Exploring the Role of Phenylalanine Residues in Modulating the Flexibility and Topography of the Active Site in the Peroxygenase Variant PaDa-I
Abstract
1. Introduction
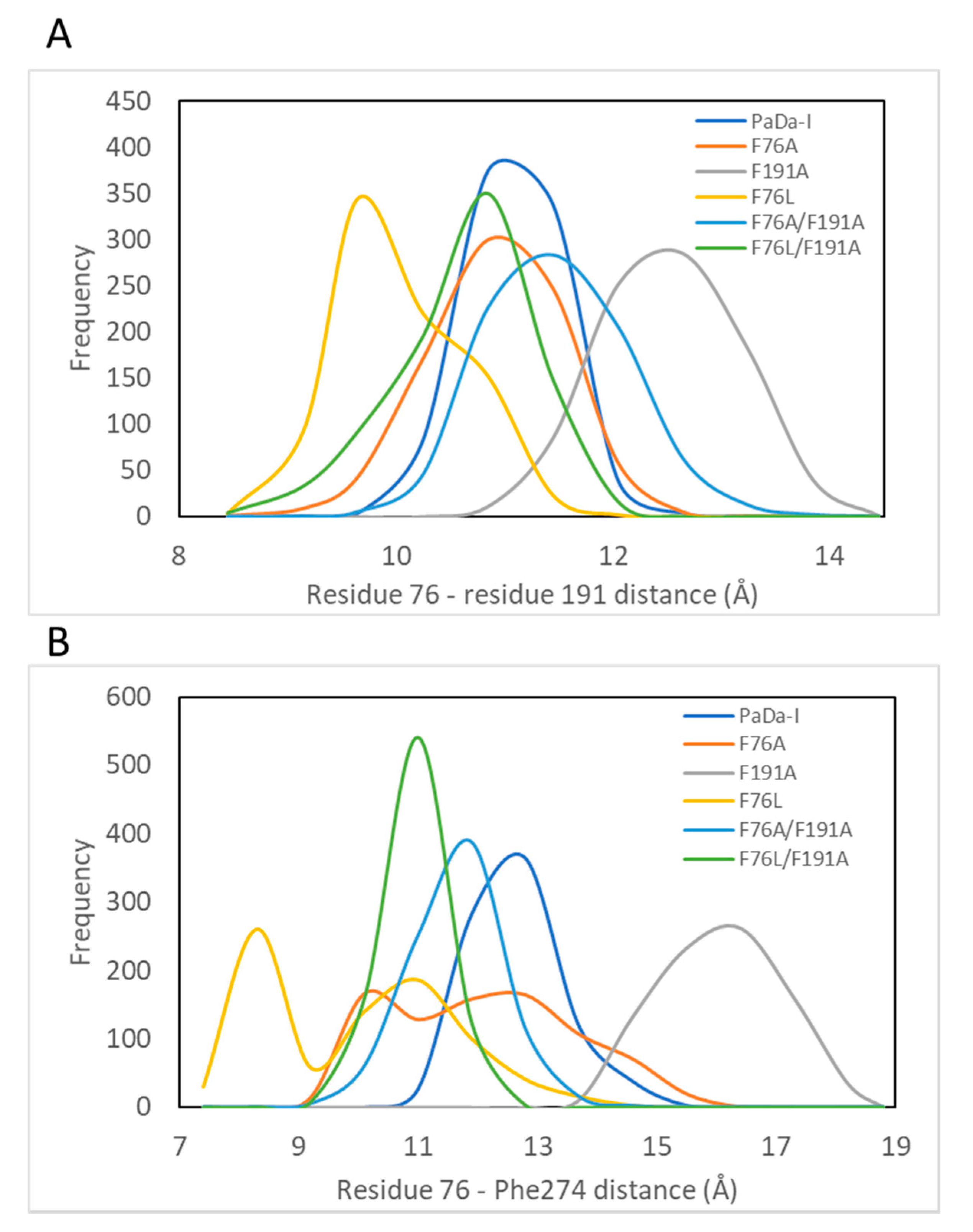
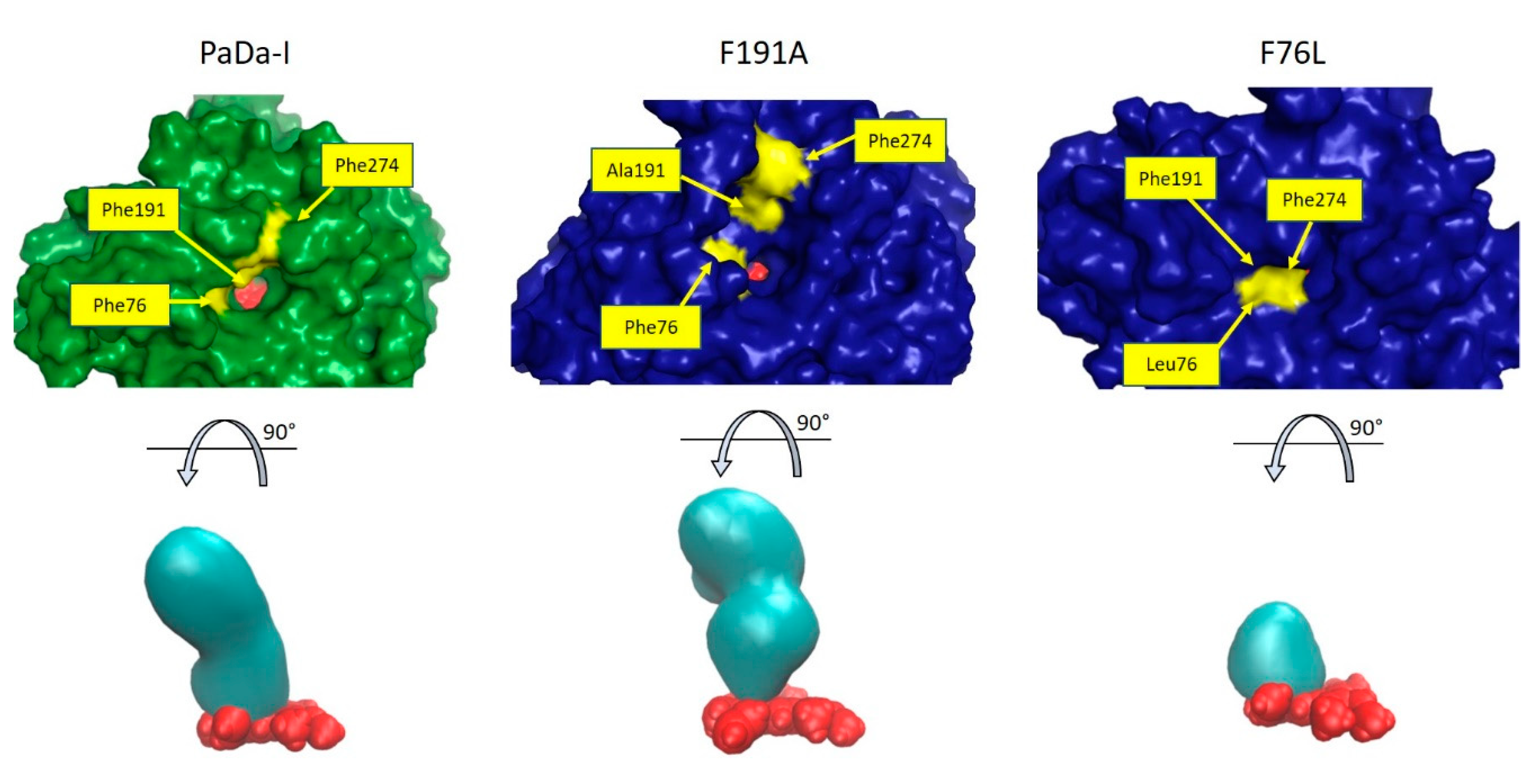
2. Results and Discussion
3. Materials and Methods
3.1. Computational Methods
3.1.1. System Preparation
3.1.2. Molecular Dynamics Simulations
3.2. Experimental Section
3.2.1. Site-Directed Mutagenesis
3.2.2. Enzyme Production
3.2.3. Enzyme Purification
3.2.4. Enzyme Activity Assays
3.2.5. Determination of Kinetic Parameters
3.2.6. Determination of the Total Turnover Number
3.2.7. Stability of Enzyme Variants in Hydrogen Peroxide
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ullrich, R.; Nüske, J.; Scheibner, K.; Spantzel, J.; Hofrichter, M. Novel haloperoxidase from the agaric basidiomycete Agrocybe aegerita oxidizes aryl alcohols and aldehydes. Appl. Environ. Microbiol. 2004, 70, 4575–4581. [Google Scholar] [CrossRef] [PubMed]
- Ullrich, R.; Hofrichter, M. The haloperoxidase of the agaric fungus Agrocybe aegerita hydroxylates toluene and naphthalene. FEBS Lett. 2005, 579, 6247–6250. [Google Scholar] [CrossRef] [PubMed]
- Hofrichter, M.; Ullrich, R. Oxidations catalyzed by fungal peroxygenases. Curr. Opin. Chem. Biol. 2014, 19, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Kluge, M.; Ullrich, R.; Dolge, C.; Scheibner, K.; Hofrichter, M. Hydroxylation of naphthalene by aromatic peroxygenase from Agrocybe aegerita proceeds via oxygen transfer from H2O2 and intermediary epoxidation. Appl. Microbiol. Biotechnol. 2009, 81, 1071–1076. [Google Scholar] [CrossRef]
- Aranda, E.; Ullrich, R.; Hofrichter, M. Conversion of polycyclic aromatic hydrocarbons, methyl naphthalenes and dibenzofuran by two fungal peroxygenases. Biodegradation 2010, 21, 267–281. [Google Scholar] [CrossRef]
- Peter, S.; Kinne, M.; Wang, X.; Ullrich, R.; Kayser, G.; Groves, J.T.; Hofrichter, M. Selective hydroxylation of alkanes by an extracellular fungal peroxygenase. FEBS J. 2011, 278, 3667–3675. [Google Scholar] [CrossRef]
- Kinne, M.; Poraj-Kobielska, M.; Ralph, S.A.; Ullrich, R.; Hofrichter, M.; Hammel, K.E. Oxidative Cleavage of Diverse Ethers by an Extracellular Fungal Peroxygenase. J. Biol. Chem. 2009, 284, 29343–29349. [Google Scholar] [CrossRef]
- Babot, E.D.; del Río, J.C.; Cañellas, M.; Sancho, F.; Lucas, F.; Guallar, V.; Kalum, L.; Lund, H.; Gröbe, G.; Scheibner, K.; et al. Steroid Hydroxylation by Basidiomycete Peroxygenases: A Combined Experimental and Computational Study. Appl. Environ. Microbiol. 2015, 81, 4130–4142. [Google Scholar] [CrossRef]
- Hofrichter, M.; Ullrich, R. Heme-thiolate haloperoxidases: Versatile biocatalysts with biotechnological and environmental significance. Appl. Microbiol. Biotechnol. 2006, 71, 276–288. [Google Scholar] [CrossRef]
- Ahn, D.H.; Ullrich, R.; Benndorf, D.; Svatos, A.; Muck, A.; Hofrichter, M. The Coprophilous Mushroom Coprinus radians Secretes a Haloperoxidase That Catalyzes Aromatic Peroxygenation. Appl. Environ. Microbiol. 2007, 73, 5477–5485. [Google Scholar]
- Gröbe, G.; Ullrich, R.; Pecyna, M.J.; Kapturska, D.; Friedrich, S.; Hofrichter, M.; Scheibner, K. High-yield production of aromatic peroxygenase by the agaric fungus Marasmius rotula. AMB Express 2011, 1, 31–41. [Google Scholar] [CrossRef]
- Kiebist, J.; Schmidtke, K.; Zimmermann, J.; Kellner, H.; Jehmlich, N.; Ullrich, R.; Zänder, D.; Hofrichter, M.; Scheibner, K. A Peroxygenase from Chaetomium globosum Catalyzes the Selective Oxygenation of Testosterone. ChemBioChem 2017, 18, 563–569. [Google Scholar] [CrossRef] [PubMed]
- Faiza, M.; Huang, S.; Lan, D.; Wang, Y. New insights on unspecific peroxygenases: Superfamily reclassification and evolution. BMC Evol. Biol. 2019, 19, 76. [Google Scholar] [CrossRef] [PubMed]
- Hofrichter, M.; Kellner, H.; Herzog, R.; Karich, A.; Liers, C.; Scheibner, K.; Kimani, V.W.; Ullrich, R. Fungal peroxygenases: A phylogenetically old superfamily of heme enzymes with promiscuity for oxygen transfer reactions. In Grand Challenges in Fungal Biotechnology. Grand Challenges in Biology and Biotechnology; Nevalainen, H., Ed.; Springer: Berlin/Heidelberg, Germany, 2020; pp. 369–403. [Google Scholar]
- Piontek, K.; Strittmatter, E.; Ullrich, R.; Gröbe, G.; Pecyna, M.J.; Kluger, M.; Scheibner, K.; Hofrichter, M.; Plattner, D.A. Structural Basis of Substrate Conversion in a New Aromatic Peroxygenase: Cytochrome P450 functionality with benefits. J. Biol. Chem. 2013, 288, 34767–34776. [Google Scholar] [CrossRef] [PubMed]
- Ramirez-Escudero, M.; Molina-Espeja, P.; Gomez de Santos, P.; Hofrichter, M.; Sanz-Aparicio, J.; Alcalde, M. Structural Insights into the Substrate Promiscuity of a Laboratory-Evolved Peroxygenase. ACS Chem. Biol. 2018, 13, 3259–3268. [Google Scholar] [CrossRef] [PubMed]
- Sundaramoorthy, M.; Terner, J.; Poulos, T.L. The crystal structure of chloroperoxidase: A heme peroxidase-cytochrome P450 functional hybrid. Structure 1995, 3, 1367–1377. [Google Scholar] [CrossRef]
- Olmedo, A.; del Rio, J.C.; Kiebist, J.; Ullrich, R.; Hofrichter, M.; Scheibner, K.; Martinez, A.T.; Gutierrez, A. From Alkanes to Carboxylic Acids: Terminal Oxygenation by a Fungal Peroxygenase. Chem. Eur. J. 2017, 23, 16985–16989. [Google Scholar] [CrossRef]
- Peter, S.; Kinne, M.; Ullrich, R.; Kayser, G.; Hofrichter, M. Epoxidation of linear, branched and cyclic alkenes catalyzed by unspecific peroxygenase. Enz. Microb. Technol. 2013, 52, 370–376. [Google Scholar] [CrossRef]
- Ayala, M.; Pickard, M.A.; Vazquez-Duhalt, R. Fungal Enzymes for Environmental Purposes, a Molecular Biology Challenge. J. Mol. Microbiol. Biotechnol. 2008, 15, 172–180. [Google Scholar] [CrossRef]
- Wang, Y.; Lan, D.; Durrani, R.; Hollmann, F. Peroxygenases en route to becoming dream catalysts. What are the opportunities and challenges? Curr. Opin. Chem. Biol. 2017, 37, 1–9. [Google Scholar] [CrossRef]
- Garcia-Guevara, F.; Avelar, M.; Ayala, M.; Segovia, L. Computational Tools Applied to Enzyme Design—A review. Biocatalysis 2015, 1, 109–117. [Google Scholar] [CrossRef][Green Version]
- Molina-Espeja, P.; Canellas, M.; Plou, F.J.; Hofrichter, M.; Lucas, F.; Guallar, V.; Alcalde, M. Synthesis of 1-Naphthol by a Natural Peroxygenase Engineered by Directed Evolution. ChemBioChem 2016, 17, 341–349. [Google Scholar] [CrossRef] [PubMed]
- Gomez de Santos, P.; Canellas, M.; Tieves, F.; Younes, S.H.H.; Molina-Espeja, P.; Hofrichter, M.; Hollmann, F.; Guallar, V.; Alcalde, M. Selective Synthesis of the Human Drug Metabolite 5′-Hydroxypropranolol by an Evolved Self-Sufficient Peroxygenase. ACS Catal. 2018, 8, 4789–4799. [Google Scholar] [CrossRef]
- Molina-Espeja, P.; Garcia-Ruiz, E.; Gonzalez-Perez, D.; Ullrich, R.; Hofrichter, M.; Alcalde, M. Directed Evolution of Unspecific Peroxygenase from Agrocybe aegerita. Appl. Environ. Microbiol. 2014, 80, 3496–3507. [Google Scholar] [CrossRef] [PubMed]
- Samanta, S.K.; Singh, O.V.; Jain, R.K. Polycyclic aromatic hydrocarbons: Environmental pollution and bioremediation. Trends Biotechnol. 2002, 20, 243–248. [Google Scholar] [CrossRef]
- Ortiz de Montellano, P.R. Catalytic Mechanisms of Heme Peroxidases. In Biocatalysis Based on Heme Peroxidases; Torres, E., Ayala, M., Eds.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 79–107. [Google Scholar]
- Polizzi, K.M.; Bommarius, A.S.; Broering, J.M.; Chaparro-Riggers, J.F. Stability of biocatalysts. Curr. Op. Chem. Biol. 2007, 11, 220–225. [Google Scholar] [CrossRef]
- Valderrama, B.; Ayala, M.; Vazquez-Duhalt, R. Suicide Inactivation of Peroxidases and the Challenge of Engineering More Robust Enzymes. Chem. Biol. 2002, 9, 555–565. [Google Scholar] [CrossRef]
- Ayala, M.; Batista, C.V.; Vazquez-Duhalt, R. Heme destruction, the main molecular event during the peroxide-mediated inactivation of chloroperoxidase from Caldariomyces fumago. J. Biol. Inorg. Chem. 2011, 16, 63–68. [Google Scholar] [CrossRef]
- Karich, A.; Scheibner, K.; Ullrich, R.; Hofrichter, M. Exploring the catalase activity of unspecific peroxygenases and the mechanism of peroxide-dependent heme destruction. J. Mol. Catal. B Enzym. 2016, 134, 238–246. [Google Scholar] [CrossRef]
- Schrödinger Release 2014-2: Maestro, Schrödinger; LLC: New York, NY, USA, 2014; Available online: https://www.schrodinger.com/maestro (accessed on 9 August 2020).
- Bowers, K.J.; Chow, E.; Xu, H.; Dror, R.O.; Eastwood, M.P.; Gregersen, B.A.; Klepeis, J.L.; Kolossvary, I.; Moraes, M.A.; Sacerdoti, F.D.; et al. Scalable algorithms for molecular dynamics simulations on commodity clusters. In Proceedings of the ACM/IEEE Conference on Supercomputing (SC06), Tampa, FL, USA, 11–17 November 2006. [Google Scholar]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual molecular dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef]
- Wagner, J.R.; Sørensen, J.; Hensley, N.; Wong, C.; Zhu, C.; Perison, T.; Amaro, R.E. POVME 3.0: Software for Mapping Binding Pocket Flexibility. J. Chem. Theory Comp. 2017, 13, 4584–4592. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Perez, D.; Garcia-Ruiz, E.; Alcalde, M. Saccharomyces cerevisiae in directed evolution. An efficient tool to improve enzymes. Bioeng. Bugs 2012, 3, 172–177. [Google Scholar] [PubMed]
- Childs, R.E.; Bardsley, W.G. The steady-state kinetics of peroxidase with 2,2′-azino-di-(3-ethyl-benzthiazoline-6-sulphonic acid) as chromogen. Biochem. J. 1975, 145, 9–103. [Google Scholar] [CrossRef] [PubMed]
- Poraj-Kobielska, M.; Kinne, M.; Ullrich, R.; Scheibner, K.; Hofrichter, M. A spectrophotometric assay for the detection of fungal peroxygenases. Analyt. Biochem. 2012, 421, 327–329. [Google Scholar] [CrossRef] [PubMed]
- Kluge, M.G.; Ullrich, R.; Scheibner, K.; Hofrichter, M. Spectrophotometric assay for detection of aromatic hydroxylation catalyzed by fungal haloperoxidase–peroxygenase. Appl. Microbiol. Biotechnol. 2007, 75, 1473–1478. [Google Scholar] [CrossRef]
- Noble, R.W.; Gibson, Q.H. The Reaction of Ferrous Horseradish Peroxidase with Hydrogen Peroxide. J. Biol. Chem. 1970, 245, 2409–2413. [Google Scholar]
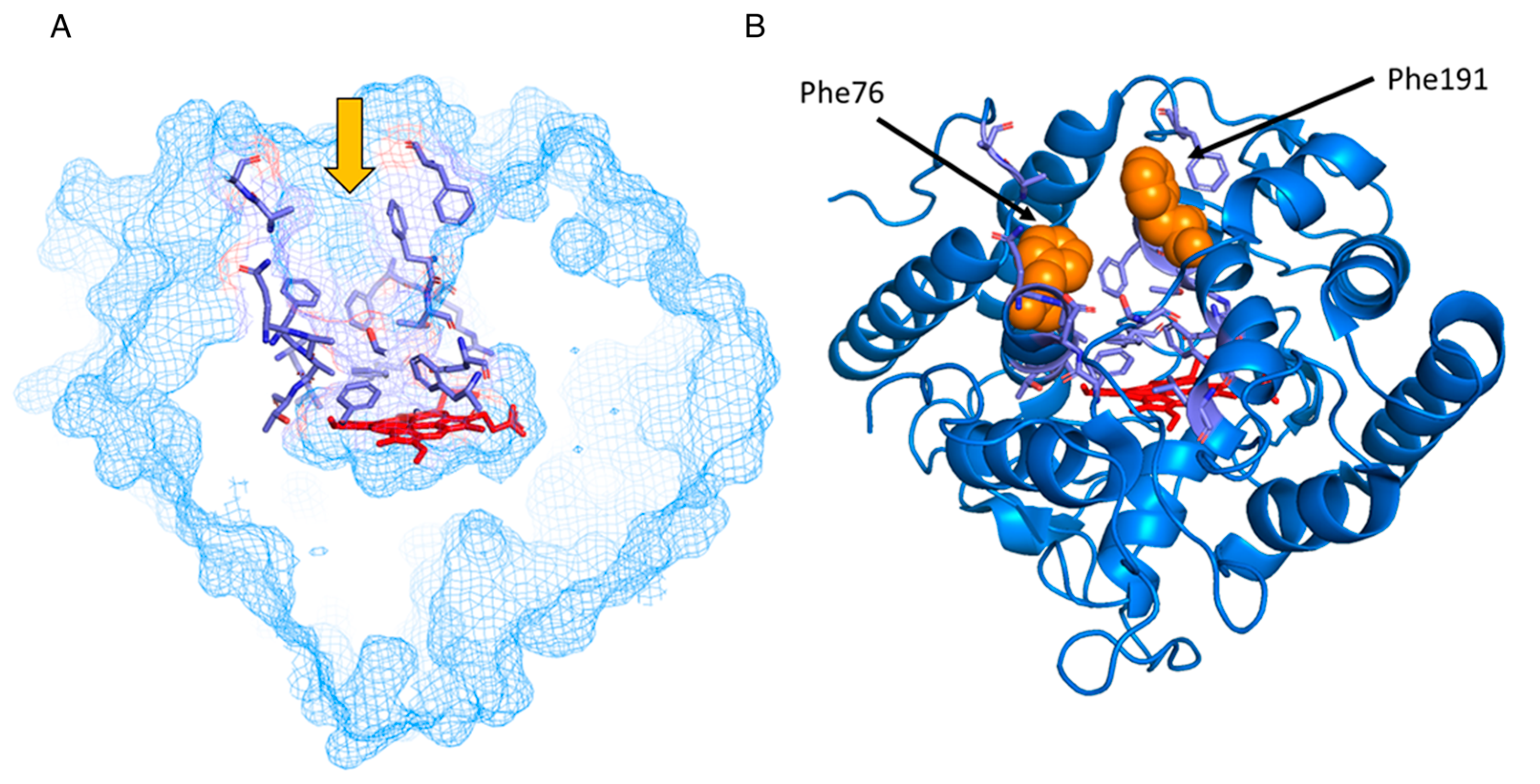
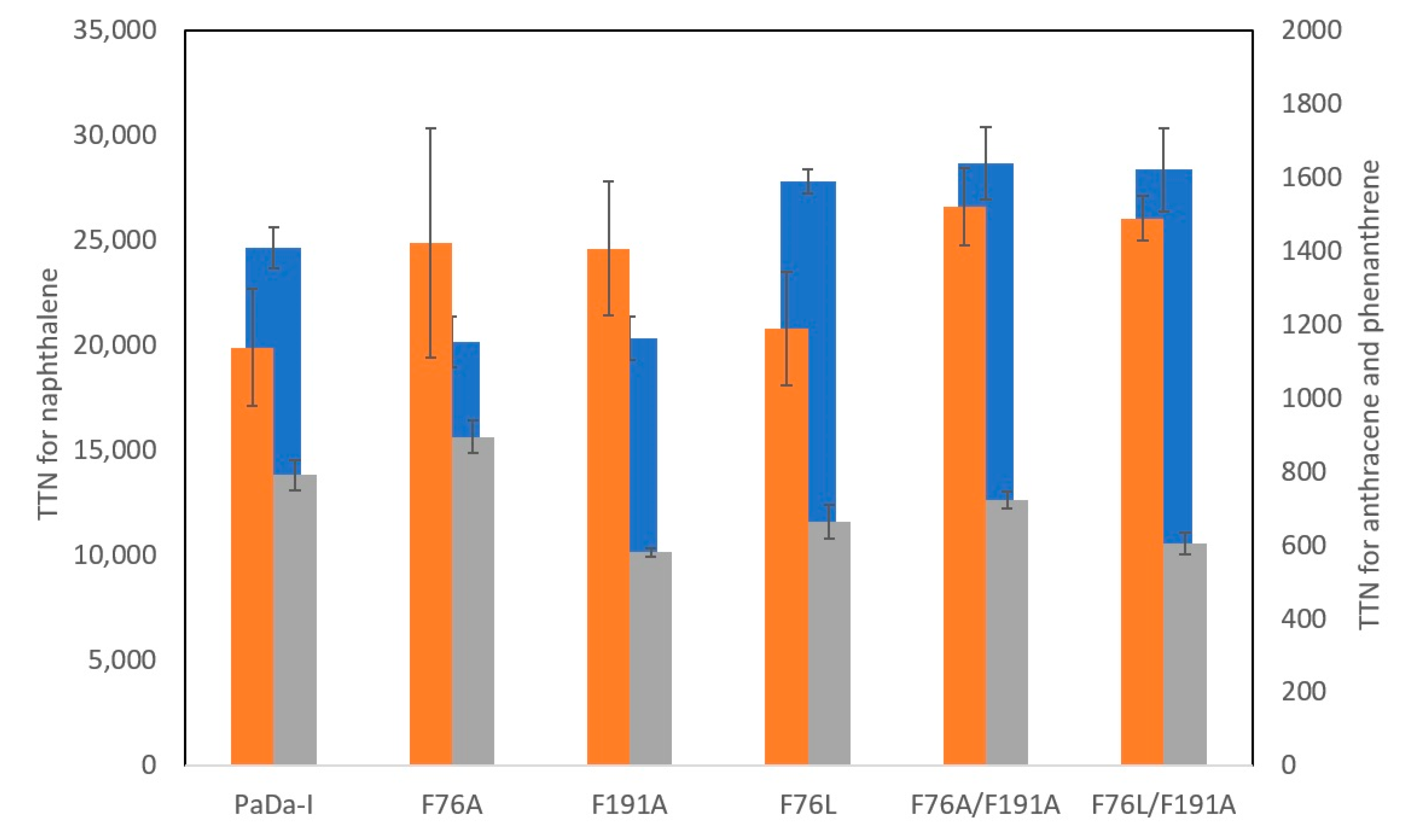
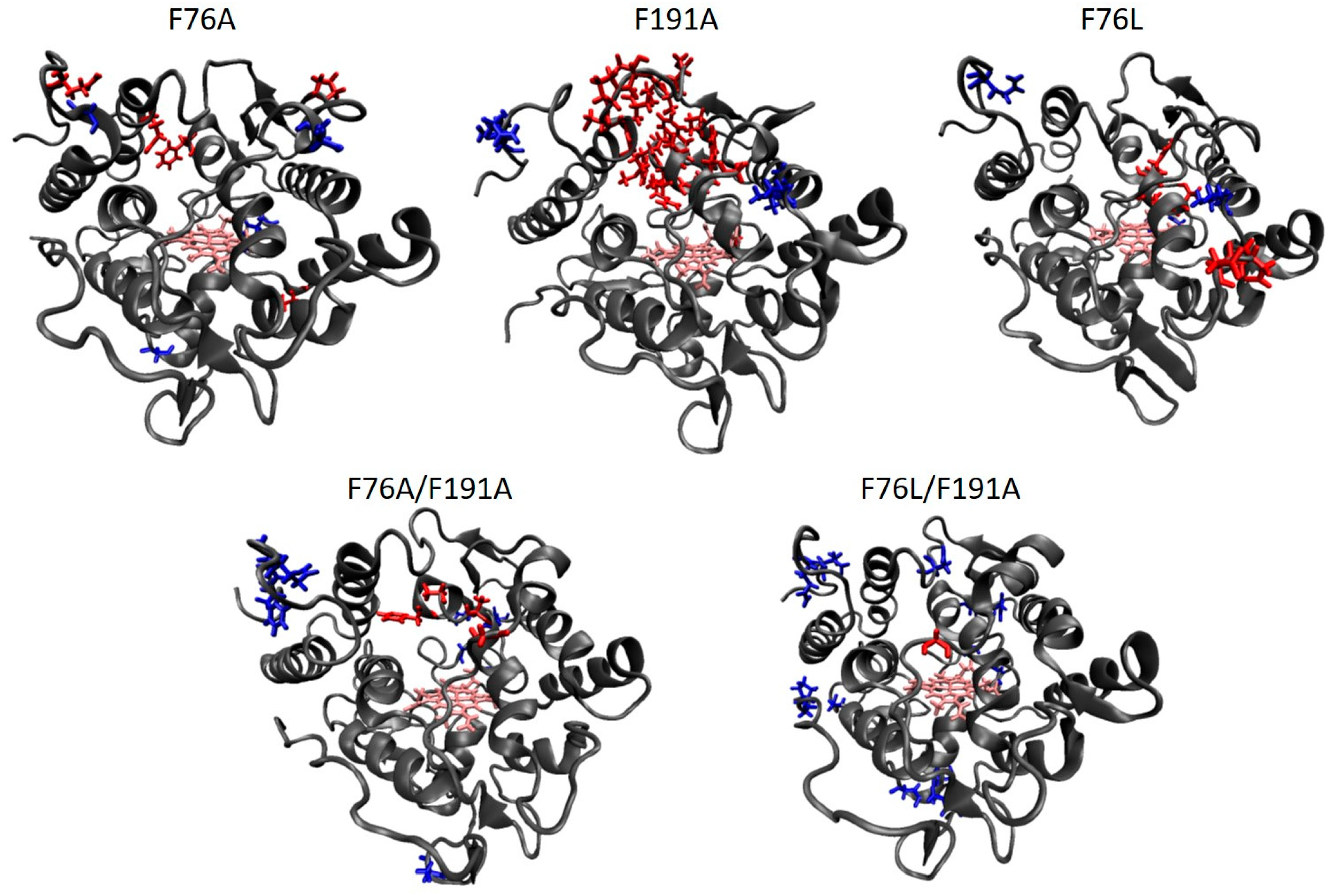
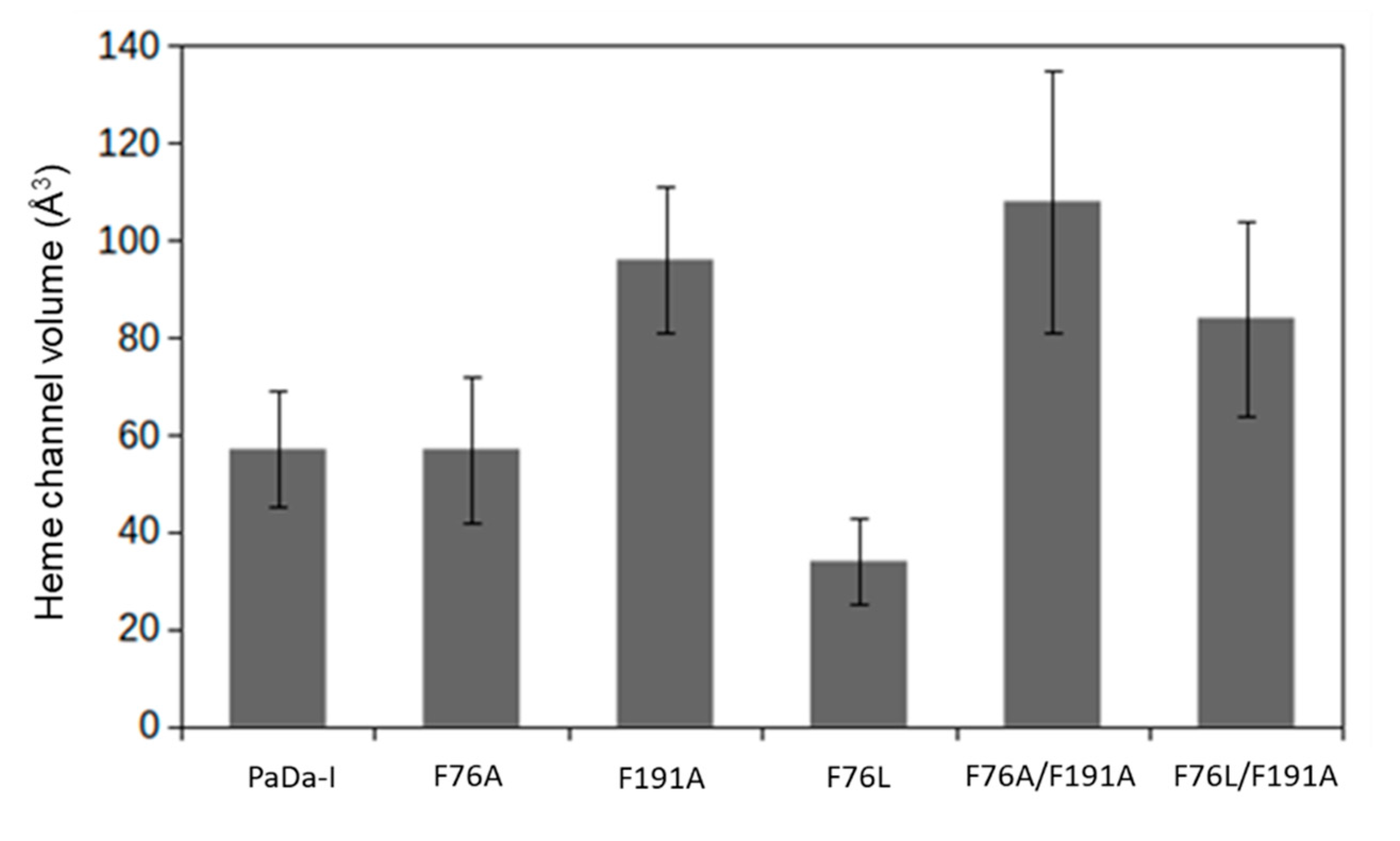
| Substrate | Kinetic Parameter * | PaDaI | F76A | F191A | F76L | F76A/F191A | F76L/F191A |
|---|---|---|---|---|---|---|---|
| NBD | kcat | 286 ± 18 | 356 ± 30 | 279 ± 18 | 454 ± 22 | 258 ± 15 | 332 ± 33 |
| Km | 736 ± 87 | 1386 ± 176 | 525 ± 73 | 890 ± 81 | 1389 ± 119 | 1552 ± 155 | |
| kcat /Km | 3.89 × 105 | 2.57 × 105 | 5.31 × 105 | 5.10 × 105 | 1.86 × 105 | 2.14 × 105 | |
| Naphthalene | kcat | 308 ± 25 | 305 ± 13 | 512 ± 28 | 341 ± 10 | 327 ± 17 | 485 ± 39 |
| Km | 289 ± 23 | 544 ± 58 | 740 ± 41 | 466 ± 36 | 627 ± 78 | 486 ± 39 | |
| kcat /Km | 1.07 × 106 | 5.61 × 105 | 6.92 × 105 | 7.33 × 105 | 5.22 × 105 | 9.98 × 105 | |
| ABTS | kcat | 1620 ± 67 | 751 ± 77 | 1039 ± 33 | 1122 ± 49 | 702 ± 15 | 926 ± 65 |
| Km | 186 ± 15 | 298 ± 51 | 93 ± 8 | 80 ± 9 | 136 ± 7 | 55 ± 12 | |
| kcat / Km | 8.71 × 106 | 2.52 × 106 | 1.12 × 107 | 1.40 × 107 | 5.16 × 106 | 1.68 × 107 |
| Variant | kinact (min−1) | t1/2 (min) | Catalase Specific Activity (U mg−1) |
|---|---|---|---|
| PaDa-I | 0.181 ± 0.014 | 3.8 ± 0.3 | 704 ± 4 |
| F76A | 0.368 ± 0.048 | 1.9 ± 0.3 | 517 ± 29 |
| F191A | 0.204 ± 0.012 | 3.4 ± 0.2 | 686 ± 32 |
| F76L | 0.322 ± 0.062 | 2.2 ± 0.4 | 518 ± 10 |
| F76A/F191A | 0.250 ± 0.014 | 2.8 ± 0.2 | 595 ± 7 |
| F76L/F191A | 0.267 ± 0.012 | 2.6 ± 0.1 | 1349 ± 234 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramirez-Ramirez, J.; Martin-Diaz, J.; Pastor, N.; Alcalde, M.; Ayala, M. Exploring the Role of Phenylalanine Residues in Modulating the Flexibility and Topography of the Active Site in the Peroxygenase Variant PaDa-I. Int. J. Mol. Sci. 2020, 21, 5734. https://doi.org/10.3390/ijms21165734
Ramirez-Ramirez J, Martin-Diaz J, Pastor N, Alcalde M, Ayala M. Exploring the Role of Phenylalanine Residues in Modulating the Flexibility and Topography of the Active Site in the Peroxygenase Variant PaDa-I. International Journal of Molecular Sciences. 2020; 21(16):5734. https://doi.org/10.3390/ijms21165734
Chicago/Turabian StyleRamirez-Ramirez, Joaquin, Javier Martin-Diaz, Nina Pastor, Miguel Alcalde, and Marcela Ayala. 2020. "Exploring the Role of Phenylalanine Residues in Modulating the Flexibility and Topography of the Active Site in the Peroxygenase Variant PaDa-I" International Journal of Molecular Sciences 21, no. 16: 5734. https://doi.org/10.3390/ijms21165734
APA StyleRamirez-Ramirez, J., Martin-Diaz, J., Pastor, N., Alcalde, M., & Ayala, M. (2020). Exploring the Role of Phenylalanine Residues in Modulating the Flexibility and Topography of the Active Site in the Peroxygenase Variant PaDa-I. International Journal of Molecular Sciences, 21(16), 5734. https://doi.org/10.3390/ijms21165734






