Abstract
Sucrose, an important sugar, is transported from source to sink tissues through the phloem, and plays important role in the development of important traits in plants. However, the SUT gene family is still not well characterized in pomegranate. In this study, we first identified the pomegranate sucrose transporter (SUT) gene family from the whole genome. Then, the phylogenetic relationship of SUT genes, gene structure and their promoters were analyzed. Additionally, their expression patterns were detected during the development of the seed. Lastly, genetic transformation and cytological observation were used to study the function of PgL0145810.1. A total of ten pomegranate SUT genes were identified from the whole genome of pomegranate ‘Tunisia’. The promoter region of all the pomegranate SUT genes contained myeloblastosis (MYB) elements. Four of the SUT genes, PgL0328370.1, PgL0099690.1, PgL0145810.1 and PgL0145770.1, were differentially expressed during seed development. We further noticed that PgL0145810.1 was expressed most prominently in the stem parts in transgenic plants compared to other tissue parts (leaves, flowers and silique). The cells in the xylem vessels were small and lignin content was lower in the transgenic plants as compared to wild Arabidopsis plants. In general, our result suggests that the MYB cis-elements in the promoter region might regulate PgL0145810.1 expression to control the structure of xylem, thereby affecting seed hardness in pomegranate.
1. Introduction
Pomegranate (Punica granatum L.) is one of the oldest fruits known to mankind, and it thrives better in tropical and sub-tropical regions [1]. It used to be placed in the monogeneric family Punicaceae [2] but now has been shifted to family Lythraceae by the Angiosperm Phylogeny Group [3] on the basis of phylogenetic studies. The fruits have a medicinal value and are a good source of compounds like antioxidant phenolics and anthocyanins, which depends upon the diversity of the pomegranate genotypes. The fruit is regarded as a “superfruit” for its health benefits [4]. This has created awareness globally for an increase in the production area as well as the demand for consumption [5,6]. The prominent increase in the consumption of the fruits has increased the demand for the fruits in the market, which encourages researchers and breeders to develop and breed ideal types of varieties [7], such as a soft-seeded cultivar. In the past, breeding fruit crops was traditional and time-consuming as the fruit crops have a long generation time, large size and a long juvenile phase for seedlings, and it takes a long time to obtain the fruits ready for marketing [8,9]. Mapping the soft-seeded genes and adopting marker assisted selection (MAS) will accelerate the progress of soft-seeded cultivar breeding.
Although there are some research works on seed hardness traits in pomegranate, there are no clear molecular mechanisms or identification of genes responsible for seed hardness. The seed coat of pomegranate contains high levels of lignin [10]. The lignin content in the seed coat has a positive correlation with the characteristics of seed hardness, and amount [11,12]. Based on the transcriptomic analysis, WRKY, AP2 like, MYC, MYB and NAC transcription factors (TFs) are expressed differently in soft- and hard-seed pomegranate varieties [13,14]. The miRNA–mRNA influence seed hardness in pomegranate by acting on the cell wall. NAC1, WRKY and MYC are involved in seed hardness due to differentially expressed mdm-miR164e and mdm-miR172b. The miRNA–mRNA network results in a complex biological process [15]. This cell wall biosynthesis and degradation might play a role in seed hardness in pomegranate [16,17]. In another study, between “Tunisia” soft-seeded and “Sanbai” hard-seeded varieties, a single nucleotide polymorphism (SNP) (T-C) at the 166bp position was non-synonymously substituted with lysine and replaced by glutamic acid. The lignin biosynthesis and seed hardness in pomegranate is due to the NAC transcription factor, PgSND1-like gene [18].
Sucrose plays a key role in plant growth, development and crop yield [19,20,21,22]. SUT/SUC genes have a key role for transporting sucrose from source to sink organs. The sucrose produced in the source leaves is the major photosynthetic product that is transported to the storage organs (sink tissues), roots, stems, flowers, fruits and seeds, in the plants through the vascular tissue [23,24,25,26,27]. It is regarded as the major product that is necessary for the growth and development of the plants [28,29,30]. Depending on the plant species, sucrose is transported in different ways in the plant system to the phloem, apoplastically over short distances through the plasma membrane and symplastically through plasmodesmata [31,32]. Apart from short distance loading, sucrose is transported to sink organs, which is the final consumption location, and storage organs in plants [29,30,33,34]. The sucrose transporter genes regulate the transport activities to adapt to changes in the external environment, such as temperature, photoperiod, pathogens, etc. [35,36].
The first SUT gene in plants, SoSUT, was reported from spinach [37] then, a large number of SUT genes were found in rice [38] and Arabidopsis [39,40,41]. The translocation of sucrose from the leaves to the seeds plays an important role in the maximization of the yield in maize [42]. Similarly, in cotton during the seed development stage, sucrose is transported to enhance fiber formation, development and elongation [43]. Additionally, in rapeseed, BnA7SUT1 was significantly associated with the number of effective branches, siliques per plant and seed weight [44]. In an antisense transformation study in a tomato crop, the SUT genes played an important role in the retardation of sucrose translocation, which reduced the fruit size and even the fertility was lowered [45,46]. Basic research and crop improvement activities in pomegranate crops are limited [47]. However, there is no information about the SUT gene family in pomegranate. We have no idea about the function of SUT genes, and the relationship between the SUT genes and soft-seeded phenotypes. Therefore, the present study was designed to identify the SUT genes and analyze their functions in the development of seed hardness in pomegranate.
2. Results
2.1. Identification of SUT Gene Family
Through sequence analysis from the “Tunisia” pomegranate genome database, a total of 10 members of the SUT gene family were obtained on Chr1, Chr3, Chr4, Chr5, Chr6 and Chr7 (Table 1). Among them, the SUT genes were distributed more (three each) in Chr3 and Chr6. The analysis of the results shows that the gene length varies greatly, with an average length of 4406.7bp. The maximum gene length was observed in PgL0237030.1 with 8014bp and the minimum in PgL0281810.1 with 279bp. The highest and the lowest differences among the longest and the shortest sequences of SUT genes of pomegranate are 7735bp and 158bp. The average adenine, cytosine, guanine and thymine contents in the pomegranate SUT genes were 28.50%, 21.58%, 22.77% and 27.15%, respectively. However, in the promoter sequences, the average adenine and thymine content was higher than that of the gene sequences. The average adenine and thymine contents in promoter sequences were 30.51% and 30.71%, which are higher by 7% and 11.31%, respectively. Meanwhile, the average cytosine, guanine and GC contents were 19.17%, 19.62% and 38.79% in the promoter sequences, which were lower than those of gene sequences (Table 2).

Table 1.
Gene location, position, length and nucleotide composition of SUT genes in pomegranate.

Table 2.
Nucleotide composition of SUT promoters in pomegranate.
We analyzed the SUT proteins and their amino acid composition and found that the protein sequence length varied by 92–1251 amino acids (Table S1). Meanwhile, the variation in molecular weight was 10260.37–142472.24 Dalton. The proteins of PgL0281820.1 and PgL0281800.1 had no tryptophan and the protein of PgL0281810.1 was without cysteine acid. In general, the average leucine content of the SUT protein sequences was the highest at 10.69, and the average cysteine content, 0.82, was the lowest. These structural features of the genes and proteins may relate to the functional diversity among the SUT genes.
2.2. Phylogenetic and Gene Structure Analysis of Pomegranate SUT Genes
From the phylogenetic tree analysis, we conclude that the pomegranate SUT genes are mainly divided into three major categories: group1, group2 and group3 (Figure 1). Group1 and group3 contained four and five SUT genes, respectively. PgL0237030.1 forms a separate branch. Among all the SUT genes, PgL0237030.1 from group2 contained the largest number of exons and introns, followed by PgL0099690.1 and PgL0233780.1 from group3. Meanwhile, in the other SUT genes from group1, there are fewer than ten exons and introns. Thus, the variation of the number of exons and introns was largely in accordance with the structure categories.
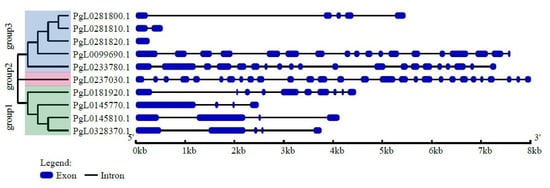
Figure 1.
Phylogenetic tree of SUT genes of pomegranate and their exon/intron structure analysis. All pomegranate SUT protein sequences were determined with hidden Markov model (HMM) and were classified into three groups based on phylogenetic analysis. MEGA5.0 was used to construct a phylogenetic tree. The blue boxes represent exons and the black lines are introns.
2.3. Promoter Analysis of Pomegranate SUT Genes
PLACE analysis revealed that promoter regions of all the pomegranate SUT genes contained multiple MYB elements (Figure 2). These MYB elements are mainly MYB2AT, MYB2CONSENSUSAT, MYBCORE, MYBCOREATCYCB1, MYBGAHV, MYBPLANT, MYBPZM, MYB1AT and MYB1LEPR. Among the ten SUT genes, PgL0145810.1, PgL0237030.1 and PgL0181920.1 contained more than twenty MYB elements and PgL0099690.1 contained the least, with seven MYB elements.
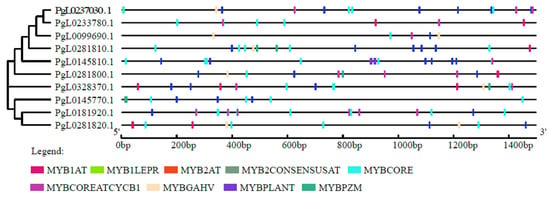
Figure 2.
Promoters of SUT genes of pomegranate and their cis-acting element analysis. The online tool PlantCARE was used to predict the cis-acting elements and the Gene Structure Display Server (GSDS) 2.0 tool was used for constructing the cis-acting structure. The colored boxes represent different MYB elements.
2.4. Expression Analysis of the SUT Genes in Seed Development
Previous transcriptomic data [13,14,15] showed that the expression of four SUT genes, PgL0328370.1, PgL0099690.1, PgL0145810.1 and PgL0145770.1, may be involved in regulating the development process of pomegranate seeds (Figure S1). A qRT-PCR analysis further proved that the relative expression levels of PgL0145810.1 were normally higher in the mature seed of soft-seeded varieties (ZSL8, Yi3 and Tunisia) compared to the hard-seeded varieties (Jiu Zi Hong, Yi2000-1 and Sanbai) (Figure 3A; Table S2). The result indicated that PgL0145810.1 may negatively regulate the development of seed hardness in pomegranate.
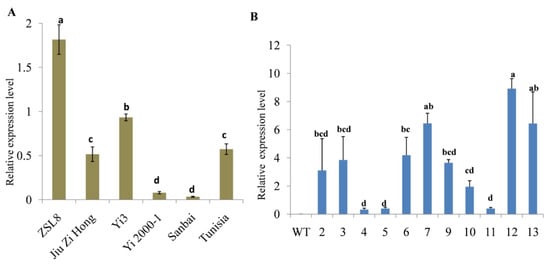
Figure 3.
Relative expression level of the PgL0145810.1. Expression level of the gene in the seeds of different pomegranate varieties (A). Expression level of the gene in different transgenic Arabidopsis lines (B). Error bars indicate standard deviations from three replicates (n = 3). Values are means ± SD (n = 3). WT: Wild type and 2, 3, 4, 5, 6, 7, 9, 10, 11, 12 and 13: transgenic Arabidopsis lines. The letters indicate significant differences (p < 0.05).
2.5. Growth Phenotype and Expression of PgL0145810.1 in Transgenic Lines
To further investigate the function of PgL0145810.1, we cloned PgL0145810.1 and dipped the flower buds of Arabidopsis in an Agrobacterium cell suspension. The transgenic Arabidopsis lines were confirmed through qRT-PCR analysis (Figure 3B; Table S3). The transgenic lines were grown for three generations (T3) to obtain homozygous plants for the transferred gene.
Growth phenotypes of the PgL0145810.1 transgenic lines were highly different from the wild type in terms of plant height, number of leaves, number of stems and number of seeds per siliques (Figure 4C; Figure S2). Additionally, the growth phenotypes of the PgL0145810.1 transgenic line L4 was comparable to that of L12. Further expression analysis indicated that the expression level of the PgL0145810.1 gene was more prominent in the stem parts compared to other tissue parts (leaves, flowers and silique) either in L12 or L4 (Figure 4B; Table S4). However, the expression levels of PgL0145810.1 in the stem of L12 and L4 were higher than that of the wild type. This result is similar to the observation that the lignin content in the transgenic Arabidopsis plant L12 was reduced by 13.63% and in L4 by 9.37% as compared to the wild type (Figure 4D). Thus, the expression level of PgL0145810.1 is related to the stem differences between wild type and transgenic lines.
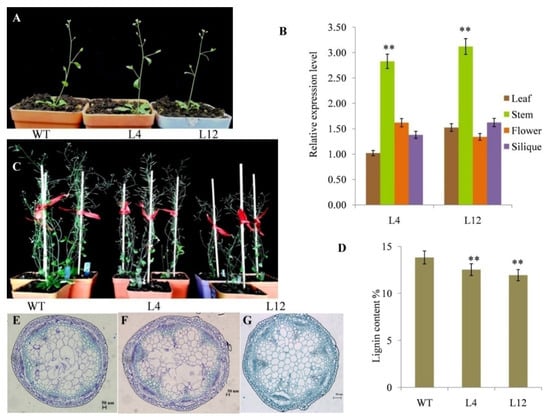
Figure 4.
Growth parameters of wildtype (WT) Arabidopsis and transgenic lines (L4 and L12). Data shown are sample plants at the 35-day-old seedling stage for cytological observation (A), relative expression level of PgL0145810.1 in different tissues of L4 and L12 (B), images of 8-week-old plants of WT, L4 and L12 (C), lignin content in the stems of WT, L4 and L12 (D), cytological observation of the flowering stems of WT, L4 and L12 (E–G). Error bars indicate standard deviations from three replicates (n =3). Values are means ± SD (n= 3). ** significant at p < 0.01 probability levels.
2.6. T-DNA Insertion Site in Transgenic Line
Genome walking was performed to locate the insertion site of T-DNA in the transgenic lines. The sequencing of the flanking genomic sequence showed that the T-DNA insertion site in transgenic lines was at Chr5: 4809575–4810941. The T-DNA insertion site was located in the coding sequence of the gene located in Chr5: 4808091–4811093, which was annotated as cyclic nucleotide-gated channel 18 (CNGC18), regulating the essential role in pollen development and pollen tube growth (Figure S3; Table S5).
2.7. Cytological Observation of the Stems
To further assess the function of PgL0145810.1, cytological observation was conducted in the tissue of stems from the thirty-five-day-old seedling stage collected from WT, L4 and L12 plants (Figure 4A). We observed that the numbers of the cells in the vascular bundle and pith were significantly higher, while the size of the cells was comparatively small in the transgenic lines L4 and L12 as compared to the WT plants (Figure 4E–G). Similarly, the length and the thickness of the xylem vessel in both of the transgenic lines, L4 and L12,were shorter and reduced as compared to the wild type plants, while the expression patterns of PgL0145810.1 in the stems of L4 and L12 were higher. Collectively, this implies that high expression of PgL0145810.1 regulated the structure of the xylem.
3. Discussion
The present study first explored all the SUT gene family members that are present in the pomegranate genome and identified a total of ten SUT genes. Phylogenetic analysis divided the pomegranate SUT genes into three major categories, and variations of the number of exons and introns were largely in accordance with the structure categories. Additionally, PgL0328370.1, PgL0145810.1 and PgL0145770.1 were proved to relate the seed development by the transcriptome, and all of them came from group1. Thus, the structure group of the SUT genes may determine gene structure and biological diversity in pomegranate.
Sucrose is the primary photosynthetic product that is translocated through the phloem in most economically important plants [48,49] from source to sink organs and constitutes a key component for carbon portioning for the whole plant [50,51,52]. SUT/SUC is a large gene family [53] and plays an important role in the biological evolution and the formation of the important traits [28,54] such as flowering, plant height and crop yield [19,20,21,22]. The rapid development of high-throughput sequencing and bioinformatics analysis technology has made transcriptome analysis easy for researchers to detect the genes associated with complex quantitative traits [55,56]. Through transcriptomic analysis, NAC1, WRKY, MYB and MYC transcription factors were proved to be responsible for the formation of seed hardness in pomegranate [13,14]. Our study first reveals that the expression of SUC genes (PgL0099690.1, PgL0145810.1, PgL0145770.1 and PgL0328370.1) is involved in the development of seeds in pomegranate. In particular, the down regulation of PgL0145810.1 is related to an increase in the seed hardness trait in pomegranate. Thus, we inferred that PgL0145810.1 may regulate the formation of seed hardness, which enhances the understanding of the function of the SUT genes in pomegranate.
Expression analysis indicated that the expression level of the PgL0145810.1 gene varied in different tissues of transgenic lines, and most prominently in the stem part. Our result is similar to that, and the accumulation of sucrose is found more in mature, fully elongated internodes (at basal rather than the apical internodes) and the stems [57,58]. Additionally, the expression levels of PgL0145810.1 in the stem of transgenic lines were higher than that of the wild type. Meanwhile, the lignin content of the stems in the transgenic Arabidopsis plants was significantly lower than in the wild type Arabidopsis. Cytological observation of the stem further verified that the length and the thickness of the xylem vessel in the transgenic lines were shorter and reduced as compared to the wild type plants. Previous studies proved that PgL0145810.1 varied between soft-seeded cultivars and hard-seeded cultivars [13]. Thus, PgL0145810.1 may play a role in the synthesis of lignin to regulate the structure of the xylem, thereby affecting seed hardness in pomegranate.
Promoter sequences that are located at the 5′ end are the DNA sequences responsible for the initiation of the transcription, with functions in the growth and development processes of plants. The promoter sequence in the navel orange sucrose synthase 1 (CsSUS1p) gene can drive foreign genes into the phloem which can be used to prevent and treat vascular bundle diseases [59]. The cis-regulatory elements within the specific gene reveal the gene expression patterns that are directly involved in environmental adaptation and further add to our understanding that they encode the proteins for plant growth and development, as well as fruit coloring in plants [60]. The MYB elements are typical cis-regulatory elements and have been proved to be related to the formation and evolution of important agronomic traits [61] and may be the main factors determining the pleiotropy of the SUT genes [20]. In this study, we identified different numbers of MYB cis-elements in the promoter region of SUT genes of pomegranate. PgL0145810.1 has the highest number of MYB cis-elements in the promoter region among SUT genes in pomegranate. Global genome comparison analysis indicated that the gene sequence showed no difference between the comparisons: Tunisia_Dabenzi and Tunisia_Taishanhong [13]. Thus, we suppose that the MYB cis-elements in the promoter region may regulate PgL0145810.1 expression to function in the development of seed hardness in pomegranate.
4. Materials and Methods
4.1. Plant Material
The pomegranate varieties, soft-seeded varieties (ZSL8, Yi3 and Tunisia) and hard-seeded varieties (Jiu Zi Hong, Yi2000-1 and Sanbai), used in this study were eleven-year-old trees grown in the nursery of the Zhengzhou Fruit Research Institute, Chinese Academy of Agricultural Sciences (CAAS). All of the pomegranate trees were robust with similar growth habits. The fruit samples from the trees were collected at 120 days after flowering (DAF). The seeds, along with the arils from the fruits, were extracted immediately and were placed in liquid nitrogen and finally frozen at −80 °C in the lab for further use.
4.2. Identification of SUT Genes from Pomegranate
We considered the whole genome sequence of pomegranate “Tunisia” [13] during our study. The Arabidopsis SUT gene sequences and protein sequences were downloaded from TAIR (https://www.arabidopsis.org/). The SUT gene family was downloaded from the TIGRFAMS database (J. Craig Venter Institute, La Jolla, CA, USA) [62]. We used the TIGR01301 [63] program to perform the sequence alignment and constructed a hidden Markov model (HMM) [64] for the structure of SUT proteins based on the alignment results. This HMM model was used to search the pomegranate protein database until the database was exhausted and, finally, pomegranate SUT protein sequences were compared with ClustalW. The seed sequences were used as the target sequences and the pomegranate protein sequences were analyzed in Blastp [65]. For the screening of the sequences, we considered E values less than 10–15 and got all the members of the SUT gene family.
4.3. Protein Sequence, Gene Structure and Promoter Analysis of SUT Genes
After the multiple sequence alignment through ClustalW, MEGA5.0 [66] was used to construct a phylogenetic tree with bootstrap set to 1000 replicates. Cluster analysis was performed on the protein sequences and promoter sequences of SUT genes. To obtain the intron numbers from each SUT gene, we extracted corresponding gff files. The 1.5kb upstream sequences of the genes were taken as the promoter sequences and we used the online tool PlantCARE (Flanders Institute for Biotechnology (VIB), Rijvisschestraat, Belgium) [67] to predict the cis-acting elements. To draw the gene structure and the cis-acting structure, we used the online tool GSDS 2.0 (http://gsds.cbi.pku.edu.cn/, GitHub, Inc., San Francisco, CA, USA) [68].
4.4. SUT Gene Protein Sequence Analysis and Isolation of PgL0145810.1
We used BioEdit software (https://bioedit.software.informer.com, BioEdit7.0.9, Informer Technologies, Inc., Carlsbad, CA, USA) [69] to analyze the pomegranate SUT genes and their upstream sequences and nucleic acid composition and calculate their GC content. The same software was used to analyze the amino acid composition and analysis of SUT protein molecular weight (M/W). The full-length sequence of PgL0145810.1 was obtained from the whole genome sequence of pomegranate “Tunisia” from the seeds of both “Tunisia” and “Sanbai” through polymerase chain reaction (PCR). We used NCBI Primer Blast software (National Library of Medicine 8600 Rockville Pike, Bethesda MD, USA) [70] to design the primers (Table S6).
4.5. Growth Conditions for Arabidopsis thaliana
The seeds of A. thaliana (ecotype Columbia) were washed with 95% ethanol (v/v in water) for 1 min for surface disinfection, followed with 50% sodium hypochlorite (v/v in water) for the next 3 min. Then, the seeds were subsequently rinsed five times with sterile deionized water under the clean bench. The disinfected seeds were placed in Petri dishes with 0.2X MS medium, pH 7.0 along with 3% sucrose and 1% plant agar. In the case of selecting the transgenic lines, the medium was supplemented with kanamycin (60 g/mL). For the uniform germination of the seeds, the Petri dishes were placed at 4 °C in the dark for 48 h and then transferred to the growth chamber. After seven days of germination, the plants were transferred to pots with substrate, top soil and perlite in the ratio 2:1:1 and were placed in the growth chamber with following conditions: 23–25 °C temperature, 70–80% relative humidity and 16:8 h light–dark photoperiod and 150 µmol m−2s−1 light intensity.
4.6. RNA Extraction and cDNA Synthesis
The total RNA from the pomegranate seeds was extracted using the cetyltrimethyl ammonium bromide (CTAB) method (Solarbio, Beijing, China). The cDNA was generated using one microgram of RNA by using a PrimeScriptTM RT Kit with gDNA Eraser (TaKaRa, Dalian, China) following the manufacturer’s protocol. A similar procedure was followed during the extraction of total RNA and cDNA from the transgenic lines of Arabidopsis.
4.7. Construction of the Vector and Genetic Transformation
The coding sequence (CDS) of the PgL0145810.1 gene from pomegranate seeds was amplified from its cDNA by PCR. After amplification, the sequence was cloned into the pEASY-Blunt Simple vector. Then, it was further cloned into the PBI121-eGFP vector with XbaI/SacI restriction sites. The vector was transformed into Agrobacterium tumefaciens (GV3101 strain), then the Agrobacterium cell suspension was introduced into A. thaliana Col-0 inflorescences through the floral dip method [71,72]. The cell suspension contained 5% sucrose (wt/vol) and 0.05% Silwet L-77 (vol/vol) for an efficient absorption of the Agrobacterium by the female gametes [73,74]. After the floral dip, the plants were placed in the dark for 12 h and then transferred to light conditions in the growth chamber. Finally, the seeds were collected from the fully matured siliques.
4.8. Gene Expression Analysis through Quantitative Real-Time PCR (qRT-PCR)
Quantitative real-time PCR was conducted by using a Roche Light Cycler 480 Real Time PCR system and a Roche Light Cycler 480 SYBR Green I Master. The procedure during the qRT-PCR was as per the manufacturer’s protocol. The relative quantitative expression level was calculated by the 2−∆∆CT method [75]. PgActin was used as an endogenous reference gene in sample tests to analyze the expression of genes in the different pomegranate varieties, and actin was used in the transgenic lines, performed in three replicates. The homozygous lines of transgenic three (T3) generations were used for the phenotypic expression analysis. The primers used are listed in Table S6.
4.9. Sequencing of Genomic DNA
Total genomic DNA was isolated from young leaves of transgenic lines according to the procedures previously described [76]. The T-DNA insertion sites of the transgenic lines were examined by the genomic walking method [77,78]. The sequence of the right border region was used to design specific primers for thermal asymmetric interlaced PCR. The specific primers used during the study are listed in Table S6.
4.10. Measurement of Lignin Content
The total lignin contents from the stems of the wild type Arabidopsis and the transgenic Arabidopsis plants were determined through the UV spectrophotometer method. The sample stems from the base of the plants for quantifying lignin were taken from 42-day-old seedlings. During the measurement of the lignin content, we used thioglycolic acid (TGA) assay procedures with slight modifications [79].
4.11. Cytological Analysis of Inflorescence Stem
For the cytological observation, the samples from the inflorescence stem of Arabidopsis (both wild type and transgenic) plants were taken at the 35-day-old seedling stage. The samples were placed in FAA solution (3.7% formaldehyde, 5% glacial acetic acid and 50% ethanol) under vacuum for 24 h for embedding with paraffin. The specimens were further sliced into ultrathin sections using an ultramicrotome and stained with 0.05% toluidine blue O and observed under an OLYMPUS BX51 light microscope (Hitachi, Ibaraki, Japan).
5. Conclusions
The whole genome sequence of pomegranate “Tunisia” was a reference genome from which we identified ten SUT genes. We performed genetic transformation, expression analysis and cytological observation of PgL0145810.1. The transgenic plants showed that the cells in the xylem were small and the lignin content was lower in the transgenic plants as compared to the wild type. This study provides a reference for understanding the SUT gene structure and their promoters as well as the expression of PgL0145810.1 in transgenic plants.
Supplementary Materials
Supplementary Materials can be found online at https://www.mdpi.com/1422-0067/21/18/6608/s1.
Author Contributions
S.C., X.L. and K.P. conceived the research and designed the experiments; K.P., L.C., X.X., D.J. and L.T. contributed to most of the research works (PCR, qRT-PCR, transformation); X.L. and L.C. conducted the data analysis part; K.P. wrote the manuscript; S.C., X.L. and H.L. helped to review and edit the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research work was supported by the National Natural Science Foundation of China (31901343) and the Agricultural Science and Technology Innovation Program of the Chinese Academy of Agricultural Sciences (CAAS-ASTIP-2020-ZFPRI).
Acknowledgments
The authors would like to thank all the researchers of Zhengzhou Fruit Research Institute, dry fruit research group, Chinese Academy of Agricultural Sciences.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Singh, N.V.; Abburi, V.L.; Ramajayam, D.; Kumar, R.; Chandra, R.; Sharma, K.K.; Sharma, J.; Babu, K.D.; Pal, R.K.; Mundewadikar, D.M.; et al. Genetic diversity and association mapping of bacterial blight and other horticulturally important traits with microsatellite markers in pomegranate from India. Mol. Genet. Genom. 2015, 290, 1393–1402. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; Yin, Y.; Qu, J.; Zhu, L.; Li, Y. Population Genetic Diversity in Chinese Pomegranate (Punica granatum L.) Cultivars Revealed by Fluorescent-AFLP Markers. J. Genet. Genom. 2007, 34, 1061–1071. [Google Scholar] [CrossRef]
- APG. An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG IV. Bot. J. Linn. Soc. 2016, 181, 1–20. [Google Scholar] [CrossRef]
- Zhao, X.; Yuan, Z.; Feng, L.; Fang, Y. Cloning and expression of anthocyanin biosynthetic genes in red and white pomegranate. J. Plant Res. 2015, 128, 687–696. [Google Scholar] [CrossRef]
- Faria, A.; Monteiro, R.; Mateus, N.; Azevedo, I.; Calhau, C. Effect of pomegranate (Punica granatum L.) juice intake on hepatic oxidative stress. Eur. J. Nutr. 2007, 46, 271–278. [Google Scholar] [CrossRef]
- Ozgen, M.; Durgac, C.; Serce, S.; Kaya, C. Chemical and antioxidant properties of pomegranate cultivars grown in the Mediterranean region of Turkey. Food Chem. 2008, 111, 703–706. [Google Scholar] [CrossRef]
- Zhang, S.; Chen, L.; Huang, R.; Isha, A.; Dong, L.; Flachowsky, H. Generation and analysis of expressed sequence tag sequences from a soft-seeded pomegranate cDNA library. Plant Breed. 2017, 136, 994–999. [Google Scholar] [CrossRef]
- Luby, J.J.; Shaw, D.V. Does marker-assisted selection make dollars and sense in a fruit breeding program? Hort Sci. 2001, 36, 872–879. [Google Scholar] [CrossRef]
- Rikkerink, E.H.; Oraguzie, N.C.; Gardiner, S.E. Prospects of Association Mapping in Perennial Horticultural Crops. In Association Mapping in Plants; Springer: New York, NY, USA, 2007; pp. 249–269. [Google Scholar]
- Dalimov, D.N.; Dalimova, G.N.; Bhatt, M. Chemical composition and lignins of tomato and pomegranate seeds. Chem. Nat. Compounds 2003, 39, 37–40. [Google Scholar] [CrossRef]
- Cao, S.; Niu, J.; Cao, D.; Li, H.; Xue, H.; Chen, L.; Zhang, F.L.; Zhao, D. Comparative proteomics analysis of pomegranate seeds on fruit maturation period (Punica granatum L.). J. Integr. Agric. 2015, 14, 2558–2564. [Google Scholar] [CrossRef]
- Qin, G.; Xu, C.; Ming, R.; Tang, H.; Guyot, R.; Kramer, E.M.; Hu, Y.; Yi, X.; Qi, Y.; Xu, X.; et al. The pomegranate (Punica granatum L.) genome and the genomics of punicalagin biosynthesis. Plant J. 2017, 91, 1108–1128. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Li, H.; Wu, Z.; Yao, W.; Zhao, P.; Cao, D.; Yu, H.; Li, K.; Poudel, K.; Zhao, D.; et al. The pomegranate (Punica granatum L.) draft genome dissects genetic divergence between soft and hard-seeded cultivars. Plant Biotechnol. J. 2019, 18, 955–968. [Google Scholar] [CrossRef] [PubMed]
- Xue, H.; Cao, S.; Li, H.; Zhang, J.; Niu, J.; Chen, L.; Zhang, F.; Zhao, D. De novo transcriptome assembly and quantification reveal differentially expressed genes between soft-seed and hard-seed pomegranate (Punica granatum L.). PLoS ONE 2017, 12, e0178809. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Cao, D.; Zhang, J.; Chen, L.; Xia, X.; Li, H.; Zhao, D.; Zhang, F.; Xue, H.; Chen, L.; et al. Integrated microRNA and mRNA expression profiling reveals a complex network regulating pomegranate (Punica granatum L.) seed hardness. Sci. Rep. 2018, 8, 9292. [Google Scholar] [CrossRef]
- Niu, J.; Cao, D.; Li, H.; Xue, H.; Chen, L.; Liu, B.; Cao, S. Quantitative proteomics of pomegranate varieties with contrasting seed hardness during seed development stages. Tree Genet. Genomes 2018, 14. [Google Scholar] [CrossRef]
- Zarei, A.; Zamani, Z.; Fatahi, R.; Mousavi, A.; Salami, S.A.; Avila, C.; Canovas, F.M. Differential expression of cell wall related genes in the seeds of soft and hard-seeded pomegranate genotypes. Sci. Hortic. 2016, 205, 7–16. [Google Scholar] [CrossRef]
- Xia, X.; Li, H.; Cao, D.; Luo, X.; Yang, X.; Chen, L.; Liu, B.; Wang, Q.; Jing, D.; Cao, S. Characterization of a NAC transcription factor involved in the regulation of pomegranate seed hardness (Punica granatum L.). Plant Physiol. Biochem. 2019, 139, 379–388. [Google Scholar] [CrossRef]
- Ainsworth, E.A.; Bush, D.R. Carbohydrate export from the leaf: A highly regulated process and target to enhance photosynthesis and productivity. Plant Physiol. 2011, 155, 64–69. [Google Scholar] [CrossRef]
- Julius, B.T.; Leach, K.A.; Tran, T.M.; Mertz, R.A.; Braun, D.M. Sugar Transporters in Plants: New Insights and Discoveries. Plant Cell Physiol. 2017, 58, 1442–1460. [Google Scholar] [CrossRef]
- Lemoine, R. Sucrose transporters in plants: Update and function and structure. Biochim. Biophys. Acta 2000, 1465, 246–262. [Google Scholar] [CrossRef]
- Lemoine, R.; LaCamera, S.; Atanassova, R.; Dedaldechamp, F.; Allario, T.; Pourtau, N.; Bonnemain, J.L.; Laloi, M.; Coutos-Thevenot, P.; Maurousset, L.; et al. Source-to-sink transport of sugar and regulation by environmental factors. Front. Plant Sci. 2013, 4, 272. [Google Scholar] [CrossRef] [PubMed]
- Ludewig, F.; Flugge, U.I. Role of metabolite transporters in source-sink carbon allocation. Front. Plant Sci. 2013, 4, 231. [Google Scholar] [CrossRef] [PubMed]
- Sauer, N.; Ludwig, A.; Knoblauch, A.; Rothe, P.; Gahrtz, M.; Klebl, F. AtSUC8 and AtSUC9 encode functional sucrose transporters, but the closely related AtSUC6 and AtSUC7 genes encode aberrant proteins in different Arabidopsis ecotypes. Plant J. 2004, 40, 120–130. [Google Scholar] [CrossRef]
- Shiratake, K. Genetics of Sucrose Transporter in Plants. Genes Genomes Genom. 2007, 1, 73–80. [Google Scholar]
- Sivitz, A.B.; Reinders, A.; Johnson, M.E.; Krentz, A.D.; Grof, C.P.; Perroux, J.M.; Ward, J.M. Arabidopsis sucrose transporter AtSUC9. High-affinity transport activity, intragenic control of expression, and early flowering mutant phenotype. Plant Physiol. 2007, 143, 188–198. [Google Scholar] [CrossRef] [PubMed]
- Truernit, E. Plant Physiology: Unveiling the Dark Side of Phloem Translocation. Curr Biol 2017, 27, 348–350. [Google Scholar] [CrossRef][Green Version]
- Kuhn, C.; Grof, C.P.L. Sucrose transporters of higher plants. Curr. Opin. Plant Biol. 2010, 13, 287–297. [Google Scholar] [CrossRef]
- Lalonde, S.; Wipf, D.; Frommer, W.B. Transport mechanisms for organic forms of carbon and nitrogen between source and sink. Annu. Rev. Plant Biol. 2004, 55, 341–372. [Google Scholar] [CrossRef]
- Lim, J.D.; Cho, J.I.; Park, Y.; Hahn, T.; Choi, S.; Jeon, J. Sucrose transport from source to sink seeds in rice. Physiol. Plant 2006, 126, 572–584. [Google Scholar] [CrossRef]
- Rennie, E.A.; Turgeon, R. A comprehensive picture of phloem loading strategies. Proc. Natl. Acad. Sci. USA 2009, 106, 14162–14167. [Google Scholar] [CrossRef]
- Turgeon, R.; Wolf, S. Phloem transport: Cellular pathways and molecular trafficking. Annu. Rev. Plant Biol. 2009, 60, 207–221. [Google Scholar] [CrossRef]
- Sauer, N. Molecular physiology of higher plant sucrose transporters. FEBS Lett. 2007, 581, 2309–2317. [Google Scholar] [CrossRef] [PubMed]
- Williams, L.E.; Lemoine, R.; Sauer, N. Sugar transporters in higher plants—A diversity of roles and complex regulation. Trends Plant Sci. 2000, 5, 283–290. [Google Scholar] [CrossRef]
- Berthier, A.; Desclos, M.; Amiard, V.; Morvan-Bertrand, A.; Demmig-Adams, B.; Adams, W.; Turgeon, R.; Prudhomme, M.; Noiraud-Romy, N. Activation of sucrose transport in defoliated Lolium perenne L.: An example of apoplastic phloem loading plasticity. Plant Cell Physiol. 2009, 50, 1329–1344. [Google Scholar] [CrossRef] [PubMed]
- Liesche, J.; Schulz, A.; Krugel, U.; Grimm, B.; Kuhn, C. Dimerization and endocytosis of the sucrose transporter StSUT1 in mature sieve elements. Plant Signal. Behav. 2008, 3, 1136–1137. [Google Scholar] [CrossRef][Green Version]
- Riesmeier, J.W.; Willmitzer, L.; Frommer, W.B. Isolation and characterization of a sucrose carrier cDNA from spinach by functional expression in yeast. EMBO J. 1992, 11, 4705–4713. [Google Scholar] [CrossRef]
- Aoki, N.; Hirose, T.; Scofield, G.N.; Whitfeld, P.R.; Furbank, R.T. The sucrose transporter gene family in rice. Plant Cell Physiol. 2003, 44, 223–232. [Google Scholar] [CrossRef]
- Meyer, S.; Lauterbach, C.; Niedermeier, M.; Barth, I.; Sjolund, R.D.; Sauer, N. Wounding enhances expression of AtSUC3, a sucrose transporter from Arabidopsis sieve elements and sink tissues. Plant Physiol. 2004, 134, 684–693. [Google Scholar] [CrossRef]
- Schulze, W.; Weise, A.; Frommer, W.B.; Ward, J.M. Function of the cytosolic N-terminus of sucrose transporter AtSUT2 in substrate affinity. FEBS Lett. 2000, 485, 189–194. [Google Scholar] [CrossRef]
- Sivitz, A.B.; Reinders, A.; Ward, J.M. Arabidopsis sucrose transporter AtSUC1 is important for pollen germination and sucrose-induced anthocyanin accumulation. Plant Physiol. 2008, 147, 92–100. [Google Scholar] [CrossRef]
- Bezrutczyk, M.; Yang, J.; Eom, J.S.; Prior, M.; Sosso, D.; Hartwig, T.; Szurek, B.; Oliva, R.; Vera-Cruz, C.; White, F.F.; et al. Sugar flux and signaling in plant–microbe interactions. Plant J. 2018, 93, 675–685. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Gao, Z.; Wang, J.; Huang, Y.; Chen, Y.; Li, J.; Lv, M.; Wang, J.; Luo, M.; Zuo, K. Cotton fiber elongation requires the transcription factor GhMYB212 to regulate sucrose transportation into expanding fibers. New Phytol. 2019, 222, 864–881. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Ma, C.; Wang, X.; Gao, C.; Zhang, J.; Wang, Y.; Cong, N.; Li, X.; Wen, J.; Yi, B.; et al. Characterization of sucrose transporter alleles and their association with seed yield-related traits in Brassica napus L. BMC Plant Biol. 2011, 11, 168. [Google Scholar] [CrossRef] [PubMed]
- Riesmeier, J.W.; Willmitzer, L.; Frommer, W.B. Evidence for an essential role of the sucrose transporter in phloem loading and assimilate partitioning. EMBO J. 1994, 13, 1–7. [Google Scholar] [CrossRef]
- Hackel, A.; Schauer, N.; Carrari, F.; Fernie, A.R.; Grimm, B.; Kuhn, C. Sucrose transporter LeSUT1 and LeSUT2 inhibition affects tomato fruit development in different ways. Plant J. 2006, 45, 180–192. [Google Scholar] [CrossRef]
- Yuan, Z.; Fang, Y.; Zhang, T.; Fei, Z.; Han, F.; Liu, C.; Liu, M.; Xiao, W.; Zhang, W.; Wu, S.; et al. The pomegranate (Punica granatum L.) genome provides insights into fruit quality and ovule developmental biology. Plant Biotechnol. J. 2018, 16, 1363–1374. [Google Scholar] [CrossRef]
- Gao, Z.; Maurousset, L.; Lemoine, R.; Yoo, S.D.; Van Nocker, S.; Loescher, W. Cloning, expression, and characterization of sorbitol transporters from developings our cherry fruit and leaf sink tissues. Plant Physiol. 2003, 131, 1566–1575. [Google Scholar] [CrossRef]
- Ruan, Y.L. Signaling role of sucrose metabolism in development. Mol. Plant 2012, 5, 763–765. [Google Scholar] [CrossRef]
- Barker, L.; Kuhn, C.; Weise, A.; Schulz, A.; Gebhardt, C.; Hirner, B.; Hellmann, H.; Schulze, W.; Ward, J.M.; Frommer, W.B. SUT2, a putative sucrose sensor in sieve elements. Plant Cell 2000, 12, 1153–1164. [Google Scholar] [CrossRef]
- Wang, P.; Wei, P.; Niu, F.; Liu, X.; Zhang, H.; Lyu, M.; Yuan, Y.; Wu, B. Cloning and Functional Assessments of Floral-Expressed SWEET Transporter Genes from Jasminum sambac. Int. J. Mol. Sci 2019, 20, 4001. [Google Scholar] [CrossRef]
- Doidy, J.; Grace, E.; Kuhn, C.; Simon-Plas, F.; Casieri, L.; Wipf, D. Sugar transporters in plants and in their interactions with fungi. Trends Plant Sci. 2012, 17, 413–422. [Google Scholar] [CrossRef] [PubMed]
- Chincinska, I.A.; Liesche, J.; Krugel, U.; Michalska, J.; Geigenberger, P.; Grimm, B.; Kuhn, C. Sucrose transporter StSUT4 from potato affects flowering, tuberization, and shade avoidance response. Plant Physiol. 2008, 146, 515–528. [Google Scholar] [CrossRef] [PubMed]
- Durand, M.; Mainson, D.; Porcheron, B.; Maurousset, L.; Lemoine, R.; Pourtau, N. Carbon source–sink relationship in Arabidopsis thaliana: The role of sucrose transporters. Planta 2018, 247, 587–611. [Google Scholar] [CrossRef]
- Chen, Q.; Guo, W.; Feng, L.; Ye, X.; Xie, W.; Huang, X.; Liu, J. Data for transcriptome and proteome analysis of Eucalyptus infected with Calonectria pseudoreteaudii. Data Brief 2015, 3, 24–28. [Google Scholar] [CrossRef]
- Wu, H.; Jia, H.; Ma, X.; Wang, S.; Yao, Q.; Xu, W.; Zhou, Y.G.; Gao, Z.S.; Zhan, R. Transcriptome and proteomic analysis of mango (Mangifera indica L.) fruits. J. Proteom. 2014, 105, 19–30. [Google Scholar] [CrossRef]
- Bihmidine, S.; Baker, R.F.; Hoffner, C.; Braun, D.M. Sucrose accumulation in sweet sorghum stems occurs by apoplasmic phloem unloading and doesnot involve differential sucrose transporter expression. BMC Plant Biol. 2015, 15, 186. [Google Scholar] [CrossRef] [PubMed]
- Rae, A.L.; Perroux, J.M.; Grof, C.P. Sucrose partitioning between vascular bundles and storage parenchyma in the sugarcane stem: A potential role for the ShSUT1 sucrose transporter. Planta 2005, 220, 817–825. [Google Scholar] [CrossRef] [PubMed]
- Singer, S.D.; Hily, J.M.; Cox, K.D. The sucrose synthase-1 promoter from Citrus sinensis directs expression of the β-glucuronidase reporter gene in phloem tissue and inresponse to wounding in transgenic plants. Planta 2011, 234, 623–637. [Google Scholar] [CrossRef]
- Berendzen, K.W.; Weiste, C.; Wanke, D.; Kilian, J.; Harter, K.; Droge-Laser, W. Bioinformatic cis-element analyses performed in Arabidopsis and rice disclose bZIP and MYB-related binding sites as potential AuxRE-coupling elements in auxin-mediated transcription. BMC Plant Biol. 2012, 12, 125. [Google Scholar] [CrossRef]
- Feller, A.; Machemer, K.; Braun, E.L.; Grotewold, E. Evolutionary and comparative analysis of MYB and bHLH plant transcription factors. Plant J. 2011, 66, 94–116. [Google Scholar] [CrossRef]
- TIGRFAMS. Available online: http://tigrfams.jcvi.org/cgi-bin/index.cgi (accessed on 16 May 2020).
- Thompson, J.D.; Gibson, T.J.; Higgins, D.G. Multiple sequence alignment using ClustalW and ClustalX. Curr. Protoc. Bioinform. 2003. [Google Scholar] [CrossRef] [PubMed]
- Eddy, S.R. Profile hidden Markov models. Bioinformatics 1998, 14, 755–763. [Google Scholar] [CrossRef] [PubMed]
- Kent, W.J. BLAT-The BLAST-like alignment tool. Genome Res. 2002, 12, 656–664. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Peterson, D.; Peterson, N.; Stecher, G.; Nei, M.; Kumar, S. MEGA5: Molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 2011, 28, 2731–2739. [Google Scholar] [CrossRef]
- PlantCARE. Available online: http://bioinformatics.psb.ugent.be/webtools/plantcare/html/ (accessed on 15 May 2020).
- Ison, J.; Rapacki, K.; Menager, H.; Kalas, M.; Rydza, E.; Chmura, P.; Brunak, S. Tools and data services registry: A community effort to document bioinformatics resources. Nucleic Acids Res. 2015, 44, 38–47. [Google Scholar] [CrossRef]
- Hall, T.A. BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucl. Acids. Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- National Centre for Biotechnology Information. Available online: https://www.ncbi.nlm.nih.gov/tools/primer-blast/ (accessed on 16 May 2020).
- Clough, S.J.; Bent, A.F. Floral dip: A simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 1998, 16, 735–743. [Google Scholar] [CrossRef]
- Martinez-Trujillo, M.; Limones-Briones, V.; Cabrera-Ponce, J.L.; Herrera-Estrella, L. Improving transformation efficiency of Arabidopsis thaliana by modifying the floral dip method. Plant Mol. Biol. Rep. 2004, 22, 63–70. [Google Scholar] [CrossRef]
- Bechtold, N.; Jolivet, S.; Voisin, R.; Pelletier, G. The endosperm and the embryo of Arabidopsis thaliana are independently transformed through infiltration by Agrobacterium tumefaciens. Transgenic Res. 2003, 12, 509–517. [Google Scholar] [CrossRef]
- Desfeux, C.; Clough, S.J.; Bent, A.F. Female Reproductive Tissues Are the Primary Target of Agrobacterium-Mediated Transformation by the Arabidopsis Floral-Dip Method. Plant Physiol. 2000, 123, 895. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−∆∆CT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Kasajima, I.; Ide, Y.; Ohkama-Ohtsu, N.; Hayashi, H.; Yoneyama, T.; Fujiwara, T. A Protocol for Rapid DNA Extraction from Arabidopsis thaliana for PCR Analysis. Plant Mol. Biol. 2004, 22, 49–52. [Google Scholar] [CrossRef]
- Liu, Y.G.; Whittier, R.F. Thermal Asymmetric Interlaced PCR: Automatable Amplification and Sequencing of Insert End Fragments from PI and YAC Clones for Chromosome Walking. Genomics 1995, 25, 674–681. [Google Scholar] [CrossRef]
- Zheng, S.J.; Henken, B.; Sofiari, E.; Jacobsen, E.; Krens, F.A.; Kik, C. Molecular characterization of transgenic shallots (Allium cepa L.) by adaptor ligation PCR (AL-PCR) and sequencing of genomic DNA flanking T-DNA borders. Transgenic Res. 2001, 10, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Liang, M.; Davis, E.; Gardner, D.; Cai, X.; Wu, Y. Involvement of AtLAC15 in lignin synthesis in seeds and in root elongation of Arabidopsis. Planta 2006, 224, 1185–1196. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).