] [PubMed]
- Cesari, M.; Landi, F.; Calvani, R.; Cherubini, A.; Di Bari, A.; Kortebein, P.; Del Signore, S.; Le Lain, R.; Vellas, B.; Pahor, M.; et al. SPRINTT Consortium. Rationale for a preliminary operational definition of physical frailty and sarcopenia in the SPRINTT trial. Aging Clin. Exp. Res. 2017, 29, 81–88. [Google Scholar] [CrossRef]
- Grosicki, G.J.; Fielding, R.A.; Lustgarten, M.S. Gut microbiota contribute to age-related changes in skeletal muscle size, composition, and function: Biological basis for a gut-muscle axis. Calcif. Tissue Int. 2018, 102, 433–442. [Google Scholar] [CrossRef]
- Ticinesi, A.; Lauretani, F.; Milani, C.; Nouvenne, A.; Tana, C.; Del Rio, D.; Maggio, M.; Ventura, M.; Meschi, T. Aging gut microbiota at the cross-road between nutrition, physical frailty, and sarcopenia: Is there a gut-muscle axis? Nutrients 2017, 9, 1303. [Google Scholar] [CrossRef]
- Lustgarten, M.S. The role of the gut microbiome on skeletal muscle mass and physical function: 2019 update. Front. Physiol. 2019, 10, 1435. [Google Scholar] [CrossRef]
- Vaiserman, A.M.; Koliada, A.K.; Marotta, F. Gut microbiota: A player in aging and a target for anti-aging intervention. Ageing Res. Rev. 2017, 35, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Gemikonakli, G.; Mach, J.; Hilmer, S.N. Interactions between the aging gut microbiome and common geriatric giants: Polypharmacy, frailty, and dementia. J. Gerontol. A Biol. Sci. Med. Sci. 2020. [Google Scholar] [CrossRef] [PubMed]
- Ticinesi, A.; Tana, C.; Nouvenne, A. The intestinal microbiome and its relevance for functionality in older persons. Curr. Opin. Clin. Nutr. Metab. Care 2019, 22, 4–12. [Google Scholar] [CrossRef]
- Ticinesi, A.; Nouvenne, A.; Cerundolo, N.; Catania, P.; Prati, B.; Tana, C.; Meschi, T. Gut microbiota, muscle mass and function in aging: A focus on physical frailty and sarcopenia. Nutrients 2019, 11, 1633. [Google Scholar] [CrossRef] [PubMed]
- Biagi, E.; Rampelli, S.; Turroni, S.; Quercia, S.; Candela, M.; Brigidi, P. The gut microbiota of centenarians: Signatures of longevity in the gut microbiota profile. Mech. Ageing Dev. 2017, 165, 180–184. [Google Scholar] [CrossRef] [PubMed]
- Nagpal, R.; Mainali, R.; Ahmadi, S.; Wang, S.; Singh, R.; Kavanagh, K.; Kitzman, D.W.; Kushugulova, A.; Marotta, F.; Yadav, H. Gut microbiome and aging: Physiological and mechanistic insights. Nutr. Healthy Aging 2018, 4, 267–285. [Google Scholar] [CrossRef] [PubMed]
- Arboleya, S.; Watkins, C.; Stanton, C.; Ross, R.P. Gut Bifidobacteria populations in human health and aging. Front. Microbiol. 2016, 7, 1204. [Google Scholar] [CrossRef]
- Picca, A.; Fanelli, F.; Calvani, R.; Mulè, G.; Pesce, V.; Sisto, A.; Pantanelli, C.; Bernabei, R.; Landi, F.; Marzetti, E. Gut dysbiosis and muscle aging: Searching for novel targets against sarcopenia. Mediat. Inflamm. 2018, 2018, 7026198. [Google Scholar] [CrossRef]
- Casati, M.; Ferri, E.; Azzolino, D.; Cesari, M.; Arosio, B. Gut microbiota and physical frailty through the mediation of sarcopenia. Exp. Gerontol. 2019, 124, 110639. [Google Scholar] [CrossRef]
- Siddhart, J.; Chakrabarti, A.; Pannérec, A.; Karaz, S.; Morin-Rivron, D.; Masoodi, M.; Feige, J.N.; Parkinson, S.J. Aging and sarcopenia associate with specific interactions between gut microbes, serum biomarkers and host physiology in rats. Aging 2017, 9, 1698–1720. [Google Scholar] [CrossRef]
- Uchiyama, K.; Wakino, S.; Irie, J.; Miyamoto, J.; Matsui, A.; Tajima, T.; Itoh, T.; Oshima, Y.; Yoshifuji, A.; Kimura, I.; et al. Contribution of uremic dysbiosis to insulin resistance and sarcopenia. Nephrol. Dial. Transplant. 2020. [Google Scholar] [CrossRef] [PubMed]
- Fielding, R.A.; Reeves, A.R.; Jasuja, R.; Liu, C.; Barrett, B.B.; Lustgarten, M.S. Muscle strength is increased in mice that are colonized with microbiota from high-functioning older adults. Exp. Gerontol. 2019, 127, 110722. [Google Scholar] [CrossRef] [PubMed]
- Jackson, M.A.; Jeffery, I.B.; Beaumont, M.; Bell, J.T.; Clark, A.G.; Ley, R.E.; O’Toole, P.W.; Spector, T.D.; Steves, C.J. Signatures of early frailty in the gut microbiota. Genome Med. 2016, 8, 8. [Google Scholar] [CrossRef] [PubMed]
- Picca, A.; Ponziani, F.R.; Calvani, R.; Marini, F.; Biancolillo, A.; Coelho-Júnior, H.J.; Gervasoni, J.; Primiano, A.; Putignani, L.; Del Chierico, F.; et al. Gut microbial, inflammatory and metabolic signatures in older people with physical frailty and sarcopenia: Results from the BIOSPHERE Study. Nutrients 2020, 12, 65. [Google Scholar] [CrossRef] [PubMed]
- Ni Lochlainn, M.; Bowyer, R.C.E.; Steves, C.J. Dietary protein and muscle in aging people: The potential role of the gut microbiome. Nutrients 2018, 10, 929. [Google Scholar] [CrossRef]
- Prokopidis, K.; Cervo, M.M.; Gandham, A.; Scott, D. Impact of protein intake in older adults with sarcopenia and obesity: A gut microbiota perspective. Nutrients 2020, 12, 2285. [Google Scholar] [CrossRef] [PubMed]
- Ren, G.; Zhang, J.; Li, M.; Tang, Z.; Yang, Z.; Cheng, G.; Wang, J. Gut microbiota composition influences outcomes of skeletal muscle nutritional intervention via blended protein supplementation in postttransplant patients with hematologic malignancies. Clin. Nutr. 2020. [Google Scholar] [CrossRef]
- Lordan, C.; Thapa, D.; Ross, R.P.; Cotter, P.D. Potential for enriching next-generation health-promoting gut bacteria through prebiotics and other dietary components. Gut. Microbes. 2020, 11, 1–20. [Google Scholar] [CrossRef]
- Ganesan, K.; Chung, S.K.; Vanamala, J.; Xu, B. Causal relationship between diet-induced gut microbiota changes and diabetes: A novel strategy to transplant Faecalibacterium prausnitzii in preventing diabetes. Int. J. Mol. Sci. 2018, 19, 3720. [Google Scholar] [CrossRef]
- Hiippala, K.; Jouthen, H.; Ronkainen, A.; Hartikainen, A.; Kainulainen, V.; Jalanka, J.; Satokari, R. The potential of gut commensals in reinforcing intestinal barrier function and alleviating inflammation. Nutrients 2018, 10, 988. [Google Scholar] [CrossRef]
- Machiels, K.; Joossens, M.; Sabino, J.; De Preter, V.; Arijs, I.; Eeckhaut, V.; Ballet, V.; Claes, K.; Van Immerseel, F.; Verbeke, K.; et al. A decrease of the butyrate-producing species Roseburia hominis and Faecalibacterium prausnitzi defines dysbiosis in patients with ulcerative colitis. Gut 2014, 63, 1275–1283. [Google Scholar] [CrossRef] [PubMed]
- Björkqvist, O.; Repsilber, D.; Seifert, M.; Brislawn, C.; Jansson, J.; Engstrand, L.; Rangel, I.; Halfvarson, J. Alterations in the relative abundance of Faecalibacterium prausnitzii correlate with changes in fecal calprotectin in patients with ileal Crohn’s disease: A longitudinal study. Scand. J. Gastroenterol. 2019, 54, 577–585. [Google Scholar] [CrossRef] [PubMed]
- Martín, R.; Miquel, S.; Chain, F.; Natividad, J.M.; Jury, J.; Lu, J.; Sokol, H.; Theodorou, V.; Becik, P.; Verdu, E.F.; et al. Faecalibacterium prausnitzii prevents physiological damages in a chronic low-grade inflammation murine model. BMC Microbiol. 2015, 15, 67. [Google Scholar] [CrossRef] [PubMed]
- Munukka, E.; Rintala, A.; Toivonen, R.; Nylund, M.; Tang, B.; Takanen, A.; Hänninen, A.; Vuopio, J.; Huovinen, P.; Jalkanen, S.; et al. Faecalibacterium prausnitzii treatment improves hepatic health and reduces adipose tissue inflammation in high-fat fed mice. ISME J. 2017, 11, 1667–1679. [Google Scholar] [CrossRef] [PubMed]
- Canfora, E.E.; Jocken, J.W.; Blaak, E.E. Short-chain fatty acids in control of body weight and insulin sensitivity. Nat. Rev. Endocrinol. 2015, 11, 577–591. [Google Scholar] [CrossRef]
- Nay, K.; Jollet, M.; Goustard, B.; Baati, N.; Vernus, B.; Pontones, M.; Lefeuvre-Orfila, L.; Bendavid, C.; Rué, O.; Mariadassou, M.; et al. Gut bacteria are critical for optimal muscle function: A potential link with glucose homeostasis. Am. J. Physiol. Endocrinol. Metab. 2019, 317, E158–E171. [Google Scholar] [CrossRef]
- Walsh, M.E.; Bhattacharya, A.; Sataranatarajan, K.; Qaisar, R.; Sloane, L.; Rahman, M.M.; Kinter, M.; Van Remmen, H. The histone acetylase inhibitor butyrate improves metabolism and reduces muscle atrophy during aging. Aging Cell 2015, 14, 957–970. [Google Scholar] [CrossRef]
- Lahiri, S.; Kim, H.; Garcia-Perez, M.; Martin, K.A.; Kundu, P.; Cox, L.M.; Selkrig, J.; Posma, J.M.; Zhang, H.; Padmanabhan, P.; et al. The gut microbiota influences skeletal muscle mass and function in mice. Sci. Transl. Med. 2019, 11, eaan5662. [Google Scholar] [CrossRef]
- Lin, T.Y.; Wu, P.H.; Lin, Y.T.; Hung, S.C. Characterization of gut microbiota composition in hemodialysis patients with normal weight obesity. J. Clin. Endocrinol. Metab. 2020, 105, dgaa166. [Google Scholar] [CrossRef]
- Scott, K.P.; Martin, J.C.; Campbell, G.; Mayer, C.D.; Flint, H.J. Whole-genome transcription profiling reveals genes up-regulated by growth on fucose in the human gut bacterium “Roseburia inulinivorans”. J. Bacteriol. 2006, 188, 4340–4349. [Google Scholar] [CrossRef]
- Reichardt, N.; Duncan, S.H.; Young, P.; Belenguer, A.; McWilliam Leitch, C.; Scott, K.P.; Flint, H.J.; Louis, P. Phylogenic distribution of three pathways for propionate production within the human gut microbiota. ISME J. 2014, 8, 1323–1335. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Nishida, A.; Fujimoto, T.; Fujii, M.; Shioya, M.; Imaeda, H.; Inatomi, O.; Bamba, S.; Sugimoto, M.; Andoh, A. Reduced abundance of butyrate-producing bacteria species in the fecal microbial community in Crohn’s disease. Digestion 2016, 93, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Parker, B.J.; Wearsch, P.A.; Veloo, A.C.M.; Rodriguez-Palacios, A. The genus Alistipes: Gut bacteria with emerging implications to inflammation, cancer, and mental health. Front. Immunol. 2020, 11, 906. [Google Scholar] [CrossRef] [PubMed]
- Milani, C.; Ticinesi, A.; Gerritsen, J.; Nouvenne, A.; Lugli, G.A.; Mancabelli, L.; Turroni, F.; Duranti, S.; Mangifesta, M.; Viappiani, A.; et al. Gut microbiota composition and Clostridium difficile infection in hospitalized elderly individuals: A metagenomic study. Sci. Rep. 2016, 6, 25945. [Google Scholar] [CrossRef]
- Luan, Z.; Sun, G.; Huang, Y.; Yang, Y.; Yang, R.; Li, C.; Wang, T.; Tan, D.; Qi, S.; Jun, C.; et al. Metagenomics study reveals changes in gut microbiota in centenarians: A cohort study of Hinan centenarians. Front. Microbiol. 2020, 11, 1474. [Google Scholar] [CrossRef]
- Verhoog, S.; Taneri, P.E.; Roa Díaz, Z.M.; Marques-Vidal, P.; Troup, J.P.; Bally, L.; Franco, O.H.; Glisic, M.; Muka, T. Dietary factors and modulation of bacteria strains of Akkermansia muciniphila and Faeclaibacterium prausnitzii: A systematic review. Nutrients 2019, 11, 1565. [Google Scholar] [CrossRef]
- Rios-Covian, D.; Gueimonde, M.; Duncan, S.H.; Flint, H.J.; de los Reyes-Gavilan, C.G. Enhanced butyrate formation by cross-feeding between Faecalibacterium prausnitzii and Bifidobacterium adolescentis. FEMS Microbiol. Lett. 2015, 362, fnv176. [Google Scholar] [CrossRef]
- Moens, F.; Weckx, S.; De Vuyst, L. Bifidobacterial inulin-type fructan degradation capacity determines cross-feeding interactions between bifidobacteria and Faecalibacterium prausnitzii. Int. J. Food Microbiol. 2016, 231, 76–85. [Google Scholar] [CrossRef]
- Marzani, B.; Balage, M.; Vénien, A.; Astruc, T.; Papet, I.; Dardevet, D.; Mosoni, L. Antioxidant supplementation restores defective leucine stimulation of protein synthesis in skeletal muscle from old rats. J. Nutr. 2008, 138, 2205–2211. [Google Scholar] [CrossRef]
- Welch, A.A.; Jennings, A.; Kelaiditi, E.; Skinner, J.; Steves, C.J. Cross-sectional associations between dietary antioxidant vitamins C, E and carotenoid intakes and sarcopenic indices in women aged 18–79 years. Calcif. Tissue Int. 2020, 106, 331–342. [Google Scholar] [CrossRef]
- Semba, R.D.; Blaum, C.; Guralnik, J.M.; Totin Moncrief, D.; Ricks, M.O.; Fried, L.P. Carotenoid and vitamin E status are associated with indicators of sarcopenia among older women living in the community. Aging Clin. Exp. Res. 2003, 15, 482–487. [Google Scholar] [CrossRef] [PubMed]
- Semba, R.D.; Lauretani, F.; Ferrucci, L. Carotenoids as protection against sarcopenia in older adults. Arch. Biochem. Biophys. 2007, 458, 141–145. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, C.J.; Hermans, W.J.H.; Holwerda, A.M.; Smeets, J.S.J.; Senden, J.M.; van Kranenburg, J.; Gijsen, A.P.; Wodzig, W.K.H.W.; Schierbeek, H.; Verdijk, L.B.; et al. Branched-chain amino acid and branched-chain ketoacid ingestion increases muscle protein synthesis rates in vivo in older adults: A double-blind, randomized trial. Am. J. Clin. Nutr. 2019, 110, 862–872. [Google Scholar] [CrossRef] [PubMed]
- Ter Borg, S.; Luiking, Y.C.; van Helvoort, A.; Boirie, Y.; Schols, J.M.G.A.; de Groot, C.P.G.M. Low levels of branched chain amino acids, eicosapentaenoic acid and micronutrients are associated with low muscle mass, strength and function in community-dwelling older adults. J. Nutr. Health Aging 2019, 23, 27–34. [Google Scholar] [CrossRef]
- Del Favero, S.; Roschel, H.; Solis, M.Y.; Hayashi, A.P.; Artioli, G.G.; Otaduy, M.C.; Benatti, F.B.; Harris, R.C.; Wise, J.A.; Leite, C.C.; et al. Beta-alanine (Carnosyn™) supplementation in elderly subjects (60–80 years): Effects on muscle carnosine content and physical capacity. Amino Acids 2012, 43, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, M.; Kitano, T.; Kawata, N.; Sugihira, T.; Kitakaze, T.; Harada, N.; Yamaji, R. Daidzein down-regulates ubiquitin-specific protease 19 expression through estrogen receptor β and increases skeletal muscle mass in young female mice. J. Nutr. Biochem. 2017, 49, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Cuesta, F.; Formiga, F.; Lopez-Soto, A.; Masanes, F.; Ruiz, D.; Artaza, I.; Salvà, A.; Serra-Rexach, J.A.; Luque, X.R.I.; Cruz-Jentoft, A.J. Prevalence of sarcopenia in patients attending outpatient geriatric clinics: The ELLI study. Age Ageing 2015, 44, 807–809. [Google Scholar] [CrossRef][Green Version]
- Zambone, M.A.; Liberman, S.; Garcia, M.L.B. Anthropometry, bioimpedance and densitometry: Comparative methods for lean mass body analysis in elderly outpatients from a tertiary hospital. Exp. Gerontol. 2020, 138, 111020. [Google Scholar] [CrossRef]
- Janssen, I.; Baumgartner, R.N.; Ross, R.; Rosenberg, I.H.; Roubenoff, R. Skeletal muscle cutpoints associated with elevated physical disability risk in older men and women. Am. J. Epidemiol. 2004, 159, 413–421. [Google Scholar] [CrossRef]
- Janssen, I.; Heymsfield, S.B.; Ross, R. Low relative skeletal muscle mass (sarcopenia) in older persons is associated with functional impairment and physical disability. J. Am. Geriatr. Soc. 2002, 50, 889–896. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Baeyens, J.P.; Bauer, J.M.; Boirie, Y.; Cederholm, T.; Landi, F.; Martin, F.C.; Michel, J.P.; Rolland, Y.; Schneider, S.M.; et al. Sarcopenia: European consensus on definition and diagnosis. Age Ageing 2010, 39, 412–423. [Google Scholar] [CrossRef] [PubMed]
- Guralnik, J.M.; Simonsick, E.M.; Ferrucci, L.; Glynn, J.R.; Berkman, L.F.; Blazer, D.G.; Scherr, P.A.; Wallace, R.B. A short physical performance battery assessing lower extremity function: Association with self-reported disability and prediction of mortality and nursing home admission. J. Gerontol. 1994, 49, M85–M94. [Google Scholar] [CrossRef]
- Pala, V.; Sieri, S.; Palli, D.; Salvini, S.; Berrino, F.; Bellegotti, M.; Frasca, G.; Tumino, R.; Sacerdote, C.; Fiorini, L.; et al. Diet in the Italian EPIC cohorts: Presentation of data and methodological issues. Tumori 2003, 89, 594–607. [Google Scholar] [CrossRef] [PubMed]
- Pedone, C.; Napoli, N.; Pozzilli, P.; Rossi, F.F.; Lauretani, F.; Bandinelli, S.; Ferrucci, L.; Antonelli-Incalzi, R. Dietary pattern and bone density changes in elderly women: A longitudinal study. J. Am. Coll. Nutr. 2011, 30, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Ticinesi, A.; Milani, C.; Guerra, A.; Allegri, F.; Lauretani, F.; Nouvenne, A.; Mancabelli, L.; Lugli, G.A.; Turroni, F.; Duranti, S.; et al. Understanding the gut-kidney axis in nephrolithiasis: An analysis of the gut microbiota composition and functionality of stone formers. Gut 2018, 67, 2097–2106. [Google Scholar] [CrossRef] [PubMed]
- Pisani, P.; Faggiano, F.; Krogh, V.; Palli, D.; Vineis, P.; Berrino, F. Relative validity and reproducibility of a food frequency dietary questionnaire for use in the Italian EPIC centres. Int. J. Epidemiol. 1997, 26 (Suppl. 1), S152–S160. [Google Scholar] [CrossRef]
- Milani, C.; Casey, E.; Lugli, G.A.; Moore, R.; Kaczorowska, J.; Feehily, C.; Mangifesta, M.; Mancabelli, L.; Duranti, S.; Turroni, F.; et al. Tracing mother-infant transmission of bacteriophages by means of a novel analytical tool for shotgun metagenomics datasets: METAnnotatorX. Microbiome 2018, 6, 145. [Google Scholar] [CrossRef]
- Chen, Y.; Ye, W.; Zhang, Y.; Xu, Y. High speed BLASTN: An accelerated MegaBLAST search tool. Nucleic Acids Res. 2015, 43, 7762–7768. [Google Scholar] [CrossRef]
- Ciufo, S.; Kannan, S.; Sharma, S.; Badretdin, A.; Clark, K.; Turner, S.; Brover, S.; Schoch, C.L.; Kimchi, A.; DiCuccio, M. Using average nucleotide identity to improve taxonomic assignments in prokaryotic genomes at the NCBI. Int. J. Syst. Evol. Microbiol. 2018, 68, 2386–2392. [Google Scholar] [CrossRef]
- Richter, M.; Rosselló-Móra, R. Shifting the genomic gold standard for the prokaryotic species definition. Proc. Natl. Acad. Sci. USA 2009, 106, 19126–19131. [Google Scholar] [CrossRef]
- Caspi, R.; Billington, R.; Ferrer, L.; Foerster, H.; Fulcher, C.A.; Keseler, I.M.; Kothari, A.; Krummenacker, M.; Latendresse, M.; Mueller, L.A.; et al. The MetaCyc database of metabolic pathways and enzymes and the BioCyc collection of pathway/genome database. Nucleic Acids Res. 2016, 44, D471–D480. [Google Scholar] [CrossRef] [PubMed]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Peña, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef] [PubMed]
- Bokulich, N.A.; Kaehler, B.D.; Rideout, J.R.; Dillon, M.; Bolyen, E.; Knight, R.; Huttley, G.A.; Caporaso, J.G. Optimizing taxonomic classification of marker-gene amplicon sequences with QIIME 2’s q2-feature-classifier plugin. Microbiome 2018, 6, 90. [Google Scholar] [CrossRef] [PubMed]
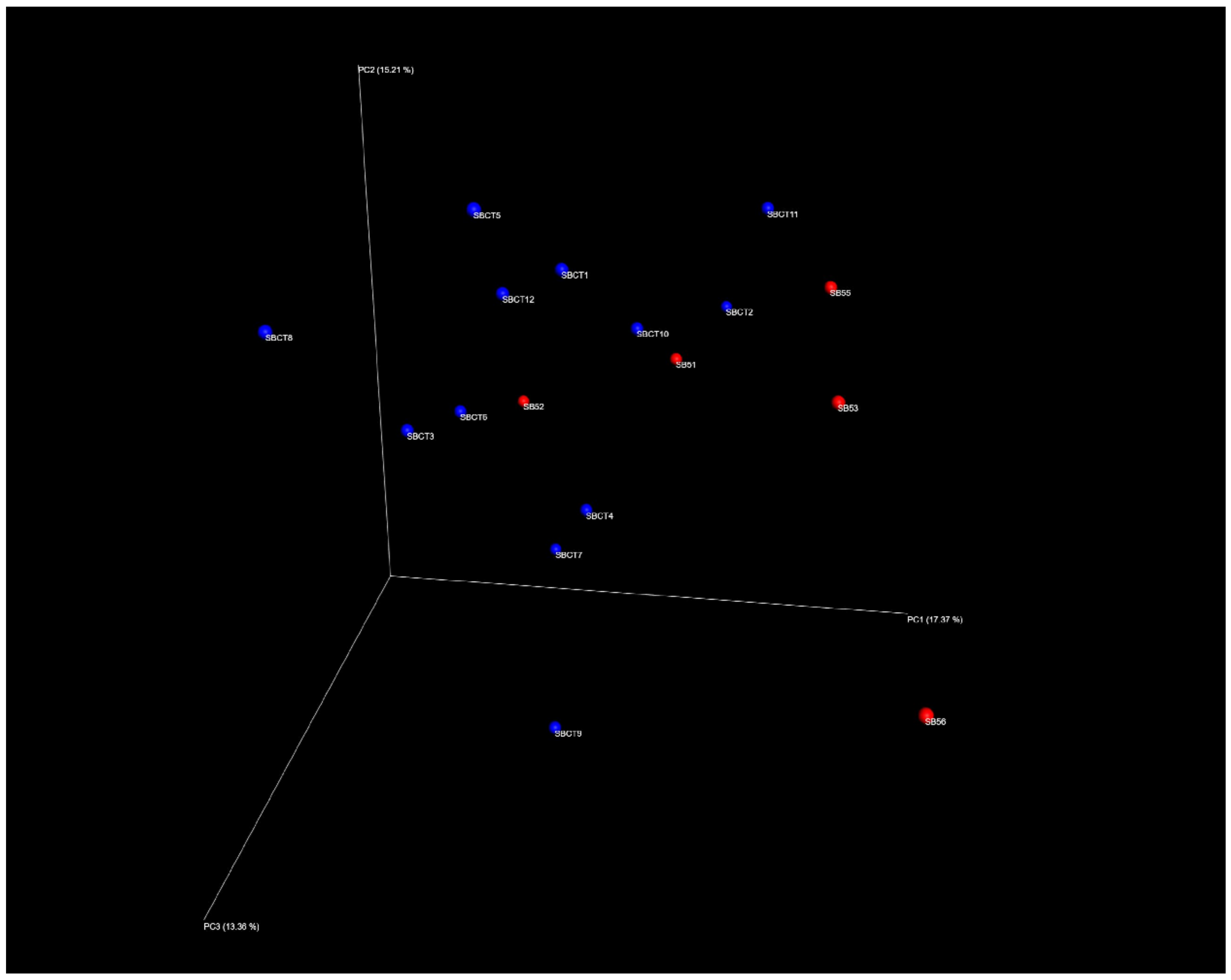
| Variable | Sarcopenic Subjects (n = 5) | Non-Sarcopenic Controls (n = 12) | p * |
|---|---|---|---|
| Age, years | 77 (75.5–86) | 71.5 (70–75) | 0.08 |
| SPPB, points | 6 (3–8) | 11 (10–12) | <0.001 |
| SMM, Kg | 14.6 (13.7–15.8) | 18.2 (17.1–23.5) | <0.001 |
| SMI, Kg/m2 | 6.40 (6.33–6.47) | 7.24 (7.04–9.44) | <0.001 |
| Weight, Kg | 59.5 (45.1–70.4) | 66.1 (61.3–78.5) | 0.165 |
| BMI, Kg/m2 | 24.3 (20.9–26.7) | 27.4 (24.5–29.1) | 0.075 |
| Bristol Stool Scale, points | 4 (1.5–5.5) | 3 (2.5–5) | 0.970 |
| Variable/Nutrient | Sarcopenic Subjects (n = 5) | Non-Sarcopenic Controls (n = 12) | p * |
|---|---|---|---|
| Total proteins, g | 55.5 (46.1–92.9) | 76.6 (56.7–93.5) | 0.69 |
| Animal proteins, g | 30.4 (27.7–58.3) | 42.6 (22.2–64.9) | 0.99 |
| Vegetal proteins, g | 27.8 (17.1–34.5) | 25.3 (22.3–32.3) | 0.89 |
| Total lipids, g | 79.8 (46.2–105.4) | 89.2 (75.4–96.5) | 0.70 |
| Animal lipids, g | 30.7 (27.6–51.6) | 32.1 (21.5–48.6) | 0.60 |
| Vegetal lipids, g | 49.2 (18.8–53.9) | 55.2 (44.4–67.5) | 0.29 |
| Total saturated lipids, g | 24.7 (17.5–35.0) | 25.2 (18.6–30.5) | 0.99 |
| Total polyunsaturated lipids, g | 10.4 (5.8–13.8) | 10.8 (8.7–13.6) | 0.79 |
| Cholesterol, mg | 216 (196–305) | 228 (168–323) | 0.99 |
| Sugars, g | 238 (169–290) | 229 (184–271) | 0.89 |
| Fibers, g | 22.2 (14.1–30.0) | 21.5 (18.4–23.5) | 0.89 |
| Energy, Kcal | 1873 (1236–2460) | 1971 (1714–2257) | 0.69 |
| Iron, mg | 8.77 (5.61–14.00) | 9.90 (8.89–12.64) | 0.43 |
| Calcium, mg | 548 (496–1064) | 891 (616–992) | 0.44 |
| Sodium, mg | 1915 (1547–2959) | 1806 (1596–2161) | 0.90 |
| Potassium, mg | 2587 (1637–3792) | 2989 (2818–3748) | 0.44 |
| Zinc, mg | 6.6 (5.8–10.4) | 8.9 (6.6–11.7) | 0.40 |
| Tiamin, mg | 0.58 (0.40–0.96) | 0.92 (0.85–1.40) | 0.43 |
| Riboflavin, mg | 0.63 (0.52–1.02) | 0.92 (0.85–1.40) | 0.08 |
| Niacin, mg | 12.09 (8.47–20.53) | 18.17 (13.41–21.65) | 0.43 |
| Vitamin C, mg | 140 (92–177) | 159 (111–177) | 0.44 |
| Vitamin B6, mg | 1.29 (0.70–1.91) | 1.64 (1.34–2.29) | 0.24 |
| Folic acid, μg | 231 (147–343) | 276 (239–348) | 0.51 |
| Beta-carotene, μg | 2729 (808–3403) | 3652 (2868–5147) | 0.11 |
| Vitamin E, μg | 13.1 (6.2–16.5) | 16.0 (13.6–19.9) | 0.24 |
| Vitamin D, mg | 1.39 (1.27–3.32) | 2.41 (1.29–4.21) | 0.60 |
| Bacterial Species | Sarcopenic Subjects (n = 5) | Non-Sarcopenic Controls (n = 12) | p * |
|---|---|---|---|
| Akkermansia muciniphila | 0.00% (0.00–8.62) | 0.00% (0.00–0.09) | 0.99 |
| Alistipes onderdonkii | 0.29% (0.00–12.35) | 0.60% (0.14–1.40) | 0.57 |
| Alistipes shahii | 0.00% (0.00–0.20) | 0.88% (0.16–1.70) | 0.019 |
| Bacteroides caccae | 0.44% (0.00–5.50) | 1.01% (0.08–2.44) | 0.87 |
| Bacteroides dorei | 0.16% (0.00–0.68) | 0.46% (0.18–1.97) | 0.23 |
| Bacteroides fragilis | 0.42% (0.09–11.39) | 0.26% (0.04–1.59) | 0.50 |
| Bacteroides uniformis | 13.02% (6.93–20.78) | 6.33% (2.26–14.78) | 0.19 |
| Bacteroides vulgatus | 1.67% (0.17–2.34) | 3.78% (1.47–9.13) | 0.08 |
| Barnesiella intestinihominis | 0.12% (0.00–3.61) | 2.38% (0.11–2.93) | 0.44 |
| Bifidobacterium longum | 0.42% (0.19–1.08) | 0.00% (0.00–0.38) | 0.13 |
| Escherichia coli | 0.26% (0.06–3.83) | 0.00% (0.00–0.28) | 0.16 |
| Faecalibacterium prausnitzii | 0.15% (0.07–3.93) | 5.56% (1.79–9.87) | 0.019 |
| Flavonifractor plautii | 0.93% (0.61–1.42) | 0.52% (0.31–0.94) | 0.23 |
| Parabacteroides distasonis | 2.94% (1.68–18.49) | 1.06% (0.61–3.92) | 0.32 |
| Parabacteroides merdae | 1.22% (0.17–3.21) | 1.14% (0.17–2.16) | 0.87 |
| Roseburia intestinalis | 0.29% (0.00–0.41) | 0.32% (0.03–1.70) | 0.51 |
| Roseburia inulinivorans | 0.00% (0.00–0.00) | 0.32% (0.12–0.97) | 0.006 |
| Ruminococcus bromii | 0.89% (0.00–1.50) | 0.32% (0.04–1.43) | 0.99 |
| Ruminococcus gnavus | 0.33% (0.07–3.28) | 0.14% (0.00–0.25) | 0.19 |
| Subdoligranulum unknown species | 0.17% (0.00–0.44) | 0.21% (0.12–0.36) | 0.64 |
| Variable/Nutrient | Sarcopenic Subjects (n = 5) | Non-Sarcopenic Controls (n = 12) | p * |
|---|---|---|---|
| Alpha-carotene biosynthesis | 0.13 ± 0.03 | 0.18 ± 0.04 | 0.049 |
| Beta-alanine biosynthesis | 0.02 ± 0.02 | 0.06 ± 0.03 | 0.023 |
| Acetyl-CoA fermentation to butanoate | 0.23 ± 0.06 | 0.32 ± 0.08 | 0.036 |
| Daidzein conjugates interconversion | 0.34 ± 0.03 | 0.41 ± 0.07 | 0.048 |
| Flavin biosynthesis | 0.05 ± 0.04 | 0.11 ± 0.04 | 0.018 |
| Glycolysis I (from glucose-6-phosphate) | 0.85 ± 0.21 | 0.64 ± 0.09 | 0.009 |
| L-glutamine degradation | 0.59 ± 0.08 | 0.70 ± 0.08 | 0.013 |
| L-isoleucine degradation | 0.05 ± 0.04 | 0.10 ± 0.03 | 0.013 |
| L-methionine biosynthesis | 0.11 ± 0.05 | 0.17 ± 0.05 | 0.046 |
| Piruvate fermentation to acetate | 0.01 ± 0.01 | 0.02 ± 0.02 | 0.034 |
| Succinate fermentation to butanoate | 0.08 ± 0.03 | 0.17 ± 0.03 | <0.001 |
| Superpathway of glycolysis, pyruvate dehydrogenase, TCA, and glyoxylate bypass | 1.15 ± 0.20 | 0.84 ± 0.12 | <0.001 |
| Superpathway of L-homoserine and L-methionine biosynthesis | 0.18 ± 0.06 | 0.25 ± 0.06 | 0.044 |
| Superpathway of L-lysine, L-threonine and L-methionine biosynthesis II | 0.03 ± 0.02 | 0.05 ± 0.02 | 0.023 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ticinesi, A.; Mancabelli, L.; Tagliaferri, S.; Nouvenne, A.; Milani, C.; Del Rio, D.; Lauretani, F.; Maggio, M.G.; Ventura, M.; Meschi, T. The Gut-Muscle Axis in Older Subjects with Low Muscle Mass and Performance: A Proof of Concept Study Exploring Fecal Microbiota Composition and Function with Shotgun Metagenomics Sequencing. Int. J. Mol. Sci. 2020, 21, 8946. https://doi.org/10.3390/ijms21238946
Ticinesi A, Mancabelli L, Tagliaferri S, Nouvenne A, Milani C, Del Rio D, Lauretani F, Maggio MG, Ventura M, Meschi T. The Gut-Muscle Axis in Older Subjects with Low Muscle Mass and Performance: A Proof of Concept Study Exploring Fecal Microbiota Composition and Function with Shotgun Metagenomics Sequencing. International Journal of Molecular Sciences. 2020; 21(23):8946. https://doi.org/10.3390/ijms21238946
Chicago/Turabian StyleTicinesi, Andrea, Leonardo Mancabelli, Sara Tagliaferri, Antonio Nouvenne, Christian Milani, Daniele Del Rio, Fulvio Lauretani, Marcello Giuseppe Maggio, Marco Ventura, and Tiziana Meschi. 2020. "The Gut-Muscle Axis in Older Subjects with Low Muscle Mass and Performance: A Proof of Concept Study Exploring Fecal Microbiota Composition and Function with Shotgun Metagenomics Sequencing" International Journal of Molecular Sciences 21, no. 23: 8946. https://doi.org/10.3390/ijms21238946
APA StyleTicinesi, A., Mancabelli, L., Tagliaferri, S., Nouvenne, A., Milani, C., Del Rio, D., Lauretani, F., Maggio, M. G., Ventura, M., & Meschi, T. (2020). The Gut-Muscle Axis in Older Subjects with Low Muscle Mass and Performance: A Proof of Concept Study Exploring Fecal Microbiota Composition and Function with Shotgun Metagenomics Sequencing. International Journal of Molecular Sciences, 21(23), 8946. https://doi.org/10.3390/ijms21238946









