Significance of 5-S-Cysteinyldopa as a Marker for Melanoma
Abstract
1. Introduction
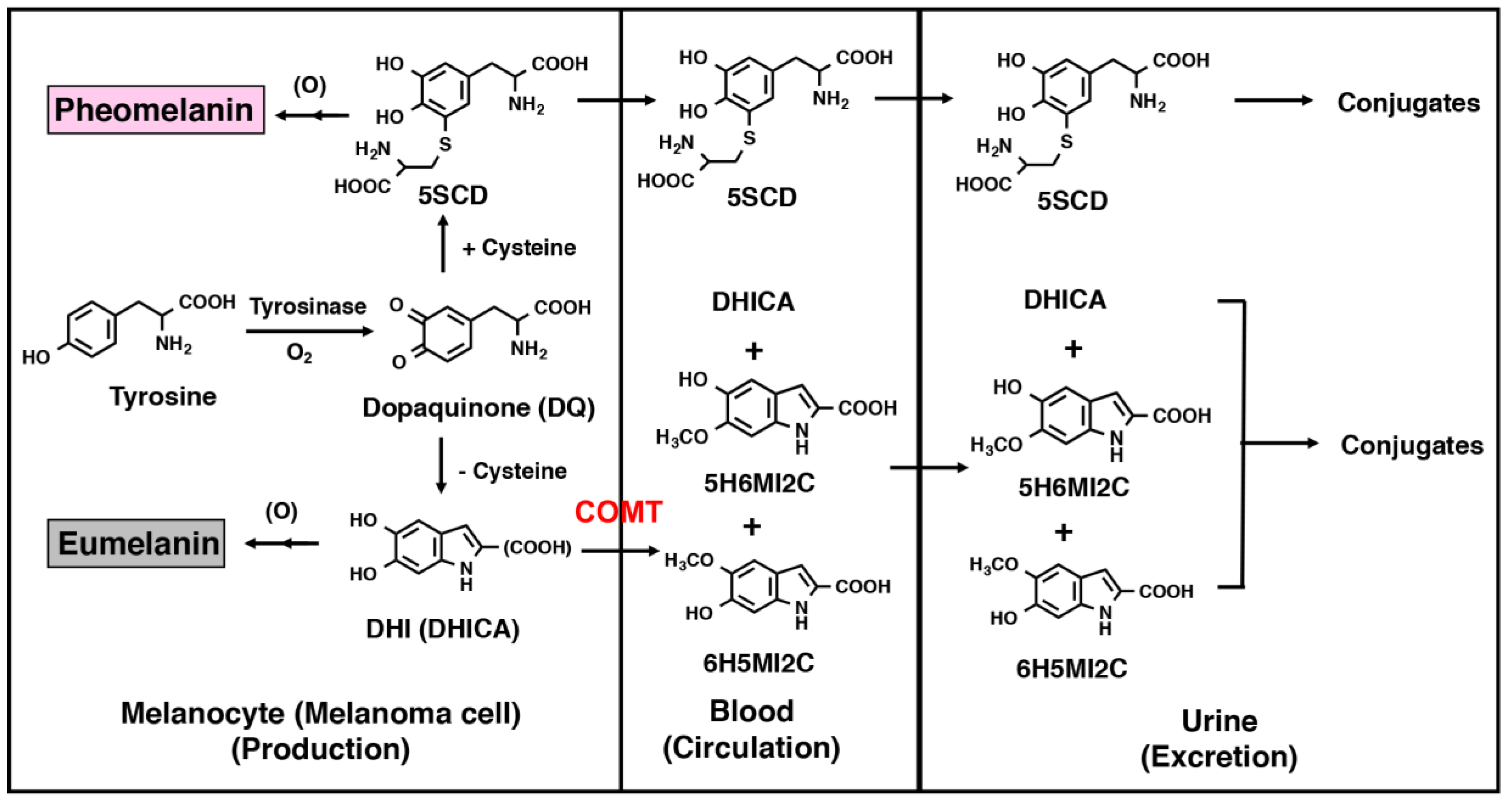
2. Intermediate Metabolites of Melanin Synthesis as Melanoma Biomarkers
2.1. Urinary Excretion and Serum Concentrations of Intermediate Metabolites in Normal Subjects
2.2. Melanin Intermediate Metabolites in Melanoma Patients
3. SCD as a Marker of Melanoma Progression
4. Comparison of 5SCD with Other Melanoma Markers
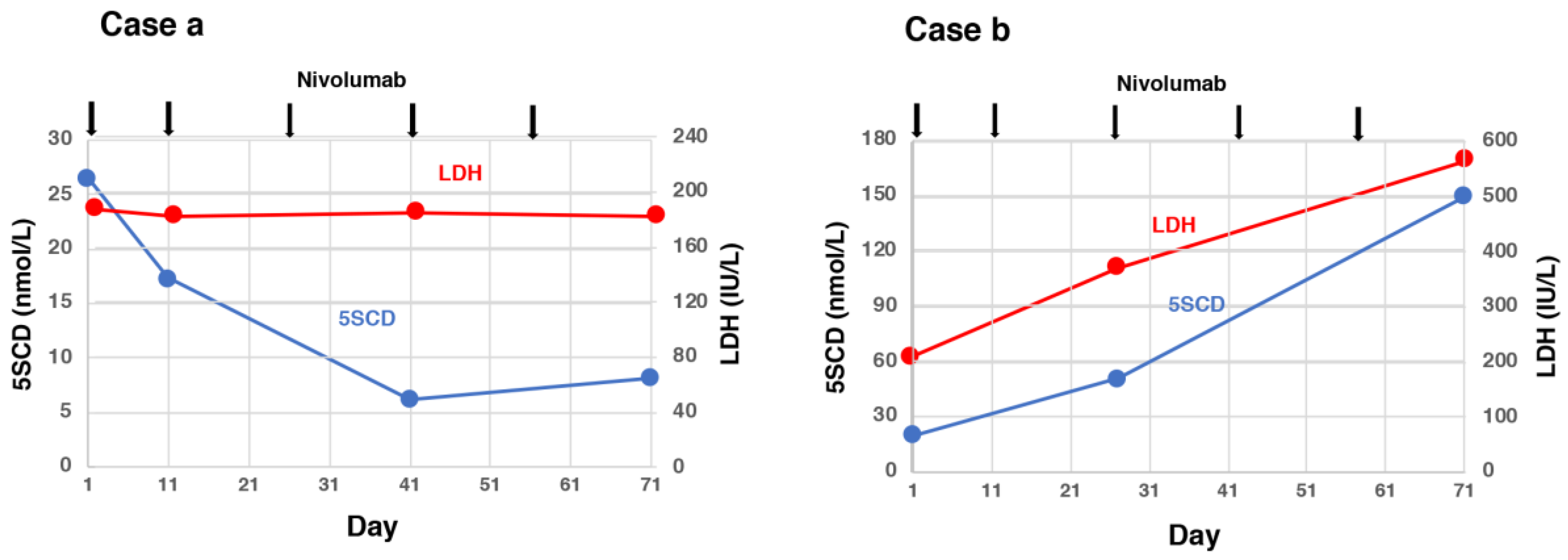
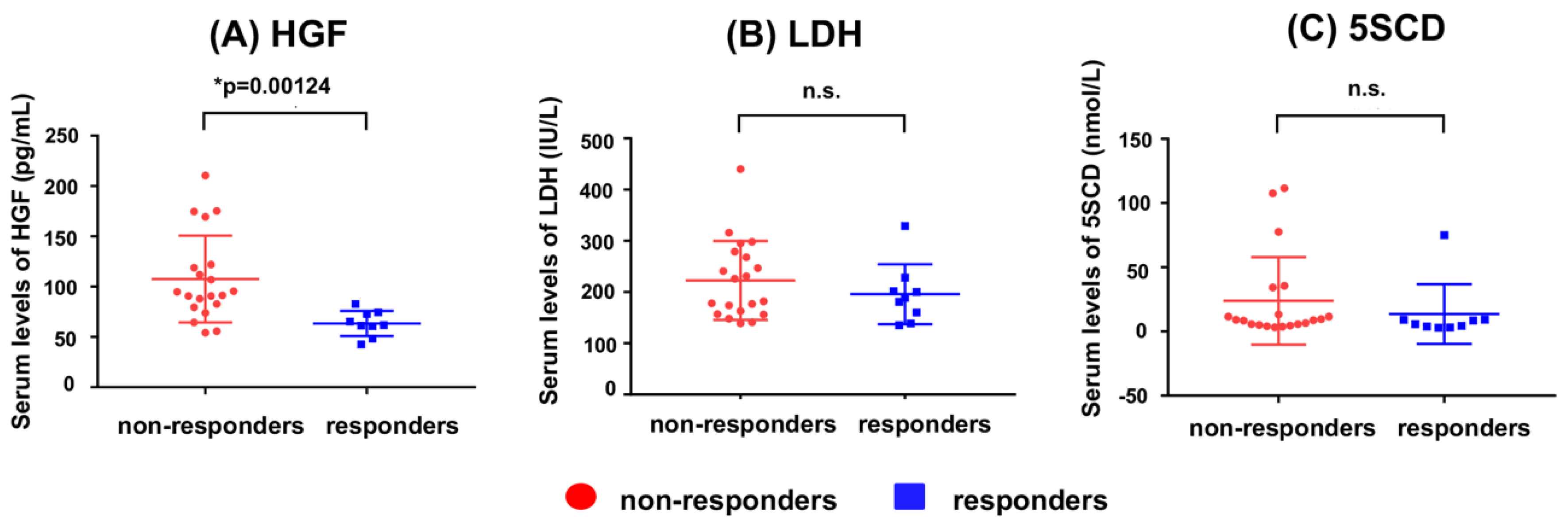
5. Usefulness of 5SCD in Monitoring Treatments for Melanoma
6. SCD Levels in Patients with Non-Cutaneous Melanomas
7. Other Applications Influencing the 5SCD Levels
8. Discussion and Future Perspectives
9. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| 4-AHP | 4-Amino-3-hydroxyphenylalanine |
| Anti-PD | Anti-programmed cell death protein |
| BRAF | B-Raf proto-oncogene serine/threonine kinase |
| CMC | Circulating melanoma cells |
| COMT | Catechol-O-methyl transferase |
| CSF | Cerebrospinal fluid |
| DHI | 5,6-Dihydroxyindole |
| DHICA | 5,6-Dihydroxyindole-2-carboxylic acid |
| EI | Erythema index |
| HD | Hemodialysis |
| HMB-45 | Human melanoma black-45 |
| HGF | Hepatocyte growth factor |
| 5H6MI2C | 5-Hydroxy-6-methoxyindole-2-carboxylic acid |
| 6H5MI2C | 6-Hydroxy-5-methoxyindole-2-carboxylic acid |
| ICAM-1 | Intercellular adhesion molecule-I |
| LDH | Lactate dehydrogenase |
| MI | Melanin index |
| MIA | Melanoma inhibitory activity |
| miRNA | microRNA |
| Nivo | Nivolumab |
| PB-DOPA | Protein-bound 3,4-dihydroxyphenylalanine |
| PB-5SCD | Protein-bound (PB)-5SCD |
| PD | Peritoneal dialysis |
| PD-1 | Anti-programmed cell death protein |
| RT | Radiotherapy |
| PMMB | Primary malignant melanoma of the breast |
| PMME | Primary melanoma of the esophagus |
| RT-PCR | Reverse transcription polymerase chain reaction |
| S100B | S100 calcium-binding protein B |
| 5SCD | 5-S-Cysteinyldopa |
| UV | Ultraviolet |
References
- Landreville, S.; Agapova, O.A.; Harbour, J.W. Emerging insights into the molecular pathogenesis of uveal melanoma. Future Oncol. 2008, 4, 629–636. [Google Scholar] [CrossRef] [PubMed]
- Akaraviputh, T.; Arunakul, S.; Lohsiriwat, V.; Iramaneerat, C.; Trakarnsanga, A. Surgery for gastrointestinal malignant melanoma: Experience from surgical training center. World J. Gastroenterol. 2010, 16, 745–748. [Google Scholar] [CrossRef] [PubMed]
- Bakalian, S.; Marshall, J.C.; Logan, P.; Faingold, D.; Maloney, S.; Di Cesare, S.; Martins, C.; Fernandes, B.F.; Burnier, M.N., Jr. Molecular pathways mediating liver metastasis in patients with uveal melanoma. Clin. Cancer Res. 2008, 14, 951–956. [Google Scholar] [CrossRef] [PubMed]
- Rigel, D.S.; Russak, J.; Friedman, R. The evolution of melanoma diagnosis: 25 years beyond the ABCDs. CA A Cancer J. Clin. 2010, 60, 301–316. [Google Scholar] [CrossRef] [PubMed]
- Seetharamu, N.; Ott, P.A.; Pavlick, A.C. Mucosal melanoma: A case-based review of the literature. Oncologist 2010, 15, 772–781. [Google Scholar] [CrossRef] [PubMed]
- Gladfelter, P.; Darwish, N.H.E.; Mousa, S.A. Current status and future direction in the management of malignant melanoma. Melanoma Res. 2017, 27, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Lens, M.B.; Dawes, M. Global perspectives of contemporary epidemiological trends of cutaneous malignant melanoma. Br. J. Dermatol. 2004, 150, 179–185. [Google Scholar] [CrossRef]
- Hu, D.N.; Yu, G.; McCormick, S.A.; Finger, P.T. Population-based incidence of conjunctival melanoma in various races and ethnic groups and comparison with other melanoma. Am. J. Ophthalmol. 2008, 145, 418–423. [Google Scholar] [CrossRef]
- Jemal, A.; Siegel, R.; Xu, J.; Ward, E. Cancer statistics, 2010. CA Cancer J. Clin. 2010, 60, 277–300. [Google Scholar] [CrossRef]
- Markovic, S.N.; Erickson, L.A.; Rao, R.D.; Weenig, R.H.; Pockaj, B.A.; Bardia, A.; Vachon, C.M.; Schild, S.E.; McWilliams, R.R.; Hand, J.L.; et al. Melanoma Study Group of the Mayo Clinic Cancer Center. Malignant melanoma in the 21st century, part 1: Epidemiology, risk factors, screening, prevention, and diagnosis. Mayo Clin. Proc. 2007, 82, 364–380. [Google Scholar] [CrossRef]
- Schulman, J.M.; Fisher, D.E. Indoor ultraviolet tanning and skin cancer: Health risks and opportunities. Curr. Opin. Oncol. 2009, 21, 144–149. [Google Scholar] [CrossRef] [PubMed]
- Alessi, C.; Scapulatempo Neto, C.; Viana, C.R.; Vazquez, V.L. PD-1/PD-L1 and VEGF-A/VEGF-C expression in lymph node microenvironment and association with melanoma metastasis and survival. Melanoma Res. 2017, 27, 565–572. [Google Scholar] [CrossRef] [PubMed]
- Kurniawan, F.; Kartasasmita, R.E.; Yoshioka, N.; Mutalib, A.; Tjahjono, D.H. Computational Study of Imidazolylporphyrin Derivatives as a Radiopharmaceutical Ligand for Melanoma. Curr. Comput. Alded Drug Des. 2018, 14, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, K.; Masataka Umitsu, M.; De Silva, D.M.; Arpita Roy, A.; Bottaro, D.P. Hepatocyte growth factor/MET in cancer progression and biomarker discovery. Cancer Sci. 2017, 108, 296–307. [Google Scholar] [CrossRef]
- Graziani, G.; Pedro, M.; Lacal, P.M. Neuropilin-1 as therapeutic target for malignant melanoma. Front. Oncol. 2015, 5, 125. [Google Scholar] [CrossRef]
- Campos, L.S.; Rodriguez, Y.I.; Leopoldino, A.M.; Hait, N.C.; Bergami, P.L.; Castro, M.G.; Sanchez, E.S.; Maceyka, M.; Spiegel, S.; Alvareza, S.E. Filamin A expression negatively regulates sphingosine-1-phosphate-induced NF-κB activation in melanoma cells by inhibition of Akt signaling. Mol. Cell. Biol. 2016, 36, 320–329. [Google Scholar] [CrossRef]
- Jenkins, M.H.; Brinckerhoff, C.E.; David, W.; Mullins, D.W. CXCR3 signaling in BRAFWT melanoma increases IL-8 expression and tumorigenicity. PLoS ONE 2015, 10, e0121140. [Google Scholar] [CrossRef]
- Tsuji, T.; Kawada, Y.; Kai-Murozono, M.; Komatsu, S.; Han, S.A.; Takeuchi, K.; Mizushima, H.; Miyazaki, K.; Irimura, T. Regulation of melanoma cell migration and invasion by laminin-5 and alpha3beta1 integrin (VLA-3). Clin. Exp. Metastasis 2002, 19, 127–134. [Google Scholar] [CrossRef]
- Kiss, T.; Ecsedi, S.; Vizkeleti, L.; Koroknai, V.; Emri, G.; Kovács, N.; Adany, R.; Balazs, M. The role of osteopontin expression in melanoma progression. Tumor Biol. 2015, 36, 7841–7847. [Google Scholar] [CrossRef]
- Esmeralda Carrillo, E.; Prados, J.; Marchal, J.A.; Boulaiz, H.; Martínez, A.; Rodríguez-Serrano, F.; Caba, O.; Serrano, S.; Aránega, A. Prognostic value of RT-PCR tyrosinase detection in peripheral blood of melanoma patients. Dis. Markers 2006, 22, 175–181. [Google Scholar] [CrossRef][Green Version]
- Maeng, H.; Terabe, M.; Berzofsky, J.A. Cancer Vaccines: Translation from mice to human clinical trials. Curr. Opin. Immunol. 2018, 51, 111–122. [Google Scholar] [CrossRef] [PubMed]
- Bresnick, A.R.; Weber, D.J.; Zimmer, D.B. S100 proteins in cancer. Nat. Rev. Cancer 2015, 15, 96–109. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Sheng, J.; Yong Fan, Y.; Zhu, X.; Tao, Q.; He, Y.; Wang, S. Association between serum amyloid A levels and cancers: A systematic review and meta-analysis. Postgrad. Med. J. 2018, 94, 499–507. [Google Scholar] [CrossRef] [PubMed]
- Fang, S.; Wang, Y.; Sui, D.; Liu, H.; Ross, M.I.; Gershenwald, J.E.; Cormier, J.N.; Royal, R.E.; Lucci, A.; Schacherer, C.W.; et al. C-reactive protein as a marker of melanoma progression. J. Clin. Oncol. 2015, 33, 1389–1396. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Merbs, S.L.; Sokoll, L.J.; Chan, D.W.; Zhang, Z. A multiplex immunoassay of serum biomarkers for the detection of uveal melanoma. Clin. Proteom. 2019, 16, 10. [Google Scholar] [CrossRef] [PubMed]
- Utikal, J.; Schadendorf, D.; Ugurel, S. Serologic and immunohistochemical prognostic biomarkers of cutaneous malignancies. Arch. Dermatol. Res. 2007, 298, 469–477. [Google Scholar] [CrossRef]
- Kelley, M.C.; Jones, R.C.; Gupta, R.K.; Yee, R.; Stern, S.; Wanek, L.; Morton, D.L. Tumor associated antigen TA-90 immune complex assay predicts subclinical metastasis and survival for patients with early stage melanoma. Cancer 1998, 83, 1355–1361. [Google Scholar] [CrossRef]
- Samarkos, M.; Papaxoinis, G.; Athanasoula, K.; Benopoulou, O.; Bouros, S.; Anastasopoulou, A.; Diamantopoulos, P.; Gogas, H.; Mantzourani, M. Significance of survivin mRNA blood levels in patients with melanoma. J. BU ON 2018, 23, 96–103. [Google Scholar]
- Vergilis, I.J.; Szarek, M.; Ferrone, S.; Reynolds, S.R. Presence and prognostic significance of melanoma-associated antigens CYT-MAA and HMW-MAA in serum of patients with melanoma. J. Investig. Dermatol. 2005, 125, 526–531. [Google Scholar] [CrossRef]
- Nangia-Makker, P.; Hogan, V.; Raz, A. Galectin-3 and cancer stemness. Glycobiology 2018, 28, 172–181. [Google Scholar] [CrossRef]
- Ohmi, Y.; Kambe, M.; Ohkawa, Y.; Hamamura, K.; Tajima, O.; Takeuchi, R.; Furukawa, K.; Furukawa, K. Differential roles of gangliosides in malignant properties of melanomas. PLoS ONE 2018, 13, e0206881. [Google Scholar] [CrossRef] [PubMed]
- Tuaeva, N.O.; Falzone, L.; Porozov, Y.B.; Nosyrev, A.E.; Trukhan, V.M.; Kovatsi, L.; Spandidos, D.A.; Drakoulis, N.; Kalogeraki, A.; Mamoulakis, C.; et al. Translational Application of Circulating DNA in Oncology: Review of the Last Decades Achievements. Cells 2019, 8, 1251. [Google Scholar] [CrossRef] [PubMed]
- Mastroianni, J.; Stickel, N.; Andrlova, H.; Hanke, K.; Melchinger, W.; Duquesne, S.; Schmidt, D.; Falk, M.; Andrieux, G.; Pfeifer, D.; et al. miR-146a controls immune response in the melanoma microenvironment. Cancer Res. 2019, 79, 183–195. [Google Scholar] [CrossRef] [PubMed]
- Richtig, G.; Ehall, B.; Richtig, E.; Aigelsreiter, A.; Tony Gutschner, T.; Pichler, M. Function and clinical implications of long non-coding RNAs in melanoma. Int. J. Mol. Sci. 2017, 18, 715. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A.; Barajon, I.; Garlanda, C. IL-1 and IL-1 Regulatory Pathways in Cancer Progression and Therapy. Immunol. Rev. 2018, 281, 57–61. [Google Scholar] [CrossRef]
- Jia, Y.; Xie, X.; Shi, X.; Li, S. Associations of common IL-4 gene polymorphisms with cancer risk: A meta-analysis. Mol. Med. Rep. 2017, 16, 1927–1945. [Google Scholar] [CrossRef]
- Mohapatra, P.; Prasad, C.P.; Andersson, T. Combination therapy targeting the elevated interleukin-6 level reduces invasive migration of BRAF inhibitor-resistant melanoma cells. Mol. Oncol. 2019, 13, 480–494. [Google Scholar] [CrossRef]
- Trifunović, J.; Miller, L.; Debeljak, Z.; Horvat, V. Pathologic patterns of interleukin 10 expression—A review. Biochem. Med. 2015, 25, 36–48. [Google Scholar] [CrossRef]
- Lasek, W.; Zagożdżon, R.; Jakobisiak, M. Interleukin 12: Still a promising candidate for tumor immunotherapy? Cancer Immunol. Immunother. 2014, 63, 419–435. [Google Scholar] [CrossRef]
- Lugowska, I.; Maria Kowalska, M.; Fuksiewicz, M.; Kotowicz, B.; Mierzejewska, E.; Koseła-Paterczyk, H.; Szamotulska, K.; Rutkowski, P. Serum markers in early-stage and locally advanced melanoma. Tumor Biol. 2015, 36, 8277–8285. [Google Scholar] [CrossRef]
- Mumford, B.; Robertson, G.P. Circulating Melanoma Cells in the Diagnosis and Monitoring of Melanoma: An Appraisal of Clinical Potential. Mol. Diagn. Ther. 2014, 18, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Wagner, N.B.; Forschner, A.; Leiter, U.; Garbe, C.; Eigentler, T.K. S100B and LDH as early prognostic markers for response and overall survival in melanoma patients treated with anti-PD-1 or combined anti-PD-1 plus anti-CTLA-4 antibodies. Br. J. Cancer 2018, 119, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, M.; Shats, I.; Krahn, J.M.; Flake, G.P.; Umbach, D.M.; Li, X.; Li, L. Glypican 6 is a putative biomarker for metastatic progression of cutaneous melanoma. PLoS ONE 2019, 14, e0218067. [Google Scholar] [CrossRef] [PubMed]
- Salvatierra, E.; Alvarez, M.J.; Leishman, C.C.; Baquero, E.R.; Lutzky, V.P.; Chuluyan, H.E.; Podhajcer, O.L. SPARC Controls Melanoma Cell Plasticity through Rac1. PLoS ONE 2015, 10, e0134714. [Google Scholar] [CrossRef]
- Chalkiadaki, G.; Nikitovic, D.; Katonis, P.; Berdiaki, A.; Tsatsakis, A.; Kotsikogianni, I.; Karamanos, N.K.; Tzanakakis, G.N. Low molecular weight heparin inhibits melanoma cell adhesion and migration through a PKCa/JNK signaling pathway inducing actin cytoskeleton changes. Cancer Lett. 2011, 312, 235–244. [Google Scholar] [CrossRef]
- Wakamatsu, K.; Kageshita, T.; Furue, M.; Hatta, N.; Kiyohara, Y.; Nakayama, J.; Ono, T.; Saida, T.; Tanaka, M.; Tsuchida, T.; et al. Evaluation of 5-S-cysteinyldopa as a marker of melanoma progression: 10 years’ experience. Melanoma Res. 2002, 12, 245–253. [Google Scholar] [CrossRef]
- Kärnell, R.; von Schoultz, E.; Hansson, L.O.; Nilsson, B.; Arstrand, K.; Kågedal, B. S100B protein, 5-S-cysteinyldopa and 6-hydroxy-5-methoxyindole-2-carboxylic acid as biochemical markers for survival prognosis in patients with malignant melanoma. Melanoma Res. 1997, 7, 393–399. [Google Scholar] [CrossRef]
- Prota, G. Recent advances in the chemistry of melanogenesis in mammals. J. Investig. Dermatol. 1980, 75, 122–127. [Google Scholar] [CrossRef]
- D’Ischia, M.; Wakamatsu, K.; Cicoira, F.; Di Mauro, E.; Garcia-Borron, J.C.; Commo, S.; Galván, I.; Ghanem, G.; Kenzo, K.; Meredith, P.; et al. Melanins and melanogenesis: From pigment cells to human health and technological applications. Pigment Cell Melanoma Res. 2015, 28, 520–544. [Google Scholar] [CrossRef]
- Ito, S.; Prota, G. A facile one-step synthesis of cysteinyldopas using mushroom tyrosinase. Experientia 1977, 33, 1118–1119. [Google Scholar] [CrossRef]
- Borovansky, J.; Mirejovsky, P.; Riley, P.A. Possible relationship between abnormal melanosome structure and cytotoxic phenomena in malignant melanoma. Neoplasma 1991, 38, 393–400. [Google Scholar] [PubMed]
- Rorsman, H.; Agrup, G.; Hansson, C.; Rogengren, E. Biochemical recorders of malignant melanoma. In Pigment Cell; MacKie, R.M., Ed.; Karger: Basel, Switzerland, 1983; Volume 6, pp. 93–115. [Google Scholar]
- Pavel, S.; Elzinga, H.; Muskiet, F.A.J.; Smit, J.M.; Mulder, N.H.; Schraffordt Koops, H. Eumelanin-related indolic compounds in the urine of treated melanoma patients. J. Clin. Chem. Clin. Biochem. 1986, 24, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Wakamatsu, K.; Ito, S.; Fujita, K. Production, circulation, and excretion of melanin-related metabolites in B16 melanoma-bearing mice. Acta Derm. Venereol. 1990, 70, 367–372. [Google Scholar] [PubMed]
- Agrup, G.; Agrup, P.; Anderson, T.; Hafström, L.; Hansson, C.; Jacobsson, S.; Jönsson, P.E.; Rorsman, H.; Rosengren, A.M.; Rosengren, E. 5 years’ experience of 5-S-cysteinyldopa in melanoma diagnosis. Acta Derm. Venereol. 1979, 59, 381–388. [Google Scholar]
- Hu, F.; Woodward, W.R.; Peterson, L.L. Plasma 5-S-cysteinyldopa correlates with tumor size in melanoma-bearing mice. J. Investig. Dermatol. 1988, 90, 149–151. [Google Scholar]
- Peterson, L.L.; Woodward, W.R.; Fletcher, W.S.; Palmquist, M.; Tucker, M.A.; Ilias, A. Plasma 5-S-cysteinyldopa differentiates patients with primary and metastatic melanoma from patients with dysplastic nevus syndrome and normal subjects. J. Am. Acad. Dermatol. 1988, 19, 509–515. [Google Scholar] [CrossRef]
- Wakamatsu, K.; Ito, S.; Fujita, K. Melanin-related metabolites in urine of B16 melanoma-bearing mice. Acta Derm. Venereol. 1988, 68, 385–389. [Google Scholar]
- Ito, S.; Wakamatsu, K.; Inoue, S.; Fujita, K. Correlation between urinary melanin-related metabolites and tumour weight in melanoma-bearing mice. Acta Derm. Venereol. 1989, 69, 380–384. [Google Scholar]
- Wakamatsu, K.; Ito, S. Identification of ester glucuronide and sulfate conjugates of 5-hydroxy-6-methoxyindole-2-carboxylic acid and 6-hydroxy-5-methoxyindole-2-carboxylic acid in melanoma urine. J. Dermatol. Sci. 1990, 1, 253–260. [Google Scholar] [CrossRef]
- Wakamatsu, K.; Ito, S.; Horikoshi, T. Normal values of urinary excretion and serum concentration of 5-S-cysteinyldopa and 6-hydroxy-5-methoxyindole-2-carboxylic acid, biochemical markers of melanoma progression. Melanoma Res. 1991, 1, 141–147. [Google Scholar] [CrossRef]
- Wakamatsu, K.; Ito, S. Seasonal variation in serum concentration of 5-S-cysteinyldopa and 6-hydroxy-5-methoxyindole-2-carboxylic acid in healthy Japanese. Pigment Cell Res. 1995, 8, 132–134. [Google Scholar] [CrossRef] [PubMed]
- Ito, S.; Jimbow, K.; Kato, T.; Kiyota, M.; Fujita, K. Protein-bound dopa and 5-S-cysteinyldopa in non-melanogenic tissues. Acta Derm. Venereol. 1983, 63, 463–467. [Google Scholar] [PubMed]
- Ito, S.; Fujita, K. Oxygen-dependent conjugation of dopa with cysteine catalyzed by iron-EDTA complex. Biochem. Parmacol. 1984, 33, 2193–2197. [Google Scholar] [CrossRef]
- Nimmo, J.E.; Hunter, J.A.; Perey-Robb, I.W.; Jay, B.; Phillips, C.I.; Taylor, W.O.G. Plasma 5-S-cysteinyldopa concentrations in oculocutaneous albinism. Acta Derm. Venereol. 1985, 63, 463–467. [Google Scholar]
- Stierner, U.; Rosdahl, I.; Augustsson, A.; Kågedal, B. Urinary excretion of 5-S-cysteinyldopa in relation to skin type. UVB-induced erythema and melanocyte proliferation in human skin. J. Investig. Dermatol. 1988, 96, 506–510. [Google Scholar] [CrossRef]
- Wakamatsu, K.; Yokochi, M.; Naito, A.; Kageshita, T.; Ito, S. Comparison of phaeomelanin and its precursor of 5-S-cysteinyldopa in the serum of melanoma patients. Melanoma Res. 2003, 13, 357–363. [Google Scholar] [CrossRef]
- Brochez, L.; Naeyaert, J.-M. Serological markers for melanoma. Br. J. Dermatol. 2000, 143, 256–268. [Google Scholar] [CrossRef]
- Rorsman, H.; Agrup, G.; Falck, B.; Rosengren, A.-M.; Rosengren, E. Exposure to sunlight and urinary excretion of 5-5-cysteinyldopa. In Pigment Cell; Riley, V., Ed.; Karger: Basel, Switzerland, 1976; Volume 2, pp. 284–289. [Google Scholar]
- Sasaki, K.; Uchikado, Y.; Omoto, I.; Amatatsu, M.; Megumi, K.; Okumura, H.; Maemura, K.; Natsugoe, S. Multidisciplinary therapy for metastatic primary malignant melanoma of the esophagus; a case report. Mol. Clin. Oncol. 2018, 8, 528–532. [Google Scholar] [CrossRef]
- Cinar, M.; Karabacak, M.; Asiri, A.M. An experimental and density functional study on conformational and spectroscopic analysis of 5-methoxyindole-2-carboxylic acid. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 137, 670–676. [Google Scholar] [CrossRef]
- Yamada, K.; Walsh, N.; Hara, H.; Jimbow, K.; Chen, H.; Ito, S. Measurement of eumelanin precursor metabolites in the urine as a new marker for melanoma metastases. Arch. Dermatol. 1992, 128, 491–494. [Google Scholar] [CrossRef]
- Valko-Rokytovská, M.; Hubková, B.; Birková, A.; Jana Mašlanková, J.; Stupák, M.; Zábavníková, M.; Čižmárová, B.; Mareková, M. Specific urinary metabolites in malignant melanoma. Medicina 2019, 55, 145. [Google Scholar] [CrossRef]
- Ito, S.; Homma, K.; Kiyota, M.; Fujiya, K.; Jimbow, K. Characterization of structural properties for morphologic differentiation of melanosomes. III. Free and protein-bound dopa and 5-S-cysteinyldopa in B16 and Harding-Passay melanoma. J. Investig. Dermatol. 1983, 80, 207–209. [Google Scholar] [CrossRef]
- Westerhof, W.; Pavel, S.; Kammeyer, A.; Beusenberg, F.D.; Cormane, R. Melanin-related metabolites as markers of the skin pigmentary system. J. Investig. Dermatol. 1987, 89, 78–81. [Google Scholar] [CrossRef] [PubMed]
- Hansson, C. Some indolic compounds as markers of the melanocyte activity. Acta Derm. Venereol. 1988, 138, 1–60. [Google Scholar]
- Ekelund, M.C.; Carstam, R.; Hansson, C.; Rorsman, H.; Rosengren, E.; Wirestrand, L.-E. Urinary excretion of 5-S-cysteinyldopa and 6-hydroxy-5-methoxyindole-2-carboxylic acid: Differences between pigmented and albino mice. Acta Derm. Venereol. 1985, 65, 437–439. [Google Scholar] [PubMed]
- Bánfalvi, T.; Gilde, K.; Boldizsar, M.; Kremmer, T.; Otto, S. Serum levels of S100 protein and 5-S-cysteinyldopa as markers of melanoma progression. Pathol. Oncol. Res. 1999, 5, 218–223. [Google Scholar] [CrossRef] [PubMed]
- Horikoshi, T.; Ito, S.; Wakamatsu, K.; Onodera, H.; Eguchi, H. Evaluation of melanin-related metabolites as markers of melanoma progression. Cancer 1994, 73, 629–636. [Google Scholar] [CrossRef]
- Kärnell, R.; Kågedal, B.; Lindholm, C.; Nilsson, B.; Arstrand, K.; Ringborg, U. The value of cysteinyldopa in the follow-up of disseminated malignant melanoma. Melanoma Res. 2000, 10, 363–369. [Google Scholar] [CrossRef] [PubMed]
- Johansson, M.; Takasaki, A.; Lenner, L.; Arstrand, K.; Kågedal, B. Quantitative relationship between pigment-related mRNA and biochemical melanoma markers in melanoma cell lines. Melanoma Res. 2002, 12, 193–200. [Google Scholar] [CrossRef]
- Umemura, H.; Yamasaki, O.; Kaji, T.; Otsuka, M.; Asagoe, K.; Takata, M.; Iwatsuki, K. Usefulness of serum 5-S-cysteinyl-dopa as a biomarker for predicting prognosis and detecting relapse in patients with advanced stage malignant melanoma. J. Dermatol. 2017, 44, 449–454. [Google Scholar] [CrossRef]
- Hasegawa, M.; Takata, M.; Hatta, N.; Wakamatsu, K.; Ito, S.; Takehara, K. Simultaneous measurement of serum 5-5-cysteinyldopa, circulating intercellular adhesion molecule-1 and soluble interleukin-2 receptor levels in Japanese patients with malignant melanoma. Melanoma Res. 1997, 7, 243–251. [Google Scholar] [CrossRef]
- Hirai, S.; Kageshita, T.; Kimura, T.; Tsujisaki, M.; Imai, K.; Wakamatsu, K.; Ito, S.; Ono, T. Serum levels of sICA-1 and 5-S-cysteinyldopa as markers of melanoma progression. Melanoma Res. 1997, 7, 58–62. [Google Scholar] [CrossRef]
- Bánfalvi, T.; Gilde, K.; Boldizsár, M.; Fejös, Z.; Horváth, B.; Liszkay, G.; Beczássy, E.; Kremmer, T. Serum concentration of 5-S-cysteinyldopa in patients with melanoma. Eur. J. Clin. Investig. 2000, 30, 900–904. [Google Scholar] [CrossRef]
- Lougheed, J.C.; Holton, J.M.; Alber, T.; Bazan, J.F.; Handle, T.M. Structure of melanoma inhibitory activity protein, a member of a recently identified family of secreted proteins. Proc. Natl. Acad. Sci. USA 2001, 98, 5515–5520. [Google Scholar] [CrossRef]
- Bosserhoff, A.K.; Echtenacher, B.; Hein, R.; Buettner, R. Functional role of melanoma inhibitory activity in regulating invasion and metastasis of malignant melanoma cells in vivo. Melanoma Res. 2001, 11, 417–421. [Google Scholar] [CrossRef]
- Schmitz, C.; Brenner, W.; Henze, E.; Christophers, E.; Hauschild, A. Comparative study on the clinical use of Protein S100B and MIA (melanoma inhibitory activity) in melanoma patients. Anticancer Res. 2000, 20, 5059–5063. [Google Scholar] [PubMed]
- Stahlecker, J.; Gauger, A.; Bosserhoff, A.; Ring, J.; Hein, R. MIA as a reliable tumor marker in the serum of patients with malignant melanoma. Anticancer Res. 2000, 20, 5041–5044. [Google Scholar] [PubMed]
- Bosserhoff, A.K.; Dreau, D.; Hein, R.; Landthaler, M.; Holder, W.D.; Buettner, R. Melanoma inhibitory activity (MlA), a serological marker of malignant melanoma. Recent Results Cancer Res. 2001, 158, 158–168. [Google Scholar] [PubMed]
- Matsushita, Y.; Hatta, N.; Wakamatsu, K.; Takehara, K.; Ito, S.; Takata, M. Melanoma inhibitory activity (MIA) as a serum marker for early detection of post-surgical relapse in melanoma patients: Comparison with 5-S-cysteinyldopa. Melanoma Res. 2002, 12, 319–323. [Google Scholar] [CrossRef] [PubMed]
- Uslu, U.; Schliep, S.; Schliep, K.; Erdmann, M.; Koch, H.U.; Parsch, H.; Rosenheinrich, S.; Anzengruber, D.; Bosserhoff, A.K.; Schler, G.; et al. Comparison of the serum tumor markers S100 and melanoma-inhibitory activity (MIA) in the monitoring of patients with metastatic melanoma receiving vaccination immunotherapy with dendritic cells. Anticancer Res. 2017, 37, 5033–5037. [Google Scholar] [PubMed]
- Feuerer, L.; Lamm, S.; Henz, I.; Kappelmann-Fenzl, M.; Haferkamp, S.; Meierjohann, S.; Hellerbrand, C.; Kuphal, S.; Bosserhoff, A.K. Role of melanoma inhibitory activity in melanocyte senescence. Pigment Cell Melanoma Res. 2019, 32, 777–791. [Google Scholar] [CrossRef] [PubMed]
- Thompson, K.J.; Lau, K.N.; Johnson, S.; Martinie, J.B.; Iannitti, D.A.; McKipop, L.H.; Sindram, D. Leptin inhibits hepatocellular carcinoma proliferation via p38-MAPK-dependent signaling. HPB 2011, 13, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Ellerhorst, J.A.; Diwan, A.H.; Dang, S.M.; Uffort, D.G.; Johnson, M.K.; Cooke, C.P.; Grimm, E.A. Promotion of melanoma growth by the metabolic hormone leptin. Oncol. Rep. 2010, 23, 901–907. [Google Scholar] [CrossRef] [PubMed]
- Mizutani, H.; Fukushima, S.; Masuguchi, S.; Yamashita, J.; Miyashita, A.; Nakahara, S.; Aoi, J.; Inoue, Y.; Jinnin, M.; Ihn, H. Serum levels of leptin receptor in patients with malignant melanoma as a new tumor marker. Exp. Dermatol. 2013, 22, 748–774. [Google Scholar] [CrossRef] [PubMed]
- Hida, T.; Yoneta, A.; Wakamatsu, K.; Yanagisawa, K.; Ishii-Osai, Y.; Kan, Y.; Kato, J.; Yamashita, T. Circulating melanoma cell is a potential biomarker to detect metastasis and evaluate prognosis. Australas. J. Dermatol. 2016, 57, 145–149. [Google Scholar] [CrossRef]
- Wimmer, I.; Meyer, J.C.; Seifert, B.; Dummer, R.; Flace, A.; Burg, G. Prognostic value of serum 5-S-cysteinyldopa for monitoring human metastatic melanoma during immunochemotherapy. Cancer Res. 1997, 57, 5073–5076. [Google Scholar]
- Nakamura, K.; Ashida, A.; Nakamura, R.; Kiniwa, Y.; Okumura, R. Dacarbazine as third-line treatment for malignant melanoma: Retrospective study of three cases. Skin Cancer 2018, 33, 196–200. [Google Scholar] [CrossRef]
- Omodaka, T.; Minagawa, A.; Uhara, H.; Wakamatsu, K.; Koizumi, T.; Yokokawa, Y.; Koga, H.; Okuyama, R. Serum 5-S-cysteinyldopa behavior in the early phase of nivolumab treatment of 12 melanoma patients. J. Dermatol. 2018, 45, 1340–1344. [Google Scholar] [CrossRef]
- Kubo, Y.; Fukushima, S.; Inamori, Y.; Tsuruta, M.; Egashira, S.; Yamada-Kanazawa, S.; Nakahara, S.; Tokuzumi, A.; Miyashita, A.; Aoi, J.; et al. Serum concentrations of HGF are correlated with response to anti-PD-1 antibody therapy in patients with metastatic melanoma. J. Dermatol. Sci. 2019, 93, 33–40. [Google Scholar] [CrossRef]
- Kilby, M.D.; Afford, S.; Li, X.F.; Strain, A.J.; Ahmed, A.; Whittle, M.J. Localisation of hepatocyte growth factor and its receptor (c-met) protein and mRNA in human term placenta. Growth Factors 1996, 13, 133–139. [Google Scholar] [CrossRef]
- Birchmeier, C.; Birchmeier, W.; Gherardi, E.; Vande Woude, G.F. Met, metastasis, motility and more. Nat. Rev. Mol. Cell Biol. 2003, 4, 915–925. [Google Scholar] [CrossRef] [PubMed]
- Umemura, H.; Kaji, T.; Tachibana, K.; Morizane, S.; Yamasaki, O. Serum 5-S-cysteinyldopa levels as predictive marker for the efficacy of nivolumab in advanced malignant melanoma. Int. J. Biol. Markers 2019, 34. [Google Scholar] [CrossRef] [PubMed]
- Petersen, R.P.; Hanish, S.I.; Haney, J.C.; Miller, C.C., 3rd; Burfeind, W.R., Jr.; Tyler, D.S.; Seigler, H.F.; Wolfe, W.; D’Amico, T.A.; Harpole, D.H., Jr. Improved survival with pulmonary metastasectomy: An analysis of 1720 patients with pulmonary metastatic melanoma. J. Thorac. Cardiovasc. Surg. 2007, 133, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Wilson, R.W.; Moran, C.A. Primary melanoma of the lung: A clinicopathologic and immunohistochemical study of eight cases. Am. J. Pathol. 1997, 21, 1196–1202. [Google Scholar] [CrossRef] [PubMed]
- Shikuma, K.; Omasa, M.; Yutaka, Y.; Okuda, M.; Taki, T. Treatment of primary melanoma of the lung monitored by 5-S-cysteinyldopa levels. Ann. Thorac. Surg. 2009, 87, 1264–1266. [Google Scholar] [CrossRef] [PubMed]
- Aoki, Y.; Soma, T.; Nakamura, Y.; Fukui, N.; Sakai, Y.; Kageyama, Y. Malignant melanoma of the male urethra with increased 5-S-cysteinyldopa: A case report. IJU Case Rep. 2019, 2, 215–217. [Google Scholar] [CrossRef]
- Goto, H.; Usui, M.; Wakamatsu, K.; Ito, S. 5-S-cysteinyldopa as diagnostic tumor marker for uveal malignant melanoma. Jpn. J. Ophthalmol. 2001, 45, 538–542. [Google Scholar] [CrossRef]
- Lai, C.F.; Kao, T.W.; Tsai, T.F.; Chen, H.Y.; Huang, K.C.; Wu, M.S.; Wu, K.D. Quantitative comparison of skin colors in patients with ESRD undergoing different dialysis modalities. Am. J. Kidney Dis. 2006, 48, 292–300. [Google Scholar] [CrossRef]
- Airaghi, L.; Garofalo, L.; Cutuli, M.G.; Delgado, R.; Carlin, A.; Demitri, M.T.; Badalamenti, S.; Graziani, G.; Lipton, J.M.; Catania, A. Plasma concentrations of α-melanocyte-stimulating hormone are elevated in patients on chronic haemodialysis. Nephrol. Dial. Transplant. 2000, 15, 1212–1216. [Google Scholar] [CrossRef][Green Version]
- Nguyen-Khoa, T.; Massy, Z.A.; De Bandt, J.P.; Kebede, M.; Salama, L.; Lambrey, G.; Witko-Sarsat, V.; Druek, T.B.; Lacour, B.; Thevenin, M. Oxidative stress and haemodialysis: Role of inflammation and duration of dialysis treatment. Nephrol. Dial. Transplant. 2001, 16, 335–340. [Google Scholar] [CrossRef]
- Köken, T.; Serteser, M.; Kahraman, A.; Gökçe, Ç.; Demir, S. Changes in serum markers of oxidative stress with varying periods of haemodialysis. Nephrology 2004, 9, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Shirai, S.; Ominato, M.; Shimazu, T.; Toyama, K.; Ogimoto, G.; Fujino, T.; Yasuda, T.; Sato, T.; Maeba, T.; Owada, S.; et al. Imbalance between production and scavenging of hydroxyl radicals in patients maintained on hemodialysis. Clin. Exp. Nephrol. 2005, 9, 310–314. [Google Scholar] [CrossRef] [PubMed]
- Murakami, K.; Wakamatsu, K.; Nakanishi, Y.; Takahashi, H.; Sugiyama, S.; Ito, S. Serum levels of pigmentation markers are elevated in patients undergoing hemodialysis. Blood Purif. 2007, 25, 483–489. [Google Scholar] [CrossRef] [PubMed]
- Sutherland, W.H.; Gieseg, S.P.; Walker, R.J.; de Jong, S.A.; Firth, C.A.; Scott, N. Serum protein-bound 3,4-dihydroxyphenylalanine and related products of protein oxidation and chronic hemodialysis. Ren. Fail. 2003, 25, 997–1009. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Murakami, K.; Nakanishi, Y.; Wakamatsu, K.; Yamamoto, K.; Kohriyama, N.; Hasegawa, M.; Tomita, M.; Nabeshima, K.; Hiki, Y.; Asano, S.; et al. Serum levels of 5-S-cysteinyldopa are correlated with skin colors in hemodialysis patients but not in peritoneal dialysis patients. Blood Purif. 2009, 28, 209–215. [Google Scholar] [CrossRef]
- Bennett, D.C.; Lamoreux, M.L. The color loci of mice: A genetic century. Pigment Cell Res. 2003, 16, 333–344. [Google Scholar] [CrossRef]
- Kadekaro, A.L.; Wakamatsu, K.; Ito, S.; Abdel-Malek, Z.A. Cutaneous photoprotection and melanoma susceptibility: Reaching beyond melanin content to the frontiers of DNA repair. Front Biosci. 2006, 11, 2157–2173. [Google Scholar] [CrossRef]
- Nagano, A.; Watanabe, A.; Nonaka, Y.; Koga, T.; Wakamatsu, K.; Ito, S.; Nakayama, J. A case of malignant melanoma increasing during pregnancy: Measurement of tissue 5-S-cysteinyldopa. Jpn. J. Dermatol. 1999, 109, 1621–1626. [Google Scholar]
- Yoshino, K.; Aoki, M.; Kawana, S. Elevation of serum 5-S-CD in a patient with malignant melanoma probably due to Agaricus blazei Murill. Jpn. J. Clin. Dermatol. 2005, 59, 1013–1015. [Google Scholar]
- Shinno, K.; Nagahiro, S.; Uno, M.; Kannuki, S.; Nakaiso, M.; Sano, N.; Horiguchi, H. Neurocutaneous melanosis associated with malignant leptomeningeal melanoma in an adult: Clinical significance of 5-S-cysteinyldopa in the cerebrospinal fluid. Neurol. Med. Chir. 2003, 43, 619–625. [Google Scholar] [CrossRef]
- Polini, B.; Carpi, S.; Romanini, A.; Breschi, M.; Nieri, P.; Podesta, A. Circulating cell-free microRNAs in cutaneous melanoma staging and recurrence or survival prognosis. Pigment Cell Melanoma Res. 2019, 32, 486–499. [Google Scholar] [CrossRef] [PubMed]
- Nakahara, S.; Fukushima, S.; Okada, E.; Morinaga, J.; Kubo, Y.; Tokuzumi, A.; Matsumoto, S.; Tsuruta-Kadohisa, M.; Kimura, T.; Kuriyama, H.; et al. MicroRNAs that predict the effectiveness of anti-PD-1 therapies in patients with advanced melanoma. J. Dermatol. Sci. 2019. [Google Scholar] [CrossRef] [PubMed]
- Czarnecka, A.M.; Teterycz, P.; Mariuk-Jarema, A.; Lugowska, I.; Rogala, P.; Dudzisz-Sledz, M.; Swaitaj, T.; Rutkowski, P. Treatment sequencing and clinical outcomes in BRAF-positive and BRAF-negative unresectable and metastatic melanoma patients treated with new systemic therapies in routine practice. Target. Oncol. 2019, 14, 729–742. [Google Scholar] [CrossRef] [PubMed]
- Koh, J.; Lee, J.; Jung, S.Y.; Kang, H.S.; Yun, T.; Kwon, Y. Primary malignant melanoma of the breast: A report of two cases. J. Pathol. Transl. Med. 2019, 53, 119–124. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wakamatsu, K.; Fukushima, S.; Minagawa, A.; Omodaka, T.; Hida, T.; Hatta, N.; Takata, M.; Uhara, H.; Okuyama, R.; Ihn, H. Significance of 5-S-Cysteinyldopa as a Marker for Melanoma. Int. J. Mol. Sci. 2020, 21, 432. https://doi.org/10.3390/ijms21020432
Wakamatsu K, Fukushima S, Minagawa A, Omodaka T, Hida T, Hatta N, Takata M, Uhara H, Okuyama R, Ihn H. Significance of 5-S-Cysteinyldopa as a Marker for Melanoma. International Journal of Molecular Sciences. 2020; 21(2):432. https://doi.org/10.3390/ijms21020432
Chicago/Turabian StyleWakamatsu, Kazumasa, Satoshi Fukushima, Akane Minagawa, Toshikazu Omodaka, Tokimasa Hida, Naohito Hatta, Minoru Takata, Hisashi Uhara, Ryuhei Okuyama, and Hironobu Ihn. 2020. "Significance of 5-S-Cysteinyldopa as a Marker for Melanoma" International Journal of Molecular Sciences 21, no. 2: 432. https://doi.org/10.3390/ijms21020432
APA StyleWakamatsu, K., Fukushima, S., Minagawa, A., Omodaka, T., Hida, T., Hatta, N., Takata, M., Uhara, H., Okuyama, R., & Ihn, H. (2020). Significance of 5-S-Cysteinyldopa as a Marker for Melanoma. International Journal of Molecular Sciences, 21(2), 432. https://doi.org/10.3390/ijms21020432






