‘Candidatus Liberibacter Asiaticus’ SDE1 Effector Induces Huanglongbing Chlorosis by Downregulating Host DDX3 Gene
Abstract
1. Introduction
2. Results
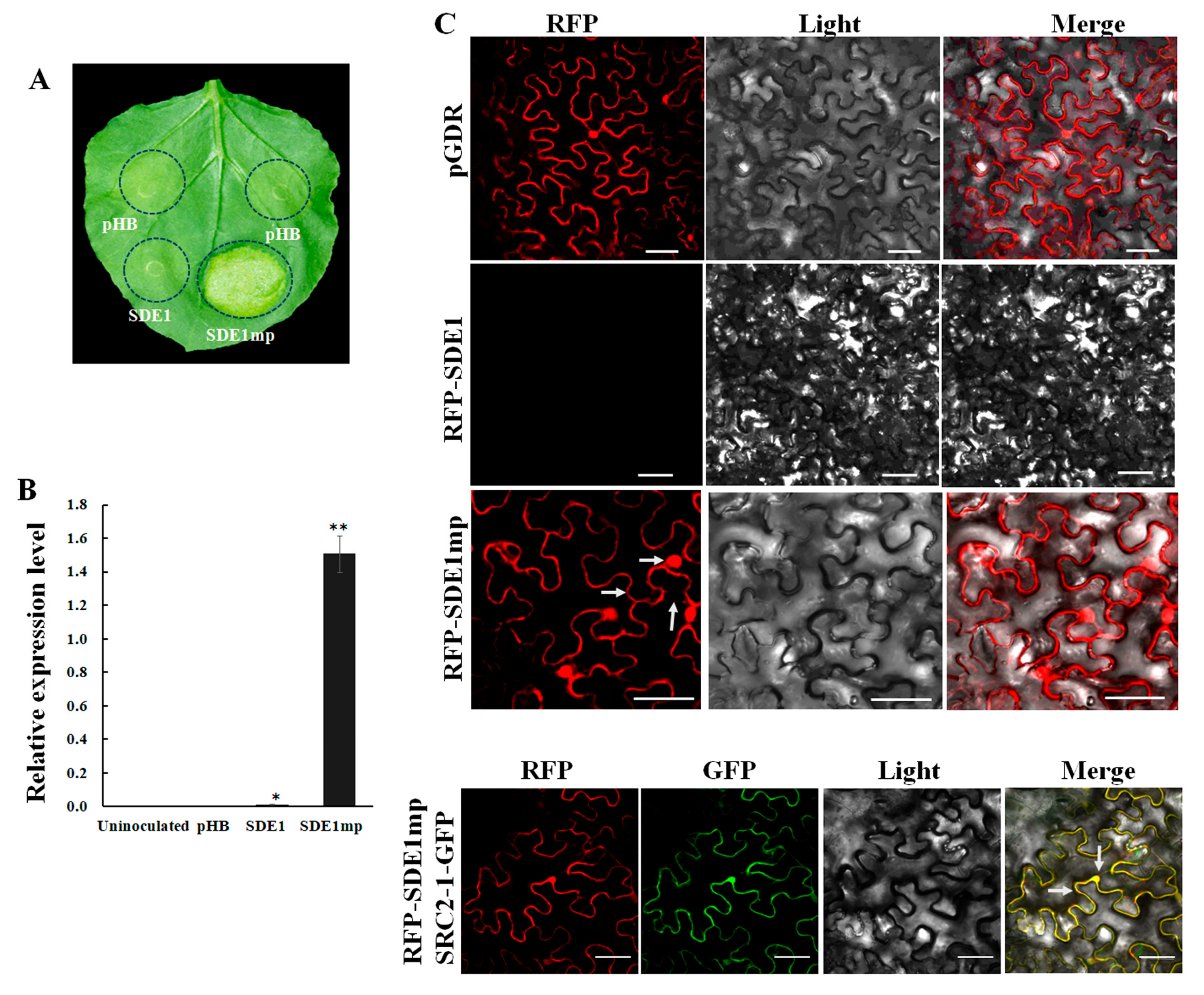
2.1. The Full-Length SDE1 Gene Is Not Transcribed during Agrobacterium-Mediated Transient Expression in N. benthamiana
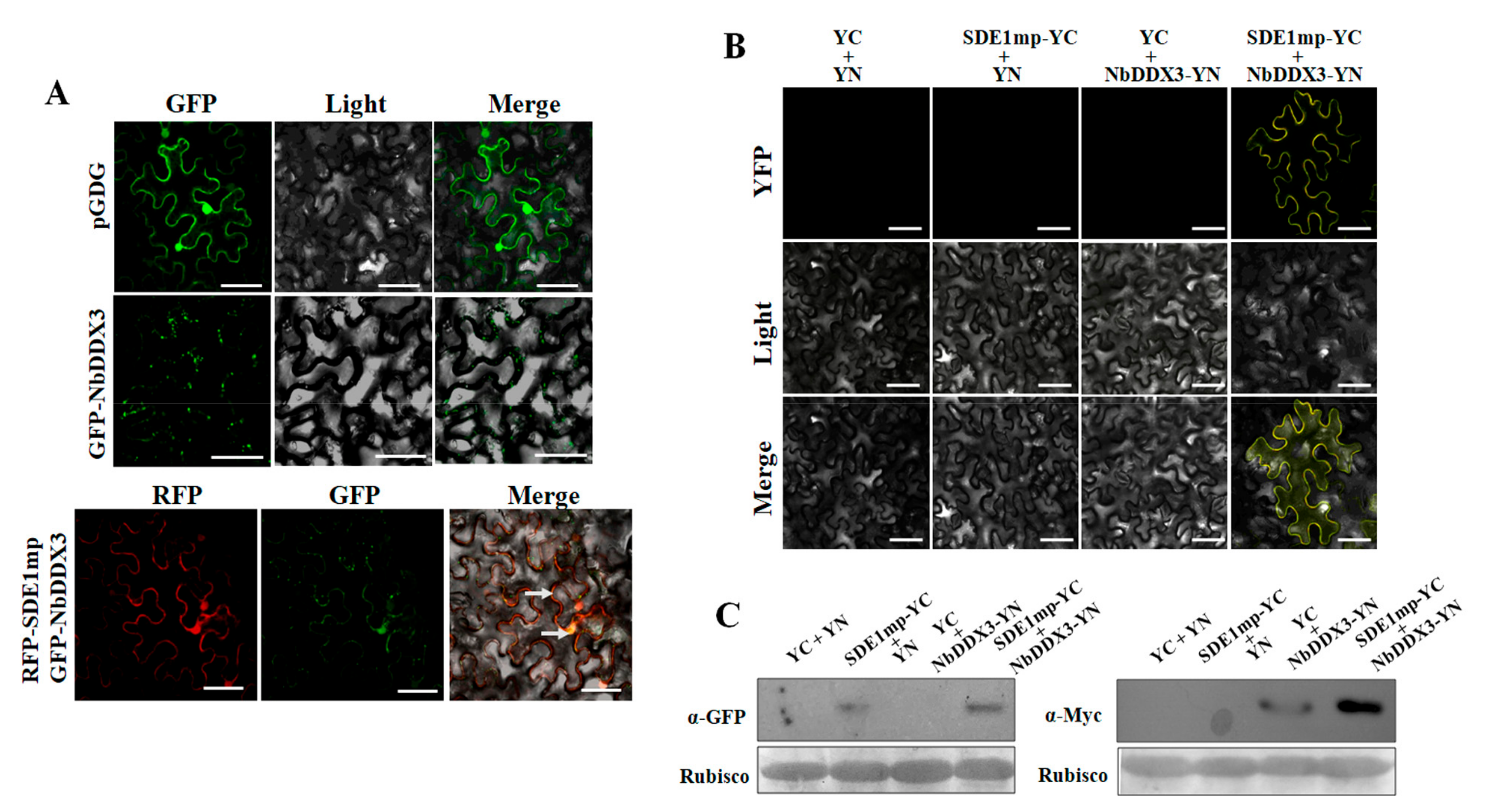
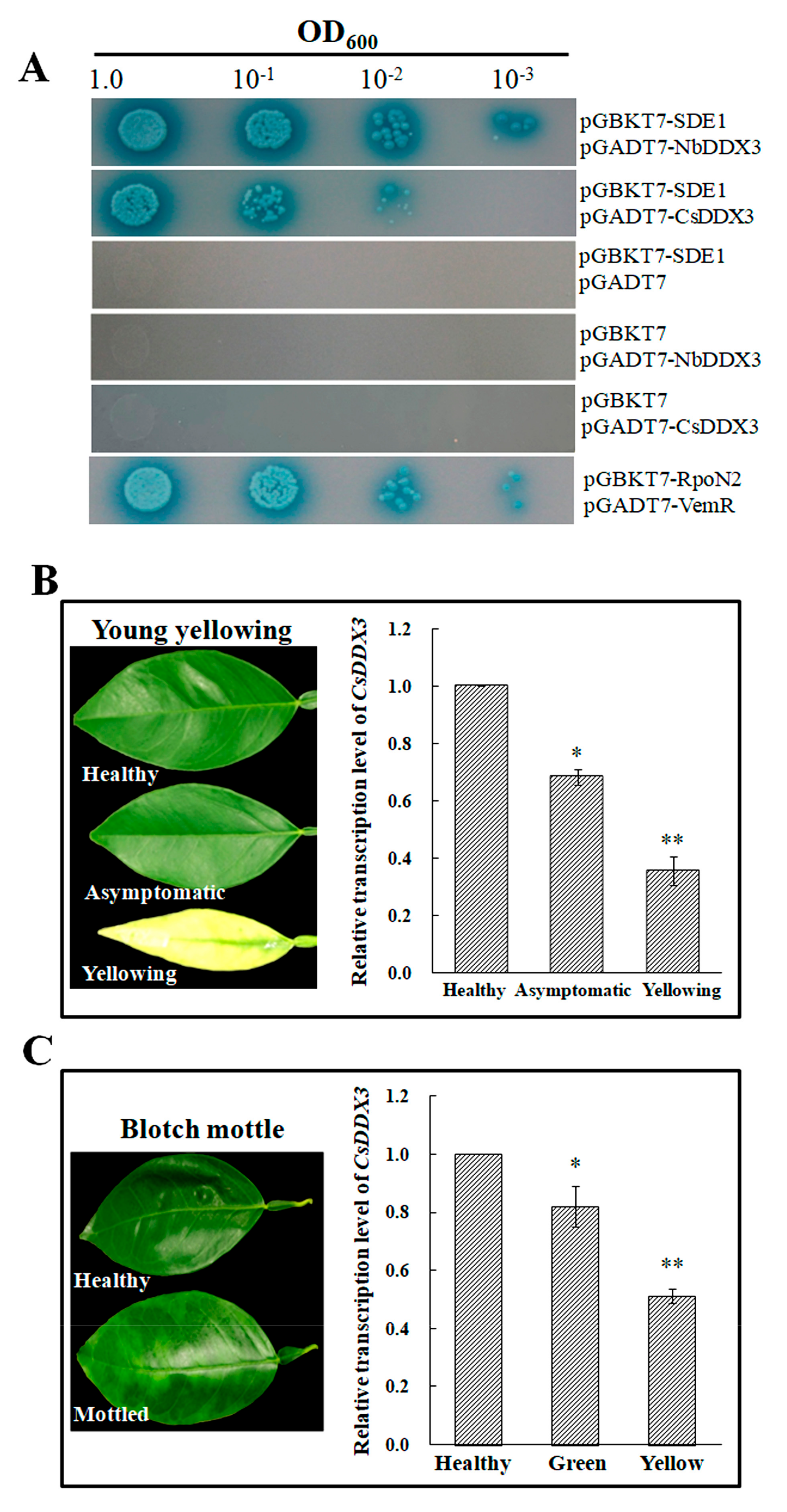
2.2. SDE1 Interacts with the N. benthamiana DDX3
2.3. Interaction between SDE1 and NbDDX3 Is Localized at the Cell Membrane
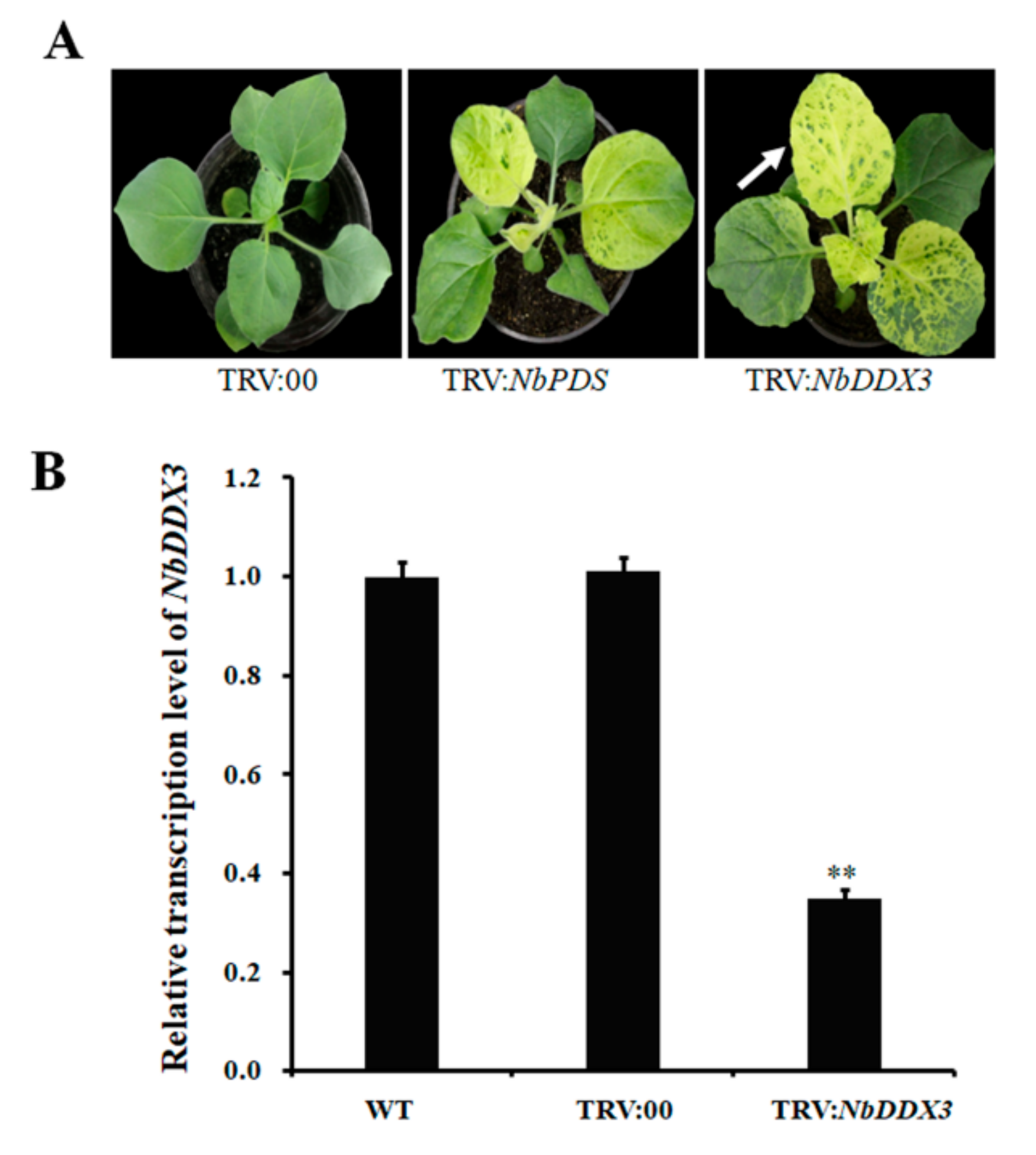
2.4. Silencing of NbDDX3 Results in Mottled Leaves with Chlorosis
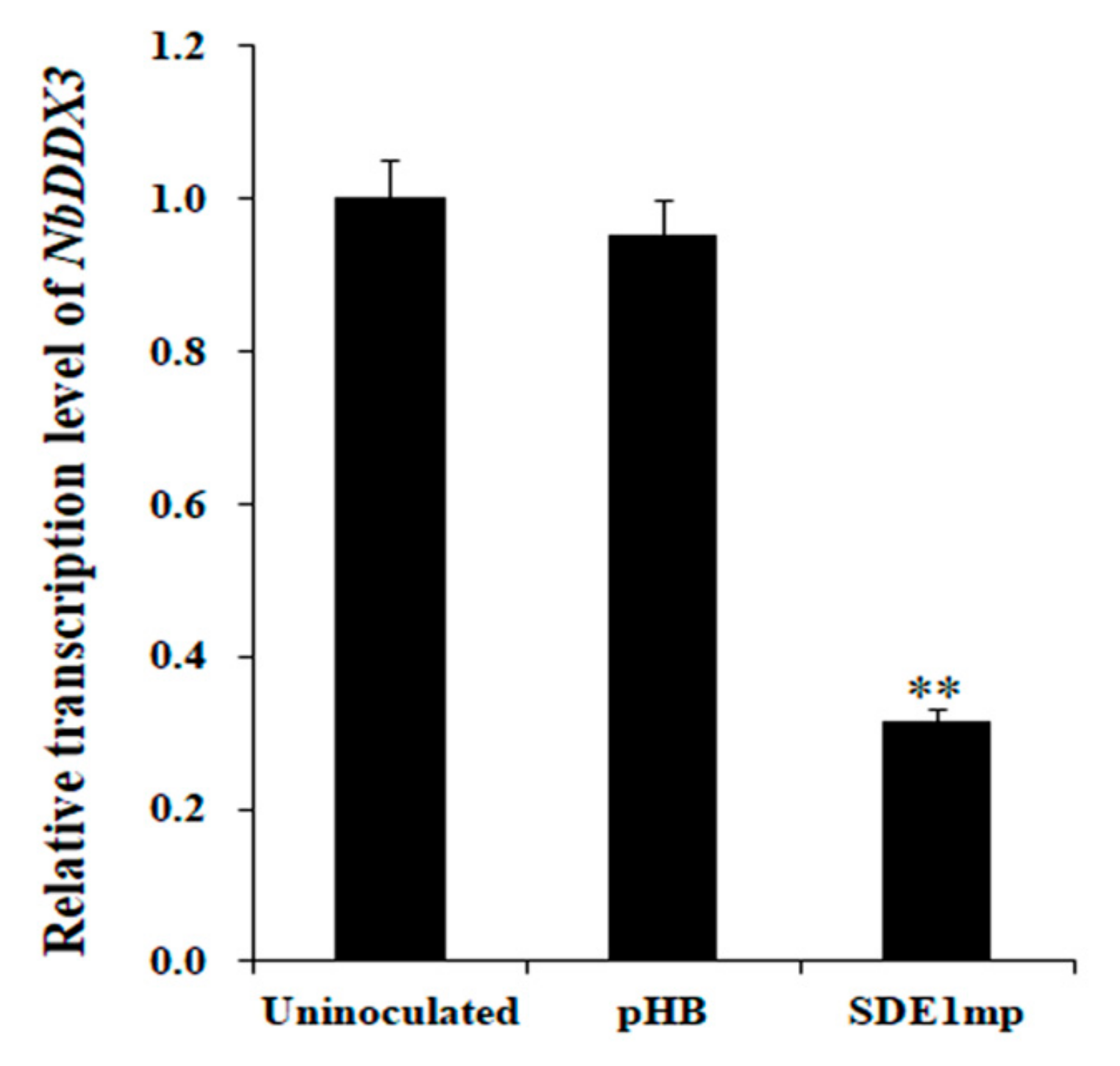
2.5. Transient Expression of SDE1 Suppresses NbDDX3 Gene Expression
2.6. The Citrus Homolog CsDDX3 Interacts with SDE1 and Shows Reduced Expression in HLB-Infected Leaf Tissue
3. Discussion
4. Materials and Methods
4.1. Plant and Bacterial Materials
4.2. DNA Manipulation and Plasmid Construction
4.3. Agrobacterium-Mediated Transient Expression
4.4. qRT-PCR Analysis
4.5. Yeast Two-Hybrid Assay
4.6. Virus-Induced Gene Silencing
4.7. Western Blot Analysis
4.8. Sequence Analysis
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Bové, J.M. Huanglongbing: A destructive, newly-emerging, century-old disease of citrus. J. Plant Pathol. 2006, 88, 7–37. [Google Scholar]
- Jagoueix, S.; Bové, J.M.; Garnier, M. The phloem-limited bacterium of greening disease of citrus is a member of the alpha subdivision of the Proteobacteria. Int. J. Syst. Bacteriol. 1994, 44, 379–486. [Google Scholar] [CrossRef]
- Duan, Y.P.; Gottwald, T.R.; Zhou, L.J.; Gabriel, D.W. First report of dodder transmission of ‘Candidatus Liberibacter a siaticus’ to tomato (Lycopersicon esculentum). Plant Dis. 2008, 92, 831. [Google Scholar] [CrossRef] [PubMed]
- Francischini, F.J.B.; Oliveira, K.D.S.; Astúa-Monge, G.; Novelli, A.; Lorenzino, R.; Matiolli, C.; Kemper, E.; Da Silva, A.C.R.; Kitajima, E.W. First report on the transmission of ‘Candidatus Liberibacter americanus’ from citrus to Nicotiana tabacum cv. Xanthi. Plant Dis. 2017, 91, 631. [Google Scholar] [CrossRef] [PubMed]
- Garnier, M.; Bové, J.M. Transmission of the organism associated with citrus greening disease from sweet orange to periwinkle by dodder. Phytopathology 1983, 73, 1358–1363. [Google Scholar] [CrossRef]
- Pitino, M.; Allen, V.; Duan, Y.P. Las∆15315 effector induces extreme starch accumulation and chlorosis as ‘Candidatus Liberibacter asiaticus’ infection in Nicotiana benthamiana. Front. Plant Sci. 2018, 9, 113. [Google Scholar] [CrossRef] [PubMed]
- Halbert, S.E.; Manjunath, K.L. Asian citrus psyllids (Sternorrhyncha: Psyllidae) and greening disease of citrus: A literature review and assessment of risk in Florida. Fla. Entomol. 2004, 87, 330–353. [Google Scholar] [CrossRef]
- Ding, F.; Duan, Y.P.; Paul, C.; Brlansky, R.H.; Hartung, J.S. Localization and distribution of ‘Candidatus Liberibacter asiaticus’ in citrus and periwinkle by direct tissue blot immuno assay with an anti-OmpA polyclonal antibody. PLoS ONE 2015, 10, e0123939. [Google Scholar] [CrossRef] [PubMed]
- Folimonova, S.Y.; Achor, D.S. Early events of citrus greening (Huanglongbing) disease development at the ultrastructural level. Phytopathology 2010, 100, 949–958. [Google Scholar] [CrossRef] [PubMed]
- Schneider, H. Anatomy of greening-diseased sweet orange shoots. Phytopathology 1968, 58, 1155–1160. [Google Scholar]
- Liao, H.L.; Burns, J.K. Gene expression in Citrus sinensis fruit tissues harvested from huanglongbing infected trees: Comparison with girdled fruit. J. Exp. Bot. 2012, 63, 3307–3319. [Google Scholar] [CrossRef] [PubMed]
- Martinelli, F.; Uratsu, S.L.; Albrecht, U.; Reagan, R.L.; Phu, M.L.; Britton, M.; Buffalo, V.; Fass, J.; Leicht, E.; Zhao, W.; et al. Transcriptome profiling of citrus fruit response to huanglongbing disease. PLoS ONE 2012, 7, e38039. [Google Scholar] [CrossRef] [PubMed]
- Masaoka, Y.; Pustika, A.; Subandiyah, S.; Okada, A.; Hanundin, E.; Purwanto, B.; Okuda, M.; Okada, Y.; Saito, A.; Holford, P.; et al. Lower concentrations of microelements in leaves of citrus infected with ‘Candidatus Liberibacter asiaticus’. Jpn. Agric. Res. Q. 2011, 45, 269–275. [Google Scholar] [CrossRef]
- Razi, M.F.; Khan, I.A.; Jaskani, M.J. Citrus plant nutritional profile in relation to Huanglongbing prevalence in Pakistan. Pak. J. Agric. Sci. 2011, 48, 299–304. [Google Scholar]
- Prasad, S.; Xu, J.; Zhang, Y.; Wang, N. Sec-translocon dependent extracytoplasmic proteins of ‘Candidatus Liberibacter asiaticus’. Front. Microbiol. 2016, 7, 1989. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Ying, X.; Shang, L.; Redfern, B.; Kypraios, N.; Xie, X.; Xu, F.; Wang, S.; Zhang, J.; Jian, H.; et al. Heterologous expression of CLIBASIA_03915/CLIBASIA_04250 by tobacco mosaic virus resulted in phloem necrosis in the senescent leaves of Nicotiana benthamiana. Int. J. Mol. Sci. 2020, 21, 1414. [Google Scholar] [CrossRef]
- Shi, Q.; Pitino, M.; Zhang, S.; Krystel, J.; Cano, L.M.; Shatters, R.G., Jr.; Hall, D.G.; Stover, E. Temporal and spatial detection of Candidatus Liberibacter asiaticus putative effector transcripts during interaction with Huanglongbing-susceptible, -tolerant, and -resistant citrus hosts. BMC Plant Biol. 2019, 19, 122. [Google Scholar] [CrossRef]
- Yan, Q.; Sreedharan, A.; Wei, S.; Wang, J.; Pelz-Stelinski, K.; Folimonova, S.; Wang, N. Global gene expression changes in ‘Candidatus Liberibacter asiaticus’ during the transmission in distinct hosts between plant and insect. Mol. Plant Pathol. 2013, 14, 391–404. [Google Scholar] [CrossRef]
- Clark, K.; Franco, J.Y.; Schwizer, S.; Pang, Z.; Hawara, E.; Liebrand, T.W.H.; Pagliaccia, D.; Zeng, L.; Gurung, F.B.; Wang, P.; et al. An effector from the Huanglongbing-associated pathogen targets citrus proteases. Nat. Commun. 2018, 9, 1718. [Google Scholar] [CrossRef]
- Pitino, M.; Armstrong, C.M.; Cano, L.M.; Duan, Y.P. Transient expression of ‘Candidatus Liberibacter asiaticus’ effector induces cell death in Nicotiana benthamiana. Front. Plant Sci. 2016, 7, 982. [Google Scholar] [CrossRef]
- Ariumi, Y. Multiple functions of DDX3 RNA helicase in gene regulation, tumorigenesis, and viral infection. Front. Genet. 2014, 5, 423. [Google Scholar] [CrossRef] [PubMed]
- Gong, Z.; Dong, C.H.; Lee, H.; Zhu, J.; Xiong, L.; Gong, D.; Stevenson, B.; Zhu, J.K. A DEAD box RNA helicase is essential for mRNA export and important for development and stress responses in Arabidopsis. Plant Cell 2005, 17, 256–267. [Google Scholar] [CrossRef]
- Khan, A.; Garbelli, A.; Grossi, S.; Florentin, A.; Batelli, G.; Acuna, T.; Zolla, G.; Kaye, Y.; Paul, L.K.; Zhu, J.K.; et al. The Arabidopsis STRESS RESPONSE SUPPRESSOR DEAD-box RNA helicases are nucleolar-and chromocenter-localized proteins that undergo stress-mediated relocalization and are involved in epigenetic gene silencing. Plant J. 2014, 79, 28–43. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Chen, G.; Dong, T.; Wang, L.; Zhang, J.; Zhao, Z.; Hu, Z. SlDEAD31, a putative DEAD-box RNA helicase gene, regulates salt and drought tolerance and stress-related genes in tomato. PLoS ONE 2015, 10, e0133849. [Google Scholar] [CrossRef]
- Sambrook, J.; Fritsch, E.F.; Maniatis, T. Molecular Cloning: A Laboratory Manual, 2nd ed.; Cold Spring Harbor Laboratory Pres: New York, NY, USA, 1989. [Google Scholar]
- Li, Y.R.; Che, Y.Z.; Zou, H.S.; Cui, Y.P.; Guo, W.; Zou, L.F.; Biddle, E.M.; Yang, C.H.; Chen, G.Y. Hpa2 required by HrpF to translocate Xanthomonas oryzae transcriptional activator-like effectors into rice for pathogenicity. Appl. Environ. Microbiol. 2011, 77, 3809–3818. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.Q.; Qiu, A.L.; Shi, L.P.; Cai, J.S.; Huang, X.Y.; Yang, S.; Wang, B.; Shen, L.; Huang, M.K.; Mou, S.L.; et al. SRC2-1 is required in PcINF1-induced pepper immunity by acting as an interacting partner of PcINF1. J. Exp. Bot. 2015, 66, 3683–3698. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Guo, J.; Zhou, Y.; Zhuo, T.; Hu, X.; Zou, H. The ColRS-regulated membrane protein gene XAC1347 is involved in copper homeostasis and hrp gene expression in Xanthomonas citri subsp citri. Front. Microbiol. 2018, 9, 1171. [Google Scholar] [CrossRef]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative C (T) method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef]
- Wu, W.; Zhao, Z.; Luo, X.; Fan, X.; Zhuo, T.; Hu, X.; Liu, J.; Zou, H. Response regulator VemR regulates the transcription of flagellar rod gene flgG by interacting with σ54 factor RpoN2 in Xanthomonas citri ssp. citri. Mol. Plant Pathol. 2019, 20, 372–381. [Google Scholar] [CrossRef]
- Liu, Y.; Schiff, M.; Dinesh-Kumar, S.P. Tobacco Rar1, EDS1 and NPR1/NIM1 like genes are required for N-mediated resistance to tobacco mosaic virus. Plant J. 2002, 30, 415–429. [Google Scholar] [CrossRef]
- Martinez-Garcia, J.F.; Monte, E.; Quail, P.H. A simple, rapid and quantitative method for preparing Arabidopsis protein extracts for immunoblot analysis. Plant J. 1999, 20, 251–257. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Y.; Wei, X.; Li, Y.; Liu, Z.; Duan, Y.; Zou, H. ‘Candidatus Liberibacter Asiaticus’ SDE1 Effector Induces Huanglongbing Chlorosis by Downregulating Host DDX3 Gene. Int. J. Mol. Sci. 2020, 21, 7996. https://doi.org/10.3390/ijms21217996
Zhou Y, Wei X, Li Y, Liu Z, Duan Y, Zou H. ‘Candidatus Liberibacter Asiaticus’ SDE1 Effector Induces Huanglongbing Chlorosis by Downregulating Host DDX3 Gene. International Journal of Molecular Sciences. 2020; 21(21):7996. https://doi.org/10.3390/ijms21217996
Chicago/Turabian StyleZhou, Yinghui, Xiangying Wei, Yanjiao Li, Zhiqin Liu, Yongping Duan, and Huasong Zou. 2020. "‘Candidatus Liberibacter Asiaticus’ SDE1 Effector Induces Huanglongbing Chlorosis by Downregulating Host DDX3 Gene" International Journal of Molecular Sciences 21, no. 21: 7996. https://doi.org/10.3390/ijms21217996
APA StyleZhou, Y., Wei, X., Li, Y., Liu, Z., Duan, Y., & Zou, H. (2020). ‘Candidatus Liberibacter Asiaticus’ SDE1 Effector Induces Huanglongbing Chlorosis by Downregulating Host DDX3 Gene. International Journal of Molecular Sciences, 21(21), 7996. https://doi.org/10.3390/ijms21217996






