Increased C-X-C Motif Chemokine Ligand 12 Levels in Cerebrospinal Fluid as a Candidate Biomarker in Sporadic Amyotrophic Lateral Sclerosis
Abstract
1. Introduction
2. Results
2.1. Proteomics Results
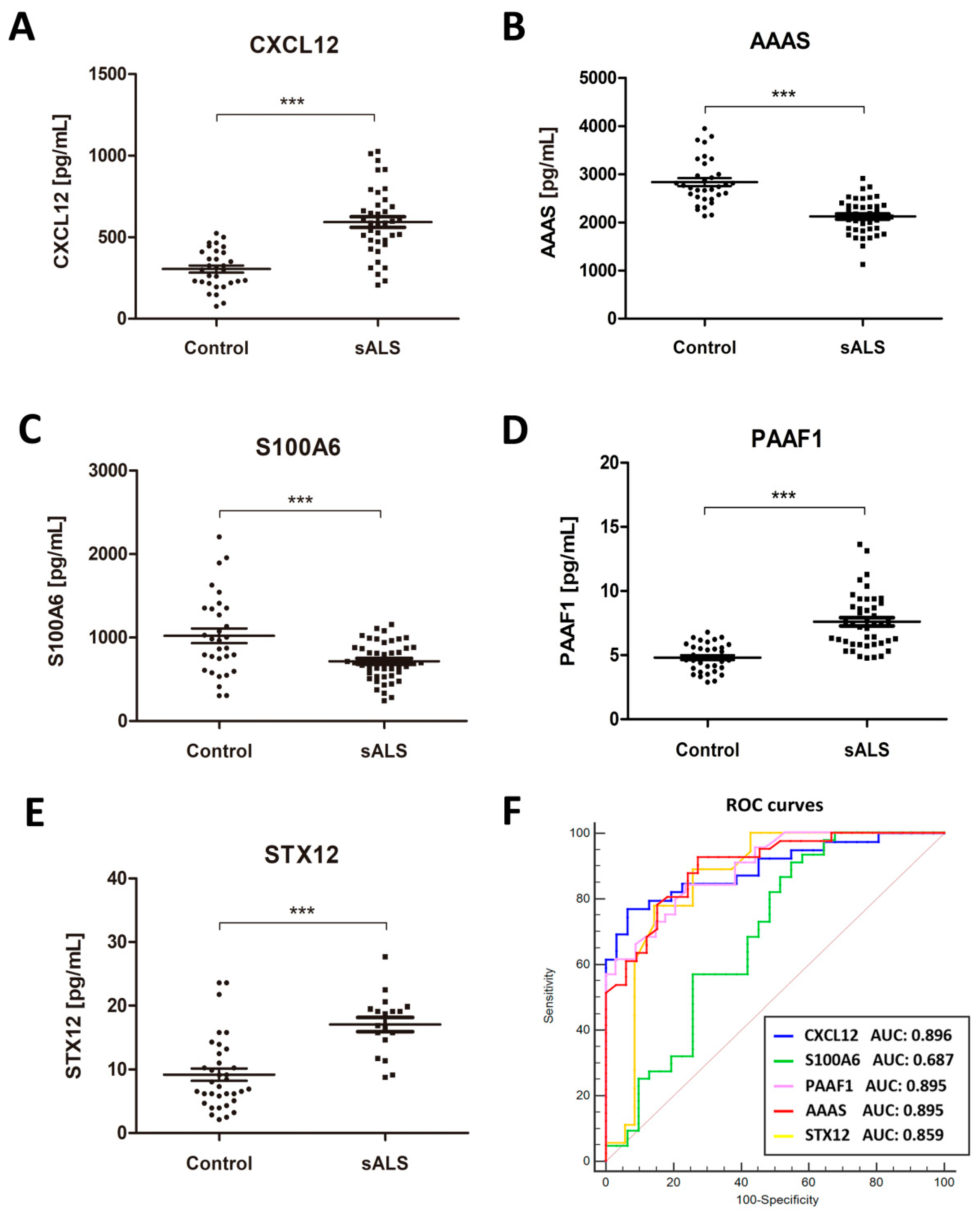
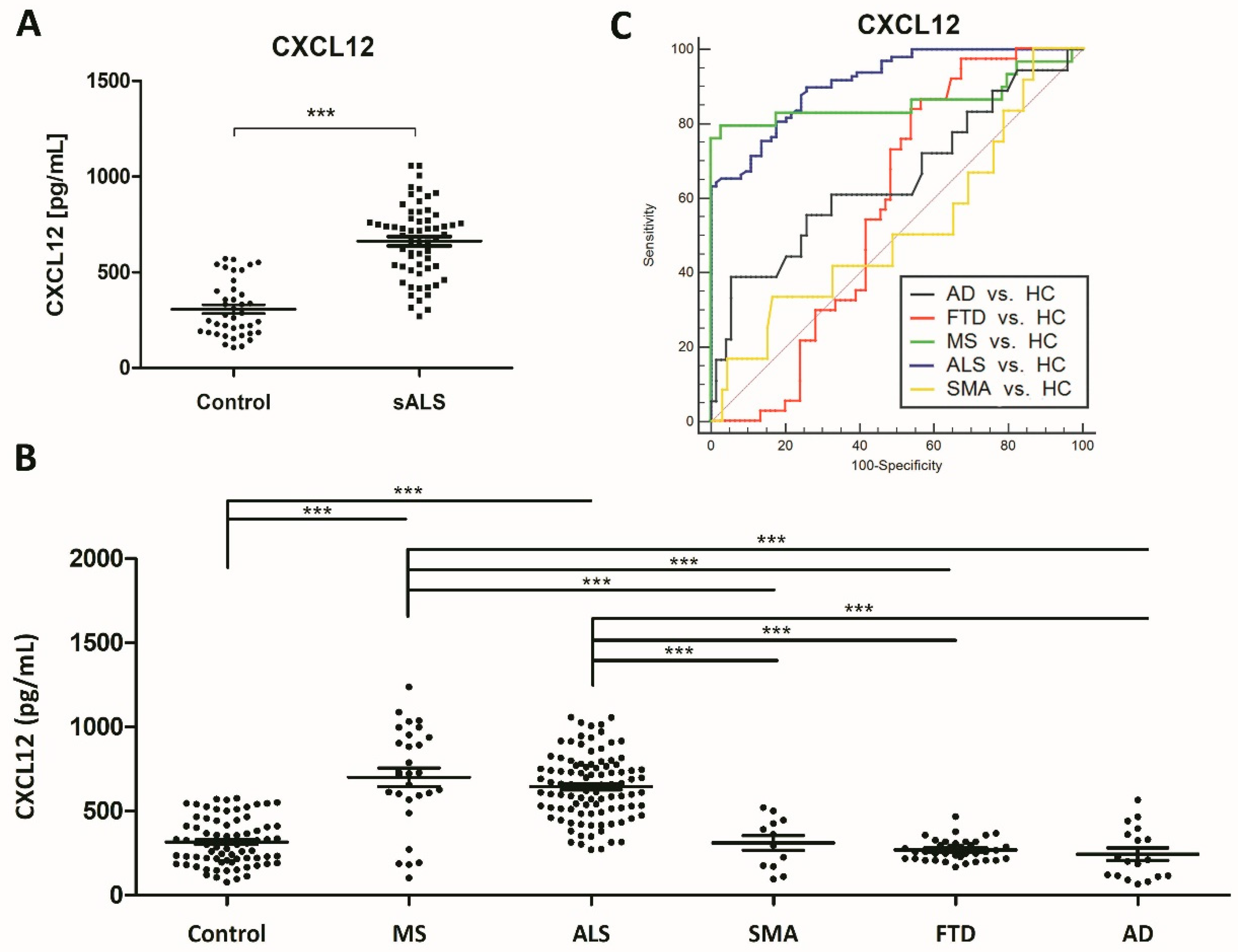
2.2. Identification of Putative Biomarker Candidates and Quantification Using ELISA
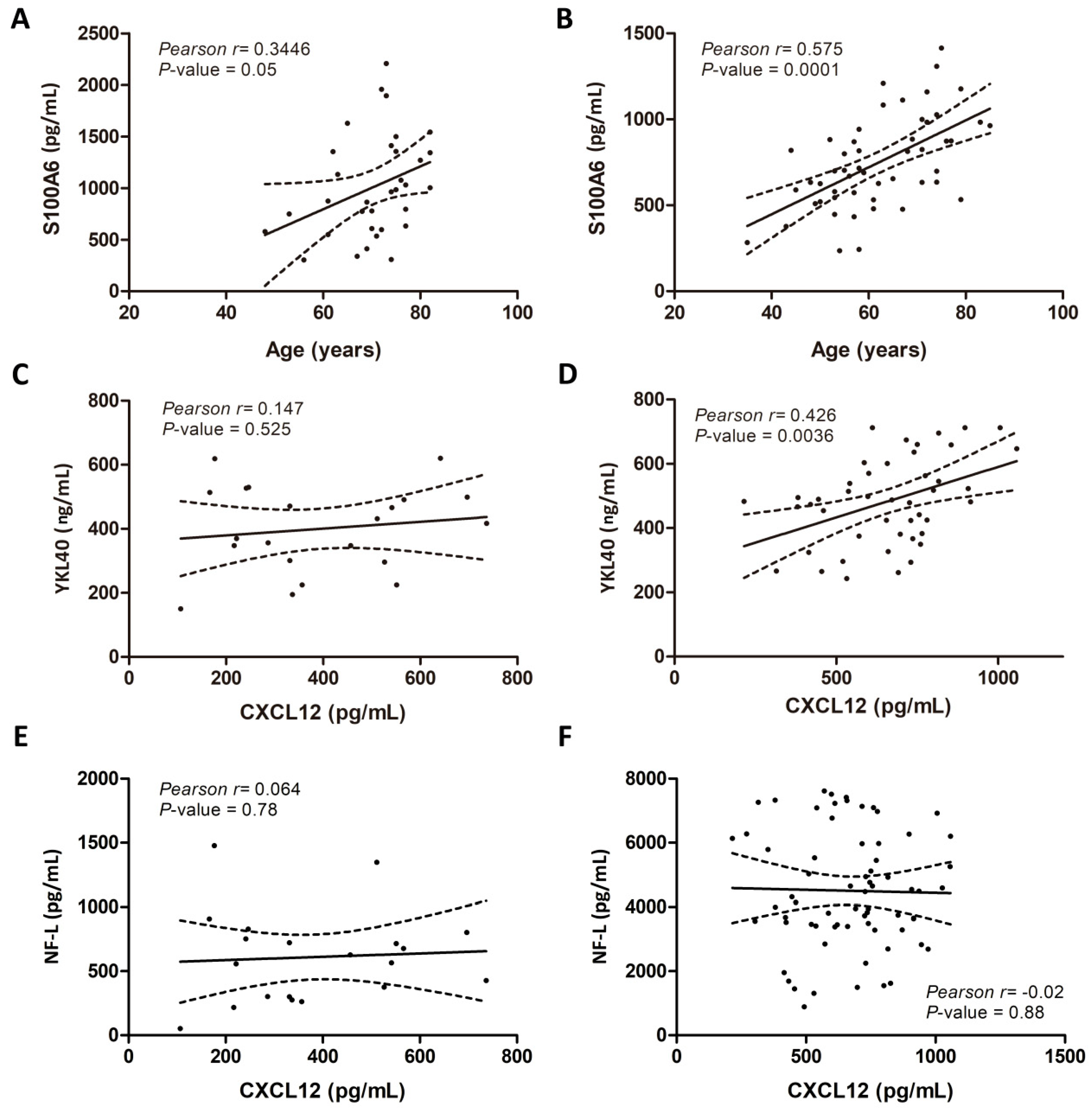
2.3. Associations of CSF Biomarker Levels, and Clinical and Biochemical Parameters
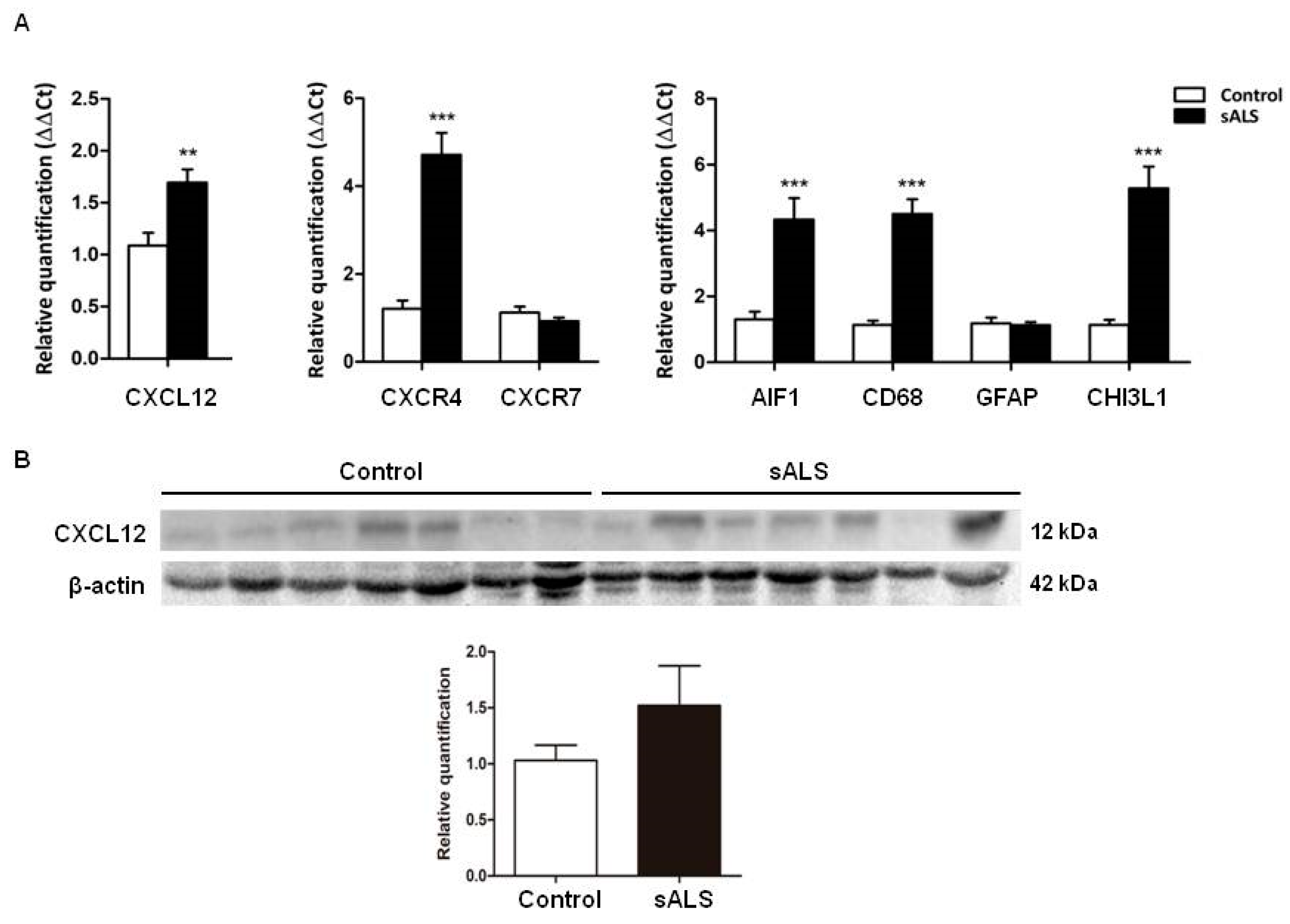
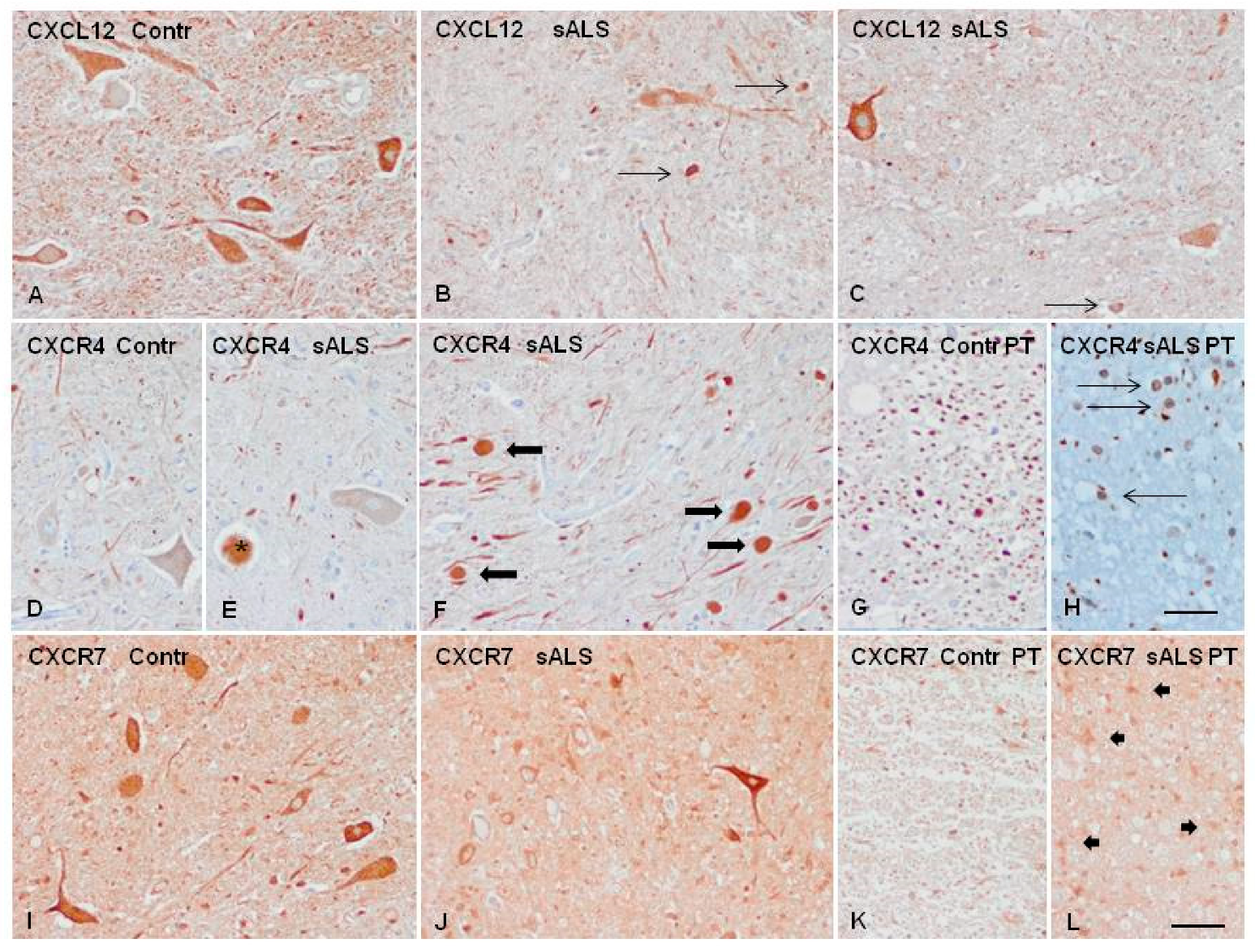
2.4. Candidate Biomarker mRNA Expression and Protein Levels in the Spinal Cord Tissue of sALS and Controls
2.5. Increased CSF CXCL12 Protein Levels Distinguish sALS (and MS) from Other Neurodegenerative Diseases
3. Discussion
4. Materials and Methods
4.1. CSF Collection
4.2. Proteomics Analysis
4.2.1. Building an MS/MS Library for SWATH Analysis
4.2.2. Quantitative Proteomics with SWATH Analysis
4.3. LC-SWATH-MS Analysis
4.4. Protein Identification and Quantification
4.5. CSF Analysis
4.6. Tissue Samples
4.7. RNA Extraction and RT-qPCR Validation
4.8. Gel Electrophoresis and Immunoblotting
4.9. Immunohistochemistry
4.10. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Ethics Approval and Consent to Participate
Availability of Data and Materials
References
- Hortobágyi, T.; Cairns, N.J.; Kovacs, G.G. Amyotrophic lateral sclerosis and frontotemporal lobar degeneration. In Neuropathology of Neurodegenerative Diseases; Cambridge University Press: Cambridge, UK, 2014; pp. 209–248. [Google Scholar]
- Schreiber, S.; Spotorno, N.; Schreiber, F.; Acosta-Cabronero, J.; Kaufmann, J.; Machts, J.; Debska-Vielhaber, G.; Garz, C.; Bittner, D.; Hensiek, N.; et al. Significance of CSF NfL and tau in ALS. J. Neurol. 2018, 265, 2633–2645. [Google Scholar]
- Abu-Rumeileh, S.; Vacchiano, V.; Zenesini, C.; Polischi, B.; De Pasqua, S.; Fileccia, E.; Mammana, A.; Di Stasi, V.; Capellari, S.; Salvi, F.; et al. Diagnostic-prognostic value and electrophysiological correlates of CSF biomarkers of neurodegeneration and neuroinflammation in amyotrophic lateral sclerosis. J. Neurol. 2020, 267, 1699–1708. [Google Scholar]
- Andrés-Benito, P.; Domínguez, R.; Colomina, M.J.; Llorens, F.; Povedano, M.; Ferrer, I. YKL40 in sporadic amyotrophic lateral sclerosis: Cerebrospinal fluid levels as a prognosis marker of disease progression. Aging 2018, 10, 2367–2382. [Google Scholar] [PubMed]
- Rossi, D.; Volanti, P.; Brambilla, L.; Colletti, T.; Spataro, R.; La Bella, V. CSF neurofilament proteins as diagnostic and prognostic biomarkers for amyotrophic lateral sclerosis. J. Neurol. 2018, 265, 510–521. [Google Scholar] [PubMed]
- Italiani, P.; Carlesi, C.; Giungato, P.; Puxeddu, I.; Borroni, B.; Bossù, P.; Migliorini, P.; Siciliano, G.; Boraschi, D. Evaluating the levels of interleukin-1 family cytokines in sporadic amyotrophic lateral sclerosis. J. Neuroinflamm. 2014, 11, 94. [Google Scholar] [PubMed]
- Tateishi, T.; Yamasaki, R.; Tanaka, M.; Matsushita, T.; Kikuchi, H.; Isobe, N.; Ohyagi, Y.; Kira, J.-I. CSF chemokine alterations related to the clinical course of amyotrophic lateral sclerosis. J. Neuroimmunol. 2010, 222, 76–81. [Google Scholar]
- Moreau, C.; Gosset, P.; Brunaud-Danel, V.; Lassalle, P.; Degonne, B.; Destée, A.; Defebvre, L.; Devos, D. CSF profiles of angiogenic and inflammatory factors depend on the respiratory status of ALS patients. Amyotroph. Lateral Scler. 2009, 10, 175–181. [Google Scholar]
- Illán-Gala, I.; Alcolea, D.; Montal, V.; Dols-Icardo, O.; Muñoz, L.; De Luna, N.; Turón-Sans, J.; Cortés-Vicente, E.; Sánchez-Saudinós, M.B.; Subirana, A.; et al. CSF sAPPβ, YKL-40, and NfL along the ALS-FTD spectrum. Neurology 2018, 91, e1619–e1628. [Google Scholar]
- Thompson, A.G.; Gray, E.; Thézénas, M.-L.; Charles, P.D.; Evetts, S.; Hu, M.T.; Talbot, K.; Fischer, R.; Kessler, B.M.; Turner, M.R. Cerebrospinal fluid macrophage biomarkers in amyotrophic lateral sclerosis. Ann. Neurol. 2018, 83, 258–268. [Google Scholar]
- Gille, B.; De Schaepdryver, M.; Dedeene, L.; Goossens, J.; Claeys, K.G.; Bosch, L.V.D.; Tournoy, J.; Van Damme, P.; Poesen, K. Inflammatory markers in cerebrospinal fluid: Independent prognostic biomarkers in amyotrophic lateral sclerosis? J. Neurol. Neurosurg. Psychiatry 2019, 90, 1338–1346. [Google Scholar]
- Llorens, F.; Thüne, K.; Tahir, W.; Kanata, E.; Diaz-Lucena, D.; Xanthopoulos, K.; Kovatsi, E.; Pleschka, C.; Garcia-Esparcia, P.; Schmitz, M.; et al. YKL-40 in the brain and cerebrospinal fluid of neurodegenerative dementias. Mol. Neurodegener. 2017, 12, 1–21. [Google Scholar]
- Tashiro, K.; Tada, H.; Heilker, R.; Shirozu, M.; Nakano, T.; Honjo, T. Signal sequence trap: A cloning strategy for secreted proteins and type I membrane proteins. Science 1993, 261, 600–603. [Google Scholar] [PubMed]
- Levoye, A.; Balabanian, K.; Baleux, F.; Bachelerie, F.; Lagane, B. CXCR7 heterodimerizes with CXCR4 and regulates CXCL12-mediated G protein signaling. Blood 2009, 113, 6085–6093. [Google Scholar] [PubMed]
- Rajagopal, S.; Kim, J.; Ahn, S.; Craig, S.; Lam, C.M.; Gerard, N.P.; Gerard, C.; Lefkowitz, R.G. Beta-arrestin but not G protein-mediated signaling by the “decoy” receptor CXCR7. Proc. Natl. Acad. Sci. USA 2010, 107, 628–632. [Google Scholar] [PubMed]
- Chen, Q.; Zhang, M.; Li, Y.; Xu, D.; Wang, Y.; Song, A.; Zhu, B.; Huang, Y.; Zheng, J.C. CXCR7 Mediates Neural Progenitor Cells Migration to CXCL12 Independent of CXCR4. Stem Cells 2015, 33, 2574–2585. [Google Scholar] [PubMed]
- Wang, Y.; Xu, P.; Qiu, L.; Zhang, M.; Huang, Y.; Zheng, J.C. CXCR7 Participates in CXCL12-mediated Cell Cycle and Proliferation Regulation in Mouse Neural Progenitor Cells. Curr. Mol. Med. 2016, 16, 738–746. [Google Scholar] [PubMed]
- Klein, R.S.; Rubin, J.B. Immune and nervous system CXCL12 and CXCR4: Parallel roles in patterning and plasticity. Trends Immunol. 2004, 25, 306–314. [Google Scholar]
- Petty, J.M.; Sueblinvong, V.; Lenox, C.C.; Jones, C.C.; Cosgrove, G.P.; Cool, C.D.; Rai, P.R.; Brown, K.K.; Weiss, D.J.; Poynter, M.E.; et al. Pulmonary Stromal-Derived Factor-1 Expression and Effect on Neutrophil Recruitment during Acute Lung Injury. J. Immunol. 2007, 178, 8148–8157. [Google Scholar]
- Li, M.; Ransohoff, R.M. Multiple roles of chemokine CXCL12 in the central nervous system: A migration from immunology to neurobiology. Prog. Neurobiol. 2008, 84, 116–131. [Google Scholar]
- Stumm, R.K.; Rummel, J.; Junker, V.; Culmsee, C.; Pfeiffer, M.; Krieglstein, J.; Höllt, V.; Schulz, S. A dual role for the SDF-1/CXCR4 chemokine receptor system in adult brain: Isoform-selective regulation of SDF-1 expression modulates CXCR4-dependent neuronal plasticity and cerebral leukocyte recruitment after focal ischemia. J. Neurosci. 2002, 22, 5865–5878. [Google Scholar]
- Bonavia, R.; Bajetto, A.; Barbero, S.; Pirani, P.; Florio, T.; Schettini, G. Chemokines and their receptors in the CNS: Expression of CXCL12/SDF-1 and CXCR4 and their role in astrocyte proliferation. Toxicol. Lett. 2003, 139, 181–189. [Google Scholar] [PubMed]
- Banisadr, G.; Skrzydelski, D.; Callewaere, C.; Desarménien, M.; Kitabgi, P.; Rostène, W. Chemokines and chemokine receptors in the brain: Possible implications in neuromodulation and neuroendocrine regulation. In Proceedings of the 34th Annual Meeting of Society for Neurosciences, SanDiego, CA, USA, 23–27 October 2004. [Google Scholar]
- Guyon, A.; Banisadr, G.; Rovere, C.; Cervantes, A.; Kitabgi, P.; Melik-Parsadaniantz, S.; Nahon, J.L. Complex effects of stromal cell-derived factor-1alpha on melanin-concentrating hormone neuronexcitability. Eur. J. Neurosci. 2005, 21, 701–710. [Google Scholar] [PubMed]
- Heinisch, S.; Kirby, L.G. SDF-1alpha/CXCL12 enhances GABA and glutamate synaptic activity at serotonin neurons in the rat dorsal raphe nucleus. Neuropharmacology 2010, 58, 501–514. [Google Scholar] [PubMed]
- Ardelt, A.A.; Bhattacharyya, B.J.; Belmadani, A.; Ren, D.; Miller, R.J. Stromal derived growth factor-1 (CXCL12) modulates synaptic transmission to immature neurons during post-ischemic cerebral repair. Exp. Neurol. 2013, 248, 246–253. [Google Scholar]
- Li, Y.; Chang, S.; Li, W.; Tang, G.; Ma, Y.; Liu, Y.; Yuan, F.; Zhang, Z.; Yang, G.-Y.; Wang, Y. cxcl12-engineered endothelial progenitor cells enhance neurogenesis and angiogenesis after ischemic brain injury in mice. Stem Cell Res. Ther. 2018, 9, 139. [Google Scholar]
- Zhang, Y.; Zhang, H.; Lin, S.; Chen, X.; Yao, Y.; Mao, X.; Shao, B.; Zhuge, Q.; Jin, K. SDF-1/CXCR7 chemokine signaling is induced in the peri-infarct regions in patients with ischemic stroke. Aging Dis. 2018, 9, 287–295. [Google Scholar]
- Jaerve, A.; Müller, H.W. Chemokines in CNS injury and repair. Cell Tissue Res. 2012, 349, 229–248. [Google Scholar]
- Mao, W.; Yi, X.; Qin, J.; Tian, M.; Jin, G. CXCL12/CXCR4 axis improves migration of neuroblasts along corpus callosum by stimulating MMP-2 secretion after traumatic brain injury in rats. Neurochem. Res. 2016, 41, 1315–1322. [Google Scholar]
- Mao, W.; Yi, X.; Qin, J.; Tian, M.; Jin, G. CXCL12 promotes proliferation of radial glia like cells after traumatic brain injury in rats. Cytokine 2020, 125, 154771. [Google Scholar]
- Li, M.; Hale, J.S.; Rich, J.N.; Ransohoff, R.M.; Lathia, J.D. Chemokine CXCL12 in neurodegenerative diseases: An SOS signal for stem cell-based repair. Trends Neurosci. 2012, 35, 619–628. [Google Scholar]
- Pashenkov, M.; Söderström, M.; Link, H. Secondary lymphoid organ chemokines are elevated in the cerebrospinal fluid during central nervous system inflammation. J. Neuroimmunol. 2003, 135, 154–160. [Google Scholar] [PubMed]
- Corcione, A.; Casazza, S.; Ferretti, E.; Giunti, D.; Zappia, E.; Pistorio, A.; Gambini, C.; Mancardi, G.L.; Uccelli, A.; Pistoia, V. Recapitulation of B cell differentiation in the central nervous system of patients with multiple sclerosis. Proc. Natl. Acad. Sci. USA 2004, 101, 11064–11069. [Google Scholar] [PubMed]
- Krumbholz, M.; Theil, D.; Cepok, S.; Hemmer, B.; Kivisäkk, P.; Ransohoff, R.M.; Hofbauer, M.; Farina, C.; Derfuss, T.; Hartle, C.; et al. Chemokines in multiple sclerosis: CXCL12 and CXCL13 up-regulation is differentially linked to CNS immune cell recruitment. Brain 2006, 129, 200–211. [Google Scholar] [PubMed]
- Magliozzi, R.; Howell, O.W.; Nicholas, R.; Cruciani, C.; Castellaro, M.; Romualdi, C.; Rossi, S.; Pitteri, M.; Benedetti, M.D.; Gajofatto, A.; et al. Inflammatory intrathecal profiles and cortical damage in multiple sclerosis. Ann. Neurol. 2018, 83, 739–755. [Google Scholar] [PubMed]
- Magliozzi, R.; Marastoni, D.; Rossi, S.; Castellaro, M.; Mazziotti, V.; Pitteri, M.; Gajofatto, A.; Monaco, S.; Benedetti, M.D.; Calabrese, M. Increase of CSF inflammatory profile in a case of highly active multiple sclerosis. BMC Neurol. 2019, 19, 1–5. [Google Scholar]
- Edwards, K.R.; Goyal, J.; Plavina, T.; Czerkowicz, J.; Goelz, S.; Ranger, A.; Cadavid, D.; Browning, J.L. Feasibility of the Use of Combinatorial Chemokine Arrays to Study Blood and CSF in Multiple Sclerosis. PLoS ONE 2013, 8, e81007. [Google Scholar]
- Calderon, T.M.; Eugenin, E.A.; Lopez, L.; Kumar, S.S.; Hesselgesser, J.; Raine, C.S.; Berman, J.W. A role for CXCL12 (SDF-1α) in the pathogenesis of multiple sclerosis: Regulation of CXCL12 expression in astrocytes by soluble myelin basic protein. J. Neuroimmunol. 2006, 177, 27–39. [Google Scholar]
- McCandless, E.E.; Wang, Q.; Woerner, B.M.; Harper, J.M.; Klein, R.S. CXCL12 Limits Inflammation by Localizing Mononuclear Infiltrates to the Perivascular Space during Experimental Autoimmune Encephalomyelitis. J. Immunol. 2006, 177, 8053–8064. [Google Scholar]
- Zilkha-Falb, R.; Kaushansky, N.; Kawakami, N.; Ben-Nun, A. Post-CNS-inflammation expression of CXCL12 promotes the endogenous myelin/neuronal repair capacity following spontaneous recovery from multiple sclerosis-like disease. J. Neuroinflamm. 2016, 13, 1–19. [Google Scholar]
- Patel, J.R.; McCandless, E.E.; Dorsey, D.; Klein, R.S. CXCR4 promotes differentiation of oligodendrocyte progenitors and remyelination. Proc. Natl. Acad. Sci. USA 2010, 107, 11062–11067. [Google Scholar]
- Carbajal, K.S.; Schaumburg, C.; Strieter, R.; Kane, J.; Lane, T.E. Migration of engrafted neural stem cells is mediated by CXCL12 signaling through CXCR4 in a viral model of multiple sclerosis. Proc. Natl. Acad. Sci. USA 2010, 107, 11068–11073. [Google Scholar] [PubMed]
- Zeis, T.; Howell, O.; Reynolds, R.; Schaeren-Wiemers, N. Molecular pathology of Multiple Sclerosis lesions reveals a heterogeneous expression pattern of genes involved in oligodendrogliogenesis. Exp. Neurol. 2018, 305, 76–88. [Google Scholar] [PubMed]
- Boroujeni, F.B.; Pasbakhsh, P.; Mortezaee, K.; Pirhajati, V.; Alizadeh, R.; Aryanpour, R.; Madadi, S.; Kashani, I.R. Intranasal delivery of SDF-1α-preconditioned bone marrow mesenchymal cells improves remyelination in the cuprizone-induced mouse model of multiple sclerosis. Cell Biol. Int. 2019, 44, 499–511. [Google Scholar]
- Cho, K.-I.; Yoon, D.; Qiu, S.; Danziger, Z.; Grill, W.M.; Wetsel, W.C.; Ferreira, P.A. Loss of Ranbp2 in motoneurons causes disruption of nucleocytoplasmic and chemokine signaling, proteostasis of hnRNPH3 and Mmp28, and development of amyotrophic lateral sclerosis-like syndromes. Dis. Model. Mech. 2017, 10, 559–579. [Google Scholar] [PubMed]
- Luo, Y.; Xue, H.; Pardo, A.C.; Mattson, M.P.; Rao, M.S.; Maragakis, N.J. Impaired SDF1/CXCR4 signaling in glial progenitors derived from SOD1(G93A) mice. J. Neurosci. Res. 2007, 85, 2422–2432. [Google Scholar] [PubMed]
- Rabinovich-Nikitin, I.; Ezra, A.; Barbiro, B.; Rabinovich-Toidman, P.; Solomon, B. Chronic administration of AMD3100 increases survival and alleviates pathology in SOD1G93A mice model of ALS. J. Neuroinflamm. 2016, 13, 123. [Google Scholar]
- Brooks, B.R.; Miller, R.G.; Swash, M.; Munsat, T.L. El Escorial revisited: Revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph. Lateral Scler. 2000, 1, 293–299. [Google Scholar]
- De Carvalho, M.; Swash, M. Awaji diagnostic algorithm increases sensitivity of El Escorial criteria for ALS diagnosis. Amyotroph. Lateral Scler. 2009, 10, 53–57. [Google Scholar]
- Rascovsky, K.; Hodges, J.R.; Knopman, D.; Mendez, M.F.; Kramer, J.H.; Neuhaus, J.; Van Swieten, J.C.; Seelaar, H.; Dopper, E.G.P.; Onyike, C.U.; et al. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain 2011, 134, 2456–2477. [Google Scholar]
- Lachén-Montes, M.; González-Morales, A.; Zelaya, M.V.; Pérez-Valderrama, E.; Ausín, K.; Ferrer, I.; Fernández-Irigoyen, J.; Santamaría, E. Olfactory bulb neuroproteomics reveals a chronological perturbation of survival routes and a disruption of prohibitin complex during Alzheimer’s disease progression. Sci. Rep. 2017, 7, 1–15. [Google Scholar]
- Lachén-Montes, M.; González-Morales, A.; Iloro, I.; Elortza, F.; Ferrer, I.; Gveric, D.; Fernández-Irigoyen, J.; Santamaría, E. Unveiling the olfactory proteostatic disarrangement in Parkinson’s disease by proteome-wide profiling. Neurobiol. Aging 2019, 73, 123–134. [Google Scholar] [PubMed]
- Gillet, L.C.; Navarro, P.; Tate, S.; Röst, H.; Selevsek, N.; Reiter, L.; Bonner, R.; Aebersold, R. Targeted Data Extraction of the MS/MS Spectra Generated by Data-independent Acquisition: A New Concept for Consistent and Accurate Proteome Analysis. Mol. Cell. Proteom. 2012, 11. [Google Scholar] [CrossRef]
- Tyanova, S.; Temu, T.; Sinitcyn, P.; Carlson, A.; Hein, M.Y.; Geiger, T.; Mann, M.; Cox, J. The Perseus computational platform for comprehensive analysis of (prote)omics data. Nat. Methods 2016, 13, 731–740. [Google Scholar] [PubMed]
- Ferrer, I. Brain Banking. In Encyclopedia of the Neurological Sciences, 2nd ed.; Aminoff, M.J., Daroff, R.B., Eds.; Academic Press: Oxford, UK, 2014; pp. 467–473. [Google Scholar]
- Strong, M.J.; Hortobágyi, T.; Okamoto, K.; Kato, S. Amyotrophic lateral sclerosis, primary lateral sclerosis, and spinal muscular atrophy. In Neurodegeneration: The Molecular Pathology of Dementia and Movement Disorders, 2nd ed.; Dickson, D.W., Weller, R.O., Eds.; Wiley-Blackwell: Oxford, UK, 2011; pp. 418–433. [Google Scholar]
- Durrenberger, P.F.; Fernando, F.S.; Magliozzi, R.; Kashefi, S.N.; Bonnert, T.P.; Ferrer, I.; Seilhean, D.; Nait-Oumesmar, B.; Schmitt, A.; Gebicke-Haerter, P.J.; et al. Selection of novel reference genes for use in the human central nervous system: A BrainNet Europe Study. Acta Neuropathol. 2012, 124, 893–903. [Google Scholar]
- Youden, W.J. Index for rating diagnostic tests. Cancer 1950, 3, 32–35. [Google Scholar]
- Delong, E.R.; Delong, D.M.; Clarke-Pearson, D.L. Comparing the Areas under Two or More Correlated Receiver Operating Characteristic Curves: A Nonparametric Approach. Biometrics 1988, 44, 837. [Google Scholar]
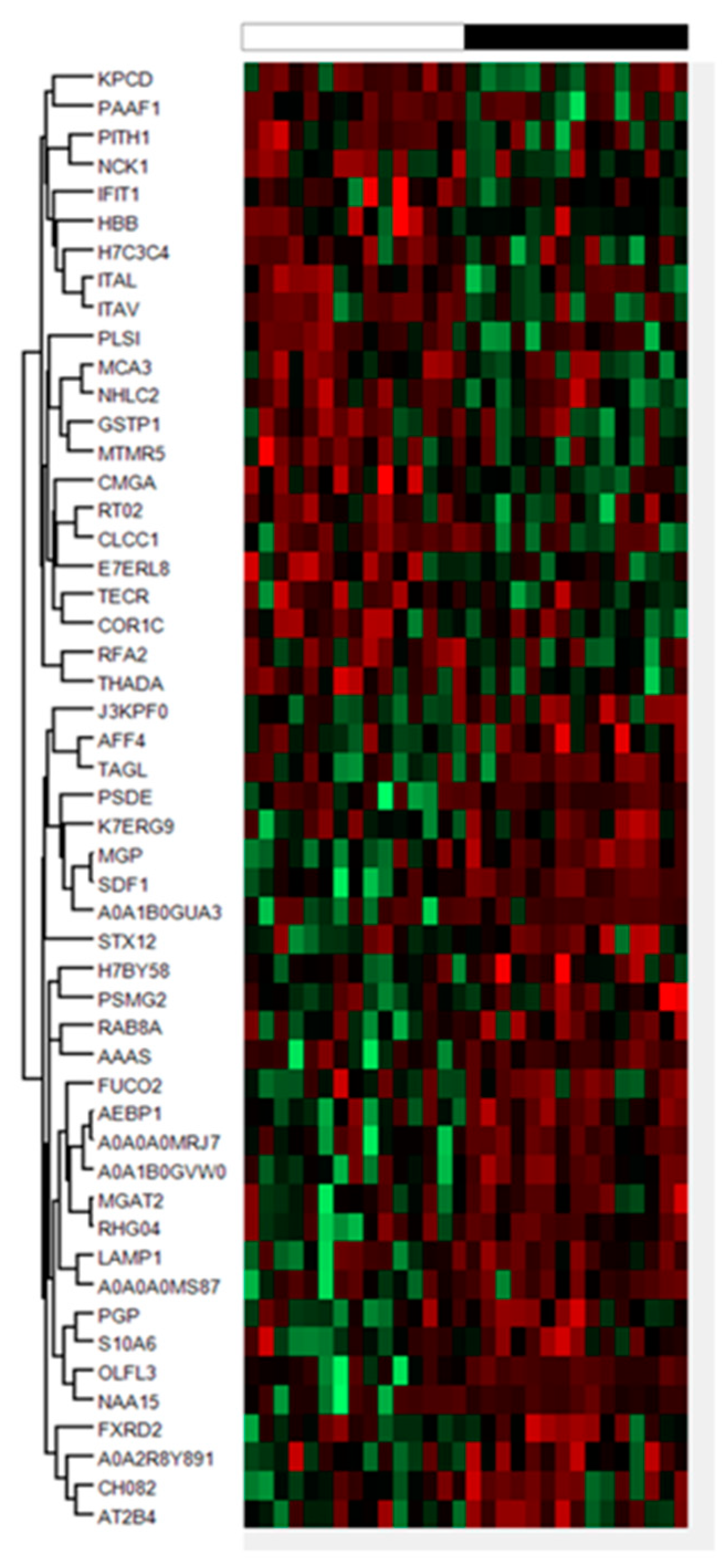
| Gene | Accesion | Peptides | %CV | FC | p-Value | |
|---|---|---|---|---|---|---|
| HBB | hemoglobin subunit beta | P68871 | 7 | 105.28 | 0.37 | 0.04 |
| CHGA | chromogranin A | P10645 | 6 | 72.40 | 0.55 | 0.00 |
| E7ERL8 | neurexin 1 | E7ERL8 | 5 | 63.37 | 0.66 | 0.02 |
| CLCC1 | chloride channel CLIC like 1 | Q96S66 | 3 | 33.26 | 0.64 | 0.04 |
| K7ERG9 | complement factor D | K7ERG9 | 3 | 40.73 | 1.41 | 0.02 |
| TECR | trans-2,3-enoyl-CoA reductase | Q9NZ01 | 2 | 42.33 | 0.67 | 0.04 |
| ARHGAP4 | Rho GTPase activating protein | P98171 | 2 | 72.50 | 1.32 | 0.02 |
| FUCA2 | alpha-L-fucosidase 2 | Q9BTY2 | 2 | 56.10 | 1.57 | 0.04 |
| A0A0A0MRJ7 | - | A0A0A0MRJ7 | 2 | 66.71 | 2.12 | 0.00 |
| NCK1 | NECK adaptor protein 1 | P16333 | 1 | 102.16 | 0.35 | 0.04 |
| MTMR5 | SBF1 (MTMR5) SET binding factor 1 | O95248 | 1 | 82.74 | 0.36 | 0.02 |
| COR1C | coronin-1C | Q9ULV4 | 1 | 98.19 | 0.40 | 0.05 |
| ITAV | integrin, alpha 5 | P06756 | 1 | 16.49 | 0.41 | 0.01 |
| PAAF1 | proteasomal ATPase associated factor 1 | Q9BRP4 | 1 | 83.56 | 0.43 | 0.03 |
| ITAL | toll-like receptor 4 | P20701 | 1 | 78.52 | 0.43 | 0.01 |
| MCA3 | eukaryotic translation elongation factor 1 epsilon-1 | O43324 | 1 | 100.07 | 0.45 | 0.04 |
| KPCD | protein kinase C delta type i | Q05655 | 1 | 41.25 | 0.46 | 0.03 |
| THADA | THADA armadillo repeat containing | Q6YHU6 | 1 | 46.64 | 0.48 | 0.01 |
| NHLC2 | NHL repeat-containing protein 2 | Q8NBF2 | 1 | 73.60 | 0.49 | 0.03 |
| PLSI | plastin 1 | Q14651 | 1 | 25.89 | 0.54 | 0.03 |
| RFA2 | replication protein A2 | P15927 | 1 | 93.57 | 0.54 | 0.01 |
| MRPS2 | mitochondrial ribosomal protein S2 | Q9Y399 | 1 | 61.86 | 0.56 | 0.02 |
| GSTP1 | glutathione S-transferase pi1 | P09211 | 1 | 30.22 | 0.57 | 0.04 |
| PITH1 | PITH domain containing 1 | Q9GZP4 | 1 | 60.84 | 0.60 | 0.02 |
| IFIT1 | interferon induced protein with tetratricopeptide repeats 1 | P09914 | 1 | 41.39 | 0.63 | 0.05 |
| H7C3C4 | - | H7C3C4 | 1 | 32.17 | 0.70 | 0.04 |
| MGAT2 | alpha-1,6-mannosyl-glycoprotein 2-beta-N-acetylglucosaminyltransferase | Q10469 | 1 | 34.5 | 1.39 | 0.02 |
| AFF4 | AF4/FMR2 familiy member 4 | Q9UHB7 | 1 | 37.83 | 1.39 | 0.03 |
| PGP | phosphoglycolate phosphatase | A6NDG6 | 1 | 20.82 | 1.45 | 0.04 |
| AEBP1 | AE binding protein 1 | Q8IUX7 | 1 | 39.01 | 1.57 | 0.00 |
| PSMG2 | proteasome assembly chaperone 2 | Q969U7 | 1 | 40.08 | 1.60 | 0.03 |
| RAB8A | RAB8A, member RAS oncogene family | P61006 | 1 | 77.36 | 1.66 | 0.01 |
| S10A6 | S100 calcium biding protein A6 | P06703 | 1 | 64.07 | 1.67 | 0.03 |
| A0A2R8Y891 | - | A0A2R8Y891 | 1 | 46.81 | 1.91 | 0.02 |
| LAMP1 | lysosomal associated membrane protein 1 | P11279 | 1 | 99.83 | 1.92 | 0.02 |
| FXRD2 | FAD-dependent oxidoreductase domain containing 2 | Q8IWF2 | 1 | 56.92 | 1.95 | 0.05 |
| SDF1 | C-X-C motif chemokine ligand 12 | P48061 | 1 | 39.23 | 1.97 | 0.00 |
| A0A0A0MS87 | - | A0A0A0MS87 | 1 | 67.51 | 1.98 | 0.04 |
| MGP | matrix Gla protein | P08493 | 1 | 28.51 | 2.00 | 0.00 |
| A0A1B0GVW0 | - | A0A1B0GVW0 | 1 | 60.77 | 2.01 | 0.00 |
| STX12 | syntaxin 12 | Q86Y82 | 1 | 60.13 | 2.01 | 0.04 |
| AT2B4 | ATPase plasma membrane Ca2+ transportin 4 | P23634 | 1 | 59.88 | 2.04 | 0.04 |
| PSDE | proteasome 26S subunit, non-ATPase 14 | O00487 | 1 | 69.53 | 2.12 | 0.04 |
| PCMT1 | protein-l-isoaspartate (D-aspartate) O-methyltransferase | H7BY58 | 1 | 50.17 | 2.14 | 0.03 |
| CH082 | Q6P1X6 | 1 | 52.14 | 2.19 | 0.03 | |
| AAAS | aladin WD repeat nucleoporin | Q9NRG9 | 1 | 59.66 | 2.19 | 0.02 |
| A0A1B0GUA3 | - | A0A1B0GUA3 | 1 | 123.34 | 2.27 | 0.03 |
| OLFL3 | olfactomedin 3 | Q9NRN5 | 1 | 62.69 | 2.57 | 0.00 |
| TAGL | transgelin | Q01995 | 1 | 136.16 | 2.95 | 0.01 |
| NAA15 | N-alpha.-acetyltransferase 15, NatA auxiliary subunit | Q9BXJ9 | 1 | 130.87 | 3.26 | 0.04 |
| J3KPF0 | - | J3KPF0 | 1 | 114.46 | 3.66 | 0.00 |
| Group | n | Age | Sex (f/m) | Disease Subtype |
|---|---|---|---|---|
| Cohort 1 | ||||
| HC | 36 | 67 ± 12.9 | (21/15) | |
| sALS | 43 | 61 ± 10.8 | (29/14) | 21 Spinal |
| 21 Bulbar | ||||
| 1 Resp | ||||
| Cohort 2 | ||||
| HC | 44 | 68 ± 09.7 | (26/18) | |
| sALS | 65 | 66 ± 12.3 | (27/38) | 43 Spinal |
| 21 Bulbar | ||||
| 1 Resp | ||||
| MS | 30 | 41 ± 10.7 | (22/8) | 18 Acute-MS |
| 12 RRMS | ||||
| SMA-III | 13 | 31 ± 7.75 | (6/7) | Type III |
| AD | 19 | 64 ± 08.6 | (14/5) | |
| FTD | 39 | 66 ± 08.5 | (18/21) | 39 bvFTD |
| Case | Age | Gender | Diagnosis | PM Delay | Site of Onset | RIN |
|---|---|---|---|---|---|---|
| 1 | 70 | M | ALS | 03 h 00 min | Resp. | 7.00 |
| 2 | 77 | M | ALS | 04 h 30 min | N/A | 7.50 |
| 3 | 83 | F | ALS | 15 h 15 min | N/A | 7.20 |
| 4 | 56 | F | ALS | 03 h 45 min | N/A | 8.10 |
| 5 | 56 | M | ALS | 10 h 50 min | N/A | 6.60 |
| 6 | 76 | M | ALS | 12 h 40 min | Spinal | 7.00 |
| 7 | 69 | M | ALS | 02 h 00 min | N/A | 7.00 |
| 8 | 63 | F | ALS | 13 h 50 min | Bulbar | 6.50 |
| 9 | N/A | M | ALS | N/A | N/A | 8.70 |
| 10 | 65 | F | ALS | 04 h 10 min | N/A | 7.70 |
| 11 | 50 | M | ALS | 10 h 10 min | Spinal | 5.30 |
| 12 | 71 | M | ALS | 03 h 25 min | N/A | 8.10 |
| 13 | 54 | M | ALS | 04 h 50 min | Spinal | 8.80 |
| 14 | 64 | M | ALS | 16 h 30 min | N/A | 6.70 |
| 15 | 75 | F | ALS | 04 h 05 min | Bulbar | 8.50 |
| 16 | 76 | F | ALS | 13 h 00 min | N/A | 8.10 |
| 17 | 57 | F | ALS | 10 h 00 min | Bulbar | 7.00 |
| 18 | 79 | F | ALS | 02 h 10 min | Bulbar | 8.10 |
| 19 | 57 | F | ALS | 04 h 00 min | Bulbar | 6.20 |
| 20 | 46 | M | ALS | 07 h 00 min | Spinal | 7.00 |
| 21 | 69 | F | ALS | 17 h 00 min | Spinal | 6.40 |
| 22 | 59 | M | ALS | 03 h 15 min | N/A | 6.80 |
| 23 | 66 | M | Control | 14 h 00 min | - | 5.00 |
| 24 | 46 | M | Control | 15 h 00 min | - | 5.70 |
| 25 | 66 | M | Control | 05 h 00 min | - | 5.40 |
| 26 | 77 | F | Control | 08 h 30 min | - | 5.10 |
| 27 | 64 | F | Control | 05 h 00 min | - | 7.00 |
| 28 | 60 | F | Control | 09 h 40 min | - | 5.80 |
| 29 | 52 | M | Control | 03 h 00 min | - | 5.00 |
| 30 | 67 | M | Control | 07 h 00 min | - | 5.50 |
| 31 | 47 | M | Control | 04 h 55 min | - | 5.60 |
| 32 | 64 | F | Control | 11 h 20 min | - | 6.20 |
| 33 | 56 | M | Control | 07 h 10 min | - | 6.10 |
| 34 | 71 | F | Control | 08 h 30 min | - | 5.90 |
| 35 | 55 | M | Control | 09 h 45 min | - | 5.30 |
| 36 | 75 | M | Control | 07 h 30 min | - | 6.60 |
| 37 | 51 | F | Control | 04 h 00 min | - | 6.30 |
| 38 | 59 | M | Control | 12 h 05 min | - | 6.40 |
| 39 | 75 | F | Control | 10 h 30 min | - | 5.20 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andrés-Benito, P.; Povedano, M.; Domínguez, R.; Marco, C.; Colomina, M.J.; López-Pérez, Ó.; Santana, I.; Baldeiras, I.; Martínez-Yelámos, S.; Zerr, I.; et al. Increased C-X-C Motif Chemokine Ligand 12 Levels in Cerebrospinal Fluid as a Candidate Biomarker in Sporadic Amyotrophic Lateral Sclerosis. Int. J. Mol. Sci. 2020, 21, 8680. https://doi.org/10.3390/ijms21228680
Andrés-Benito P, Povedano M, Domínguez R, Marco C, Colomina MJ, López-Pérez Ó, Santana I, Baldeiras I, Martínez-Yelámos S, Zerr I, et al. Increased C-X-C Motif Chemokine Ligand 12 Levels in Cerebrospinal Fluid as a Candidate Biomarker in Sporadic Amyotrophic Lateral Sclerosis. International Journal of Molecular Sciences. 2020; 21(22):8680. https://doi.org/10.3390/ijms21228680
Chicago/Turabian StyleAndrés-Benito, Pol, Mònica Povedano, Raúl Domínguez, Carla Marco, Maria J. Colomina, Óscar López-Pérez, Isabel Santana, Inês Baldeiras, Sergio Martínez-Yelámos, Inga Zerr, and et al. 2020. "Increased C-X-C Motif Chemokine Ligand 12 Levels in Cerebrospinal Fluid as a Candidate Biomarker in Sporadic Amyotrophic Lateral Sclerosis" International Journal of Molecular Sciences 21, no. 22: 8680. https://doi.org/10.3390/ijms21228680
APA StyleAndrés-Benito, P., Povedano, M., Domínguez, R., Marco, C., Colomina, M. J., López-Pérez, Ó., Santana, I., Baldeiras, I., Martínez-Yelámos, S., Zerr, I., Llorens, F., Fernández-Irigoyen, J., Santamaría, E., & Ferrer, I. (2020). Increased C-X-C Motif Chemokine Ligand 12 Levels in Cerebrospinal Fluid as a Candidate Biomarker in Sporadic Amyotrophic Lateral Sclerosis. International Journal of Molecular Sciences, 21(22), 8680. https://doi.org/10.3390/ijms21228680










