Abstract
The immune system plays a critical role in protecting hosts from the invasion of organisms. CD4 T cells, as a key component of the immune system, are central in orchestrating adaptive immune responses. After decades of investigation, five major CD4 T helper cell (Th) subsets have been identified: Th1, Th2, Th17, Treg (T regulatory), and Tfh (follicular T helper) cells. Th1 cells, defined by the expression of lineage cytokine interferon (IFN)-γ and the master transcription factor T-bet, participate in type 1 immune responses to intracellular pathogens such as mycobacterial species and viruses; Th2 cells, defined by the expression of lineage cytokines interleukin (IL)-4/IL-5/IL-13 and the master transcription factor GAΤA3, participate in type 2 immune responses to larger extracellular pathogens such as helminths; Th17 cells, defined by the expression of lineage cytokines IL-17/IL-22 and the master transcription factor RORγt, participate in type 3 immune responses to extracellular pathogens including some bacteria and fungi; Tfh cells, by producing IL-21 and expressing Bcl6, help B cells produce corresponding antibodies; whereas Foxp3-expressing Treg cells, unlike Th1/Th2/Th17/Tfh exerting their effector functions, regulate immune responses to maintain immune cell homeostasis and prevent immunopathology. Interestingly, innate lymphoid cells (ILCs) have been found to mimic the functions of three major effector CD4 T helper subsets (Th1, Th2, and Th17) and thus can also be divided into three major subsets: ILC1s, ILC2s, and ILC3s. In this review, we will discuss the differentiation and functions of each CD4 T helper cell subset in the context of ILCs and human diseases associated with the dysregulation of these lymphocyte subsets particularly caused by monogenic mutations.
1. Introduction
To defend countless pathogen invasions, mammals have developed and evolved a rigorous and precise immune system, which is built upon various kinds of cells, cytokines, chemokines, antibodies et al. [1,2]. CD4 T helper cells, as a key component of this system, play a critical role in defending infections throughout life by coordinating the actions of other immune cells as well as non-immune cells. CD4 T cells originate from common lymphoid progenitors in the bone marrow, develop in the thymus, and execute their functions mainly in peripheral tissues and various lymphoid organs [3]. The developmental process is precisely regulated so that CD4 T cells may efficiently recognize a very broad range of foreign antigens without eliciting auto-reactivity to self-antigens. For executing their effector functions, naïve CD4 T cells first need to be activated when receiving pathogen signals delivered by antigen-presenting cells (APCs), such as dendritic cells (DCs), and then differentiate into distinct T effector (Teff) populations based on the nature of infections. Effector CD4 T cells provide host defensive mechanisms against pathogens through the production of effector cytokines, which activate other immune cells such as macrophages and CD8 T cells in killing infected cells and help B cells in producing antibodies to mediate humoral immune responses [4,5,6].
Based on the expression of signature cytokines and lineage-specific master transcription factors, CD4 T helper (Th) cells are typically categorized into five major subsets (Figure 1): Th1 (interferon (IFN)-γ and T-bet), Th2 (interleukin (IL)-4/IL-5/IL-13 and GATA3), Th17 (IL-17/IL-22 and RORγt), Tfh (IL-21 and Bcl6) and Treg (T regulatory) (IL-10/transforming growth factor (TGF)-β/IL-35 and Foxp3) [7]. Some labs have also described some other subsets, such as Th3 (TGF-β) [8], Tr1 (IL-10) [9], Th9 (IL-9) [10,11], and Th22 (IL-22) [12]. Since there are still controversial issues in defining them as unique lineage subsets (particularly because the lack of a master regulator associated with each of these “lineages”), we will not further discuss these CD4 T cell subsets in this review.
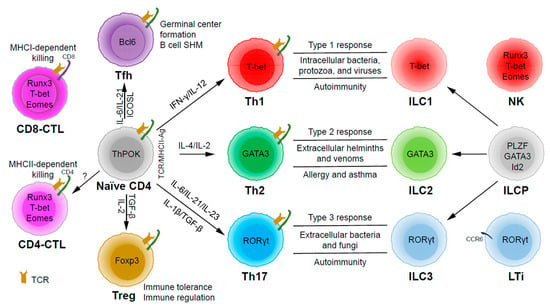
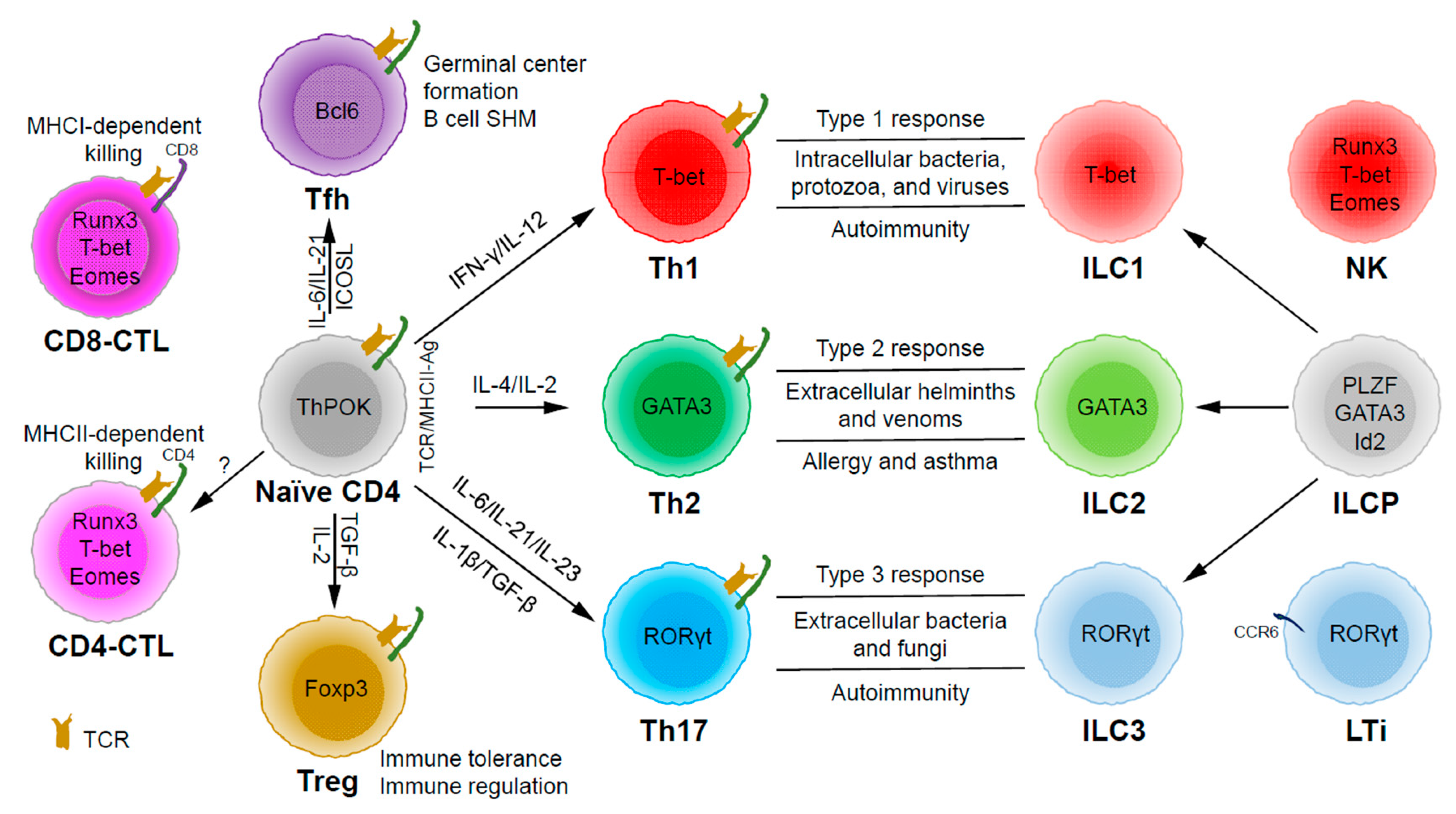
Figure 1.
The development and functions of CD4 T helper (Th) and innate lymphoid cell (ILC) subsets. Naïve CD4 T cells can differentiate into Th1, Th2, Th17, Tfh (follicular T helper), and Treg (T regulatory) subsets upon T cell receptor (TCR) activation in different cytokine milieu. T cells may also become cytotoxic CD4 T cells (CD4-CTLs) that kill their target cells in major histocompatibility complex class II (MHCII)-restricted manner. ILC1, ILC2, and ILC3 subsets are the innate counterparts of Th1, Th2, and Th17 cells and they are involved in type 1, type 2, and type 3 immune responses, respectively. ILC1, ILC2, and ILC3 subsets develop from ILC progenitors (ILCPs), which are distinct from progenitors that give rise to natural killer (NK) cells and lymphoid tissue inducers (LTis).
Through thymic selection, CD4/CD8 double positive T cells develop into either mature CD4 (helper) or CD8 (cytotoxic) T cells, which recognize major histocompatibility complex (MHC) class II (MHCII)/peptide and MHCI/peptide complexes, respectively (Figure 1). However, some mature CD4 T cells, cytotoxic CD4 T cells (CD4-CTLs or Th-CTLs), may act similarly as cytotoxic CD8 T cells in killing their target cells through the secretion of granzyme B and perforin but in an MHCII-restricted manner [13,14,15]. The transcriptional factor Eomes (a T-box transcription factor critical for the development of functional natural killer (NK) and CD8 T cells), T-bet, and Blimp1 are critical for inducing the expression of cytotoxicity-associated genes in CD4-CTLs [16,17,18]. Similar to CD8 T cells, CD4-CTLs display increased Runx3 but reduced ThPOK expression [19,20,21,22]. Furthermore, opposite to Tfh cells, CD4-CTLs express low levels of Bcl6 but high levels of Blimp-1 [23]. Two important markers for CD4-CTLs are CRTAM [24] and NKG2C/E [25]. It has been shown that the cytotoxicity executed through CD4-CTLs becomes effective when CD8-CTL activity is impaired during infections in association with virus escape strategies [14,15]. Interestingly, more CD4-CTLs have been identified in severe COVID-19 patients than in mild symptomatic patients [26], suggesting a possible inefficient CD8 response in viral control in patients with severe disease, which is an important question that needs to be tested in the future. In addition to protecting the host from viral infections, CD4-CTLs are also involved in anti-tumor responses as well as in various autoimmune conditions [19,27,28]. Strikingly, CD4-CTLs are increased in people of exceptional longevity [29]. The development and functions of CD4-CTLs have been recently reviewed [15]. In consideration of the scope of this review, we will not further discuss this interesting population below in great detail.
Among the CD4 T subsets possessing helper functions (Figure 1), Th1 cells induce type 1 responses to protect against intracellular pathogens such as bacteria, viruses, and protozoa by activating type 1 macrophages (M1) [5]. Th2 cells are involved in type 2 responses to fight infections by parasites such as helminths by activating type 2 microphages (M2), as well as the recruitment of eosinophils, basophils, and mast cells to the sites of infections [30]. Th17 cells mediate type 3 responses for the clearance of extracellular bacteria and fungi by orchestrating sustained neutrophil recruitment and an induction of antimicrobial peptide production by epithelial cells of barrier tissues such as intestines, lungs, and skin [31]. Tfh cells are found to be important in helping B cells make antibody responses by promoting germinal center formation, affinity maturation, and possibly immunoglobulin class switch recombination [32], although Tfh-independent antibody responses have also been reported [33]. Treg cells are critical for the preservation of immune tolerance and involved in preventing autoimmune diseases [34,35].
Innate lymphoid cells (ILCs) [7,36,37] that do not express antigen receptors have been recently identified as the counterparts of T helper subsets within the innate arm of the immune system. While progenitors of ILCs are found both in bone marrow and tissue, mature ILCs are largely tissue-resident [38]. Therefore, ILCs can act earlier and more quickly than CD4 T cells during an immune response and thus provide the first line of protection against pathogens through a rapid and local secretion of effector cytokines, which were previously thought to be largely produced by CD4 T helper cells. Similar to the classification of CD4 T effector subsets into three major subsets, Th1/Th2/Th17 cells, ILCs can also be categorized into three major groups (Figure 1), which produce similar effector cytokines and lineage-defining transcription factors. Thus, group 1 ILCs (ILC1s) secret IFN-γ and express T-bet; group 2 ILCs (ILC2s) secret IL-5 and IL-13, and express GATA3; group 3 ILCs (ILC3s) secret IL-17 and IL-22, and express RORγt [7]. Functionally, group 1 ILCs, similar to Th1 cells, may participate in defending intracellular pathogens, such as bacteria, viruses, and protozoa; group 2 ILCs, just as Th2 cells, respond to large extracellular pathogens, such as parasites and allergens; group 3 ILCs, like Th17 cells, combat extracellular microbes, such as bacteria and fungi [37]. ILCs, similar to Th cells, are also involved in many inflammatory diseases including autoimmunity, allergy, and asthma [39]. It is worth pointing out that within the RORγt-expressing ILCs, there is a lymphoid tissue inducer (LTi) population, and they are involved in the formation of lymphoid structures as well as in host defense [40]. Recently, ILCs were also found to be involved in non-immunological functions including tissue repair, metabolism regulation, and immune-neuronal interactions [36].
A proper and effective response to a particular microorganism invasion through the activation and differentiation of T helper subsets and ILCs protects host from that pathogen without causing excessive inflammation that damages host tissues. However, when an immune response is out of balance, either too weak or too strong, it can cause chronic infection or inflammatory diseases [5,6,32,41,42]. Inappropriate immune responses to harmless environmental agents or self-antigens may also lead to inflammation, allergy, and autoimmunity, such as inflammatory bowel disease (IBD). Immunodeficiency with reduced ILC and CD4 T cell numbers and/or diminished ILC and CD4 T cell functions can result in insufficient immune response, which may cause chronic or repeated infections. In fact, in human immunodeficiency virus (HIV)-infected patients, the CD4 T cell number is drastically reduced, which is the main cause of acquired immunodeficiency syndrome (AIDS). X-linked severe combined immune deficiency (SCID) patients have mutations in the IL2RG gene [43], which results in a lack of T cells and ILCs; these boys suffer from severe infections of bacteria, viruses, and fungi, and they do not survive beyond infancy without treatment. In this review, we will discuss the differentiation and functions of major T helper subsets and their involvement in host defense and diseases in the context of ILCs.
2. Th1 Cells and Related Diseases
2.1. Th1 Cells
The Th1/Th2 dichotomy was first proposed by Robert Coffman and Tim Mosmann in 1986 when they reported that CD4 T helper cell clones from mice can be divided into two distinct types based on their cytokine production profile [44]. Since then, the definition of a unique lineage has expanded to the expression of lineage-specific master transcription factors, cell surface markers, as well as transcriptomes and epigenomes, which is reflected by different epigenetic modifications to a certain degree [5].
Upon TCR activation in a particular cytokine milieu, naïve CD4 T cells can differentiate into Th1 cells. IL-12 secreted by APCs activates the transcription factor STAT4, and IFN-γ produced by NK cells and/or T cells themselves activates another transcription factor, STAT1; both STAT1 and STAT4 activation are capable of inducing the expression of the Th1-inducing master transcription factor T-bet [45,46]. T-bet, by cooperating with Hlx [47], Runx3 [48,49], Ets-1 [50], and Bhlhe40 [51], promotes IFN-γ production. While T-bet together with Runx3 may directly repress IL-4 transcription, T-bet also inhibits the expression of other master transcription factors including GATA3 and RORγt [7,45,52], thereby antagonizing Th2 and Th17 cell differentiation.
While Th1 cells can directly differentiate from naïve CD4 T cells, they can also derive from other T helper CD4 subsets, including Th17, Treg, and Tfh cells as a result of CD4 T cell plasticity [5,53,54]. Differentiated Th1 cells are capable of producing Th1 signature cytokine IFN-γ, which activates and/or stimulates other immune cells, including CD8 T cells, ILC1s, macrophages, and B cells during the process of eliminating pathogens [6]. An important chemokine receptor expressed by Th1 cells is CXCR3, which plays an important role in Th1 cell migration toward the inflammation sites with pathogen invasion, and it is also widely used for the identification of human Th1 cells [55].
In addition to Th1 cells, three other kinds of lymphocytes (ILC1s, CD8 T cells, and NK cells) are also involved in type 1 immunity [6]. ILC1s, probably by producing IFN-γ, may participate in immune responses to the infection of protozoa and viruses [56,57]. However, the relative importance of IFN-γ production by ILC1s or NK cells during infection remains unclear partly because of the lack of reliable ILC1-deficient models. It has been recently reported that ILC1s are essential for limiting early viral replication, which cannot be compensated by NK cell-mediated anti-viral effects in response to mouse cytomegalovirus infection of hepatic cells [57]. However, the transcription factor Zfp683 (Hobbit), which appeared to be specifically required for ILC1 development, may also have important functions in other immune cells including NK, CD8 T, and Th1 cells. Whether IFN-γ produced by ILC1s plays a role in promoting Th1 cell differentiation is currently unknown.
Whole genome analyses of Th1 cells generated both in vitro and in vivo, including RNA-Seq for assessing gene expression, ChIP-Seq of histone modifications, and transcription factor binding as well as ATAC-Seq (and/or DNase-Seq) for analyzing epigenetic status of specific gene loci, have not only confirmed the previously known Th1-related genes but also identified many more Th1-specific genes, which may help understand the mechanisms of Th1 cell differentiation as well as the heterogeneity and functions of Th1 cells after their differentiation [58,59,60,61].
2.2. Th1-Related Diseases
In addition to an essential protective effect in host defense, Th1 cells also play important roles in (or are associated with) different types of autoimmune diseases, such as rheumatoid arthritis, multiple sclerosis, Hashimoto thyroiditis, insulin-dependent diabetes mellitus, autoimmune gastritis, and other chronic inflammatory disorders, including inflammatory bowel diseases, atherosclerosis, and sarcoidosis [62]. Since Th1 cells are responsible for orchestrating type 1 immunity against intracellular pathogens, deficiencies in the generation and functions of Th1 cells may result in chronic and/or recurrent infections. However, no monogenic mutations only related to Th1 cells have been found [5]; this may reflect a complex connection and interaction network within our immune system.
Serving as the master regulator for Th1 cell differentiation and IFN-γ production, T-bet has recently been reported to be deficient in some patients with mycobacterial disease [63]; these patients have reduced IFN-γ production by most of the innate-like lymphocytes including NK, invariant NKT (iNKT), mucosal-associated invariant T (MAIT), and Vδ2+ γδ T cells. Interestingly, while non-mycobacterial reactive classic Th1 cells produced less IFN-γ, normal IFN-γ response was detected in mycobacterial reactive non-classic CCR6+ Th1 (Th1*) cells, indicating that T-bet-independent Th1-related response may be elicited and/or preserved in a situation where innate and classic adaptive IFN-γ response is inadequate. Μutations in many other genes that are related to Th1 cell differentiation and/or functions, such as IFNGR1, IFNGR2, STAT1, IL12RB1, IL12B and NEMO, have also been found in patients [64]. The vast majority of these abnormalities result in susceptibility to infections with mycobacterial species.
Since there is a similarity in the development and regulation of ILC1s, NK, and Th1 cells, some gene mutations that affect Th1 cell differentiation may also have an impact on the generation and functions of other type 1 lymphocytes. Indeed, T-bet deficiency has a broad impact on multiple lymphoid lineages in either development/maturation or IFN-γ production [63]. Th1 cells may also be involved in tumor immunology. Interestingly, the epigenetic silencing of Th1-type chemokines has been reported as a novel immune-evasion mechanism of tumors [65]; this indicates that a selective epigenetic reprogramming may enhance the clinical efficacy of cancer therapy.
3. Th2 Cells and Related Diseases
3.1. Th2 Cells
Th2 cells are defined as IL-4-producing T cells when the Th1/Th2 model was proposed. Th2 cells are mainly involved in host defense against large extracellular pathogens, such as helminths. Naïve CD4 T cells after receiving signals from DCs may differentiate into Th2 cells after their activation [30]. IL-2 and IL-4 are two important cytokines during Th2 cell differentiation, particularly in vitro. IL-2 and IL-4 activate STAT5 and STAT6, respectively, and the latter induce the expression of the Th2 master transcription factor GATA3 in activated CD4 T cells [5]. IL-4-independent Th2 cell differentiation may occur in vivo [66]; however, GATA3 is absolutely required for Th2 cell differentiation and functions both in vitro and in vivo [67,68]. GATA3 not only plays an essential role in inducing Th2 cell differentiation, it also represses the expression of other lineage transcription factors, such as T-bet and RORγt [58,69,70]. In addition to GATA3, other transcription factors including c-Maf [71], STAT3 [72], and Notch/CSL [73] may also regulate Th2 cell differentiation and functions.
Differentiated Th2 cells secret lineage cytokines including IL-4, IL-5, and IL-13. During a classic type 2 immune response, IL-4 promotes B cell antibody class switching to immunoglobin E (IgE), IL-5 recruits eosinophilia to the inflammation sites, and IL-13 promotes mucus production and goblet cell hyperplasia [30]. These cytokines can also be produced by ILC2s (although IL-4 production by ILC2s is somewhat limited), and they work together to eliminate invading pathogens [74]. Th2 cells and ILC2s also express chemokine receptors on the cell surface, including CCR3, CCR4, and CCR8, to receive migration signals from ligands (CCL11 for CCR3, CCL17 and CCL22 for CCR4, and CCL1 for CCR8) secreted by epithelial cells [75,76,77]. In addition to recruiting Th2 cells and ILC2s, these chemokines can also recruit other cells, such as DCs, eosinophils, basophils, and mast cells to the damaged epithelial sites caused by helminth infection or protease allergens [30,78]. Human Th2 cells also express CRTh2, which serves as a reliable marker for identification of human Th2 cells [79], although its expression in mouse Th2 cells has not been clearly demonstrated. Instead, T1/ST2 (an IL-33 receptor subunit) has been widely used to identify Th2 cells and ILC2s in mice [80,81].
Collaboration and interactions between the ILCs and Th cells of the same class during immune responses have been reported with the relationship between ILC2s and Th2 cells being the most certain one [30]. During a type 2 immune response, ILC2s and Th2 cells may crosstalk to each other through direct and indirect mechanisms (Figure 2). After activation by inflammatory cytokines, such as IL-33, IL-25, and TSLP, ILC2s, by secreting IL-13, may promote the migration of DCs to draining lymph nodes to induce Th2 cell differentiation [82]. Interestingly, some ILC2s also express MHCII, through which they may directly present antigens to Th2 cells [83]. On the other hand, Th2 cells by secreting IL-2 may expand ILC2s. Furthermore, by secreting IL-4 and IL-13, Th2 cells can induce/enhance the production of ILC2-activating inflammatory cytokines by epithelial cells, forming a positive feedback loop at the cellular level [84,85]. Since ILC2s and Th2 cells have similar effector functions during type 2 immune responses and both can be activated by IL-33, the relative abundance of one population over the other at a particular stage may determine the relative importance of these cells at that moment [86].
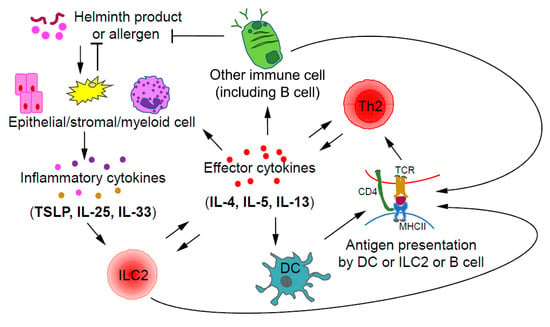
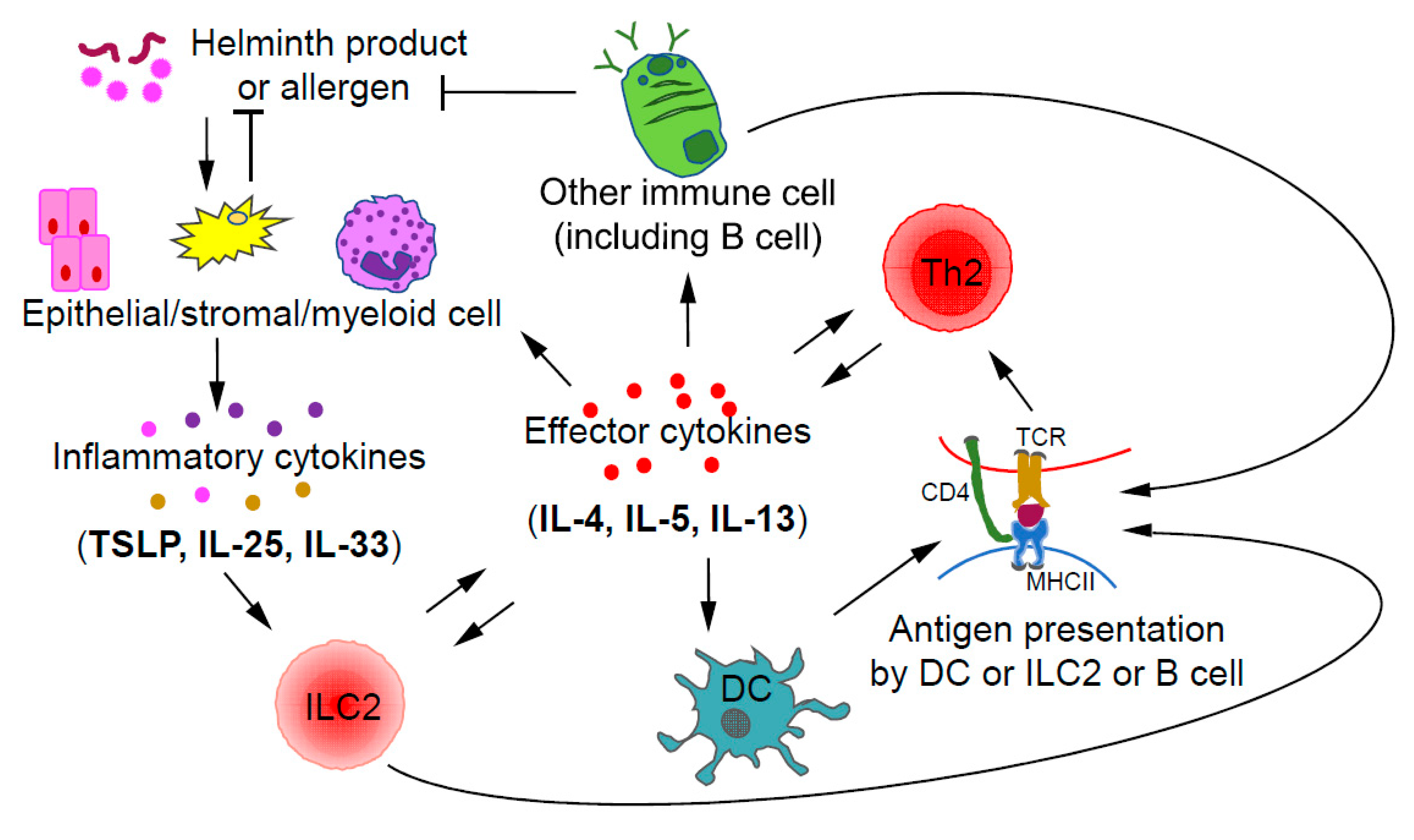
Figure 2.
Crosstalk between ILC2s and Th2 cells during type 2 immune responses. Upon stimulation by helminth products or allergens, epithelial and/or stromal cells may produce inflammatory cytokines including IL-33, IL-25, and TSLP, which are capable of activating ILC2s. By producing type 2 effector cytokines, such as IL-4 and IL-13, ILC2s may promote the migration of dendritic cells (DCs) into draining lymph nodes to induce Th2 cell differentiation. Some ILC2s also express MHCII, which allows them to directly interact with Th2 cells through antigen presentation. Thus, ILC2s could promote the further differentiation of early Th2 cells; on the other hand, Th2 cells may expand ILC2s through IL-2 production. Effector cytokines including IL-4 and IL-13 produced by Th2 cells, may also indirectly induce the expansion of ILC2s through stimulating epithelial cells to produce ILC2-activating inflammatory cytokines such as IL-25 and TSLP.
The heterogeneity of Th2 cells, particularly in vivo, has been documented [87]. In fact, even GATA3+T-bet+ and GATA3+RORγt+ CD4 T cells have been found during type 2 immune responses [88,89,90]. Such an appearance of mixed phenotype cells is possibly due to CD4 T cell plasticity [91]. Whole genome analyses of Th2 cells have also identified a collection of Th2-specific genes, many of which are shared by their expression in ILC2s. The investigation of these molecules may help further understand the mechanisms of Th2 cell differentiation and ILC2 development as well as their unique and shared functions during helminth infection and allergic responses [58,70,92,93].
3.2. Th2 Related Diseases
Th2 cells play a central role in type 2 immune responses, and together with ILC2s, Th2 cells defend against large parasites such as helminths [30]. On the other hand, the dysregulation of type 2 immune response often results in pathologic responses [30,94]. In addition to some well-known large parasites such as helminths, other stimulus derived from viruses, bacteria, and other nonmicrobial products including food allergens [95], venoms [96], interior allergens (such as house dust mites, HDM) [97], pollen allergens [98] and vaccine adjuvants (Alum, MF59) [99], may also cause allergic inflammation including asthma, atopic dermatitis, rhinitis, food allergies, and eczema. GATA3, as the master transcription factor for Th2 cells, plays an essential role in Th2 cell differentiation and functions [68]. In mice, Gata3 germline deficiency results in embryonic lethality [100]. It is probably true in humans as well. Therefore, only GATA3 heterozygous mutations that cause a haploinsufficiency of GATA3 in the hypoparathyroidism, deafness, and renal dysplasia (HDR) patients have been reported [101]. Th2 cell differentiation was reported to be partially defective in these patients’ T cells; however, they do not show any major immunologic abnormalities [102]. Consistently, another report showed that the differentiation of HDR patient T cells into Th2 cells is normal [103]. However, since ILC2 development is sensitive to the dose of GATA3 expression [104], whether the HDR patients have reduced ILC2 numbers and thus reduced ILC2-mediated immune responses remains to be tested.
RAG1 or RAG2 hypomorphic mutations are responsible for Omenn syndrome, which is an autosomal recessive disease of neonates characterized by erythroderma, chronic diarrhea, hepatosplenogaly, and lymphadenopathy [105,106]. Elevated numbers of Th2 cells, an overproduction of IL-4 and IL-5, and elevated serum IgE as well as eosinophilia were found in some of these patients. This is possibly because a partial RAG deficiency results in a limited T cell repertoire, which may lead to a Th2-biased disease. Consistent with this idea, transferring low numbers of mouse T cells (thus a reduced T cell repertoire) into a lymphopenic host also causes Th2-mediated diseases [107].
4. Th17 Cells and Related Diseases
4.1. Th17 Cells
Th17 cells are important for type 3 immune responses in defending extracellular bacteria and fungi invasion; they are also found to be involved in many autoimmune diseases, such as multiple sclerosis, because of their pathogenic effects [108]. Several key reports on the role of IL-23 versus IL-12 in autoimmunity in 2003 [109,110,111] lead to the discovery of Th17 cells in 2005 [112,113,114]. Although the discovery of Th17 cells is much later than the identification of Th1 and Th2 cells, research on this unique CD4 subset progresses rapidly. Interestingly, more regulators have been identified for Th17 cell differentiation than for Th1/Th2 cell differentiation [115,116]. Products of extracellular bacteria and fungi can activate antigen-presenting cells to produce pro-inflammatory cytokines IL-1β, IL-6, and IL-23, which in turn direct naïve CD4 T cells to differentiate into Th17 cells [6]. IL-6 and IL-23 activate STAT3 to induce the expression of Th17 master transcription factor RORγt [117,118]. In addition, TCR signaling can induce RORγt expression through the NFAT/NF-κB/AP-1 pathway [5]. RORγt, by collaborating with other transcription factors such as IRF4, BATF, and Runx1, promotes the expression of Th17 lineage-specific genes [5,119]. IL-6 and IL-21 together with TGF-β are involved in Th17 cell differentiation in vitro [5], although the TGF-β-independent pathway for Th17 cell differentiation has also been reported [120]. Differentiated Th17 cells are capable of secreting effector cytokines, such as IL-17A, IL-17F, and IL-22, to activate nonimmune and immune cells to produce matrix metallopeptidases (MMPs), nitric oxide (NO), cytokines, and antimicrobial peptides to clear extracellular pathogens [6,112,114,121,122]. In addition, CXCL8 and G-CSF produced by Th17 cells and other nonimmune cells recruit neutrophils to the inflammation sites to kill pathogens [123]. Th17 cells express a signature chemokine receptor CCR6 (inflamed tissues homing receptor and essential for homeostasis) [124], which is also considered as a unique marker in identifying human Th17 cells. In combination with CXCR3, CCR6 has been used to distinguish Th17 cells (CXCR3-CCR6+) from Th1 cells (CXCR3+CCR6-) and Th1* cells (CXCR3+CCR6+) [125,126].
ILC3s are also involved in type 3 immune responses [37]. IL-22 production by ILC3s is important for host defense against extracellular bacteria [127]. IL-22 induces the production of antimicrobial peptides by epithelial cells. In addition, ILC3-derived IL-22 may induce the expression of serum amyloid proteins, which promote IL-17 production by Th17 cells [128]. On the other hand, Th17 cells may silence IL-22 production by ILC3s through controlling commensal bacteria; the sequential activation of ILC3s and Th17 cells has been noted during normal development, while steady-state commensalism is being established in the host, and in the absence of Th17 cell differentiation, ILC3s remain constitutively active, leading to an impaired lipid metabolism in the gut [129]. Therefore, there is a crosstalk between ILC3s and Th17 cells in maintaining gut homeostasis.
4.2. CD4 T Cell Plasticity
Although cell plasticity has been found in almost all CD4 T cell subsets, Th17 cells are the most flexible in altering their phenotypes [53,130]. Now, many current studies focus on Th17 cells’ dichotomous nature (protective and pathogenic effects), plasticity, and its application in therapy [31]. Various important extracellular and intracellular regulators of Th17 cells have already been found [119]. There are several regulators, such as IL-23 [131], CD5L [132], and REV-ERBα [133], that have been found recently to be involved in regulating Th17 dual functions. Although the detailed mechanism behind their dichotomous nature is still unclear, it may have something to do with Th17 cell plasticity being influenced by the environment. Indeed, several environmental factors, such as sodium chloride [134], microbiota [135,136,137], and angiogenesis [138], may regulate the generation of pathogenic Th17 cells. In terms of cell plasticity, Th17 cells were reported to be unstable both in vitro and in vivo, and they may become Th1-like cells [53,139]. Glutamine metabolism was found to be important in regulating Th17 versus Th1 cell differentiation [140,141]. CK2 was reported to control Th17 and Treg cell differentiation through inhibiting FoxO1 [142]. Recently, it has been found that metabolic heterogeneity may underlie reciprocal fates of Th17 cell stemness and plasticity [143]. In a mouse model of autoimmune disease, it was found that Th17 cells are functionally and metabolically heterogeneous and contain two different subsets: one subset with stemness-associated features but lower anabolic metabolism, and another subset with higher metabolic activity that supports trans-differentiation into Th1-like cells. This study was also supported by another study, which reported a metabolism difference related with the degree of Th17 cell plasticity [144]. Since IL-12 and IL-23 are responsible for converting Th17 cells to Th1-like cells [52,53,145], the relationship between the production of IL-12/IL-23 and metabolic regulation requires further investigation.
4.3. Th17-Related Diseases
As discussed above, Th17 cells contribute to immunity against extracellular bacteria and fungi and the pathogenesis of multiple chronic inflammatory diseases [5,66]. Patients with mutations in the RORC (which encodes RORγt) gene suffer from chronic candidiasis consistent with a lack of Th17 cells in these patients [146]. Interestingly, these patients are also susceptible to mycobacterium infection with reduced IFNγ response. Whether this is due to a defect in thymic development in these patients (RORγt also plays an important role in T cell development) or due to a blockage of the development of Th1 cells from a transient Th17 stage requires further investigation. STAT3, as the gene encoding a key transcription factor for Th17 cell differentiation, was found to be mutated in many patients with hyper-IgE syndrome, which is a primary immunodeficiency disorder [147,148,149,150]. These patients have heightened susceptibility to be infected by bacteria, such as Staphylococcus aureus, Streptococcus pneumoniae, Candida albicans, and other fungi due to impaired Th17 cells. It was also found that mutations in DOCK8 may result in hyper-IgE syndrome [151,152], and patients with this form of mutation have a deficiency in Th17 cells. It was reported that mutations that take place in the Src homology 2 (SH2)-/tail segment (TS)-/transactivation (TA)-/DNA binding-domain of STAT1 result in loss-of-function in Th1, and patients with this form of mutation display increased susceptibility to mycobacteria and viruses [153,154]. However, mutations that take place in the coiled-coil domain of STAT1 cause enhanced Th1 but reduced Th17 cell-mediated responses; patients with this form of mutation suffered from chronic mucocutaneous candidiasis [155,156]. Mechanistically, this mutation within the coiled-coil domain of STAT1 reduces the dephosphorylation of activated STAT1 and thus increases the level of phosphorylated STAT1 in the nucleus [155]. As a result, the dominance of activated STAT1 causes an overproduction of IFN-α/β, IFN-γ, and IL-27, which induce the STAT1-mediated inhibition of Th17 cell differentiation. It has also been reported that two different genetic etiologies of chronic mucocutaneous candidiasis diseases are associated with autosomal recessive deficiency in the cytokine receptor IL-17RA and autosomal dominant deficiency in IL-17F, respectively [157]. In addition, due to the important role of IL-1β during Th17 cell differentiation and/or stabilization [117,158], mutations in IL-1β were found to cause several autoinflammatory disorders [159].
Given that Th17 cells are closely associated with multiple inflammatory and autoimmune disorders, targeting these cells or their effector cytokines is considered to be an effective way for disease treatment. For example, antibodies against IL-12p40 (the common subunit of IL-12 and IL-23) have been successfully used in treating psoriasis [160,161]. Similarly, anti-IL-17A/F, anti-IL-17RA, and RORγt inhibitors have been used in treating different types of autoimmune diseases [162,163].
5. Treg Cells and Related Diseases
5.1. Treg Cells
It has been long speculated that a specialized population of lymphocytes in mammals can perform an immune regulatory function. However, no one was able to provide strong evidence for the existence of these cells until a breakthrough report in 1995, in which Sakaguchi and his colleagues described that a subset of CD4 T cells marked by CD25 expression are essential for the maintenance of self-tolerance [164]. Another groundbreaking discovery came in 2003, when three groups independently identified Foxp3 as the master transcription factor for Treg cells [165,166,167].
Regulatory T cells can develop in the thymus during negative selection, and these Treg cells are called thymic-derived Treg (tTreg) cells [168]. tTreg cells, by recognizing self-antigens, mainly control self-reactive effector T cells that escaped negative selection to maintain immune tolerance [169]. Treg cells can also be induced in vitro from naïve CD4 T cells, and they are now called inducible Treg (iTreg) cells [170,171,172,173,174]. In contrast, Treg cells differentiated from naïve CD4 T cells in vivo outside of the thymus are now named as peripherally induced Treg (pTreg) cells. The differentiation of pTreg cells, especially in the gut [175], is promoted by retinoic acid in the environment and metabolites produced by microbiota [176,177]. Compared with tTreg cells, pTreg cells not only recognize self-antigens, but also recognize non-self-antigens including food antigens and innocuous commensal microbiota-derived antigens; therefore, they are important for the maintenance of mucosal tolerance [178]. Several markers, including Helios and neuropilin (Nrp), have been proposed to distinguish pTreg cells from tTreg cells [179,180]; however, these molecules can also be expressed by some pTreg cells, indicating that they may simply mark more differentiated Treg cells. Similarly, it has been proposed that tTreg cells are more stable than pTreg and iTreg cells, but this could also be explained by the more differentiated status of tTreg cells compared to other Treg cells [181,182]. In the gut, RORγt-expressing Treg cells may represent pTreg cells, whereas GATA3-expressing Treg cells could be largely tTreg cells [183,184].
Cytokines, especially IL-2 and TGF-β, play essential roles during the development and differentiation of Treg cells. IL-2 and TGF-β promote Foxp3 expression by activating STAT5 and SMAD2/3, respectively [185,186,187]. TCR signaling is also important and it induces Foxp3 expression through the activation of the NF-AT/NF-κB/AP-1 pathways [170].
In addition to Foxp3, many other transcription factors, such as Runx1 [188], Nrfa2 [189], Foxo family members [190], SATB1 [191], and Helios [179,192], can regulate the development and functions of Treg cells. Multiple mechanisms through which Treg cells exert their regulatory functions have been proposed: by secreting regulatory cytokines (such as IL-10, TGFβ, and IL-35) [193]; by consuming IL-2, which is important for effective CD4 T cell survival and proliferation [194,195]; by expressing cell surface receptors that transmit negative signals (such as CTLA-4, CD39, CD73) [34,196]; by expressing perforin and granzyme B to directly kill APCs [197]; and by inducing trogocytosis to strip off the antigen-MHCII complexes from APCs in an antigen-specific manner [198].
5.2. Treg Related Diseases
Since Treg cells are essential for the maintenance of immunological tolerance and Foxp3 is the master transcription factor for Treg cells, mutations in the Foxp3 gene in mice or in the FOXP3 gene in humans lead to severe autoimmune diseases and death [199,200,201]. Since the FOXP3 is located in the X chromosome, boys carrying a deleterious FOXP3 mutation will develop immunodysregulation polyendocrinopathy enteropathy X-linked (IPEX) syndrome. The syndrome of IPEX often includes insulin-dependent diabetes, elevated serum IgE with aspects of both eczema and psoriasis, and some other autoimmune symptoms include hypothyroidism, anemia, thrombocytopenia, and neutropenia. Hemizygous male mice with a mutant Foxp3 (scurfy mice) develop lethal multi-organ autoimmunity and die 16–25 days after birth. Some FOXP3 mutations cause less severe diseases. For example, patients with a point mutation in the domain swap interface of FOXP3 protein develop a Th2-related disease [202]. In addition to Foxp3 mutations, deficiency in other regulators of Treg-suppressive functions, such as TRAF3 [203], CD28 [204], Id2/Id3 [205], Ubc13 [206], Ndfip1 [207], NF-κB p65 [208], Helios [209], Th-POK/LRF [210], EZH2 [211], BACH2 [212,213], SATB1 [214], IRF-4 and Blimp-1 [215], and LKB1 [216], can also result in severe autoimmune diseases, although most of these studies were carried out in mice, and their relevance in human diseases remains to be tested.
6. Tfh Cells and Related Diseases
6.1. Tfh Cells
Tfh cells were noticed about twenty years ago but only well accepted as a distinct lineage of CD4 T cells required for the formation of germinal centers (GCs) when its master transcription factor Bcl6 was identified [217,218,219]. Tfh cells are mainly found in the B cell follicles in lymph nodes and spleen, so they may help B cells in antibody production and affinity maturation [220]. How important Tfh cells are for antibody class switching remains to be tested, since antibody class switching happens largely outside of GCs [221]. Similar to the differentiation of other CD4 T cell subsets, Tfh cell differentiation from naïve CD4 T cells is also tightly regulated by TCR signaling and cytokine milieu [32]. Possibly because of an essential role of the interaction between antigen-specific B cells and pre-Tfh cells during Tfh cell development, it is difficult to generate Tfh cells in vitro [222,223]. Nevertheless, IL-6 and IL-21 by activating STAT3 were found to induce Tfh cell differentiation. IL-6 together with TCR signaling is involved in the initiation of Tfh cell differentiation by inducing an early wave of Bcl6 expression [218,224]. IL-21 was also reported to be involved in Tfh cell differentiation [225]. Unlike IL-6 and IL-21, IL-2 [219,226] plays a negative role during Tfh cell differentiation by promoting the expression of Blimp-1, which inhibits Bcl6 expression. By producing cytokines or direct cell-cell contact, both DCs and B cells play an essential role in promoting Tfh differentiation and migration [227]. After the activation of naïve CD4 T cells in the T cell zone by DCs, these activated T cells may migrate to the T-B border through upregulating CXCR5 and downregulating CCR7 expression; with further upregulation of CXCR5 expression after in contact with B cells, Tfh cells continue to migrate into GC to become GC Tfh cells [228]. In addition to CXCR5, CCR7, PSGL1, and S1P receptors that are involved in cell migration, SLAM family receptors and integrins are also involved in regulating Tfh cell functions [32]. In addition, other regulators, including ICOSL [222], Ascl2 [229], Itch [230], VHL [231], IL-37 [232], HIF-1α [233], COX-1 [234], Th-POK [235], and Tox2 [236] are also found to be important for Tfh cell differentiation and functions. Tfh cells provide help to GC B cells in two aspects: one is to promote GC B cell maturation, and the other is to promote antibody production. CD40L expressed on Tfh cells is required for inducing the formation of high-affinity plasma cells and memory B cells [237,238]. Signature cytokine IL-21 secreted by Tfh cells may control the maintenance and optimal affinity maturation of GC responses [220,239,240].
6.2. Tfh Subsets
Although the main cytokine produced by Tfh cells is IL-21, it was found that Tfh cells can also secret cytokines that are produced by Th1, Th2, and Th17 cells; therefore, Tfh cells can be classified into Tfh1, Tfh2, and Tfh17 cell subsets [241]. Recently, it has been reported that a new subset of Tfh, which is referred to as Tfh13 cells, have an unusual cytokine profile (IL-13hi/IL-4hi/IL-5hi/IL-21lo) and co-express Bcl6 and GATA3 [242]. This subset is different from Tfh2, cells which are Bcl6/BATF co-expressing IL-4hi/IL-21hi Tfh cells. Interestingly, these Tfh13 cells are required for the production of high- but not low-affinity IgE and for subsequent allergen-induced anaphylaxis. Similarly, although class switching occurs infrequently in the germinal centers [221], IFNγ-producing cells with a history of T-bet expression have been found in the germinal centers [61]. Therefore, it is possible that germinal center IFNγ-producing Tfh cells may play an important role in the production of high-affinity IgGs.
It is likely that during activation, naive CD4 T cells may incorporate several layers of fate decisions over time and space at the stages of priming and reinforcement/terminal differentiation [4]. During priming, there are two signals essential for naïve CD4 T cells fate determination: signals downstream of TCR/co-stimulation, which affect non-Tfh versus Tfh cell fate decision, and signals downstream of cytokine receptors, which influence T helper subset decision (e.g., Th1, Th2, or Th17). Therefore, high and low TCR stimulation, which cause a differential pattern of CD25/IRF4/Blimp-1/Bcl6/CXCR5 expression, will result in non-Tfh or Tfh cell fate, respectively. Meanwhile, specific cytokine signals triggered in a specific inflamed environment may lead to the expression of the transcriptional networks that control a specific effector program (e.g., Th1/Th2/Th17 or Tfh1/Tfh2/Tfh17). During reinforcement/terminal differentiation, non-Tfh Teff cells migrate to different tissues depending on the chemokine receptors they express and produce corresponding cytokines, whereas Tfh cells migrate to the T-B border and GC for further maturation and to give help to B cells. As a result, these bifurcated parallel axes allow CD4 T cells to rapidly and effectively augment their particular effector programs in host defense [4]. Since the Tfh and non-Tfh Teff cell differentiation programs may antagonize each other, the fate determination of a particular Tfh cell subset may involve several sequential differentiation steps [223]. Alternatively, the transient expression of non-Tfh Teff cell-associated transcription factors during early Tfh cell differentiation may epigenetically program the fate of a specific subset of Tfh cells [61].
6.3. Tfh Related Diseases
Since Tfh cells are critical for the generation of effective long-lived protective antibody responses, the dysregulation of Tfh functions has been noted in various diseases such as inflammation, autoimmunity, allergy, and cancer [32]. Diseases caused by Tfh cells can be divided into two distinct types: autoimmune diseases due to an overabundance of Tfh cells and immunodeficiencies due to a defect in the generation and functions of Tfh cells. One typical representative of autoimmune diseases is systemic lupus erythematosus (SLE), and these patients are found to have increased frequencies of Tfh-like cells in peripheral blood and an amplification of pathogenic autoantibodies [243,244]. Sjogren’s syndrome (SS) [245], juvenile dermatomyositis [246], and rheumatism [247] are other common autoimmune diseases related to Tfh cells. For immunodeficient diseases, mutations of genes, such as STAT3 [248], CD40L [249,250], and ICOS [251], which are important for Tfh cell differentiation and functions, were found in immunodeficient patients with reduced numbers of Tfh cells. Given the important role of Tfh cells in helping B cell antibody production, enhancing Tfh cell-associated biology is being considered for effective vaccination, whereas targeting Tfh cells aiming to reduce antibody production could be a base for therapeutic interventions in some human autoimmune diseases.
7. Concluding Remarks
In order to protect a host from the invasion of various pathogens, the immune system has evolved a series of mechanisms to respond rapidly and effectively. CD4 T helper subsets mediate effective adaptive immune responses particularly when innate immune responses fail to eliminate pathogens; CD4 T cell responses also generate memory, which can quickly respond to the second infection of the same or similar pathogens. Different types of adaptive immune responses are executed by Th1/Th2/Th17 cells, respectively, with each subset of T helper cells possessing their unique properties in producing their signature cytokines. Interestingly, before adaptive responses take place, innate lymphoid cells, which can also be divided into ILC1/ILC2/ILC3 subsets, may provide early protection and allow time for adaptive responses, whereas memory and/or memory-phenotype CD4 T cells, by possessing some similar features of ILCs (i.e., a completed differentiation process allowing a rapid response, capable of responding to inflammatory cytokines and tissue residency), may behave similarly as ILCs, to a certain extent, during a subsequent infection [252,253].
Th1, Th2, and Th17 cells can be categorized as non-Tfh Teff cells in parallel with Tfh and Treg cells, since both Tfh cells and Treg cells may be further divided into Th1-, Th2-, and Th17-like subpopulations [7,61,183,254]. Cell plasticity may exist between non-Tfh and Tfh cells, and between Teff cells and Treg cells [130,255,256]. However, even within Teff cells, particularly for Th17 cells, cell plasticity has been well documented. Th1-like Th17 cells have been found during infections and in autoimmune settings. The heterogeneity and plasticity of CD4 T cell subsets are largely determined by the temporal and quantitative co-expression of multiple master transcription factors during the differentiation of these cell subsets [7]. Between non-Tfh and Tfh cells, their balance may determine the magnitude of cellular versus humoral responses. In contrast, the balance between Teff and Treg cells reveals the yin and yang sides of the immune system. Understanding such balances is not only important for gaining our knowledge in basic immunology in infections and inflammations, but also for providing great opportunities in treating different types of immunological diseases. In fact, more and more attention is being paid to Treg-based therapies [168], because Treg cells play critical roles in anti-inflammation, autoimmunity prevention, and cancer promotion.
With the rapid development of modern technologies, such as single-cell RNA sequencing [257], CRISPR (clustered regularly interspaced short palindromic repeats)-mediated genome editing [93,258], Cryo-EM (cryogenic electron microscopy) structure analysis [259], single-cell mass spectrometry [260], and immunoimaging [261,262,263], many important findings are being made in the identification, development, and regulation of new CD4 T cell and ILC subsets, as well as their relationship and crosstalks in diseases. In the future, it is also important to investigate how CD4 T cell memory for each subset is formed and maintained [264] and whether the plasticity of antigen cross-reactive CD4 T cell memory cells may shape the primary response to a new infection [265]. With exciting new findings in basic immunology, our better and deeper understanding of this powerful system will help us uncover innovative measures for diseases treatment, thereby improving human health.
Author Contributions
X.Z. prepared the initial draft; X.Z. and J.Z. revised and finalized the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work is supported by the Division of Intramural Research of the NIAID (1ZIAAI001169, US National Institutes of Health).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Pennington, D.J.; Vermijlen, D.; Wise, E.L.; Clarke, S.L.; Tigelaar, R.E.; Hayday, A.C. The integration of conventional and unconventional T cells that characterizes cell-mediated responses. Adv. Immunol. 2005, 87, 27–59. [Google Scholar] [CrossRef] [PubMed]
- Girardi, S.R.A.M. Conventional and Unconventional T Cells. Clin. Basic Immunodermatol. 2008, 6, 85–104. [Google Scholar]
- Kumar, B.V.; Connors, T.J.; Farber, D.L. Human T Cell Development, Localization, and Function throughout Life. Immunity 2018, 48, 202–213. [Google Scholar] [CrossRef] [PubMed]
- Ruterbusch, M.; Pruner, K.B.; Shehata, L.; Pepper, M. In Vivo CD4(+) T Cell Differentiation and Function: Revisiting the Th1/Th2 Paradigm. Annu. Rev. Immunol. 2020, 38, 705–725. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Yamane, H.; Paul, W.E. Differentiation of effector CD4 T cell populations (*). Annu. Rev. Immunol. 2010, 28, 445–489. [Google Scholar] [CrossRef]
- Annunziato, F.; Romagnani, C.; Romagnani, S. The 3 major types of innate and adaptive cell-mediated effector immunity. J. Allergy Clin. Immunol. 2015, 135, 626–635. [Google Scholar] [CrossRef]
- Fang, D.; Zhu, J. Dynamic balance between master transcription factors determines the fates and functions of CD4 T cell and innate lymphoid cell subsets. J. Exp. Med. 2017, 214, 1861–1876. [Google Scholar] [CrossRef]
- Chen, Y.; Kuchroo, V.K.; Inobe, J.; Hafler, D.A.; Weiner, H.L. Regulatory T cell clones induced by oral tolerance: Suppression of autoimmune encephalomyelitis. Science 1994, 265, 1237–1240. [Google Scholar] [CrossRef]
- Groux, H.; O’Garra, A.; Bigler, M.; Rouleau, M.; Antonenko, S.; de Vries, J.E.; Roncarolo, M.G. A CD4+ T-cell subset inhibits antigen-specific T-cell responses and prevents colitis. Nature 1997, 389, 737–742. [Google Scholar] [CrossRef]
- Dardalhon, V.; Awasthi, A.; Kwon, H.; Galileos, G.; Gao, W.; Sobel, R.A.; Mitsdoerffer, M.; Strom, T.B.; Elyaman, W.; Ho, I.C.; et al. IL-4 inhibits TGF-beta-induced Foxp3+ T cells and, together with TGF-beta, generates IL-9+ IL-10+ Foxp3(-) effector T cells. Nat. Immunol. 2008, 9, 1347–1355. [Google Scholar] [CrossRef]
- Veldhoen, M.; Uyttenhove, C.; van Snick, J.; Helmby, H.; Westendorf, A.; Buer, J.; Martin, B.; Wilhelm, C.; Stockinger, B. Transforming growth factor-beta ‘reprograms’ the differentiation of T helper 2 cells and promotes an interleukin 9-producing subset. Nat. Immunol. 2008, 9, 1341–1346. [Google Scholar] [CrossRef] [PubMed]
- Eyerich, S.; Eyerich, K.; Pennino, D.; Carbone, T.; Nasorri, F.; Pallotta, S.; Cianfarani, F.; Odorisio, T.; Traidl-Hoffmann, C.; Behrendt, H.; et al. Th22 cells represent a distinct human T cell subset involved in epidermal immunity and remodeling. J. Clin. Investig. 2009, 119, 3573–3585. [Google Scholar] [CrossRef] [PubMed]
- Devarajan, P.; Jones, M.C.; Kugler-Umana, O.; Vong, A.M.; Xia, J.; Swain, S.L. Pathogen Recognition by CD4 Effectors Drives Key Effector and Most Memory Cell Generation against Respiratory Virus. Front. Immunol. 2018, 9, 596. [Google Scholar] [CrossRef]
- Juno, J.A.; van Bockel, D.; Kent, S.J.; Kelleher, A.D.; Zaunders, J.J.; Munier, C.M. Cytotoxic CD4 T Cells-Friend or Foe during Viral Infection? Front. Immunol. 2017, 8, 19. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, A.; Saito, T. CD4 CTL, a Cytotoxic Subset of CD4(+) T Cells, Their Differentiation and Function. Front. Immunol. 2017, 8, 194. [Google Scholar] [CrossRef]
- Eshima, K.; Chiba, S.; Suzuki, H.; Kokubo, K.; Kobayashi, H.; Iizuka, M.; Iwabuchi, K.; Shinohara, N. Ectopic expression of a T-box transcription factor, eomesodermin, renders CD4(+) Th cells cytotoxic by activating both perforin- and FasL-pathways. Immunol. Lett. 2012, 144, 7–15. [Google Scholar] [CrossRef]
- Reis, B.S.; Hoytema van Konijnenburg, D.P.; Grivennikov, S.I.; Mucida, D. Transcription factor T-bet regulates intraepithelial lymphocyte functional maturation. Immunity 2014, 41, 244–256. [Google Scholar] [CrossRef]
- Sledzinska, A.; Vila de Mucha, M.; Bergerhoff, K.; Hotblack, A.; Demane, D.F.; Ghorani, E.; Akarca, A.U.; Marzolini, M.A.V.; Solomon, I.; Vargas, F.A.; et al. Regulatory T Cells Restrain Interleukin-2- and Blimp-1-Dependent Acquisition of Cytotoxic Function by CD4(+) T Cells. Immunity 2020, 52, 151–166.e156. [Google Scholar] [CrossRef]
- Cheroutre, H.; Husain, M.M. CD4 CTL: Living up to the challenge. Semin. Immunol. 2013, 25, 273–281. [Google Scholar] [CrossRef]
- Mucida, D.; Husain, M.M.; Muroi, S.; van Wijk, F.; Shinnakasu, R.; Naoe, Y.; Reis, B.S.; Huang, Y.; Lambolez, F.; Docherty, M.; et al. Transcriptional reprogramming of mature CD4(+) helper T cells generates distinct MHC class II-restricted cytotoxic T lymphocytes. Nat. Immunol. 2013, 14, 281–289. [Google Scholar] [CrossRef]
- Reis, B.S.; Rogoz, A.; Costa-Pinto, F.A.; Taniuchi, I.; Mucida, D. Mutual expression of the transcription factors Runx3 and ThPOK regulates intestinal CD4(+) T cell immunity. Nat. Immunol. 2013, 14, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Serroukh, Y.; Gu-Trantien, C.; Hooshiar Kashani, B.; Defrance, M.; Vu Manh, T.P.; Azouz, A.; Detavernier, A.; Hoyois, A.; Das, J.; Bizet, M.; et al. The transcription factors Runx3 and ThPOK cross-regulate acquisition of cytotoxic function by human Th1 lymphocytes. eLife 2018, 7, e30496. [Google Scholar] [CrossRef] [PubMed]
- Donnarumma, T.; Young, G.R.; Merkenschlager, J.; Eksmond, U.; Bongard, N.; Nutt, S.L.; Boyer, C.; Dittmer, U.; Le-Trilling, V.T.; Trilling, M.; et al. Opposing Development of Cytotoxic and Follicular Helper CD4 T Cells Controlled by the TCF-1-Bcl6 Nexus. Cell Rep. 2016, 17, 1571–1583. [Google Scholar] [CrossRef]
- Takeuchi, A.; Badr Mel, S.; Miyauchi, K.; Ishihara, C.; Onishi, R.; Guo, Z.; Sasaki, Y.; Ike, H.; Takumi, A.; Tsuji, N.M.; et al. CRTAM determines the CD4+ cytotoxic T lymphocyte lineage. J. Exp. Med. 2016, 213, 123–138. [Google Scholar] [CrossRef]
- Marshall, N.B.; Vong, A.M.; Devarajan, P.; Brauner, M.D.; Kuang, Y.; Nayar, R.; Schutten, E.A.; Castonguay, C.H.; Berg, L.J.; Nutt, S.L.; et al. NKG2C/E Marks the Unique Cytotoxic CD4 T Cell Subset, ThCTL, Generated by Influenza Infection. J. Immunol. 2017, 198, 1142–1155. [Google Scholar] [CrossRef]
- Meckiff, B.J.; Ramírez-Suástegui, C.; Fajardo, V.; Chee, S.J.; Kusnadi, A.; Simon, H.; Eschweiler, S.; Grifoni, A.; Pelosi, E.; Weiskopf, D.; et al. Imbalance of regulatory and cytotoxic SARS-CoV-2-reactive CD4+ T cells in COVID-19. Cell 2020. [Google Scholar] [CrossRef] [PubMed]
- Oh, D.Y.; Kwek, S.S.; Raju, S.S.; Li, T.; McCarthy, E.; Chow, E.; Aran, D.; Ilano, A.; Pai, C.S.; Rancan, C.; et al. Intratumoral CD4(+) T Cells Mediate Anti-tumor Cytotoxicity in Human Bladder Cancer. Cell 2020, 181, 1612–1625. [Google Scholar] [CrossRef] [PubMed]
- Mattoo, H.; Stone, J.H.; Pillai, S. Clonally expanded cytotoxic CD4(+) T cells and the pathogenesis of IgG4-related disease. Autoimmunity 2017, 50, 19–24. [Google Scholar] [CrossRef]
- Hashimoto, K.; Kouno, T.; Ikawa, T.; Hayatsu, N.; Miyajima, Y.; Yabukami, H.; Terooatea, T.; Sasaki, T.; Suzuki, T.; Valentine, M.; et al. Single-cell transcriptomics reveals expansion of cytotoxic CD4 T cells in supercentenarians. Proc. Natl. Acad. Sci. USA 2019, 116, 24242–24251. [Google Scholar] [CrossRef]
- Gurram, R.K.; Zhu, J. Orchestration between ILC2s and Th2 cells in shaping type 2 immune responses. Cell. Mol. Immunol. 2019, 16, 225–235. [Google Scholar] [CrossRef] [PubMed]
- Stockinger, B.; Omenetti, S. The dichotomous nature of T helper 17 cells. Nat. Rev. Immunol. 2017, 17, 535–544. [Google Scholar] [CrossRef]
- Crotty, S. T Follicular Helper Cell Biology: A Decade of Discovery and Diseases. Immunity 2019, 50, 1132–1148. [Google Scholar] [CrossRef] [PubMed]
- Miyauchi, K.; Sugimoto-Ishige, A.; Harada, Y.; Adachi, Y.; Usami, Y.; Kaji, T.; Inoue, K.; Hasegawa, H.; Watanabe, T.; Hijikata, A.; et al. Protective neutralizing influenza antibody response in the absence of T follicular helper cells. Nat. Immunol. 2016, 17, 1447–1458. [Google Scholar] [CrossRef] [PubMed]
- Dominguez-Villar, M.; Hafler, D.A. Regulatory T cells in autoimmune disease. Nat. Immunol. 2018, 19, 665–673. [Google Scholar] [CrossRef]
- Shevach, E.M. CD4+ CD25+ suppressor T cells: More questions than answers. Nat. Rev. Immunol. 2002, 2, 389–400. [Google Scholar] [CrossRef] [PubMed]
- Vivier, E.; Artis, D.; Colonna, M.; Diefenbach, A.; Di Santo, J.P.; Eberl, G.; Koyasu, S.; Locksley, R.M.; McKenzie, A.N.J.; Mebius, R.E.; et al. Innate Lymphoid Cells: 10 Years On. Cell 2018, 174, 1054–1066. [Google Scholar] [CrossRef] [PubMed]
- Artis, D.; Spits, H. The biology of innate lymphoid cells. Nature 2015, 517, 293–301. [Google Scholar] [CrossRef]
- Cherrier, D.E.; Serafini, N.; Di Santo, J.P. Innate Lymphoid Cell Development: A T Cell Perspective. Immunity 2018, 48, 1091–1103. [Google Scholar] [CrossRef]
- Ebbo, M.; Crinier, A.; Vely, F.; Vivier, E. Innate lymphoid cells: Major players in inflammatory diseases. Nat. Rev. Immunol. 2017, 17, 665–678. [Google Scholar] [CrossRef]
- Zhong, C.; Zheng, M.; Zhu, J. Lymphoid tissue inducer-A divergent member of the ILC family. Cytokine Growth Factor Rev. 2018, 42, 5–12. [Google Scholar] [CrossRef]
- Sallusto, F. Heterogeneity of Human CD4(+) T Cells Against Microbes. Annu. Rev. Immunol. 2016, 34, 317–334. [Google Scholar] [CrossRef] [PubMed]
- Hirahara, K.; Poholek, A.; Vahedi, G.; Laurence, A.; Kanno, Y.; Milner, J.D.; O’Shea, J.J. Mechanisms underlying helper T-cell plasticity: Implications for immune-mediated disease. J. Allergy Clin. Immunol. 2013, 131, 1276–1287. [Google Scholar] [CrossRef]
- Leonard, W.J.; Lin, J.X.; O’Shea, J.J. The gammac Family of Cytokines: Basic Biology to Therapeutic Ramifications. Immunity 2019, 50, 832–850. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T.R.; Cherwinski, H.; Bond, M.W.; Giedlin, M.A.; Coffman, R.L. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J. Immunol. 1986, 136, 2348–2357. [Google Scholar]
- Zhu, J.; Jankovic, D.; Oler, A.J.; Wei, G.; Sharma, S.; Hu, G.; Guo, L.; Yagi, R.; Yamane, H.; Punkosdy, G.; et al. The transcription factor T-bet is induced by multiple pathways and prevents an endogenous Th2 cell program during Th1 cell responses. Immunity 2012, 37, 660–673. [Google Scholar] [CrossRef] [PubMed]
- Kanno, Y.; Vahedi, G.; Hirahara, K.; Singleton, K.; O’Shea, J.J. Transcriptional and epigenetic control of T helper cell specification: Molecular mechanisms underlying commitment and plasticity. Annu. Rev. Immunol. 2012, 30, 707–731. [Google Scholar] [CrossRef] [PubMed]
- Mullen, A.C.; Hutchins, A.S.; High, F.A.; Lee, H.W.; Sykes, K.J.; Chodosh, L.A.; Reiner, S.L. Hlx is induced by and genetically interacts with T-bet to promote heritable T(H)1 gene induction. Nat. Immunol. 2002, 3, 652–658. [Google Scholar] [CrossRef]
- Djuretic, I.M.; Levanon, D.; Negreanu, V.; Groner, Y.; Rao, A.; Ansel, K.M. Transcription factors T-bet and Runx3 cooperate to activate Ifng and silence Il4 in T helper type 1 cells. Nat. Immunol. 2007, 8, 145–153. [Google Scholar] [CrossRef]
- Yagi, R.; Junttila, I.S.; Wei, G.; Urban, J.F., Jr.; Zhao, K.; Paul, W.E.; Zhu, J. The transcription factor GATA3 actively represses RUNX3 protein-regulated production of interferon-gamma. Immunity 2010, 32, 507–517. [Google Scholar] [CrossRef]
- Grenningloh, R.; Kang, B.Y.; Ho, I.C. Ets-1, a functional cofactor of T-bet, is essential for Th1 inflammatory responses. J. Exp. Med. 2005, 201, 615–626. [Google Scholar] [CrossRef]
- Yu, F.; Sharma, S.; Jankovic, D.; Gurram, R.K.; Su, P.; Hu, G.; Li, R.; Rieder, S.; Zhao, K.; Sun, B.; et al. The transcription factor Bhlhe40 is a switch of inflammatory versus antiinflammatory Th1 cell fate determination. J. Exp. Med. 2018, 215, 1813–1821. [Google Scholar] [CrossRef] [PubMed]
- Lazarevic, V.; Chen, X.; Shim, J.H.; Hwang, E.S.; Jang, E.; Bolm, A.N.; Oukka, M.; Kuchroo, V.K.; Glimcher, L.H. T-bet represses T(H)17 differentiation by preventing Runx1-mediated activation of the gene encoding RORgammat. Nat. Immunol. 2011, 12, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.K.; Turner, H.; Maynard, C.L.; Oliver, J.R.; Chen, D.; Elson, C.O.; Weaver, C.T. Late developmental plasticity in the T helper 17 lineage. Immunity 2009, 30, 92–107. [Google Scholar] [CrossRef] [PubMed]
- Wei, G.; Wei, L.; Zhu, J.; Zang, C.; Hu-Li, J.; Yao, Z.; Cui, K.; Kanno, Y.; Roh, T.Y.; Watford, W.T.; et al. Global mapping of H3K4me3 and H3K27me3 reveals specificity and plasticity in lineage fate determination of differentiating CD4+ T cells. Immunity 2009, 30, 155–167. [Google Scholar] [CrossRef]
- Bonecchi, R.; Bianchi, G.; Bordignon, P.P.; D’Ambrosio, D.; Lang, R.; Borsatti, A.; Sozzani, S.; Allavena, P.; Gray, P.A.; Mantovani, A.; et al. Differential expression of chemokine receptors and chemotactic responsiveness of type 1 T helper cells (Th1s) and Th2s. J. Exp. Med. 1998, 187, 129–134. [Google Scholar] [CrossRef]
- Klose, C.S.; Flach, M.; Mohle, L.; Rogell, L.; Hoyler, T.; Ebert, K.; Fabiunke, C.; Pfeifer, D.; Sexl, V.; Fonseca-Pereira, D.; et al. Differentiation of type 1 ILCs from a common progenitor to all helper-like innate lymphoid cell lineages. Cell 2014, 157, 340–356. [Google Scholar] [CrossRef]
- Weizman, O.E.; Adams, N.M.; Schuster, I.S.; Krishna, C.; Pritykin, Y.; Lau, C.; Degli-Esposti, M.A.; Leslie, C.S.; Sun, J.C.; O’Sullivan, T.E. ILC1 Confer Early Host Protection at Initial Sites of Viral Infection. Cell 2017, 171, 795–808. [Google Scholar] [CrossRef]
- Wei, G.; Abraham, B.J.; Yagi, R.; Jothi, R.; Cui, K.; Sharma, S.; Narlikar, L.; Northrup, D.L.; Tang, Q.; Paul, W.E.; et al. Genome-wide analyses of transcription factor GATA3-mediated gene regulation in distinct T cell types. Immunity 2011, 35, 299–311. [Google Scholar] [CrossRef]
- Hu, G.; Tang, Q.; Sharma, S.; Yu, F.; Escobar, T.M.; Muljo, S.A.; Zhu, J.; Zhao, K. Expression and regulation of intergenic long noncoding RNAs during T cell development and differentiation. Nat. Immunol. 2013, 14, 1190–1198. [Google Scholar] [CrossRef]
- Lund, R.J.; Loytomaki, M.; Naumanen, T.; Dixon, C.; Chen, Z.; Ahlfors, H.; Tuomela, S.; Tahvanainen, J.; Scheinin, J.; Henttinen, T.; et al. Genome-wide identification of novel genes involved in early Th1 and Th2 cell differentiation. J. Immunol. 2007, 178, 3648–3660. [Google Scholar] [CrossRef]
- Fang, D.; Cui, K.; Mao, K.; Hu, G.; Li, R.; Zheng, M.; Riteau, N.; Reiner, S.L.; Sher, A.; Zhao, K.; et al. Transient T-bet expression functionally specifies a distinct T follicular helper subset. J. Exp. Med. 2018, 215, 2705–2714. [Google Scholar] [CrossRef] [PubMed]
- Romagnani, S. The Th1/Th2 paradigm. Immunol. Today 1997, 18, 263–266. [Google Scholar] [CrossRef]
- Yang, R.; Mele, F.; Worley, L.; Langlais, D.; Rosain, J.; Benhsaien, I.; Elarabi, H.; Croft, C.A.; Doisne, J.M.; Zhang, P.; et al. Human T-bet governs innate and innate-like adaptive IFN-g immunity against mycobacteria. BioRxiv Prep. 2020. [Google Scholar] [CrossRef]
- Filipe-Santos, O.; Bustamante, J.; Chapgier, A.; Vogt, G.; de Beaucoudrey, L.; Feinberg, J.; Jouanguy, E.; Boisson-Dupuis, S.; Fieschi, C.; Picard, C.; et al. Inborn errors of IL-12/23- and IFN-gamma-mediated immunity: Molecular, cellular, and clinical features. Semin. Immunol. 2006, 18, 347–361. [Google Scholar] [CrossRef]
- Peng, D.; Kryczek, I.; Nagarsheth, N.; Zhao, L.; Wei, S.; Wang, W.; Sun, Y.; Zhao, E.; Vatan, L.; Szeliga, W.; et al. Epigenetic silencing of TH1-type chemokines shapes tumour immunity and immunotherapy. Nature 2015, 527, 249–253. [Google Scholar] [CrossRef]
- Paul, W.E.; Zhu, J. How are T(H)2-type immune responses initiated and amplified? Nat. Rev. Immunol. 2010, 10, 225–235. [Google Scholar] [CrossRef]
- Zhu, J.; Min, B.; Hu-Li, J.; Watson, C.J.; Grinberg, A.; Wang, Q.; Killeen, N.; Urban, J.F., Jr.; Guo, L.; Paul, W.E. Conditional deletion of Gata3 shows its essential function in T(H)1-T(H)2 responses. Nat. Immunol. 2004, 5, 1157–1165. [Google Scholar] [CrossRef]
- Zhu, J. Seventeen-Year Journey Working with a Master. Front. Immunol. 2018, 9, 960. [Google Scholar] [CrossRef]
- Wohlfert, E.A.; Grainger, J.R.; Bouladoux, N.; Konkel, J.E.; Oldenhove, G.; Ribeiro, C.H.; Hall, J.A.; Yagi, R.; Naik, S.; Bhairavabhotla, R.; et al. GATA3 controls Foxp3(+) regulatory T cell fate during inflammation in mice. J. Clin. Investig. 2011, 121, 4503–4515. [Google Scholar] [CrossRef]
- Fang, D.; Cui, K.; Hu, G.; Gurram, R.K.; Zhong, C.; Oler, A.J.; Yagi, R.; Zhao, M.; Sharma, S.; Liu, P.; et al. Bcl11b, a novel GATA3-interacting protein, suppresses Th1 while limiting Th2 cell differentiation. J. Exp. Med. 2018, 215, 1449–1462. [Google Scholar] [CrossRef]
- Kim, J.I.; Ho, I.C.; Grusby, M.J.; Glimcher, L.H. The transcription factor c-Maf controls the production of interleukin-4 but not other Th2 cytokines. Immunity 1999, 10, 745–751. [Google Scholar] [CrossRef]
- Stritesky, G.L.; Muthukrishnan, R.; Sehra, S.; Goswami, R.; Pham, D.; Travers, J.; Nguyen, E.T.; Levy, D.E.; Kaplan, M.H. The transcription factor STAT3 is required for T helper 2 cell development. Immunity 2011, 34, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Amsen, D.; Blander, J.M.; Lee, G.R.; Tanigaki, K.; Honjo, T.; Flavell, R.A. Instruction of distinct CD4 T helper cell fates by different notch ligands on antigen-presenting cells. Cell 2004, 117, 515–526. [Google Scholar] [CrossRef]
- Zhu, J. T helper 2 (Th2) cell differentiation, type 2 innate lymphoid cell (ILC2) development and regulation of interleukin-4 (IL-4) and IL-13 production. Cytokine 2015, 75, 14–24. [Google Scholar] [CrossRef] [PubMed]
- Sekiya, T.; Miyamasu, M.; Imanishi, M.; Yamada, H.; Nakajima, T.; Yamaguchi, M.; Fujisawa, T.; Pawankar, R.; Sano, Y.; Ohta, K.; et al. Inducible expression of a Th2-type CC chemokine thymus- and activation-regulated chemokine by human bronchial epithelial cells. J. Immunol. 2000, 165, 2205–2213. [Google Scholar] [CrossRef] [PubMed]
- Panina-Bordignon, P.; Papi, A.; Mariani, M.; Di Lucia, P.; Casoni, G.; Bellettato, C.; Buonsanti, C.; Miotto, D.; Mapp, C.; Villa, A.; et al. The C-C chemokine receptors CCR4 and CCR8 identify airway T cells of allergen-challenged atopic asthmatics. J. Clin. Investig. 2001, 107, 1357–1364. [Google Scholar] [CrossRef]
- Knipfer, L.; Schulz-Kuhnt, A.; Kindermann, M.; Greif, V.; Symowski, C.; Voehringer, D.; Neurath, M.F.; Atreya, I.; Wirtz, S. A CCL1/CCR8-dependent feed-forward mechanism drives ILC2 functions in type 2-mediated inflammation. J. Exp. Med. 2019, 216, 2763–2777. [Google Scholar] [CrossRef]
- Liu, Y.J. TSLP in epithelial cell and dendritic cell cross talk. Adv. Immunol. 2009, 101, 1–25. [Google Scholar] [CrossRef]
- Cosmi, L.; Annunziato, F.; Iwasaki, M.; Galli, G.; Manetti, R.; Maggi, E.; Nagata, K.; Romagnani, S. CRTH2 is the most reliable marker for the detection of circulating human type 2 Th and type 2 T cytotoxic cells in health and disease. Eur. J. Immunol. 2000, 30, 2972–2979. [Google Scholar] [CrossRef]
- Xu, D.; Chan, W.L.; Leung, B.P.; Huang, F.; Wheeler, R.; Piedrafita, D.; Robinson, J.H.; Liew, F.Y. Selective expression of a stable cell surface molecule on type 2 but not type 1 helper T cells. J. Exp. Med. 1998, 187, 787–794. [Google Scholar] [CrossRef]
- Zhong, C.; Zheng, M.; Cui, K.; Martins, A.J.; Hu, G.; Li, D.; Tessarollo, L.; Kozlov, S.; Keller, J.R.; Tsang, J.S.; et al. Differential Expression of the Transcription Factor GATA3 Specifies Lineage and Functions of Innate Lymphoid Cells. Immunity 2020, 52, 83–95.e84. [Google Scholar] [CrossRef]
- Halim, T.Y.; Steer, C.A.; Matha, L.; Gold, M.J.; Martinez-Gonzalez, I.; McNagny, K.M.; McKenzie, A.N.; Takei, F. Group 2 innate lymphoid cells are critical for the initiation of adaptive T helper 2 cell-mediated allergic lung inflammation. Immunity 2014, 40, 425–435. [Google Scholar] [CrossRef] [PubMed]
- Oliphant, C.J.; Hwang, Y.Y.; Walker, J.A.; Salimi, M.; Wong, S.H.; Brewer, J.M.; Englezakis, A.; Barlow, J.L.; Hams, E.; Scanlon, S.T.; et al. MHCII-mediated dialog between group 2 innate lymphoid cells and CD4(+) T cells potentiates type 2 immunity and promotes parasitic helminth expulsion. Immunity 2014, 41, 283–295. [Google Scholar] [CrossRef] [PubMed]
- Von Moltke, J.; Ji, M.; Liang, H.E.; Locksley, R.M. Tuft-cell-derived IL-25 regulates an intestinal ILC2-epithelial response circuit. Nature 2016, 529, 221–225. [Google Scholar] [CrossRef]
- Dewas, C.; Chen, X.; Honda, T.; Junttila, I.; Linton, J.; Udey, M.C.; Porcella, S.F.; Sturdevant, D.E.; Feigenbaum, L.; Koo, L.; et al. TSLP expression: Analysis with a ZsGreen TSLP reporter mouse. J. Immunol. 2015, 194, 1372–1380. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Huang, Y.; Chen, X.; Hu-Li, J.; Urban, J.F., Jr.; Paul, W.E. Innate immunological function of TH2 cells in vivo. Nat. Immunol. 2015, 16, 1051–1059. [Google Scholar] [CrossRef]
- Liang, H.E.; Reinhardt, R.L.; Bando, J.K.; Sullivan, B.M.; Ho, I.C.; Locksley, R.M. Divergent expression patterns of IL-4 and IL-13 define unique functions in allergic immunity. Nat. Immunol. 2012, 13, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Peine, M.; Rausch, S.; Helmstetter, C.; Frohlich, A.; Hegazy, A.N.; Kuhl, A.A.; Grevelding, C.G.; Hofer, T.; Hartmann, S.; Lohning, M. Stable T-bet(+)GATA-3(+) Th1/Th2 hybrid cells arise in vivo, can develop directly from naive precursors, and limit immunopathologic inflammation. PLoS Biol. 2013, 11, e1001633. [Google Scholar] [CrossRef]
- Wang, Y.H.; Voo, K.S.; Liu, B.; Chen, C.Y.; Uygungil, B.; Spoede, W.; Bernstein, J.A.; Huston, D.P.; Liu, Y.J. A novel subset of CD4(+) T(H)2 memory/effector cells that produce inflammatory IL-17 cytokine and promote the exacerbation of chronic allergic asthma. J. Exp. Med. 2010, 207, 2479–2491. [Google Scholar] [CrossRef]
- Irvin, C.; Zafar, I.; Good, J.; Rollins, D.; Christianson, C.; Gorska, M.M.; Martin, R.J.; Alam, R. Increased frequency of dual-positive TH2/TH17 cells in bronchoalveolar lavage fluid characterizes a population of patients with severe asthma. J. Allergy Clin. Immunol. 2014, 134, 1175–1186.e1177. [Google Scholar] [CrossRef]
- Hegazy, A.N.; Peine, M.; Helmstetter, C.; Panse, I.; Frohlich, A.; Bergthaler, A.; Flatz, L.; Pinschewer, D.D.; Radbruch, A.; Lohning, M. Interferons direct Th2 cell reprogramming to generate a stable GATA-3(+)T-bet(+) cell subset with combined Th2 and Th1 cell functions. Immunity 2010, 32, 116–128. [Google Scholar] [CrossRef] [PubMed]
- Yagi, R.; Zhong, C.; Northrup, D.L.; Yu, F.; Bouladoux, N.; Spencer, S.; Hu, G.; Barron, L.; Sharma, S.; Nakayama, T.; et al. The transcription factor GATA3 is critical for the development of all IL-7Ralpha-expressing innate lymphoid cells. Immunity 2014, 40, 378–388. [Google Scholar] [CrossRef] [PubMed]
- Henriksson, J.; Chen, X.; Gomes, T.; Ullah, U.; Meyer, K.B.; Miragaia, R.; Duddy, G.; Pramanik, J.; Yusa, K.; Lahesmaa, R.; et al. Genome-wide CRISPR Screens in T Helper Cells Reveal Pervasive Crosstalk between Activation and Differentiation. Cell 2019, 176, 882–896.e818. [Google Scholar] [CrossRef]
- Pulendran, B.; Artis, D. New paradigms in type 2 immunity. Science 2012, 337, 431–435. [Google Scholar] [CrossRef] [PubMed]
- Helm, R.M.; Burks, A.W. Mechanisms of food allergy. Curr. Opin. Immunol. 2000, 12, 647–653. [Google Scholar] [CrossRef]
- Palm, N.W.; Rosenstein, R.K.; Medzhitov, R. Allergic host defences. Nature 2012, 484, 465–472. [Google Scholar] [CrossRef]
- Thomas, W.R.; Hales, B.J. T and B cell responses to HDM allergens and antigens. Immunol. Res. 2007, 37, 187–199. [Google Scholar] [CrossRef]
- Mariani, V.; Gilles, S.; Jakob, T.; Thiel, M.; Mueller, M.J.; Ring, J.; Behrendt, H.; Traidl-Hoffmann, C. Immunomodulatory mediators from pollen enhance the migratory capacity of dendritic cells and license them for Th2 attraction. J. Immunol. 2007, 178, 7623–7631. [Google Scholar] [CrossRef]
- Coffman, R.L.; Sher, A.; Seder, R.A. Vaccine adjuvants: Putting innate immunity to work. Immunity 2010, 33, 492–503. [Google Scholar] [CrossRef]
- Ting, C.N.; Olson, M.C.; Barton, K.P.; Leiden, J.M. Transcription factor GATA-3 is required for development of the T-cell lineage. Nature 1996, 384, 474–478. [Google Scholar] [CrossRef]
- Van Esch, H.; Groenen, P.; Nesbit, M.A.; Schuffenhauer, S.; Lichtner, P.; Vanderlinden, G.; Harding, B.; Beetz, R.; Bilous, R.W.; Holdaway, I.; et al. GATA3 haplo-insufficiency causes human HDR syndrome. Nature 2000, 406, 419–422. [Google Scholar] [CrossRef] [PubMed]
- Skapenko, A.; Leipe, J.; Niesner, U.; Devriendt, K.; Beetz, R.; Radbruch, A.; Kalden, J.R.; Lipsky, P.E.; Schulze-Koops, H. GATA-3 in human T cell helper type 2 development. J. Exp. Med. 2004, 199, 423–428. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, M.G.; Leiding, J.W.; Lyons, J.J.; Hsu, A.P.; Nelson, C.C.; Jones, N.; Fitzgerald, A.; Chien, W.W.; Workman, L.; Platts-Mills, T.A.; et al. GATA3 haploinsufficiency does not block allergic sensitization or atopic disease. J. Allergy Clin. Immunol. 2016, 137, 627–629.e622. [Google Scholar] [CrossRef]
- Klein Wolterink, R.G.; Serafini, N.; van Nimwegen, M.; Vosshenrich, C.A.; de Bruijn, M.J.; Fonseca Pereira, D.; Veiga Fernandes, H.; Hendriks, R.W.; Di Santo, J.P. Essential, dose-dependent role for the transcription factor Gata3 in the development of IL-5+ and IL-13+ type 2 innate lymphoid cells. Proc. Natl. Acad. Sci. USA 2013, 110, 10240–10245. [Google Scholar] [CrossRef] [PubMed]
- Marrella, V.; Poliani, P.L.; Sobacchi, C.; Grassi, F.; Villa, A. Of Omenn and mice. Trends Immunol. 2008, 29, 133–140. [Google Scholar] [CrossRef]
- Villa, A.; Notarangelo, L.D. RAG gene defects at the verge of immunodeficiency and immune dysregulation. Immunol. Rev. 2019, 287, 73–90. [Google Scholar] [CrossRef]
- Milner, J.D.; Ward, J.M.; Keane-Myers, A.; Paul, W.E. Lymphopenic mice reconstituted with limited repertoire T cells develop severe, multiorgan, Th2-associated inflammatory disease. Proc. Natl. Acad. Sci. USA 2007, 104, 576–581. [Google Scholar] [CrossRef]
- Dolff, S.; Witzke, O.; Wilde, B. Th17 cells in renal inflammation and autoimmunity. Autoimmun. Rev. 2019, 18, 129–136. [Google Scholar] [CrossRef]
- Aggarwal, S.; Ghilardi, N.; Xie, M.H.; de Sauvage, F.J.; Gurney, A.L. Interleukin-23 promotes a distinct CD4 T cell activation state characterized by the production of interleukin-17. J. Biol. Chem. 2003, 278, 1910–1914. [Google Scholar] [CrossRef]
- Cua, D.J.; Sherlock, J.; Chen, Y.; Murphy, C.A.; Joyce, B.; Seymour, B.; Lucian, L.; To, W.; Kwan, S.; Churakova, T.; et al. Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature 2003, 421, 744–748. [Google Scholar] [CrossRef]
- Murphy, C.A.; Langrish, C.L.; Chen, Y.; Blumenschein, W.; McClanahan, T.; Kastelein, R.A.; Sedgwick, J.D.; Cua, D.J. Divergent pro- and antiinflammatory roles for IL-23 and IL-12 in joint autoimmune inflammation. J. Exp. Med. 2003, 198, 1951–1957. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Li, Z.X.; Yang, X.O.; Chang, S.H.; Nurieva, R.; Wang, Y.H.; Wang, Y.; Hood, L.; Zhu, Z.; Tian, Q.; et al. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat. Immunol. 2005, 6, 1133–1141. [Google Scholar] [CrossRef] [PubMed]
- Bettelli, E.; Carrier, Y.J.; Gao, W.D.; Korn, T.; Strom, T.B.; Oukka, M.; Weiner, H.L.; Kuchroo, V.K. Reciprocal developmental pathways for the generation of pathogenic effector T(H)17 and regulatory T cells. Nature 2006, 441, 235–238. [Google Scholar] [CrossRef] [PubMed]
- Harrington, L.E.; Hatton, R.D.; Mangan, P.R.; Turner, H.; Murphy, T.L.; Murphy, K.M.; Weaver, C.T. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat. Immunol. 2005, 6, 1123–1132. [Google Scholar] [CrossRef]
- Gaublomme, J.T.; Yosef, N.; Lee, Y.; Gertner, R.S.; Yang, L.V.; Wu, C.; Pandolfi, P.P.; Mak, T.; Satija, R.; Shalek, A.K.; et al. Single-Cell Genomics Unveils Critical Regulators of Th17 Cell Pathogenicity. Cell 2015, 163, 1400–1412. [Google Scholar] [CrossRef]
- Ciofani, M.; Madar, A.; Galan, C.; Sellars, M.; Mace, K.; Pauli, F.; Agarwal, A.; Huang, W.; Parkurst, C.N.; Muratet, M.; et al. A Validated Regulatory Network for Th17 Cell Specification. Cell 2012, 151, 289–303. [Google Scholar] [CrossRef]
- Chung, Y.; Chang, S.H.; Martinez, G.J.; Yang, X.O.; Nurieva, R.; Kang, H.S.; Ma, L.; Watowich, S.S.; Jetten, A.M.; Tian, Q.; et al. Critical regulation of early Th17 cell differentiation by interleukin-1 signaling. Immunity 2009, 30, 576–587. [Google Scholar] [CrossRef]
- Guo, L.; Wei, G.; Zhu, J.; Liao, W.; Leonard, W.J.; Zhao, K.; Paul, W. IL-1 family members and STAT activators induce cytokine production by Th2, Th17, and Th1 cells. Proc. Natl. Acad. Sci. USA 2009, 106, 13463–13468. [Google Scholar] [CrossRef]
- Dong, C. TH17 cells in development: An updated view of their molecular identity and genetic programming. Nat. Rev. Immunol. 2008, 8, 337–348. [Google Scholar] [CrossRef]
- Ghoreschi, K.; Laurence, A.; Yang, X.P.; Tato, C.M.; McGeachy, M.J.; Konkel, J.E.; Ramos, H.L.; Wei, L.; Davidson, T.S.; Bouladoux, N.; et al. Generation of pathogenic T(H)17 cells in the absence of TGF-beta signalling. Nature 2010, 467, 967–971. [Google Scholar] [CrossRef]
- Pawlak, M.; Ho, A.W.; Kuchroo, V.K. Cytokines and transcription factors in the differentiation of CD4+ T helper cell subsets and induction of tissue inflammation and autoimmunity. Curr. Opin. Immunol. 2020, 67, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Veldhoen, M.; Hocking, R.J.; Atkins, C.J.; Locksley, R.M.; Stockinger, B. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity 2006, 24, 179–189. [Google Scholar] [CrossRef]
- Matsuzaki, G.; Umemura, M. Interleukin-17 as an effector molecule of innate and acquired immunity against infections. Microbiol. Immunol. 2007, 51, 1139–1147. [Google Scholar] [CrossRef]
- Kim, C.H. Migration and function of Th17 cells. Inflamm. Allergy Drug Targets 2009, 8, 221–228. [Google Scholar] [CrossRef]
- Acosta-Rodriguez, E.V.; Rivino, L.; Geginat, J.; Jarrossay, D.; Gattorno, M.; Lanzavecchia, A.; Sallusto, F.; Napolitani, G. Surface phenotype and antigenic specificity of human interleukin 17-producing T helper memory cells. Nat. Immunol. 2007, 8, 639–646. [Google Scholar] [CrossRef]
- Annunziato, F.; Cosmi, L.; Santarlasci, V.; Maggi, L.; Liotta, F.; Mazzinghi, B.; Parente, E.; Fili, L.; Ferri, S.; Frosali, F.; et al. Phenotypic and functional features of human Th17 cells. J. Exp. Med. 2007, 204, 1849–1861. [Google Scholar] [CrossRef]
- Rankin, L.C.; Girard-Madoux, M.J.; Seillet, C.; Mielke, L.A.; Kerdiles, Y.; Fenis, A.; Wieduwild, E.; Putoczki, T.; Mondot, S.; Lantz, O.; et al. Complementarity and redundancy of IL-22-producing innate lymphoid cells. Nat. Immunol. 2016, 17, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Sano, T.; Huang, W.; Hall, J.A.; Yang, Y.; Chen, A.; Gavzy, S.J.; Lee, J.Y.; Ziel, J.W.; Miraldi, E.R.; Domingos, A.I.; et al. An IL-23R/IL-22 Circuit Regulates Epithelial Serum Amyloid A to Promote Local Effector Th17 Responses. Cell 2015, 163, 381–393. [Google Scholar] [CrossRef] [PubMed]
- Mao, K.; Baptista, A.P.; Tamoutounour, S.; Zhuang, L.; Bouladoux, N.; Martins, A.J.; Huang, Y.; Gerner, M.Y.; Belkaid, Y.; Germain, R.N. Innate and adaptive lymphocytes sequentially shape the gut microbiota and lipid metabolism. Nature 2018, 554, 255–259. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Paul, W.E. Heterogeneity and plasticity of T helper cells. Cell Res. 2010, 20, 4–12. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Awasthi, A.; Yosef, N.; Quintana, F.J.; Xiao, S.; Peters, A.; Wu, C.; Kleinewietfeld, M.; Kunder, S.; Hafler, D.A.; et al. Induction and molecular signature of pathogenic TH17 cells. Nat. Immunol. 2012, 13, 991–999. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Yosef, N.; Gaublomme, J.; Wu, C.; Lee, Y.; Clish, C.B.; Kaminski, J.; Xiao, S.; Meyer Zu Horste, G.; Pawlak, M.; et al. CD5L/AIM Regulates Lipid Biosynthesis and Restrains Th17 Cell Pathogenicity. Cell 2015, 163, 1413–1427. [Google Scholar] [CrossRef] [PubMed]
- Amir, M.; Chaudhari, S.; Wang, R.; Campbell, S.; Mosure, S.A.; Chopp, L.B.; Lu, Q.; Shang, J.; Pelletier, O.B.; He, Y.; et al. REV-ERBalpha Regulates TH17 Cell Development and Autoimmunity. Cell Rep. 2018, 25, 3733–3749.e3738. [Google Scholar] [CrossRef] [PubMed]
- Kleinewietfeld, M.; Manzel, A.; Titze, J.; Kvakan, H.; Yosef, N.; Linker, R.A.; Muller, D.N.; Hafler, D.A. Sodium chloride drives autoimmune disease by the induction of pathogenic TH17 cells. Nature 2013, 496, 518–522. [Google Scholar] [CrossRef] [PubMed]
- Wilck, N.; Matus, M.G.; Kearney, S.M.; Olesen, S.W.; Forslund, K.; Bartolomaeus, H.; Haase, S.; Mahler, A.; Balogh, A.; Marko, L.; et al. Salt-responsive gut commensal modulates TH17 axis and disease. Nature 2017, 551, 585–589. [Google Scholar] [CrossRef]
- Bacher, P.; Hohnstein, T.; Beerbaum, E.; Rocker, M.; Blango, M.G.; Kaufmann, S.; Rohmel, J.; Eschenhagen, P.; Grehn, C.; Seidel, K.; et al. Human Anti-fungal Th17 Immunity and Pathology Rely on Cross-Reactivity against Candida albicans. Cell 2019, 176, 1340–1355.e1315. [Google Scholar] [CrossRef]
- Britton, G.J.; Contijoch, E.J.; Mogno, I.; Vennaro, O.H.; Llewellyn, S.R.; Ng, R.; Li, Z.; Mortha, A.; Merad, M.; Das, A.; et al. Microbiotas from Humans with Inflammatory Bowel Disease Alter the Balance of Gut Th17 and RORgammat(+) Regulatory T Cells and Exacerbate Colitis in Mice. Immunity 2019, 50, 212–224.e214. [Google Scholar] [CrossRef] [PubMed]
- Yoo, S.A.; Kim, M.; Kang, M.C.; Kong, J.S.; Kim, K.M.; Lee, S.; Hong, B.K.; Jeong, G.H.; Lee, J.; Shin, M.G.; et al. Placental growth factor regulates the generation of TH17 cells to link angiogenesis with autoimmunity. Nat. Immunol. 2019, 20, 1348–1359. [Google Scholar] [CrossRef]
- Harbour, S.N.; Maynard, C.L.; Zindl, C.L.; Schoeb, T.R.; Weaver, C.T. Th17 cells give rise to Th1 cells that are required for the pathogenesis of colitis. Proc. Natl. Acad. Sci. USA 2015, 112, 7061–7066. [Google Scholar] [CrossRef]
- Johnson, M.O.; Wolf, M.M.; Madden, M.Z.; Andrejeva, G.; Sugiura, A.; Contreras, D.C.; Maseda, D.; Liberti, M.V.; Paz, K.; Kishton, R.J.; et al. Distinct Regulation of Th17 and Th1 Cell Differentiation by Glutaminase-Dependent Metabolism. Cell 2018, 175, 1780–1795.e1719. [Google Scholar] [CrossRef]
- Kono, M.; Yoshida, N.; Maeda, K.; Tsokos, G.C. Transcriptional factor ICER promotes glutaminolysis and the generation of Th17 cells. Proc. Natl. Acad. Sci. USA 2018, 115, 2478–2483. [Google Scholar] [CrossRef] [PubMed]
- Gibson, S.A.; Yang, W.; Yan, Z.; Qin, H.; Benveniste, E.N. CK2 Controls Th17 and Regulatory T Cell Differentiation Through Inhibition of FoxO1. J. Immunol. 2018, 201, 383–392. [Google Scholar] [CrossRef]
- Karmaus, P.W.F.; Chen, X.; Lim, S.A.; Herrada, A.A.; Nguyen, T.M.; Xu, B.; Dhungana, Y.; Rankin, S.; Chen, W.; Rosencrance, C.; et al. Metabolic heterogeneity underlies reciprocal fates of TH17 cell stemness and plasticity. Nature 2019, 565, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Omenetti, S.; Bussi, C.; Metidji, A.; Iseppon, A.; Lee, S.; Tolaini, M.; Li, Y.; Kelly, G.; Chakravarty, P.; Shoaie, S.; et al. The Intestine Harbors Functionally Distinct Homeostatic Tissue-Resident and Inflammatory Th17 Cells. Immunity 2019, 51, 77–89.e76. [Google Scholar] [CrossRef]
- Wang, Y.; Godec, J.; Ben-Aissa, K.; Cui, K.; Zhao, K.; Pucsek, A.B.; Lee, Y.K.; Weaver, C.T.; Yagi, R.; Lazarevic, V. The transcription factors T-bet and Runx are required for the ontogeny of pathogenic interferon-gamma-producing T helper 17 cells. Immunity 2014, 40, 355–366. [Google Scholar] [CrossRef]
- Okada, S.; Markle, J.G.; Deenick, E.K.; Mele, F.; Averbuch, D.; Lagos, M.; Alzahrani, M.; Al-Muhsen, S.; Halwani, R.; Ma, C.S.; et al. IMMUNODEFICIENCIES. Impairment of immunity to Candida and Mycobacterium in humans with bi-allelic RORC mutations. Science 2015, 349, 606–613. [Google Scholar] [CrossRef]
- Buckley, R.H. The hyper-IgE syndrome. Clin. Rev. Allergy Immunol. 2001, 20, 139–154. [Google Scholar]
- Holland, S.M.; DeLeo, F.R.; Elloumi, H.Z.; Hsu, A.P.; Uzel, G.; Brodsky, N.; Freeman, A.F.; Demidowich, A.; Davis, J.; Turner, M.L.; et al. STAT3 mutations in the hyper-IgE syndrome. N. Engl. J. Med. 2007, 357, 1608–1619. [Google Scholar]
- Minegishi, Y.; Saito, M.; Tsuchiya, S.; Tsuge, I.; Takada, H.; Hara, T.; Kawamura, N.; Ariga, T.; Pasic, S.; Stojkovic, O.; et al. Dominant-negative mutations in the DNA-binding domain of STAT3 cause hyper-IgE syndrome. Nature 2007, 448, 1058–1062. [Google Scholar] [PubMed]
- Deng, Y.; Li, T.; Xie, X.; Xia, D.; Ding, L.; Xiang, H.; Ma, J.J.; Li, W. Hyper IgE syndrome associated with novel and recurrent STAT3 mutations: Two case reports. Medicine 2019, 98, e14003. [Google Scholar] [CrossRef]
- Engelhardt, K.R.; McGhee, S.; Winkler, S.; Sassi, A.; Woellner, C.; Lopez-Herrera, G.; Chen, A.; Kim, H.S.; Lloret, M.G.; Schulze, I.; et al. Large deletions and point mutations involving the dedicator of cytokinesis 8 (DOCK8) in the autosomal-recessive form of hyper-IgE syndrome. J. Allergy Clin. Immunol. 2009, 124, 1289–1302.e1284. [Google Scholar] [CrossRef]
- Zhang, Q.; Davis, J.C.; Lamborn, I.T.; Freeman, A.F.; Jing, H.; Favreau, A.J.; Matthews, H.F.; Davis, J.; Turner, M.L.; Uzel, G.; et al. Combined immunodeficiency associated with DOCK8 mutations. N. Engl. J. Med. 2009, 361, 2046–2055. [Google Scholar] [CrossRef] [PubMed]
- Dupuis, S.; Dargemont, C.; Fieschi, C.; Thomassin, N.; Rosenzweig, S.; Harris, J.; Holland, S.M.; Schreiber, R.D.; Casanova, J.L. Impairment of mycobacterial but not viral immunity by a germline human STAT1 mutation. Science 2001, 293, 300–303. [Google Scholar] [CrossRef] [PubMed]
- Dupuis, S.; Jouanguy, E.; Al-Hajjar, S.; Fieschi, C.; Al-Mohsen, I.Z.; Al-Jumaah, S.; Yang, K.; Chapgier, A.; Eidenschenk, C.; Eid, P.; et al. Impaired response to interferon-alpha/beta and lethal viral disease in human STAT1 deficiency. Nat. Genet. 2003, 33, 388–391. [Google Scholar] [CrossRef]
- Liu, L.; Okada, S.; Kong, X.F.; Kreins, A.Y.; Cypowyj, S.; Abhyankar, A.; Toubiana, J.; Itan, Y.; Audry, M.; Nitschke, P.; et al. Gain-of-function human STAT1 mutations impair IL-17 immunity and underlie chronic mucocutaneous candidiasis. J. Exp. Med. 2011, 208, 1635–1648. [Google Scholar] [CrossRef] [PubMed]
- Van de Veerdonk, F.L.; Plantinga, T.S.; Hoischen, A.; Smeekens, S.P.; Joosten, L.A.; Gilissen, C.; Arts, P.; Rosentul, D.C.; Carmichael, A.J.; Smits-van der Graaf, C.A.; et al. STAT1 mutations in autosomal dominant chronic mucocutaneous candidiasis. N. Engl. J. Med. 2011, 365, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Puel, A.; Cypowyj, S.; Bustamante, J.; Wright, J.F.; Liu, L.; Lim, H.K.; Migaud, M.; Israel, L.; Chrabieh, M.; Audry, M.; et al. Chronic mucocutaneous candidiasis in humans with inborn errors of interleukin-17 immunity. Science 2011, 332, 65–68. [Google Scholar] [CrossRef]
- Ben-Sasson, S.Z.; Hu-Li, J.; Quiel, J.; Cauchetaux, S.; Ratner, M.; Shapira, I.; Dinarello, C.A.; Paul, W.E. IL-1 acts directly on CD4 T cells to enhance their antigen-driven expansion and differentiation. Proc. Natl. Acad. Sci. USA 2009, 106, 7119–7124. [Google Scholar] [CrossRef]
- Masters, S.L.; Simon, A.; Aksentijevich, I.; Kastner, D.L. Horror autoinflammaticus: The molecular pathophysiology of autoinflammatory disease (*). Annu. Rev. Immunol. 2009, 27, 621–668. [Google Scholar] [CrossRef]
- Ma, H.L.; Liang, S.; Li, J.; Napierata, L.; Brown, T.; Benoit, S.; Senices, M.; Gill, D.; Dunussi-Joannopoulos, K.; Collins, M.; et al. IL-22 is required for Th17 cell-mediated pathology in a mouse model of psoriasis-like skin inflammation. J. Clin. Investig. 2008, 118, 597–607. [Google Scholar] [CrossRef]
- Torres, T.; Faria, R. Ustekinumab: The “New Kid on the Block” in the Treatment of Psoriatic Arthritis. Drug Dev. Res. 2015, 76, 428–431. [Google Scholar] [CrossRef] [PubMed]
- McGeachy, M.J.; Cua, D.J.; Gaffen, S.L. The IL-17 Family of Cytokines in Health and Disease. Immunity 2019, 50, 892–906. [Google Scholar] [CrossRef] [PubMed]
- Huh, J.R.; Littman, D.R. Small molecule inhibitors of RORgammat: Targeting Th17 cells and other applications. Eur. J. Immunol. 2012, 42, 2232–2237. [Google Scholar] [CrossRef] [PubMed]
- Sakaguchi, S.; Sakaguchi, N.; Asano, M.; Itoh, M.; Toda, M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J. Immunol. 1995, 155, 1151–1164. [Google Scholar]
- Fontenot, J.D.; Gavin, M.A.; Rudensky, A.Y. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat. Immunol. 2003, 4, 330–336. [Google Scholar] [CrossRef]
- Hori, S.; Nomura, T.; Sakaguchi, S. Control of regulatory T cell development by the transcription factor Foxp3. Science 2003, 299, 1057–1061. [Google Scholar] [CrossRef]
- Khattri, R.; Cox, T.; Yasayko, S.A.; Ramsdell, F. An essential role for Scurfin in CD4+CD25+ T regulatory cells. Nat. Immunol. 2003, 4, 337–342. [Google Scholar] [CrossRef]
- Raffin, C.; Vo, L.T.; Bluestone, J.A. Treg cell-based therapies: Challenges and perspectives. Nat. Rev. Immunol. 2020, 20, 158–172. [Google Scholar] [CrossRef] [PubMed]
- Shevach, E.M.; Thornton, A.M. tTregs, pTregs, and iTregs: Similarities and differences. Immunol. Rev. 2014, 259, 88–102. [Google Scholar] [CrossRef] [PubMed]
- Kanamori, M.; Nakatsukasa, H.; Okada, M.; Lu, Q.; Yoshimura, A. Induced Regulatory T Cells: Their Development, Stability, and Applications. Trends Immunol. 2016, 37, 803–811. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Jin, W.; Hardegen, N.; Lei, K.J.; Li, L.; Marinos, N.; McGrady, G.; Wahl, S.M. Conversion of peripheral CD4+CD25- naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J. Exp. Med. 2003, 198, 1875–1886. [Google Scholar] [CrossRef] [PubMed]
- Fantini, M.C.; Becker, C.; Monteleone, G.; Pallone, F.; Galle, P.R.; Neurath, M.F. Cutting edge: TGF-beta induces a regulatory phenotype in CD4+CD25- T cells through Foxp3 induction and down-regulation of Smad7. J. Immunol. 2004, 172, 5149–5153. [Google Scholar] [CrossRef] [PubMed]
- Fu, S.; Zhang, N.; Yopp, A.C.; Chen, D.; Mao, M.; Chen, D.; Zhang, H.; Ding, Y.; Bromberg, J.S. TGF-beta induces Foxp3 + T-regulatory cells from CD4 + CD25—Precursors. Am. J. Transpl. 2004, 4, 1614–1627. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.G.; Wang, J.H.; Gray, J.D.; Soucier, H.; Horwitz, D.A. Natural and induced CD4+CD25+ cells educate CD4+CD25- cells to develop suppressive activity: The role of IL-2, TGF-beta, and IL-10. J. Immunol. 2004, 172, 5213–5221. [Google Scholar] [CrossRef] [PubMed]
- Schiering, C.; Krausgruber, T.; Chomka, A.; Frohlich, A.; Adelmann, K.; Wohlfert, E.A.; Pott, J.; Griseri, T.; Bollrath, J.; Hegazy, A.N.; et al. The alarmin IL-33 promotes regulatory T-cell function in the intestine. Nature 2014, 513, 564–568. [Google Scholar] [CrossRef]
- Sun, C.M.; Hall, J.A.; Blank, R.B.; Bouladoux, N.; Oukka, M.; Mora, J.R.; Belkaid, Y. Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 T reg cells via retinoic acid. J. Exp. Med. 2007, 204, 1775–1785. [Google Scholar] [CrossRef]
- Coombes, J.L.; Siddiqui, K.R.; Arancibia-Carcamo, C.V.; Hall, J.; Sun, C.M.; Belkaid, Y.; Powrie, F. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-beta and retinoic acid-dependent mechanism. J. Exp. Med. 2007, 204, 1757–1764. [Google Scholar] [CrossRef]
- Lathrop, S.K.; Bloom, S.M.; Rao, S.M.; Nutsch, K.; Lio, C.W.; Santacruz, N.; Peterson, D.A.; Stappenbeck, T.S.; Hsieh, C.S. Peripheral education of the immune system by colonic commensal microbiota. Nature 2011, 478, 250–254. [Google Scholar] [CrossRef]
- Thornton, A.M.; Korty, P.E.; Tran, D.Q.; Wohlfert, E.A.; Murray, P.E.; Belkaid, Y.; Shevach, E.M. Expression of Helios, an Ikaros transcription factor family member, differentiates thymic-derived from peripherally induced Foxp3+ T regulatory cells. J. Immunol. 2010, 184, 3433–3441. [Google Scholar] [CrossRef]
- Yadav, M.; Louvet, C.; Davini, D.; Gardner, J.M.; Martinez-Llordella, M.; Bailey-Bucktrout, S.; Anthony, B.A.; Sverdrup, F.M.; Head, R.; Kuster, D.J.; et al. Neuropilin-1 distinguishes natural and inducible regulatory T cells among regulatory T cell subsets in vivo. J. Exp. Med. 2012, 209, 1713–1722. [Google Scholar] [CrossRef]
- Floess, S.; Freyer, J.; Siewert, C.; Baron, U.; Olek, S.; Polansky, J.; Schlawe, K.; Chang, H.D.; Bopp, T.; Schmitt, E.; et al. Epigenetic control of the foxp3 locus in regulatory T cells. PLoS Biol. 2007, 5, e38. [Google Scholar] [CrossRef] [PubMed]
- Polansky, J.K.; Kretschmer, K.; Freyer, J.; Floess, S.; Garbe, A.; Baron, U.; Olek, S.; Hamann, A.; von Boehmer, H.; Huehn, J. DNA methylation controls Foxp3 gene expression. Eur. J. Immunol. 2008, 38, 1654–1663. [Google Scholar] [CrossRef] [PubMed]
- Ohnmacht, C.; Park, J.H.; Cording, S.; Wing, J.B.; Atarashi, K.; Obata, Y.; Gaboriau-Routhiau, V.; Marques, R.; Dulauroy, S.; Fedoseeva, M.; et al. MUCOSAL IMMUNOLOGY. The microbiota regulates type 2 immunity through RORgammat(+) T cells. Science 2015, 349, 989–993. [Google Scholar] [CrossRef] [PubMed]
- Sefik, E.; Geva-Zatorsky, N.; Oh, S.; Konnikova, L.; Zemmour, D.; McGuire, A.M.; Burzyn, D.; Ortiz-Lopez, A.; Lobera, M.; Yang, J.; et al. MUCOSAL IMMUNOLOGY. Individual intestinal symbionts induce a distinct population of RORgamma(+) regulatory T cells. Science 2015, 349, 993–997. [Google Scholar] [CrossRef]
- Takimoto, T.; Wakabayashi, Y.; Sekiya, T.; Inoue, N.; Morita, R.; Ichiyama, K.; Takahashi, R.; Asakawa, M.; Muto, G.; Mori, T.; et al. Smad2 and Smad3 are redundantly essential for the TGF-beta-mediated regulation of regulatory T plasticity and Th1 development. J. Immunol. 2010, 185, 842–855. [Google Scholar] [CrossRef]
- Zorn, E.; Nelson, E.A.; Mohseni, M.; Porcheray, F.; Kim, H.; Litsa, D.; Bellucci, R.; Raderschall, E.; Canning, C.; Soiffer, R.J.; et al. IL-2 regulates FOXP3 expression in human CD4+CD25+ regulatory T cells through a STAT-dependent mechanism and induces the expansion of these cells in vivo. Blood 2006, 108, 1571–1579. [Google Scholar] [CrossRef]
- Burchill, M.A.; Yang, J.; Vogtenhuber, C.; Blazar, B.R.; Farrar, M.A. IL-2 receptor beta-dependent STAT5 activation is required for the development of Foxp3+ regulatory T cells. J. Immunol. 2007, 178, 280–290. [Google Scholar] [CrossRef]
- Rudra, D.; Egawa, T.; Chong, M.M.; Treuting, P.; Littman, D.R.; Rudensky, A.Y. Runx-CBFbeta complexes control expression of the transcription factor Foxp3 in regulatory T cells. Nat. Immunol. 2009, 10, 1170–1177. [Google Scholar] [CrossRef]
- Sekiya, T.; Kashiwagi, I.; Inoue, N.; Morita, R.; Hori, S.; Waldmann, H.; Rudensky, A.Y.; Ichinose, H.; Metzger, D.; Chambon, P.; et al. The nuclear orphan receptor Nr4a2 induces Foxp3 and regulates differentiation of CD4+ T cells. Nat. Commun. 2011, 2, 269. [Google Scholar] [CrossRef]
- Kerdiles, Y.M.; Stone, E.L.; Beisner, D.R.; McGargill, M.A.; Ch’en, I.L.; Stockmann, C.; Katayama, C.D.; Hedrick, S.M. Foxo transcription factors control regulatory T cell development and function. Immunity 2010, 33, 890–904. [Google Scholar] [CrossRef]
- Kitagawa, Y.; Ohkura, N.; Kidani, Y.; Vandenbon, A.; Hirota, K.; Kawakami, R.; Yasuda, K.; Motooka, D.; Nakamura, S.; Kondo, M.; et al. Guidance of regulatory T cell development by Satb1-dependent super-enhancer establishment. Nat. Immunol. 2017, 18, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Getnet, D.; Grosso, J.F.; Goldberg, M.V.; Harris, T.J.; Yen, H.R.; Bruno, T.C.; Durham, N.M.; Hipkiss, E.L.; Pyle, K.J.; Wada, S.; et al. A role for the transcription factor Helios in human CD4(+)CD25(+) regulatory T cells. Mol. Immunol. 2010, 47, 1595–1600. [Google Scholar] [CrossRef] [PubMed]
- Shevach, E.M. Mechanisms of foxp3+ T regulatory cell-mediated suppression. Immunity 2009, 30, 636–645. [Google Scholar] [CrossRef] [PubMed]
- Spolski, R.; Li, P.; Leonard, W.J. Biology and regulation of IL-2: From molecular mechanisms to human therapy. Nat. Rev. Immunol. 2018, 18, 648–659. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Gerner, M.Y.; Van Panhuys, N.; Levine, A.G.; Rudensky, A.Y.; Germain, R.N. Immune homeostasis enforced by co-localized effector and regulatory T cells. Nature 2015, 528, 225–230. [Google Scholar] [CrossRef] [PubMed]
- Onishi, Y.; Fehervari, Z.; Yamaguchi, T.; Sakaguchi, S. Foxp3+ natural regulatory T cells preferentially form aggregates on dendritic cells in vitro and actively inhibit their maturation. Proc. Natl. Acad. Sci. USA 2008, 105, 10113–10118. [Google Scholar] [CrossRef]
- Grossman, W.J.; Verbsky, J.W.; Barchet, W.; Colonna, M.; Atkinson, J.P.; Ley, T.J. Human T regulatory cells can use the perforin pathway to cause autologous target cell death. Immunity 2004, 21, 589–601. [Google Scholar] [CrossRef]
- Akkaya, B.; Oya, Y.; Akkaya, M.; Al Souz, J.; Holstein, A.H.; Kamenyeva, O.; Kabat, J.; Matsumura, R.; Dorward, D.W.; Glass, D.D.; et al. Regulatory T cells mediate specific suppression by depleting peptide-MHC class II from dendritic cells. Nat. Immunol. 2019, 20, 218–231. [Google Scholar] [CrossRef]
- Bennett, C.L.; Christie, J.; Ramsdell, F.; Brunkow, M.E.; Ferguson, P.J.; Whitesell, L.; Kelly, T.E.; Saulsbury, F.T.; Chance, P.F.; Ochs, H.D. The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of FOXP3. Nat. Genet. 2001, 27, 20–21. [Google Scholar] [CrossRef]
- Brunkow, M.E.; Jeffery, E.W.; Hjerrild, K.A.; Paeper, B.; Clark, L.B.; Yasayko, S.A.; Wilkinson, J.E.; Galas, D.; Ziegler, S.F.; Ramsdell, F. Disruption of a new forkhead/winged-helix protein, scurfin, results in the fatal lymphoproliferative disorder of the scurfy mouse. Nat. Genet. 2001, 27, 68–73. [Google Scholar] [CrossRef]
- Wildin, R.S.; Ramsdell, F.; Peake, J.; Faravelli, F.; Casanova, J.L.; Buist, N.; Levy-Lahad, E.; Mazzella, M.; Goulet, O.; Perroni, L.; et al. X-linked neonatal diabetes mellitus, enteropathy and endocrinopathy syndrome is the human equivalent of mouse scurfy. Nat. Genet. 2001, 27, 18–20. [Google Scholar] [CrossRef] [PubMed]
- Van Gool, F.; Nguyen, M.L.T.; Mumbach, M.R.; Satpathy, A.T.; Rosenthal, W.L.; Giacometti, S.; Le, D.T.; Liu, W.; Brusko, T.M.; Anderson, M.S.; et al. A Mutation in the Transcription Factor Foxp3 Drives T Helper 2 Effector Function in Regulatory T Cells. Immunity 2019, 50, 362–377.e366. [Google Scholar] [CrossRef]
- Chang, J.H.; Hu, H.; Jin, J.; Puebla-Osorio, N.; Xiao, Y.; Gilbert, B.E.; Brink, R.; Ullrich, S.E.; Sun, S.C. TRAF3 regulates the effector function of regulatory T cells and humoral immune responses. J. Exp. Med. 2014, 211, 137–151. [Google Scholar] [CrossRef]
- Zhang, R.; Huynh, A.; Whitcher, G.; Chang, J.; Maltzman, J.S.; Turka, L.A. An obligate cell-intrinsic function for CD28 in Tregs. J. Clin. Investig. 2013, 123, 580–593. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, M.; Miyazaki, K.; Chen, S.; Itoi, M.; Miller, M.; Lu, L.F.; Varki, N.; Chang, A.N.; Broide, D.H.; Murre, C. Id2 and Id3 maintain the regulatory T cell pool to suppress inflammatory disease. Nat. Immunol. 2014, 15, 767–776. [Google Scholar] [CrossRef]
- Chang, J.H.; Xiao, Y.; Hu, H.; Jin, J.; Yu, J.; Zhou, X.; Wu, X.; Johnson, H.M.; Akira, S.; Pasparakis, M.; et al. Ubc13 maintains the suppressive function of regulatory T cells and prevents their conversion into effector-like T cells. Nat. Immunol. 2012, 13, 481–490. [Google Scholar] [CrossRef]
- Layman, A.A.K.; Deng, G.; O’Leary, C.E.; Tadros, S.; Thomas, R.M.; Dybas, J.M.; Moser, E.K.; Wells, A.D.; Doliba, N.M.; Oliver, P.M. Ndfip1 restricts mTORC1 signalling and glycolysis in regulatory T cells to prevent autoinflammatory disease. Nat. Commun. 2017, 8, 15677. [Google Scholar] [CrossRef] [PubMed]
- Oh, H.; Grinberg-Bleyer, Y.; Liao, W.; Maloney, D.; Wang, P.; Wu, Z.; Wang, J.; Bhatt, D.M.; Heise, N.; Schmid, R.M.; et al. An NF-kappaB Transcription-Factor-Dependent Lineage-Specific Transcriptional Program Promotes Regulatory T Cell Identity and Function. Immunity 2017, 47, 450–465.e455. [Google Scholar] [CrossRef] [PubMed]
- Sebastian, M.; Lopez-Ocasio, M.; Metidji, A.; Rieder, S.A.; Shevach, E.M.; Thornton, A.M. Helios Controls a Limited Subset of Regulatory T Cell Functions. J. Immunol. 2016, 196, 144–155. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, A.C.; Wohlfert, E.; Chopp, L.B.; Vacchio, M.S.; Nie, J.; Zhao, Y.; Shetty, J.; Xiao, Q.; Deng, C.; Tran, B.; et al. Control of Regulatory T Cell Differentiation by the Transcription Factors Thpok and LRF. J. Immunol. 2017, 199, 1716–1728. [Google Scholar] [CrossRef]
- DuPage, M.; Chopra, G.; Quiros, J.; Rosenthal, W.L.; Morar, M.M.; Holohan, D.; Zhang, R.; Turka, L.; Marson, A.; Bluestone, J.A. The chromatin-modifying enzyme Ezh2 is critical for the maintenance of regulatory T cell identity after activation. Immunity 2015, 42, 227–238. [Google Scholar] [CrossRef] [PubMed]
- Roychoudhuri, R.; Hirahara, K.; Mousavi, K.; Clever, D.; Klebanoff, C.A.; Bonelli, M.; Sciume, G.; Zare, H.; Vahedi, G.; Dema, B.; et al. BACH2 represses effector programs to stabilize T(reg)-mediated immune homeostasis. Nature 2013, 498, 506–510. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.H.; Gasper, D.J.; Lee, S.H.; Plisch, E.H.; Svaren, J.; Suresh, M. Bach2 regulates homeostasis of Foxp3+ regulatory T cells and protects against fatal lung disease in mice. J. Immunol. 2014, 192, 985–995. [Google Scholar] [CrossRef] [PubMed]
- Kondo, M.; Tanaka, Y.; Kuwabara, T.; Naito, T.; Kohwi-Shigematsu, T.; Watanabe, A. SATB1 Plays a Critical Role in Establishment of Immune Tolerance. J. Immunol. 2016, 196, 563–572. [Google Scholar] [CrossRef]
- Cretney, E.; Xin, A.; Shi, W.; Minnich, M.; Masson, F.; Miasari, M.; Belz, G.T.; Smyth, G.K.; Busslinger, M.; Nutt, S.L.; et al. The transcription factors Blimp-1 and IRF4 jointly control the differentiation and function of effector regulatory T cells. Nat. Immunol. 2011, 12, 304–311. [Google Scholar] [CrossRef]
- Yang, K.; Blanco, D.B.; Neale, G.; Vogel, P.; Avila, J.; Clish, C.B.; Wu, C.; Shrestha, S.; Rankin, S.; Long, L.; et al. Homeostatic control of metabolic and functional fitness of Treg cells by LKB1 signalling. Nature 2017, 548, 602–606. [Google Scholar] [CrossRef]
- Yu, D.; Rao, S.; Tsai, L.M.; Lee, S.K.; He, Y.; Sutcliffe, E.L.; Srivastava, M.; Linterman, M.; Zheng, L.; Simpson, N.; et al. The transcriptional repressor Bcl-6 directs T follicular helper cell lineage commitment. Immunity 2009, 31, 457–468. [Google Scholar] [CrossRef]
- Nurieva, R.I.; Chung, Y.; Martinez, G.J.; Yang, X.O.; Tanaka, S.; Matskevitch, T.D.; Wang, Y.H.; Dong, C. Bcl6 mediates the development of T follicular helper cells. Science 2009, 325, 1001–1005. [Google Scholar] [CrossRef]
- Johnston, R.J.; Poholek, A.C.; DiToro, D.; Yusuf, I.; Eto, D.; Barnett, B.; Dent, A.L.; Craft, J.; Crotty, S. Bcl6 and Blimp-1 are reciprocal and antagonistic regulators of T follicular helper cell differentiation. Science 2009, 325, 1006–1010. [Google Scholar] [CrossRef]
- Vinuesa, C.G.; Linterman, M.A.; Yu, D.; MacLennan, I.C. Follicular Helper T Cells. Annu. Rev. Immunol. 2016, 34, 335–368. [Google Scholar] [CrossRef]
- Roco, J.A.; Mesin, L.; Binder, S.C.; Nefzger, C.; Gonzalez-Figueroa, P.; Canete, P.F.; Ellyard, J.; Shen, Q.; Robert, P.A.; Cappello, J.; et al. Class-Switch Recombination Occurs Infrequently in Germinal Centers. Immunity 2019, 51, 337–350.e337. [Google Scholar] [CrossRef]
- Nurieva, R.I.; Chung, Y.; Hwang, D.; Yang, X.O.; Kang, H.S.; Ma, L.; Wang, Y.H.; Watowich, S.S.; Jetten, A.M.; Tian, Q.; et al. Generation of T follicular helper cells is mediated by interleukin-21 but independent of T helper 1, 2, or 17 cell lineages. Immunity 2008, 29, 138–149. [Google Scholar] [CrossRef] [PubMed]
- Lu, K.T.; Kanno, Y.; Cannons, J.L.; Handon, R.; Bible, P.; Elkahloun, A.G.; Anderson, S.M.; Wei, L.; Sun, H.; O’Shea, J.J.; et al. Functional and epigenetic studies reveal multistep differentiation and plasticity of in vitro-generated and in vivo-derived follicular T helper cells. Immunity 2011, 35, 622–632. [Google Scholar] [CrossRef] [PubMed]
- Fazilleau, N.; McHeyzer-Williams, L.J.; Rosen, H.; McHeyzer-Williams, M.G. The function of follicular helper T cells is regulated by the strength of T cell antigen receptor binding. Nat. Immunol. 2009, 10, 375–384. [Google Scholar] [CrossRef] [PubMed]
- Spolski, R.; Leonard, W.J. Interleukin-21: A double-edged sword with therapeutic potential. Nat. Rev. Drug Discov. 2014, 13, 379–395. [Google Scholar] [CrossRef]
- DiToro, D.; Winstead, C.J.; Pham, D.; Witte, S.; Andargachew, R.; Singer, J.R.; Wilson, C.G.; Zindl, C.L.; Luther, R.J.; Silberger, D.J.; et al. Differential IL-2 expression defines developmental fates of follicular versus nonfollicular helper T cells. Science 2018, 361, eaao2933. [Google Scholar] [CrossRef] [PubMed]
- Crotty, S. T follicular helper cell differentiation, function, and roles in disease. Immunity 2014, 41, 529–542. [Google Scholar] [CrossRef]
- Ma, C.S.; Deenick, E.K.; Batten, M.; Tangye, S.G. The origins, function, and regulation of T follicular helper cells. J. Exp. Med. 2012, 209, 1241–1253. [Google Scholar] [CrossRef]
- Liu, X.; Chen, X.; Zhong, B.; Wang, A.; Wang, X.; Chu, F.; Nurieva, R.I.; Yan, X.; Chen, P.; van der Flier, L.G.; et al. Transcription factor achaete-scute homologue 2 initiates follicular T-helper-cell development. Nature 2014, 507, 513–518. [Google Scholar] [CrossRef]
- Xiao, N.M.; Eto, D.; Elly, C.; Peng, G.Y.; Crotty, S.; Liu, Y.C. The E3 ubiquitin ligase Itch is required for the differentiation of follicular helper T cells. Nat. Immunol. 2014, 15, 657–666. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhao, Y.; Zou, L.; Zhang, D.; Aki, D.; Liu, Y.C. The E3 ligase VHL promotes follicular helper T cell differentiation via glycolytic-epigenetic control. J. Exp. Med. 2019, 216, 1664–1681. [Google Scholar] [CrossRef]
- Liu, Z.; Zhu, L.; Lu, Z.; Chen, H.; Fan, L.; Xue, Q.; Shi, J.; Li, M.; Li, H.; Gong, J.; et al. IL-37 Represses the Autoimmunity in Myasthenia Gravis via Directly Targeting Follicular Th and B Cells. J. Immunol. 2020, 204, 1736–1745. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.H.; Raybuck, A.L.; Blagih, J.; Kemboi, E.; Haase, V.H.; Jones, R.G.; Boothby, M.R. Hypoxia-inducible factors in CD4(+) T cells promote metabolism, switch cytokine secretion, and T cell help in humoral immunity. Proc. Natl. Acad. Sci. USA 2019, 116, 8975–8984. [Google Scholar] [CrossRef]
- Liu, T.; Yang, Q.; Cao, Y.J.; Yuan, W.M.; Lei, A.H.; Zhou, P.; Zhou, W.; Liu, Y.D.; Shi, M.H.; Yang, Q.; et al. Cyclooxygenase-1 Regulates the Development of Follicular Th Cells via Prostaglandin E2. J. Immunol. 2019, 203, 864–872. [Google Scholar] [CrossRef]
- Vacchio, M.S.; Ciucci, T.; Gao, Y.; Watanabe, M.; Balmaceno-Criss, M.; McGinty, M.T.; Huang, A.; Xiao, Q.; McConkey, C.; Zhao, Y.; et al. A Thpok-Directed Transcriptional Circuitry Promotes Bcl6 and Maf Expression to Orchestrate T Follicular Helper Differentiation. Immunity 2019, 51, 465–478.e466. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Zhao, X.; Wang, X.; Feng, H.; Gou, M.; Jin, W.; Wang, X.; Liu, X.; Dong, C. The Transcription Factor Tox2 Drives T Follicular Helper Cell Development via Regulating Chromatin Accessibility. Immunity 2019, 51, 826–839.e825. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, Y.; Dutta, P.R.; Cerasoli, D.M.; Kelsoe, G. In situ studies of the primary immune response to (4-hydroxy-3-nitrophenyl)acetyl. V. Affinity maturation develops in two stages of clonal selection. J. Exp. Med. 1998, 187, 885–895. [Google Scholar] [CrossRef]
- Han, S.; Hathcock, K.; Zheng, B.; Kepler, T.B.; Hodes, R.; Kelsoe, G. Cellular interaction in germinal centers. Roles of CD40 ligand and B7-2 in established germinal centers. J. Immunol. 1995, 155, 556–567. [Google Scholar] [PubMed]
- Zotos, D.; Coquet, J.M.; Zhang, Y.; Light, A.; D’Costa, K.; Kallies, A.; Corcoran, L.M.; Godfrey, D.I.; Toellner, K.M.; Smyth, M.J.; et al. IL-21 regulates germinal center B cell differentiation and proliferation through a B cell-intrinsic mechanism. J. Exp. Med. 2010, 207, 365–378. [Google Scholar] [CrossRef] [PubMed]
- Linterman, M.A.; Beaton, L.; Yu, D.; Ramiscal, R.R.; Srivastava, M.; Hogan, J.J.; Verma, N.K.; Smyth, M.J.; Rigby, R.J.; Vinuesa, C.G. IL-21 acts directly on B cells to regulate Bcl-6 expression and germinal center responses. J. Exp. Med. 2010, 207, 353–363. [Google Scholar] [CrossRef]
- Van Besouw, N.M.; Mendoza Rojas, A.; Baan, C.C. The role of follicular T helper cells in the humoral alloimmune response after clinical organ transplantation. HLA 2019, 94, 407–414. [Google Scholar] [CrossRef]
- Gowthaman, U.; Chen, J.S.; Zhang, B.; Flynn, W.F.; Lu, Y.; Song, W.; Joseph, J.; Gertie, J.A.; Xu, L.; Collet, M.A.; et al. Identification of a T follicular helper cell subset that drives anaphylactic IgE. Science 2019, 365, eaaw6433. [Google Scholar] [CrossRef] [PubMed]
- Simpson, N.; Gatenby, P.A.; Wilson, A.; Malik, S.; Fulcher, D.A.; Tangye, S.G.; Manku, H.; Vyse, T.J.; Roncador, G.; Huttley, G.A.; et al. Expansion of circulating T cells resembling follicular helper T cells is a fixed phenotype that identifies a subset of severe systemic lupus erythematosus. Arthritis Rheum. 2010, 62, 234–244. [Google Scholar] [CrossRef]
- Faliti, C.E.; Gualtierotti, R.; Rottoli, E.; Gerosa, M.; Perruzza, L.; Romagnani, A.; Pellegrini, G.; De Ponte Conti, B.; Rossi, R.L.; Idzko, M.; et al. P2X7 receptor restrains pathogenic Tfh cell generation in systemic lupus erythematosus. J. Exp. Med. 2019, 216, 317–336. [Google Scholar] [CrossRef]
- Psianou, K.; Panagoulias, I.; Papanastasiou, A.D.; de Lastic, A.L.; Rodi, M.; Spantidea, P.I.; Degn, S.E.; Georgiou, P.; Mouzaki, A. Clinical and immunological parameters of Sjogren’s syndrome. Autoimmun. Rev. 2018, 17, 1053–1064. [Google Scholar] [CrossRef] [PubMed]
- Morita, R.; Schmitt, N.; Bentebibel, S.E.; Ranganathan, R.; Bourdery, L.; Zurawski, G.; Foucat, E.; Dullaers, M.; Oh, S.; Sabzghabaei, N.; et al. Human blood CXCR5(+)CD4(+) T cells are counterparts of T follicular cells and contain specific subsets that differentially support antibody secretion. Immunity 2011, 34, 108–121. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Wei, Y.; Fonseca, V.R.; Graca, L.; Yu, D. T follicular helper cells and T follicular regulatory cells in rheumatic diseases. Nat. Rev. Rheumatol. 2019, 15, 475–490. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.S.; Avery, D.T.; Chan, A.; Batten, M.; Bustamante, J.; Boisson-Dupuis, S.; Arkwright, P.D.; Kreins, A.Y.; Averbuch, D.; Engelhard, D.; et al. Functional STAT3 deficiency compromises the generation of human T follicular helper cells. Blood 2012, 119, 3997–4008. [Google Scholar] [CrossRef]
- Lee, W.I.; Torgerson, T.R.; Schumacher, M.J.; Yel, L.; Zhu, Q.; Ochs, H.D. Molecular analysis of a large cohort of patients with the hyper immunoglobulin M (IgM) syndrome. Blood 2005, 105, 1881–1890. [Google Scholar] [CrossRef]
- Du, X.; Tang, W.; Chen, X.; Zeng, T.; Wang, Y.; Chen, Z.; Xu, T.; Zhou, L.; Tang, X.; An, Y.; et al. Clinical, genetic and immunological characteristics of 40 Chinese patients with CD40 ligand deficiency. Scand. J. Immunol. 2019, 90, e12798. [Google Scholar] [CrossRef]
- Bossaller, L.; Burger, J.; Draeger, R.; Grimbacher, B.; Knoth, R.; Plebani, A.; Durandy, A.; Baumann, U.; Schlesier, M.; Welcher, A.A.; et al. ICOS deficiency is associated with a severe reduction of CXCR5+CD4 germinal center Th cells. J. Immunol. 2006, 177, 4927–4932. [Google Scholar] [CrossRef]
- Strutt, T.M.; McKinstry, K.K.; Dibble, J.P.; Winchell, C.; Kuang, Y.; Curtis, J.D.; Huston, G.; Dutton, R.W.; Swain, S.L. Memory CD4+ T cells induce innate responses independently of pathogen. Nat. Med. 2010, 16, 558–564. [Google Scholar] [CrossRef]
- Kawabe, T.; Jankovic, D.; Kawabe, S.; Huang, Y.; Lee, P.H.; Yamane, H.; Zhu, J.; Sher, A.; Germain, R.N.; Paul, W.E. Memory-phenotype CD4(+) T cells spontaneously generated under steady-state conditions exert innate TH1-like effector function. Sci. Immunol. 2017, 2. [Google Scholar] [CrossRef]
- Yu, F.; Sharma, S.; Edwards, J.; Feigenbaum, L.; Zhu, J. Dynamic expression of transcription factors T-bet and GATA-3 by regulatory T cells maintains immunotolerance. Nat. Immunol. 2015, 16, 197–206. [Google Scholar] [CrossRef]
- Zhou, L.; Chong, M.M.; Littman, D.R. Plasticity of CD4+ T cell lineage differentiation. Immunity 2009, 30, 646–655. [Google Scholar] [CrossRef]
- Sakaguchi, S.; Vignali, D.A.; Rudensky, A.Y.; Niec, R.E.; Waldmann, H. The plasticity and stability of regulatory T cells. Nat. Rev. Immunol. 2013, 13, 461–467. [Google Scholar] [CrossRef] [PubMed]
- Stubbington, M.J.T.; Rozenblatt-Rosen, O.; Regev, A.; Teichmann, S.A. Single-cell transcriptomics to explore the immune system in health and disease. Science 2017, 358, 58–63. [Google Scholar] [CrossRef]
- Ledford, H. Quest to use CRISPR against disease gains ground. Nature 2020, 577, 156. [Google Scholar] [CrossRef]
- Renaud, J.P.; Chari, A.; Ciferri, C.; Liu, W.T.; Remigy, H.W.; Stark, H.; Wiesmann, C. Cryo-EM in drug discovery: Achievements, limitations and prospects. Nat. Rev. Drug Discov. 2018, 17, 471–492. [Google Scholar] [CrossRef] [PubMed]
- Bendall, S.C.; Simonds, E.F.; Qiu, P.; El-ad, D.A.; Krutzik, P.O.; Finck, R.; Bruggner, R.V.; Melamed, R.; Trejo, A.; Ornatsky, O.I.; et al. Single-cell mass cytometry of differential immune and drug responses across a human hematopoietic continuum. Science 2011, 332, 687–696. [Google Scholar] [CrossRef] [PubMed]
- Germain, R.N.; Robey, E.A.; Cahalan, M.D. A decade of imaging cellular motility and interaction dynamics in the immune system. Science 2012, 336, 1676–1681. [Google Scholar] [CrossRef] [PubMed]
- Qi, H.; Kastenmuller, W.; Germain, R.N. Spatiotemporal basis of innate and adaptive immunity in secondary lymphoid tissue. Annu. Rev. Cell Dev. Biol. 2014, 30, 141–167. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; van Panhuys, N.; Kastenmuller, W.; Germain, R.N. The future of immunoimaging—Deeper, bigger, more precise, and definitively more colorful. Eur. J. Immunol. 2013, 43, 1407–1412. [Google Scholar] [CrossRef]
- Schreiner, D.; King, C.G. CD4+ Memory T Cells at Home in the Tissue: Mechanisms for Health and Disease. Front. Immunol. 2018, 9, 2394. [Google Scholar] [CrossRef] [PubMed]
- Jameson, S.C.; Masopust, D. Understanding Subset Diversity in T Cell Memory. Immunity 2018, 48, 214–226. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).