Blocking of the IL-33/ST2 Signaling Axis by a Single-Chain Antibody Variable Fragment (scFv) Specific to IL-33 with a Defined Epitope
Abstract
1. Introduction
2. Results
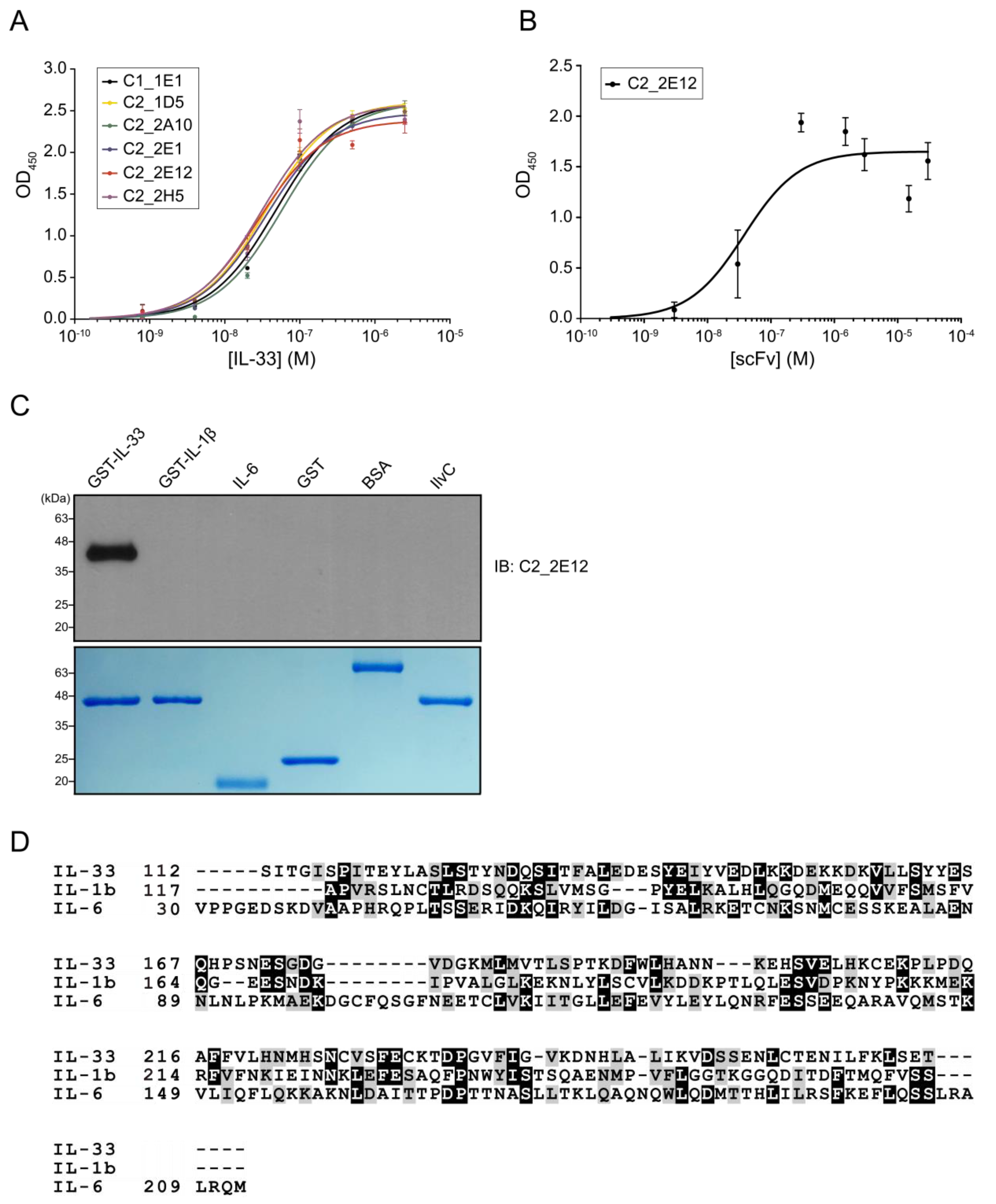
2.1. Selection of scFvs Specific to IL-33
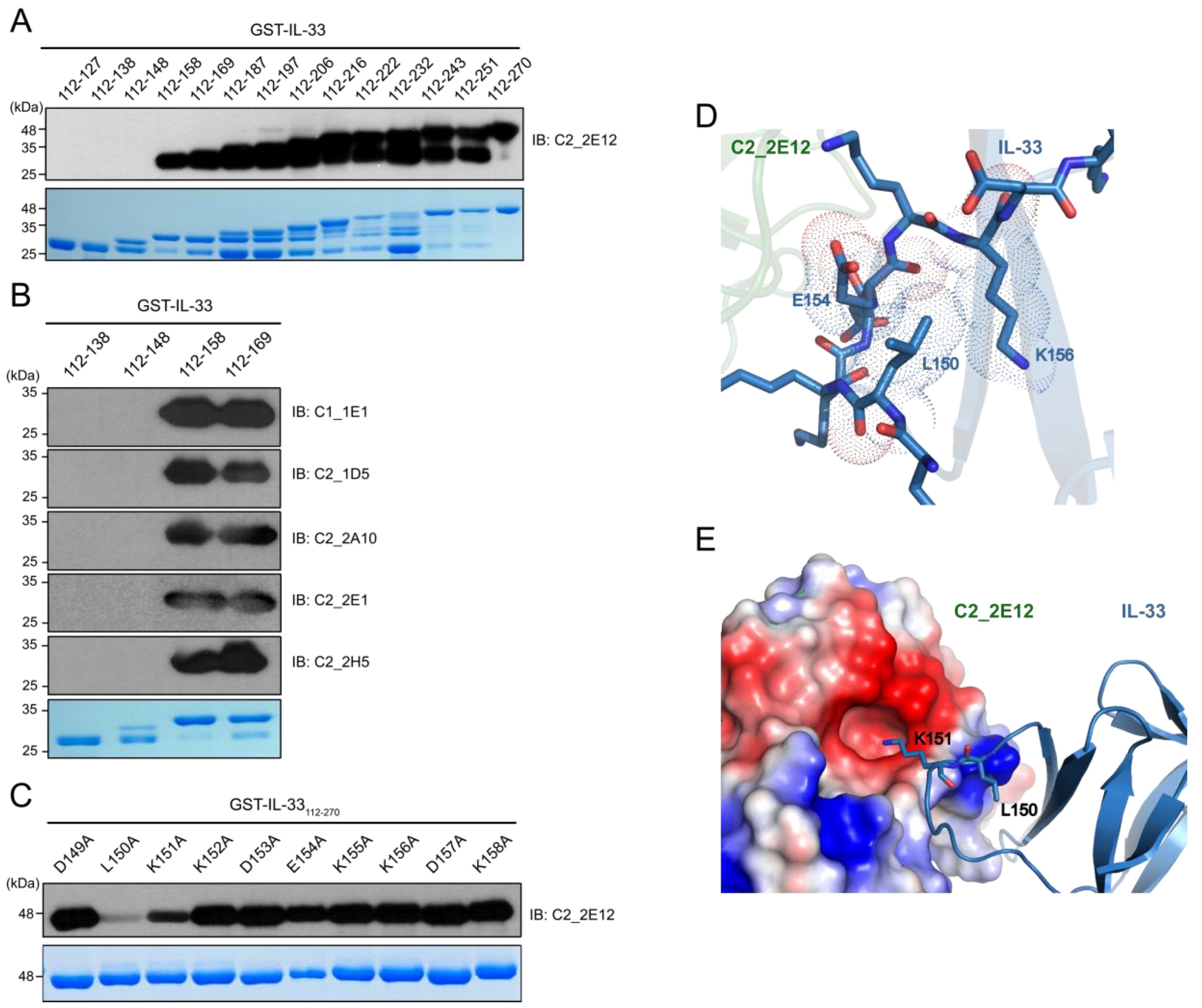
2.2. Epitope Mapping
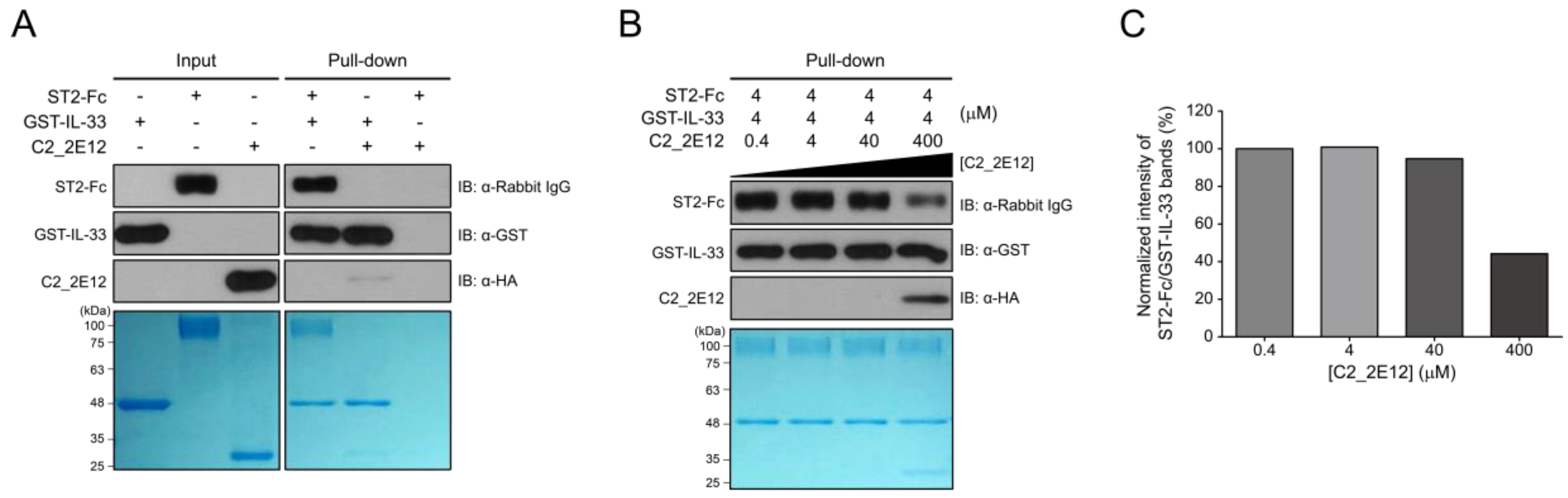
2.3. Competitive Binding of C2_2E12 to IL-33:ST2 Complex
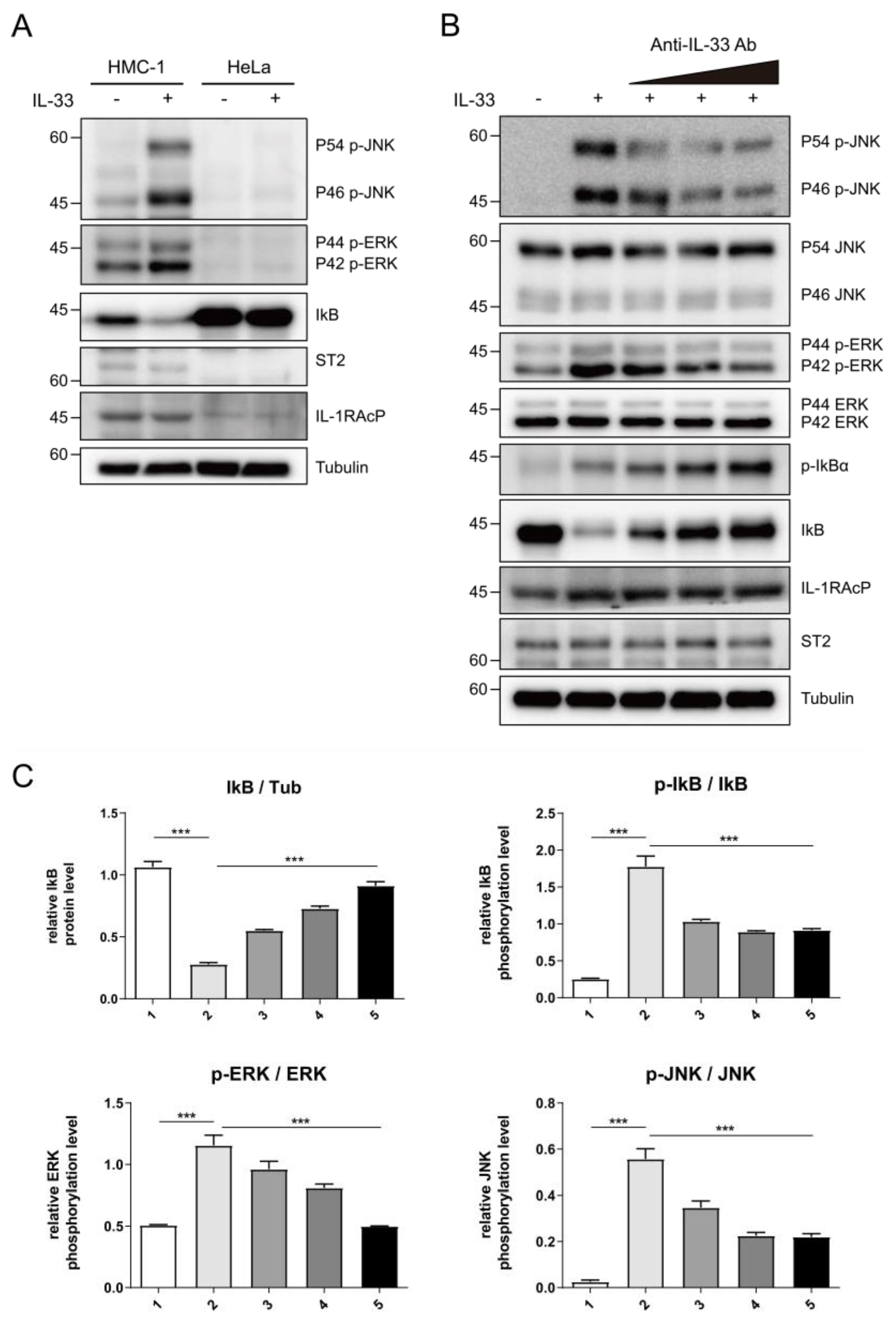
2.4. C2_2E12 Can Neutralize IL-33/ST2 Axis Driving Downstream Signaling Pathway in Human Cell Line
3. Discussion
4. Materials and Methods
4.1. Plasmid Constructs Cloning
4.2. Expression and Purification of Recombinant Proteins
4.3. Biopanning Using Phage Display
4.4. Expression and Purification of scFvs in E. coli
4.5. Reformatting, Expression, and Purification of C2_2E12 in Mammalian Cells
4.6. Immunoblot
4.7. Enzyme-Linked Immunosorbent Assay (ELISA)
4.8. Biolayer Interferometry (BLI)
4.9. Structural Modeling
4.10. Pull-Down Assay
4.11. Cell Signaling Analysis
5. Patent
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| IL-33 | Interleukin-33 |
| ST2 | ST2, suppressor of tumorigenicity 2 |
| scFv GST Fc | Single-chain antibody variable fragment glutathione S-transferase Antibody fragment crystallizable |
References
- Dinarello, C.A. Interleukin-1 in the pathogenesis and treatment of inflammatory diseases. Blood 2011, 117, 3720–3732. [Google Scholar] [CrossRef]
- Garlanda, C.; Dinarello, C.A.; Mantovani, A. The interleukin-1 family: Back to the future. Immunity 2013, 39, 1003–1018. [Google Scholar] [CrossRef]
- Schmitz, J.; Owyang, A.; Oldham, E.; Song, Y.; Murphy, E.; McClanahan, T.K.; Zurawski, G.; Moshrefi, M.; Qin, J.; Li, X.; et al. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity 2005, 23, 479–490. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.K.; Gupta, K.; Dwivedi, P.D. Pathophysiology of IL-33 and IL-17 in allergic disorders. Cytokine Growth Factor Rev. 2017, 38, 22–36. [Google Scholar] [CrossRef]
- Stolarski, B.; Kurowska-Stolarska, M.; Kewin, P.; Xu, D.; Liew, F.Y. IL-33 exacerbates eosinophil-mediated airway inflammation. J. Immunol. 2010, 185, 3472–3480. [Google Scholar] [CrossRef]
- Savinko, T.; Matikainen, S.; Saarialho-Kere, U.; Lehto, M.; Wang, G.; Lehtimaki, S.; Karisola, P.; Reunala, T.; Wolff, H.; Lauerma, A.; et al. IL-33 and ST2 in atopic dermatitis: Expression profiles and modulation by triggering factors. J. Investig. Dermatol. 2012, 132, 1392–1400. [Google Scholar] [CrossRef]
- Chan, B.C.L.; Lam, C.W.K.; Tam, L.S.; Wong, C.K. IL33: Roles in allergic inflammation and therapeutic perspectives. Front. Immunol. 2019, 10, 364. [Google Scholar] [CrossRef] [PubMed]
- Liew, F.Y.; Girard, J.P.; Turnquist, H.R. Interleukin-33 in health and disease. Nat. Rev. Immunol. 2016, 16, 676–689. [Google Scholar] [CrossRef]
- Griesenauer, B.; Paczesny, S. The ST2/IL-33 Axis in immune cells during inflammatory diseases. Front. Immunol. 2017, 8, 475. [Google Scholar] [CrossRef]
- Townsend, M.J.; Fallon, P.G.; Matthews, D.J.; Jolin, H.E.; McKenzie, A.N. T1/ST2-deficient mice demonstrate the importance of T1/ST2 in developing primary T helper cell type 2 responses. J. Exp. Med. 2000, 191, 1069–1076. [Google Scholar] [CrossRef]
- Milovanovic, M.; Volarevic, V.; Radosavljevic, G.; Jovanovic, I.; Pejnovic, N.; Arsenijevic, N.; Lukic, M.L. IL-33/ST2 axis in inflammation and immunopathology. Immunol. Res. 2012, 52, 89–99. [Google Scholar] [CrossRef]
- Das, J.; Chen, C.H.; Yang, L.; Cohn, L.; Ray, P.; Ray, A. A critical role for NF-kappa B in GATA3 expression and TH2 differentiation in allergic airway inflammation. Nat. Immunol. 2001, 2, 45–50. [Google Scholar] [CrossRef]
- Pinto, S.M.; Subbannayya, Y.; Rex, D.A.B.; Raju, R.; Chatterjee, O.; Advani, J.; Radhakrishnan, A.; Prasad, K.T.S.; Wani, M.R.; Pandey, A. A network map of IL-33 signaling pathway. J. Cell Commun. Signal 2018, 12, 615–624. [Google Scholar] [CrossRef]
- Haenuki, Y.; Matsushita, K.; Futatsugi-Yumikura, S.; Ishii, K.J.; Kawagoe, T.; Imoto, Y.; Fujieda, S.; Yasuda, M.; Hisa, Y.; Akira, S.; et al. A critical role of IL-33 in experimental allergic rhinitis. J. Allergy Clin. Immunol. 2012, 130, 184–194. [Google Scholar] [CrossRef]
- Yuan, Q.; Huang, L.; Wang, X.; Wu, Y.; Gao, Y.; Li, C.; Nian, S. Construction of human nonimmune library and selection of scFvs against IL-33. Appl. Biochem. Biotechnol. 2012, 167, 498–509. [Google Scholar] [CrossRef]
- Kim, Y.H.; Yang, T.Y.; Park, C.S.; Ahn, S.H.; Son, B.K.; Kim, J.H.; Lim, D.H.; Jang, T.Y. Anti-IL-33 antibody has a therapeutic effect in a murine model of allergic rhinitis. Allergy 2012, 67, 183–190. [Google Scholar] [CrossRef]
- Chen, W.Y.; Tsai, T.H.; Yang, J.L.; Li, L.C. Therapeutic strategies for targeting IL-33/ST2 signalling for the treatment of inflammatory diseases. Cell Physiol. Biochem. 2018, 49, 349–358. [Google Scholar] [CrossRef]
- Liu, X.; Li, M.; Wu, Y.; Zhou, Y.; Zeng, L.; Huang, T. Anti-IL-33 antibody treatment inhibits airway inflammation in a murine model of allergic asthma. Biochem. Biophys. Res. Commun. 2009, 386, 181–185. [Google Scholar] [CrossRef]
- Ye, Y.; Nian, S.; Xu, W.; Wu, T.; Wang, X.; Gao, Y.; Yuan, Q. Construction and expression of human scFv-Fc against interleukin-33. Protein Expr. Purif. 2015, 114, 58–63. [Google Scholar] [CrossRef]
- Haraldsen, G.; Balogh, J.; Pollheimer, J.; Sponheim, J.; Kuchler, A.M. Interleukin-33—Cytokine of dual function or novel alarmin? Trends Immunol. 2009, 30, 227–233. [Google Scholar] [CrossRef]
- Cohen, E.S.; Scott, I.C.; Majithiya, J.B.; Rapley, L.; Kemp, B.P.; England, E.; Rees, D.G.; Overed-Sayer, C.L.; Woods, J.; Bond, N.J.; et al. Oxidation of the alarmin IL-33 regulates ST2-dependent inflammation. Nat. Commun. 2015, 6, 8327. [Google Scholar] [CrossRef] [PubMed]
- Cayrol, C.; Girard, J.P. Interleukin-33 (IL-33): A nuclear cytokine from the IL-1 family. Immunol. Rev. 2018, 281, 154–168. [Google Scholar] [CrossRef]
- Liu, X.; Hammel, M.; He, Y.; Tainer, J.A.; Jeng, U.S.; Zhang, L.; Wang, S.; Wang, X. Structural insights into the interaction of IL-33 with its receptors. Proc. Natl. Acad. Sci. USA 2013, 110, 14918–14923. [Google Scholar] [CrossRef]
- Waterhouse, A.; Bertoni, M.; Bienert, S.; Studer, G.; Tauriello, G.; Gumienny, R.; Heer, F.T.; de Beer, T.A.P.; Rempfer, C.; Bordoli, L.; et al. SWISS-MODEL: Homology modelling of protein structures and complexes. Nucleic Acids Res. 2018, 46, W296–W303. [Google Scholar] [CrossRef]
- Mjosberg, J.M.; Trifari, S.; Crellin, N.K.; Peters, C.P.; van Drunen, C.M.; Piet, B.P.; Fokkens, W.J.; Cupedo, T.; Spits, H. Human IL-25- and IL-33-responsive type 2 innate lymphoid cells are defined by expression of CRTH2 and CD161. Nat. Immunol. 2011, 12, 1055–1062. [Google Scholar] [CrossRef]
- Sheffield, P.; Garrard, S.; Derewenda, Z. Overcoming expression and purification problems of RhoGDI using a family of “parallel” expression vectors. Protein Expr. Purif. 1999, 15, 34–39. [Google Scholar] [CrossRef]
- Yang, H.Y.; Kang, K.J.; Chung, J.E.; Shim, H. Construction of a large synthetic human scFv library with six diversified CDRs and high functional diversity. Mol. Cells 2009, 27, 225–235. [Google Scholar] [CrossRef]
- Kim, D.; Jang, S.; Oh, J.; Han, S.; Park, S.; Ghosh, P.; Rhee, D.K.; Lee, S. Molecular characterization of single-chain antibody variable fragments (scFv) specific to Pep27 from Streptococcus pneumoniae. Biochem. Biophys. Res. Commun. 2018, 501, 718–723. [Google Scholar] [CrossRef]
- Van Zundert, G.C.P.; Rodrigues, J.; Trellet, M.; Schmitz, C.; Kastritis, P.L.; Karaca, E.; Melquiond, A.S.J.; van Dijk, M.; de Vries, S.J.; Bonvin, A. The HADDOCK2.2 web server: User-friendly integrative modeling of biomolecular complexes. J. Mol. Biol. 2016, 428, 720–725. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, S.B.; Kim, S.-J.; Cho, S.W.; Choi, C.Y.; Lee, S. Blocking of the IL-33/ST2 Signaling Axis by a Single-Chain Antibody Variable Fragment (scFv) Specific to IL-33 with a Defined Epitope. Int. J. Mol. Sci. 2020, 21, 6953. https://doi.org/10.3390/ijms21186953
Park SB, Kim S-J, Cho SW, Choi CY, Lee S. Blocking of the IL-33/ST2 Signaling Axis by a Single-Chain Antibody Variable Fragment (scFv) Specific to IL-33 with a Defined Epitope. International Journal of Molecular Sciences. 2020; 21(18):6953. https://doi.org/10.3390/ijms21186953
Chicago/Turabian StylePark, Soo Bin, Sun-Jick Kim, Sang Woo Cho, Cheol Yong Choi, and Sangho Lee. 2020. "Blocking of the IL-33/ST2 Signaling Axis by a Single-Chain Antibody Variable Fragment (scFv) Specific to IL-33 with a Defined Epitope" International Journal of Molecular Sciences 21, no. 18: 6953. https://doi.org/10.3390/ijms21186953
APA StylePark, S. B., Kim, S.-J., Cho, S. W., Choi, C. Y., & Lee, S. (2020). Blocking of the IL-33/ST2 Signaling Axis by a Single-Chain Antibody Variable Fragment (scFv) Specific to IL-33 with a Defined Epitope. International Journal of Molecular Sciences, 21(18), 6953. https://doi.org/10.3390/ijms21186953




