Abstract
Male fertility preservation is required when treatment with an aggressive chemo-/-radiotherapy, which may lead to irreversible sterility. Due to new and efficient protocols of cancer treatments, surviving rates are more than 80%. Thus, these patients are looking forward to family life and fathering their own biological children after treatments. Whereas adult men can cryopreserve their sperm for future use in assistance reproductive technologies (ART), this is not an option in prepubertal boys who cannot produce sperm at this age. In this review, we summarize the different technologies for male fertility preservation with emphasize on prepubertal, which have already been examined and/or demonstrated in vivo and/or in vitro using animal models and, in some cases, using human tissues. We discuss the limitation of these technologies for use in human fertility preservation. This update review can assist physicians and patients who are scheduled for aggressive chemo-/radiotherapy, specifically prepubertal males and their parents who need to know about the risks of the treatment on their future fertility and the possible present option of fertility preservation.
1. Spermatogenesis
Spermatogenesis is the process of spermatogonial stem cell (SSC) proliferation and differentiation through meiotic stages to generate sperm [1,2,3,4]. Endocrine and paracrine systems are involved in spermatogenesis regulation [1,2,3,4,5,6]. The main endocrine factors are the GnRH and gonadotropic hormones FSH and LH. FSH directly and specifically affects Sertoli cells in order to produce different factors, such as androgen binding proteins (ABP), transferrin, inhibin, androgen receptor, while LH specifically affects Leydig cells to produce testosterone. Because germ cells do not express receptors for FSH and LH, their effects are indirect on germ cells through various factors/substances that are secreted by Sertoli, Leydig, and other affected cells in the testes [1,2,3,4,5,6].
Adult stem cells in mammals have the capacity of self-renewal and the production of differentiated daughter cells (they can replicate indefinitely). The progenitor cells are an intermediate cell population between stem and differentiated cells. These cells have only the capacity of self-renewal and differentiation (they can divide only a limited number of times) and, thus, play a homeostatic role in maintaining the development of complete spermatogenesis [1,2,3,4,5,6].
In rodents, spermatogonial cells are grouped into type A, whereas in the mouse, seven types of A spermatogonia have been described [A (single), A (pair), A (aligned), A1–A4] when A (single) cells are considered to be the SSCs. However, in human, spermatogonial cells are grouped into Adark and Apale, when Adark is considered as SSCs. The SSCs have the capacity of self-renewing with a low rate of proliferation when compared to their progeny of spermatogonial cells (Apair and Aaligned in the mouse and Apale in human) that have the capacity to self-renew, but with a high rate of proliferation [1,2,3,4,5,6].
2. Cancer, Chemotherapy, and Male Infertility
2.1. Cancer and Male Infertility
It was shown that different types of cancers, including leukemia, lymphoma, testicular cancer, non-Hodgkin’s disease, gastrointestinal malignancy, and musculoskeletal malignancy, affect sperm parameters and the quality of thawed cryopreserved sperm [7,8]. Recently, using a mature animal model of leukemia, we showed that acute myeloid leukemia (AML) significantly decreased sperm parameters, increased spontaneous acrosome reaction, and decreased both male fertility capacity and number of offspring [9]. Thus, cancer may lead to male subfertility/infertility.
2.2. Cancer Patients and Chemotherapy
In recent years, the number of young male cancer survivors has increased drastically (>75%). This is mainly due to early diagnosis and improved cancer treatment protocols [10]. Therefore, the issue of infertility treatment to maintain the ability to genetically father one’s own children is a major concern for those young survivors who were treated with gonadotoxic agents [4,11,12].
Fertility is often impaired after chemotherapy and radiation therapy and may lead to azoospermia [4,12]. Cryopreservation of semen before the commencement of cancer treatment is currently the only method of preserving future male fertility. Obviously, this technique is not an option for prepubertal male cancer patients, since they do not yet generate sperm. Until now, fertility preservation for these patients has not been an option [4,10].
2.3. Gonadotoxic Agents and Male Infertility
Cells of the seminiferous tubules (dividing spermatogonial cells) are the most sensitive to damage that is caused by gonadotoxic agents, such as chemotherapy and radiotherapy. Restoration of spermatogenesis depends on the type, dose, and duration of treatment of the drugs and radiation used. As a result, young patients cured of cancer often suffer a prolonged and/or permanent decrease in sperm parameters or even azoospermia and are infertile [4,13,14]. The most sensitive testicular cells to chemotherapeutic drugs and irradiation are those that undergo constant proliferation and differentiation into spermatogonia [15,16,17,18]. The death/depletion of these cells results in loss of the following and developed stages of germ cells and, thus, to a decrease/loss in sperm counts. Therefore, the restoration of spermatogenesis and fertility depends on the survival of active SSCs and their ability to differentiate into spermatozoa [15].
Rapid restoration of complete spermatogenesis following gonadotoxic treatment depends on the presence of active SSCs and their microenvironment (somatic cell compartment) [17]. However, if some of these cells are destroyed, as occurs with irradiation and some chemotherapeutic agents, recovery is more gradual [19,20]. In mice, it was shown that the recovery of spermatogenesis is directly proportional to the number of SSCs destroyed. It was also demonstrated that gonadotoxic agents could damage somatic cells in the testis. In rat testes, no recovery of the seminiferous epithelium was demonstrated, even though numerous SSCs survived cytotoxic treatment [21,22].
If the damage is severe (as a result of high doses of chemo-/radiotherapy and/or a combination of these treatments), all of the SSCs commit to apoptosis or, alternatively, damaged Sertoli cells are unable to support the maintenance, growth, and development of SSCs. These events may lead to the complete depletion of the pool of SSCs in the seminiferous tubules, resulting in permanent sterility [14,23].
2.4. Recovery of Human Spermatogenesis after Cancer Therapy
The restoration of sperm development in adults following gonadotoxic treatments depends on the presence of quiescent or biologically active SSCs (Adark) that mitotically divide to generate a progeny of more differentiating cells (Apale). As has been mentioned, the possibility of spermatogenesis recovery is affected by the dose and type of gonadotoxic therapy used and individual response [20]. Up to now, studies in primates to develop strategies to protect gonads from these therapies have failed [4,14,23].
3. Options for Male Fertility Preservation
Very few and limited options are available for bypassing/minimizing the loss of fertility in male cancer patients following treatment with chemo-/radiotherapy, and most of the suggested options are still under study (Table 1 and Figure 1).

Table 1.
Options for fertility preservation and restoration in males.
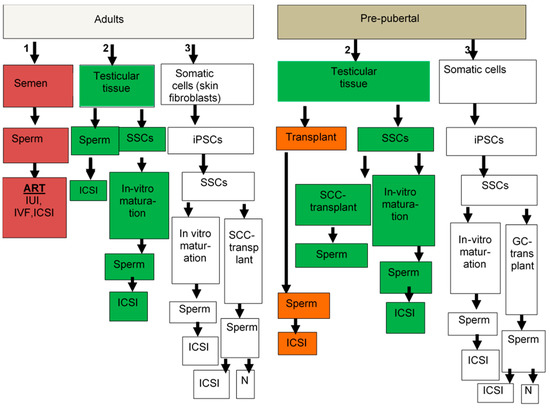
Figure 1.
Suggested options for male fertility preservation. Adult patients, if they have sperm in the semen (option #1), can cryopreserve it for ART. If they do not have sperm in their semen, the second option is to use testicular tissue (option #2). If sperm is found in their testicular tissue, it can be used for ICSI. If no sperm is found, then isolated spermatogonia stem cells (SSCs) could be used for in-vitro maturation to generate sperm for use in ICSI. If this technology will not work, or SSCs are not found, then somatic cells could be used to generate iPSC (option #3) and to develop SSCs, which can be used for in-vitro maturation to generate sperm for ICSI or for germ cell transplantation, which leads to generation of sperm in the testes that can normally (N) fertilize oocytes and lead to pregnancy, or if very little sperm is generated, it can be used in ICSI. In pre-pubertal patients, only options #2 and #3 can be used; they do not generate sperm at this age and therefore option #1 is excluded. In pre-pubertal patients, only options #2 and #3 can be used. Testicular biopsies if they contain SSCs (option #2), can be used for transplantation to develop sperm for ICSI, or isolated SSCs can be used either to develop sperm in the testes by germ cell transplantation, or used for in-vitro maturation to develop sperm for ICSI. If testicular tissues do not contain SSCs, then somatic cells (option #3) can be used to generate iPSCs to develop SSCs and used as mentioned above for adult patients.
3.1. Cryopreservation of Sperm
Cryopreservation of sperm has been extensively used for post pubertal adolescents and adults, and success rates have dramatically improved due to the introduction of intra-cytoplasmic sperm injection (ICSI) combined with mature oocyte retrieval. Unfortunately, only about 24% of men in this age group have cryopreserved semen prior to oncologic treatment [24,25]. In some of the azoospermic patients, sperm can be recovered surgically from small focal areas of spermatogenesis in the testes using testicular sperm extraction (TESE) methods. The freshly isolated testicular sperm can be used to fertilize oocytes by ICSI or freezing them for future ICSI.
It was previously shown that the live birth rate (using assistance reproductive technologies (ART)) was similar between cancer and non-cancer male infertility patients. These results indicate the value of sperm cryopreservation before starting cancer treatments, and the importance for oncologists to consult with young cancer patients to cryopreserve sperm prior to starting these treatments [26].
The main limitation of this technology in humans is that these techniques are only applicable for adult patients who can generate mature sperm cells, and not for prepubertal or other males who cannot produce mature sperm. Furthermore, sperm quantity is limited and quality is impaired.
3.2. Cryopreservation of Human Testicular Tissue
Because prepubertal boys cannot generate sperm, a potential alternative approach for preserving their fertility involves cryopreservation of their testicular tissue. This approach is also appropriate for adolescent and adult patients who may already be found to be azoospermic at the time of cancer diagnosis or post chemo-/radiotherapy [4,14,25]. Testicular cells can be cryopreserved as a cell suspension or in the form of tissue. The optimal method of storage is still unclear due to limited research that compared between cryopreserved testicular tissue and cell suspension. However, cryopreservation of testicular tissue provides the future option to use it for both tissue (such as testicular grafting or organ culture) or cell therapy (such as germ cell transplantation and in-vitro maturation), whereas the cryopreservation of testicular cell suspension can be used in the future only for cell therapy. In addition, cryopreservation of testicular tissue will maintain the microenvironment niche of the SSCs (mainly the somatic cells, Sertoli, Leydig, and peritubular cells), which may increase the viability and functionality of the SSCs following tissue thawing when compared to thawed cryopreserved cells.
The cryopreserved testicular cells or tissue could be used for prepubertal human male fertility preservation with different approaches that are already used in rodents [27,28,29,30]. The main limitation of using autologous testicular tissue or cells in human therapy from cancer patients is the possibility of the presence of residual cancer cells, which may restore and evoke the disease.
The survival of spermatogonia has been demonstrated after immature testicular tissue (ITT) freezing, which is considered to be ethically acceptable. The majority of cryopreserved samples showed reproductive potential [31]. Additionally, testicular growth of the biopsied testis was not impeded in comparison to the non-biopsied contralateral testis up until one year after surgery [32]. Cancer incidence was not increased, and the long-term survival rate was not altered after the transplantation of in-vitro propagated murine spermatogonial stem cells (SSCs) in busulfan-treated recipients as compared to non-transplanted busulfan-treated controls [33].
3.3. Germ Cell Transplantation
This technology was developed by Brinster et al. in 1994 [34,35]. It includes the injection (transplantation) of isolated testicular germ cells from lacZ transgenic mice into the seminiferous tubules of infertile mice. After a few months, spermatogenesis developed, and the mice became fertile. Additionally, their progeny contained the lac Z gene [34]. This technology was also successful using other species, such as rats, goats, sheep, dogs, pigs, and monkeys of different ages, and after different periods of germ cell cryopreservation [34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52]. The recovery of spermatogenesis in mice using SSC transplantation was demonstrated even after 14 years post cryopreservation [27,53]. This may suggest that SSCs remain biologically active, even after a long period of cryopreservation. Recently, the restoration of functional sperm production in irradiated pubertal rhesus monkeys by spermatogonial stem cell transplantation was demonstrated even though the success rate of this procedure was low [54]. In humans, it was reported that cryopreserved testicular cells from non-Hodgkin’s lymphoma patients were transplanted into their testes after recovery from cancer [55,56]; however, follow-up for these patients was not reported. Xeno-germ cell transplantation of baboons, marmosets, and humans into mice only colonized, but did not differentiate [28]. In addition, a limitation of xeno-germ cell transplantation is the risk of possible retrovirus transmission to humans if the developed cells are used for fertility therapy. The general limitations of this technology include the need for millions of testicular cells in order to contain enough SSCs to restore spermatogenesis. Nagano et al., suggested that transplantation of one-million of mouse testicular cells induced spermatogenesis by 19 SSCs [57]. It is well known that the number of SSCs in the testis is very low. In adult mouse testes, these are around 0.03% [58], and spermatogonial cells comprised about 3% of the cells in testis of prepubertal boys [59]. Therefore, the development of efficient methods to isolate/enrich SSCs and increase their numbers (proliferation) in vitro are crucial to the successful use of this technology. In addition, the efficiency of SSC transplantation is very low and needed several months (more than eight months in busulfan treated adult mice and five months in W adult mice) to restore fertility [35,60,61]. However, when the recipients were immature W mice, the efficiency was significantly improved, and fertility was demonstrated after three months [61].
The main limitation of this technology in humans is the possible contamination of testicular cells with cancer cells that may reintroduce malignancy to the patient after recovery. Today, there is no accurate and safe method that isolates pure SSCs from the testis of patients [50,62,63], in addition to the very small amount of testicular biopsy that could be used, it also contains very small numbers of these cells [4,14,24,27,29,58,64,65].
3.4. Testicular Tissue Autograft/Xenograft
The use of testicular tissue to induce spermatogonial cells of the donor to complete spermatogenesis in vivo or in vitro (organ culture) is based on the availability of the somatic cells to permit proper SSC development. This microenvironment that provides optimal cell–cell interactions and the spatial environment for complete spermatogenesis is produced by the seminiferous tubules and interstitial compartment. This technology was proven successful in both autografting/xenografting and in vitro as organ culture [27,28,29,30]. Testicular tissue xenograft was successfully performed in immunodeficient mice while using testicular tissue from different species, which was grafted under the skin, producing complete spermatogenesis. Xenografting of testis tissue from immature males of different mammalian species, such as hamsters, goats, monkeys, bulls, pigs, and cats, into immunodeficient mice also resulted in complete spermatogenesis [4,14,64,66]. Likewise, live offspring were reported from sperm generated from testicular grafts of immature mice [67] and fertilization-competent sperm from testicular xenografts of immature rhesus monkeys into mice [37]. It was reported that the autotransplantation of fresh testicular tissue from prepubertal marmosets into the scrotum led to the development of full spermatogenesis [68]. Recently, autologous grafting of cryopreserved prepubertal rhesus testis under the back skin or scrotal skin of castrated pubertal rhesus macaques matured to produce functional sperm and offspring [25]. Unfortunately, experiments involving the xenografting of human prepubertal cryopreserved testicular tissue into immunodeficient mice has not yet demonstrated the development of spermatid differentiation [25,27,69,70,71,72]. It was shown that successful spermatogenesis in testicular tissue xenografts are affected by development through spermatogenesis, in addition to the timing of development and efficiency [64]. An additional factor that affects testes’ xenograft survival and development is the age or developmental stage of the donor [64].
It should be noted that testicular xenografts in mice may lead to the transmission of viruses to the human germ line. Furthermore, testicular autografts in human cancer patients have the limitation of possibly reseeding malignant cells, promoting cancer recurrence. However, this technology might be considered in non-cancer patients.
3.5. Testicular Cell Xenograft
When compared to testicular tissue xenografting, this technique may allow for more exposure of testicular cells to the new environment. Structural organization of the seminiferous tubules might influence the development of xenograft testicular tissue [4,14,64,66]. Xenotransplantation of testicular cells (a combination of somatic and germ cells in different percentages) from neonate porcine (under the skin of nude mice) led to the development of complete spermatogenesis (after 30 weeks), including the generation of sperm (in 10% of the formed seminiferous tubules) [73]. In another study, ectopically grafting testicular cells from embryonic or neonatal mice and rats to mice led to the development of round spermatids. Using ICSI technology, these cells were able to develop embryos, resulting in the birth of normal pups [74]. In addition, the xenotransplantation of a heterogeneous cell suspension of sexually immature lambs (two weeks old) under the dorsal skin of nude mice led to the development of complete spermatogenesis after 40 weeks [75]. The transplantation of testicular cells from prepubertal rhesus monkeys into the testes of irradiated adult monkeys formed seminiferous tubules with full spermatogenesis nine months post transplantation [76]. To the best of our knowledge, this system has not yet been performed in humans.
3.6. Organ Culture
As mentioned above, the main benefit of using testicular fragments to develop spermatogenesis in vitro is the presence of testicular somatic cells and the three-dimension (3D) microenvironment that is suitable for the optimal development of SSCs to complete spermatogenesis [25,26,27,28,29,77]. Spermatogenesis development using organ culture to proceed to the meiotic stages was performed in the past without success [77,78,79,80]. However, Sato et al. recently succeeded in inducing the development of fertile sperm using an in-vitro culture of small fragments (around 3 mm) of testicular tissue from immature mice [81]. This system led to the development of flagellated spermatozoa from cryopreserved testicular tissues of immature mice [80,81]. It was shown that, for the effective development of human spermatogonial cells, it is suggested to grow the testicular human organ at 34 °C and in the presence of gonadotropins, which may increase the long-term period of Sertoli cell survival [82,83,84,85].
The successful application of this system using human testicular tissue could circumvent testicular auto-transplantation to generate sperm and the possibility of reintroducing cancer cells to cured patients. However, neither fertilization nor implantation of human tissue has been reported.
3.7. In-Vitro Cultures of SSCs
3.7.1. Two- and Three-Dimensional Culture Systems
In-vitro development of complete spermatogenesis from spermatogonial cells may overcome the limitations of restoration of cancer using organ culture or germ cell transplantation from cancer patients, and possible retrovirus transmission by using xenotransplantation techniques. In addition, the induction of proliferation of spermatogonial cells in vitro may promote the use of autologous human germ cell transplantation.
Several in-vitro culture systems have been used to induce proliferation and differentiation of SSCs from different species to the meiotic and post-meiotic stages. These systems included the addition of adherent cells, conditioned media, or recombinant growth factors while using different matrices, such as collagen, laminin, and others, to mimic seminiferous tubules in in-vivo conditions [1,77,86,87]. However, these systems could mainly induce the proliferation of SSCs and not complete the spermatogenesis process. The induction of proliferation of human SSCs isolated from both normal men and prepubertal cancer and azoospermic patients using an in-vitro culture system was reported [88,89]. To date, factors that are capable of inducing in-vitro differentiation of SSCs from any species have not yet been defined. Our group was the first to suggest the use of a methylcellulose culture system (MCS) and soft-agar-culture system (SACS) as possible three-dimensional (3D) matrices to grow and develop spermatogonial cells in vitro [87,88,89,90,91,92]. Using these two novel 3D culture systems (MCS and SACS), which are more representative of in-vivo conditions, we could induce the proliferation and differentiation of spermatogonial cells from normal and busulfan-treated immature mice to the meiotic and post-meiotic stages, and even the generation of sperm-like cells (elongated spermatid with head, neck, and tail) [87,90,92,93]. The development of meiotic and post-meiotic stages was examined after 3–6 weeks of culture. However, the efficiency of this system to generate sperm-like cells was very low, in addition to the inability to examine the capacity of their fertility. We also demonstrated the capacity to induce the proliferation and differentiation of spermatogonial cells from prepubertal monkeys to meiotic (crem-1 positive cells) and post-meiotic (acrosin positive cells) stages in vitro using MCS [91]. Recently, we showed the induction of the proliferation of spermatogonial cells from biopsies without sperm of azoospermic patients and their differentiation into meiotic cells in MCS [94,95]. We also demonstrated the presence of spermatogonial cells in testicular biopsies of prepubertal cancer patients before aggressive chemotherapy and their differentiation in MCS to meiotic and post-meiotic cells and, in one case, the generation of sperm-like cells [96,97]. We suggest that 3D culture systems may provide the SSCs with a spatial and microenvironment similar to those present in the seminiferous tubules and crucial for the development of spermatogenesis such as the presence of testicular somatic cells and the 3D tubular structure. This 3D in-vitro culture system still requires optimization in order to efficiently generate sperm.
3.7.2. 3D Bioprinted Scaffold
Although in-vitro spermatogenesis has mainly been achieved (not efficiently) in rodent organ culture and/or 3D in-vitro culture systems [27,28,29,30,31,90,91,92,93], the optimal conditions that provide an optimal 3D spatial environment with similar cellular composition of the seminiferous tubule to efficiently induce the development of spermatogonial cells in order to complete maturation and generation sperm was not yet published. A new culture system that provides alginate-based hydrogel and 3D bioprinting was developed to control scaffold design and cell deposition in order to preserve testicular cells in their native 3D spatial and cellular microenvironment to induce complete in-vitro spermatogenesis. Recently, the first report of in-vitro spermatogenesis in mouse testicular constructs generated by culturing single cell suspensions on 3D bioprinted cell-laden scaffolds and cell-free scaffolds was published [98]. Cell spheres were generated, but tubule-like structures did not develop. However, patches of differentiated post meiotic cells, including round and elongated spermatids, have been demonstrated [98]. The fertility capacity of the developed spermatids was not examined. This system still needs to be optimized at the different levels of the scaffold and cultured cells for providing tubule-like structures and more efficient spermatid development.
3.7.3. Testicular Organoids
The term organoid is used to describe 3D structures (up to two millimeters) that are composed of aggregates of cells that reorganize after cell dissociation of specific tissue [99,100,101]. These new structures show some histology and biological activity similar to the original tissue [99]. The development and functionality of these organoids depends on optimal conditioned media, nutrients, and oxygen provided in vitro, since they do not form the vasculature system [99,100,101,102]. It is possible that the development/formation of these organoids may assist researchers in understanding the cell–cell interactions and functionality of the original tissue. The development of functional organoids without the formation of testis-like histology from adult human SSCs was recently demonstrated [103]. They were co-cultured with Leydig and Sertoli cells in the presence of extracellular matrix (ECM) from human testis and led to the development of haploid cells after three weeks of culture [103]. Another study showed the development of active human testicular organoids after four weeks of culture (without similarity to the histology of the testis). These organoids did produce testosterone and inhibin B, but did not induce differentiation of the proliferative spermatogonial cells [104]. Additionally, culturing testicular cells from immature rats in a three-layer gradient system led to the development of organoids (composed mainly of Sertoli and germ cells) with tubule-like structures after seven days of culture. These structures showed the development of a functional testicular blood barrier between the Sertoli cells without differentiation of the spermatogonial cells [105]. An additional study showed the development of mouse spermatogonial cells to meiotic and post meiotic cells in organoids when cultured in fabricated testis-derived scaffolds [106].
3.7.4. Microfluid System and Organ-on-Chip Technology
The lack of circulatory system is one of the main limitations of 3D in-vitro systems. Therefore, organ-on-chip in a microfluid device may overcome this limitation and provide a dynamic condition of nutrient and gas circulation mimicking the in-vivo conditions. This system enabled the use of small amounts of media and control of their composition, diffusion, and temperatures very close to the cells/tissue present in the device. Thus, the use of the 3D in-vitro culture system may enable the capacity of spermatogonial cells to complete/efficient maturation and the generation of sperm in testicular tissue or isolated tubular cells cultured in such a system. Recently, the development of organ-on-chip of neonate mouse testicular tissue was reported [107,108,109]. In this system, a continuous perfusion of media was supplemented to the design chambers through vasculature-like structures. The media arrived to the testicular tissues only by diffusion. The testicular tissue showed efficient spermatogenesis for around six months, with the ability to develop round spermatids with fertility capacity, as demonstrated by the generation of healthy offspring following spermatid injection to oocytes [107]. Recently, our group developed a mouse microfluid system with two chambers that contained isolated cells from seven-day-old mice in methylcellulose. These chambers were provided by continuous diffused conditioned media similar to MCS. Our preliminary and unpublished data show the development of organoids with seminiferous tubule-like structures (with a histology of seminiferous tubules that contain peritubular cells and active Sertoli cells) after 5–7 weeks of culture, as examined by confocal microscope. These organoids contained, in addition to premeiotic cells (VASA and PLZF positive stained cells), developed meiotic (CREM positive stained cells) and post meiotic cells (ACROSIN positive stained cells), as examined by confocal and immunofluorescence microscopes. This microfluid system showed a significant increase in cell viability and the percentages of developed 1N cells when compared to MCS, as examined by trypan blue staining and FACS analysis, respectively.
The success of these systems in humans, including sperm generation, may overcome limitations, such as the small testicular biopsies used with a very low number of SSCs and the concerns of the possible restoration of cancer cells to cured patients. This may benefit not only prepubertal cancer patients, but also non-obstructive azoospermic patients who do not have sperm in their biopsies, but in whom spermatogonial cells are present. On the other hand, the capacity of fertility of the generated sperm in this system still needs to be confirmed, in addition to genetic and epigenetic stability, as in all in-vitro systems.
3.8. Induced Pluripotent Stem Cells
The development of induced pluripotent stem cells (iPSC) from somatic cells is possible by their transfection with a combination of transcription factors [4,110,111,112]. One of the approaches for male fertility preservation includes the development of generated iPSCs from somatic cells, such as dermal fibroblasts, blood cells, or keratinocytes, from infertile patients to primordial germ cells (PGCs). These PGCs can either be auto-transplanted into the testes (in vivo) or used in vitro in order to induce the development of gametes [113,114,115,116]. Recently, iPSCs were developed from Klinefelter and NOA patients [117,118]. It was shown that iPSC from rodents, monkeys, and humans can be differentiated into male germ cells [4,85,112,118,119,120,121,122,123,124,125,126,127,128]. The addition of bone morphogenetic proteins (BMPs) to human iPSC induced their differentiation into primordial germ and meiotic cells [129]. Additionally, manipulation of human iPSC with RNA-binding proteins induced the differentiation of their derived germ cells to meiotic stages [112,130]. The addition of conditioned media and retinoic acid to human iPSC cultures led to their differentiation into pre-meiotic, meiotic, and post meiotic (round spermatids) stages [112,131]. It should also be noted that the addition of BMP4 to mouse iPSC cultures led to the development of germ cells. The transplantation of these germ cells into seminiferous tubules of infertile mice induced complete spermatogenesis, including the generation of fertile sperm [125].
Following intra-cytoplasmic sperm injection (ICSI) and transfer of the embryos to recipient females, some of the live offspring developed cancer in the neck and died prematurely [125]. This may indicate that this system may not be safe and requires further research and optimization. The main concern of iPSC use in translational medicine is the transfection of somatic cells with oncogenes, which may lead to the development of tumors in offspring, in addition to the transition of these genes and vectors to the germ line. Additionally, in addition to genetic and epigenetic stability, fertility of the developed human sperm should be clarified (ethical issue). Recently, direct differentiation of human iPSC into pre-meiotic, meiotic, and post meiotic stages, including the development of spermatid-like cells in vitro without genetic manipulation was demonstrated [120]. However, this study needs further validation.
3.9. Protection of Spermatogenesis In Vivo from Harmful Gonadotoxic Therapy
Gonadotoxic therapy for male cancer patients may lead to permanent sterility [4,13,14,120]. This could be related not only to the cytotoxic effect on proliferating spermatogonial cells, but also to testicular somatic compartment damage [13,22]. It was demonstrated that gonadotropins and testosterone suppression in rats and mice during or after gonadotoxic treatments induced the development of spermatogenesis (from residual survival endogenous SSCs) and restored fertility [13,22,132,133]. In one human study, hormone suppression after chemo-/radiotherapy preserved spermatogenesis, while, in another study, it did not [133,134]. In addition, hormonal suppression was shown to induce colony formation and the differentiation of transplanted germ cells into the testes of chemo-/radiotherapy treated rats and mice [22,135,136,137,138] and, in some cases, restored their fertility [139,140]. The combination of hormonal suppression (using GnRH-antagonist) and germ cell auto-transplantation or allogeneic transplantation of cryopreserved testicular cells into irradiated monkey testes showed spermatogenesis recovery and a significant increase in sperm counts when compared to irradiated-only testes [54,132]. It is suggested that androgen suppression may lead to efficient effect on the permeability of Sertoli cell barrier, passage of spermatogonial cells through tight junction and the niche of the spermatogonial cells [132,140,141]. It is also possible that androgen suppression increases the homing of spermatogonial cells by decreasing tight junction proteins, and during their proliferation stage by increasing the Wnt5a expression [142].
This system needs to be optimized in order to increase its low efficiency. In addition, further elucidation of the mechanism of action of the hormonal suppression may improve our understanding of the action of the involved hormones in the process of spermatogenesis recovery. The safety issue of using germ cell auto-transplantation still needs to be considered, as mentioned above.
3.10. Pharmacological Agents
3.10.1. AS101
The immunomodulator AS101 is a synthetic organic tellurium compound and non-toxic agent [143]. It was shown to increase the survival of mice following chemo-/radiotherapy treatments, indicating an anti-tumor effect, acting synergistically with some chemotherapy in mouse tumor models [144,145]. In humans, a combination of AS101 with chemotherapy significantly reduced the severity of hematopoiesis affected by the chemotherapy [146,147]. Its effect was related to its capacity to decrease the production of anti-inflammatory cytokines, such as interleukin-10, which leads to an increase in GDNF production [148]. The co-treatment of AS101 with cyclophosphamide significantly decreased the harmful effect of cyclophosphamide on the seminiferous tubules in mice and increased the number of offspring compared to the control [149]. The mechanism of its action was suggested to be through decreased DNA fragmentation and an increase in Akt and glycogen synthase kinase-3b (GSK-3b) phosphorylation [149].
To the best of our knowledge, the effect of AS101 has not yet been evaluated in human males in regard to fertility preservation.
3.10.2. Growth Factors/Cytokines
Granulocyte colony-stimulating factor (G-CSF) is a member of the hematopoietic growth factor family, which regulates the proliferation, differentiation, and survival of hematopoietic progenitor cells [150]. Initial studies of the effects of G-CSF on either irradiation- or anticancer drug-induced myelosuppressed animals demonstrated that it can shorten the duration of myelosuppression and increase the absolute numbers of functionally active neutrophils [151,152]. Recently, the radioprotective effects of G-CSF were further investigated with respect to the testicular system. In one study, its administration to adult male mice for three consecutive days before pelvic irradiation protected about 50% of testicular germ cells from radiation induced apoptosis [153]. The study suggested that G-CSF protected from radiation-induced testicular dysfunction via an anti-apoptotic effect that might lead to the recovery of spermatogenesis. In one study, it was reported that treatment of busulfan-injected adult mice with G-CSF for seven days led (10 weeks later) to a significant restoration in spermatogenesis and number of generated sperm in the epididymis as compared to busulfan-injected mice. G-CSF led to increased numbers of PLZF spermatogonia, and G-CSF receptor (Csf3r) was found in undifferentiated spermatogonia. In another study, it was shown that treatment with G-CSF of adult mice before or after busulfan treatment was protective for spermatogenesis for a relatively long time. The G-CSF treatment increased the proliferation of the survival spermatogonial cells [154]. Using a rat model that was treated with G-CSF before busulfan injection, it was demonstrated that the G-CSF-treated animals showed an increase in testis weight and sperm count and viability, along with high expressions of DDX4, DAZL, TP2, PCNA, and BrdU [155]. It was suggested that G-CSF could be used as a possible strategy for future preservation of male fertility in busulfan-treated cancer patients [156].
However, the suggested protocol needs to be optimized and confirmed in human systems. In addition, G-CSF induces granulopoiesis through the induction of bone-marrow stem cell proliferation. Thus, its combination with chemotherapy (cytotoxic to proliferating cells) may lead to a reverse effect.
Both AS101 and G-CSF may affect spermatogenesis also through autophagy. Autophagy is a process in which cytoplasm and organelles of the cells are degraded by proteolytic mechanism. It plays important role in maintaining cellular homeostasis, self-renewal, and differentiation [155,156,157,158,159,160,161]. Autophagy is mediated by mechanistic target of rapamycin (mTOR) signaling pathway [161,162]. mTOR is a key regulator of cell growth, which involves transcription, translation, and autophagy [160,163]. Autophagy is considered to be a crucial process for the formation of important structures and regulation the depletion of other cellular components [161,162]. It was demonstrated that rapamycin (an antiproliferative and immunosuppressant drug) suppressed spermatogenesis (inhibiting spermatogonial cell proliferation that led to sperm reduction) by changing the status of autophagy through inhibiting mTOR-p70S6 kinase signaling pathway in rats [164]. Additionally, AS101 was demonstrated to regulate the phosphorylation of mTOR in mesangial cells [165]. The importance of mTOR in the process of differentiation of mouse myeloid progenitor cells to neutrophil by G-CSF was reported [166]. Therefore, it is possible that some of the functions of AS101 and G-CSF in protection of spermatogenesis might be performed through the regulation mTOR and autophagy process.
4. Conclusions and Remarks
Today, options for treatment of infertile men who cannot generate sperm are not yet available. The technologies in the field that have been proven in animal models are not yet safe for use in humans. On the other hand, different technologies and approaches are being studied in different models including auto-transplantation, hormonal therapy, gene therapy, protection against gonadotoxic agents, pharmacological agents, and in-vitro culture models. The success of these technologies/approaches and their translation to humans offer hope for new strategies to treat male infertility.
Author Contributions
The authors, M.H. and E.L. wrote and edited the manuscript, collected and summarized the data and the information. All authors have read and agreed to the published version of the manuscript.
Funding
This study was partially supported by REPRODUCTION HUB, Faculty of Health Sciences, Ben-Gurion University, Binational Science Foundation (BSF) (#2011111), Israel Science Foundation (ISF) (#1418/19).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Huleihel, M.; Abu Elhija, M.; Lunenfeld, E. In vitro culture of testicular germ cells: Regulatory factors and limitations. Growth Factors 2007, 25, 236–252. [Google Scholar]
- De Kretser, D.M.; de Loveland, K.L.; Meinhardt, A.; Simorangkir, D.; Wreford, N. Spermatogenesis. Hum. Reprod. 1998, 13, 1–8. [Google Scholar]
- Ogawa, T. Spermatogonial transplantation: The principle and possible applications. J. Mol. Med. 2001, 79, 368–374. [Google Scholar]
- Gassei, K.; Orwig, K.E. Experimental methods to preserve male fertility and treat male factor infertility. Fertil. Steril. 2016, 105, 256–266. [Google Scholar]
- Auharek, S.A.; de França, L.R. Postnatal testis development, Sertoli cell proliferation and number of different spermatogonial types in C57BL/6J mice made transiently hypo- and hyperthyroidic during the neonatal period. J. Anat. 2010, 216, 577–588. [Google Scholar]
- Ehmcke, J.; Wistuba, J.; Schlatt, S. Spermatogonial stem cells. Questions, models and perspectives. Hum. Reprod. Update 2006, 12, 275–282. [Google Scholar]
- Michailov, Y.; Lunenfeld, E.; Kapelushnik, J.; Huleihel, M. Leukemia and male infertility: Past, present, and future. Leuk. Lymphoma 2019, 60, 1126–1135. [Google Scholar]
- Williams, D.H.; Karpman, E.; Sander, J.C.; Spiess, P.E.; Pisters, L.L.; Lipshultz, L.I. Pretreatment semen parameters in men with cancer. J. Urol. 2009, 181, 736–740. [Google Scholar]
- Michailov, Y.; Lunenfeld, E.; Kapilushnik, J.; Friedler, S.; Meese, E.; Huleihel, M. Acute Myeloid Leukemia Affects Mouse Sperm Parameters, Spontaneous Acrosome Reaction, and Fertility Capacity. Int. J. Mol. Sci. 2019, 20, 219. [Google Scholar]
- Dohle, G.R. Male infertility in cancer patients: Review of the literature. Int. J. Urol. 2010, 17, 327–331. [Google Scholar]
- Schover, L.R. Motivation for parenthood after cancer: A review. J. Natl. Cancer Inst. Monogr. 2005, 34, 2–5. [Google Scholar]
- Delessard, M.; Saulnier, J.; Rives, A.; Dumont, L.; Rondanino, C.; Rives, N. Exposure to chemotherapy during childhood or adulthood and consequences on spermatogenesis ad male fertility. Int. J. Mol. Sci. 2020, 21, 1454. [Google Scholar]
- Meistrich, M.L. Effects of chemotherapy and radiotherapy on spermatogenesis in humans. Fertil. Steril. 2013, 100, 1180–1186. [Google Scholar]
- Jahnukainen, K.; Ehmcke, J.; Hou, M.; Schlatt, S. Testicular function and fertility preservation in male cancer patients. Best Pract. Res. Clin. Endocrinol. Metab. 2011, 25, 287–302. [Google Scholar]
- Drumond, A.L.; Weng, C.C.; Wang, G.; Chiarini-Garcia, H.; Eras-Garcia, L.; Meistrich, M.L. Effects of multiple doses of cyclophosphamide on mouse testes: Accessing the germ cells lost, and the functional damage of stem cells. Reprod. Toxicol. 2011, 32, 395–406. [Google Scholar]
- Dym, M.; Clermont, Y. Role of spermatogonia in the repair of the seminiferous epithelium following x-irradiation of the rat testis. Am. J. Anat. 1970, 128, 265–282. [Google Scholar]
- Lu, C.C.; Meistrich, M.L. Cytotoxic effects of chemotherapeutic drugs on mouse testis cell. Cancer Res. 1979, 39, 3575–3582. [Google Scholar]
- Kangasniemi, M.; Veromaa, T.I.; Kulmala, J.; Kaipia, A.; Parvinen, M.; Toppari, I. DNA flow cytometry of defined stages of rat seminiferous epithelium: Effects of 3 Gv of high-energy X-irradiation. J. Androl. 1990, 11, 312–317. [Google Scholar]
- Meistrich, M.L.; Hunter, N.R.; Suzuki, N.; Trostle, P.K.; Withers, H.R. Gradual regeneration of mouse testicular stem cells after exposure to ionizing radiation. Radiat. Res. 1978, 74, 349–362. [Google Scholar]
- Meistrich, M.L. Quantitative relation between testicular stem cell survival, sperm production, and fertility in the mouse after treatment with different cytotoxic agents. J. Androl. 1982, 3, 58–68. [Google Scholar]
- Kangasniemi, M.; Huhtaniemi, I.; Meistrich, M.L. Failure of spermatogenesis to recover despite the presence of a spermatogonia in the irradiated LBNF1 rat. Biol. Reprod. 1996, 54, 1200–1208. [Google Scholar]
- Zhang, Z.; Shao, S.; Meistrich, M.L. The radiation-induced block in spermatogonial differentiation is due to damage to the somatic environment, not the germ cells. J. Cell Physiol. 2007, 211, 149–158. [Google Scholar]
- de Rooij, D.G.; van de Kant, H.J.; Dol, R.; Wagemaker, G.; van Buul, P.P.; van Duijn-Goedhart, A.; de Jong, F.H.; Broerse, J.J. Long-term effects of irradiation before adulthood on reproductive function in the male rhesus monkey. Biol. Reprod. 2002, 66, 486–494. [Google Scholar]
- Schover, L.R.; Brey, K.; Lichtin, A.; Lipshultz, L.I.; Jeha, S. Knowledge and experience regarding cancer, infertility, and sperm banking in younger male survivors. J. Clin Oncol. 2002, 20, 1880–1889. [Google Scholar]
- Holoch, P.; Wald, M. Current options for preservation of fertility in the male. Fertil. Steril. 2011, 96, 286–290. [Google Scholar]
- Garcia, A.; Herrero, M.B.; Holzer, H.; Tulandi, T.; Chan, P. Assisted reproductive outcomes of male cancer survivors. J. Cancer Surviv. 2015, 9, 208–214. [Google Scholar]
- Pelzman, D.L.; Orwig, K.E.; Hwang, K. Progress in Translational Reproductive Science: Testicular Tissue Transplantation and in Vitro Spermatogenesis. Fertil. Steril. 2020, 113, 500–509. [Google Scholar] [CrossRef]
- Sharma, S.; Wistuba, J.; Pock, T.; Schlatt, S.; Neuhaus, N. Spermatogonial Stem Cells: Updates from Specification to Clinical Relevance. Hum. Reprod. Update 2019, 25, 275–297. [Google Scholar] [CrossRef]
- Komeya, M.; Sato, T.; Ogawa, T. In Vitro Spermatogenesis: A Century-Long Research Journey, Still Half Way Around. Reprod. Med. Biol. 2018, 17, 407–420. [Google Scholar] [CrossRef]
- Zarandi, N.P.; Galdon, G.; Kogan, S.; Atala, A.; Sadri-Ardekani, H. Cryostorage of immature and mature human testis tissue to preserve spermatogonial stem cells (SSCs): A systematic review of current experiences toward clinical applications. Stem. Cells Cloning 2018, 11, 23–38. [Google Scholar] [CrossRef]
- Wyns, C.; Curaba, M.; Petit, S.; Vanabelle, B.; Laurent, P.; Wese, J.F.; Donnez, J. Management of Fertility Preservation in Prepubertal Patients: 5 Years’ Experience at the Catholic University of Louvain. Hum. Reprod. 2011, 26, 737–747. [Google Scholar] [CrossRef]
- Uijldert, M.; Meißner, A.; De Melker, A.A.; Van Pelt, A.M.M.; Van De Wetering, M.D.; Van Rijn, R.R.; Van Wely, M.; Van Der Veen, F.; Repping, S. Development of the Testis in Pre-Pubertal Boys With Cancer After Biopsy for Fertility Preservation. Hum. Reprod. 2017, 32, 2366–2372. [Google Scholar] [CrossRef]
- Mulder, C.L.; Catsburg, L.A.; Zheng, Y.; de Winter-Korver, C.M.; Van Daalen, S.K.; Van Wely, M.; Pals, S.; Repping, S.; Van Pelt, A.M. Long-term Health in Recipients of Transplanted in Vitro Propagated Spermatogonial. Stem. Cells Hum. Reprod. 2018, 33, 81–90. [Google Scholar] [CrossRef]
- Brinster, R.L.; Avarbock, M.R. Germline transmission of donor haplotype following spermatogonial transplantation. Proc. Natl. Acad. Sci. USA 1994, 91, 11303–11307. [Google Scholar]
- Brinster, R.L.; Zimmermann, J.W. Spermatogenesis following male germ-cell transplantation. Proc. Natl. Acad. Sci. USA 1994, 91, 11298–11302. [Google Scholar]
- Honaramooz, A.; Megee, S.O.; Dobrinski, I. Germ cell transplantation in pigs. Biol. Reprod. 2002, 66, 21–28. [Google Scholar]
- Honaramooz, A.; Li, M.; Penedo, C.T.; Meyers, S.; Dobrinski, I. Accelerated maturation of primate testis by xenografting into mice. Biol. Reprod. 2004, 70, 1500–1503. [Google Scholar]
- Schlatt, S.; Foppiani, L.; Rolf, C.; Weinbauer, G.F.; Nieschlag, E. Germ cell transplantation into X-irradiated monkey testes. Hum. Reprod. 2002, 17, 55–62. [Google Scholar]
- Shinohara, T.; Inoue, K.; Ogonuki, N.; Kanatsu-Shinohara, M.; Miki, H.; Nakata, K.; Kurome, M.; Nagashima, H.; Toyokuni, S.; Kogishi, K.; et al. Birth of offspring following transplantation of cryopreserved immature testicular pieces and in vitro microinsemination. Hum. Reprod. 2002, 17, 3039–3045. [Google Scholar]
- Oatley, J.M.; de Avila, D.M.; Reeves, J.J.; McLean, D.J. Spermatogenesis and germ cell transgene expression in xenografted bovine testicular tissue. Biol. Reprod. 2004, 71, 494–501. [Google Scholar]
- Oatley, J.M.; Reeves, J.J.; McLean, D.J. Establishment of spermatogenesis in neonatal bovine testicular tissue following ectopic xenografting varies with donor age. Biol. Reprod. 2005, 72, 358–364. [Google Scholar]
- Snedaker, A.K.; Honaramooz, A.; Dobrinski, I. A game of cat and mouse: Xenografting of testis tissue from domestic kittens results in complete cat spermatogenesis in a mouse host. J. Androl. 2004, 25, 926–930. [Google Scholar]
- Rathi, R.; Honaramooz, A.; Zeng, W.; Turner, R.; Dobrinski, I. Germ cell development in equine testis tissue xenografted into mice. Reproduction 2006, 131, 1091–1098. [Google Scholar]
- Shinohara, T.; Orwig, K.E.; Avarbock, M.R.; Brinster, R. Remodeling of the postnatal mouse testis is accompanied by dramatic changes in stem cell number and niche accessibility. Proc. Natl. Acad. Sci. USA 2001, 98, 6186–6191. [Google Scholar]
- HHermann, B.P.; Sukhwani, M.; Winkler, F.; Pascarella, J.N.; Peters, K.A.; Sheng, Y.; Valli, H.; Rodriguez, M.; Ezzelarab, M.; Dargo, G.; et al. Spermatogonial stem cell transplantation into rhesus testes regenerates spermatogenesis producing functional sperm. Cell Stem Cell 2012, 11, 715–726. [Google Scholar]
- Ryu, B.Y.; Orwig, K.E.; Avarbock, M.R.; Brinster, R.L. Stem cell and niche development in the postnatal rat testis. Dev. Biol. 2003, 263, 253–263. [Google Scholar]
- Dobrinski, I.; Avarbock, M.R.; Brinster, R.L. Transplantation of germ cells from rabbits and dogs into mouse testes. Biol. Reprod. 1999, 61, 1331–1339. [Google Scholar]
- Dobrinski, I.; Avarbock, M.R.; Brinster, R.L. Germ cell transplantation from large domestic animals into mouse testes. Mol. Reprod. Dev. 2000, 57, 270–279. [Google Scholar]
- Hermann, B.P.; Sukhwani, M.; Lin, C.C.; Sheng, Y.; Tomko, J.; Rodriguez, M.; Shuttleworth, J.J.; McFarland, D.; Hobbs, R.M.; Pandolfi, P.P.; et al. Characterization, cryopreservation, and ablation of spermatogonial stem cells in adult rhesus macaques. Stem. Cells 2007, 25, 2330–2338. [Google Scholar]
- Dovey, S.L.; Valli, H.; Hermann, B.P.; Sukhwani, M.; Donohue, J.; Castro, C.A.; Chu, T.; Sanfilippo, J.S.; Orwig, K.E. Eliminating malignant contamination from therapeutic human spermatogonial stem cells. J. Clin. Invest. 2013, 123, 1833–1843. [Google Scholar]
- Valli, H.; Sukhwani, M.; Dovey, S.L.; Peters, K.A.; Donohue, J.; Castro, C.A.; Chu, T.; Marshall, G.R.; Orwig, K.E. Fluorescence- and magnetic-activated cell sorting strategies to isolate and enrich human spermatogonial stem cells. Fertil. Steril. 2014, 102, 566–580. [Google Scholar]
- Wu, X.; Goodyear, S.M.; Abramowitz, L.K.; Bartolomei, M.S.; Tobias, J.W.; Avarbock, M.R.; Brinster, R.L. Fertile offspring derived from mouse spermatogonial stem cells cryopreserved for more than 14 years. Hum. Reprod. 2012, 27, 1249–1259. [Google Scholar]
- Kubota, H.; Brinster, R.L. Spermatogonial stem cells. Biol. Reprod. 2018, 99, 52–74. [Google Scholar]
- Shetty, G.; Mitchell, J.M.; Meyer, J.M.; Wu, Z.; Lam, T.N.; Phan, T.T.; Zhang, J.; Hill, L.; Tailor, R.C.; Peters, K.A.; et al. Restoration of Functional Sperm Production in Irradiated Pubertal Rhesus Monkeys by Spermatogonial Stem Cell Transplantation. Andrology 2020. [Google Scholar] [CrossRef]
- Brook, P.F.; Radford, J.A.; Shalet, S.M.; Joyce, A.D.; Gosden, R.G. solation of germ cells from human testicular tissue for low temperature storage and autotransplantation. Fertil. Steril. 2001, I75, 269–274. [Google Scholar]
- Radford, J. Restoration of fertility after treatment for cancer. Horm. Res. 2003, 59 (Suppl. 1), 21–23. [Google Scholar]
- Nagano, M.; Avarbock, M.R.; Brinster, R.L. Pattern and kinetics of mouse donor spermatogonial stem cell colonization in recipient testes. Biol. Reprod. 1999, 60, 1429–1436. [Google Scholar]
- Nagano, M.; Ryu, B.Y.; Brinster, C.J.; Avarbock, M.R.; Brinster, R.L. Maintenance of mouse male germ line stem cells in vitro. Biol. Reprod. 2003, 68, 2207–2214. [Google Scholar]
- Wu, X.; Schmidt, J.A.; Avarbock, M.R.; Tobias, J.W.; Carlson, C.A.; Kolon, T.F.; Ginsberg, J.P.; Brinster, R.L. Prepubertal human spermatogonia and mouse gonocytes share conserved gene expression of germline stem cell regulatory molecules. Proc. Natl. Acad. Sci. USA 2009, 106, 21672–21677. [Google Scholar]
- Takashima, S.; Shinohara, T. Culture and transplantation of spermatogonial stem cells. Stem Cell Res. 2018, 29, 46–55. [Google Scholar]
- Ogawa, T.; Dobrinski, I.; Avarbock, M.R.; Brinster, R.L. Transplantation of male germ line stem cells restores fertility in infertile mice. Nat. Med. 2000, 6, 29–34. [Google Scholar]
- Fujita, K.; Tsujimura, A.; Miyagawa, Y.; Kiuchi, H.; Matsuoka, Y.; Takao, T.; Takada, S.; Nonomura, N.; Okuyama, A. Isolation of germ cells from leukemia and lymphoma cells in a human in vitro model: Potential clinical application for restoring human fertility after anticancer therapy. Cancer Res. 2006, 66, 11166–11171. [Google Scholar]
- Geens, M.; Goossens, E.; Tournaye, H. Cell selection by selective matrix adhesion is not sufficiently efficient for complete malignant cell depletion from contaminated human testicular cell suspensions. Fertil. Steril. 2011, 787, 91–95. [Google Scholar]
- Rodriguez-Sos, J.R.; Dobrinski, I. Recent development in testis tissue xenografting. Reproduction 2009, 138, 187–194. [Google Scholar]
- Galdon, G.; Atala, A.; Sadri-Ardekani, H. In Vitro Spermatogenesis: How Far from Clinical Application? Curr Urol Rep. 2016, 17, 49. [Google Scholar] [CrossRef]
- Honaramooz, A.; Snedaker, A.; Boiani, M.; Scholer, H.; Dobrinski, I.; Schlatt, S. Sperm from neonatal mammalian testes grafted in mice. Nature 2002, 418, 778–781. [Google Scholar]
- Luetjens, C.M.; Stukenborg, J.B.; Nieschlag, E.; Simoni, M.; Wistuba, J. Complete spermatogenesis in orthotopic but not in ectopic transplants of autologously grafted marmoset testicular tissue. Endocrinology 2008, 149, 1736–1747. [Google Scholar]
- Fayomi, A.P.; Peters, K.; Sukhwani, M.; Valli-Pulaski, H.; Shetty, G.; Meistrich, M.L.; Houser, L.; Robertson, N.; Roberts, V.; Ramsey, C.; et al. Autologous grafting of cryopreserved prepubertal rhesus testis produces sperm and offspring. Science 2019, 363, 1314–1319. [Google Scholar]
- Wyns, C.; Van Langendonckt, A.; Wese, F.X.; Donnez, J.; Curaba, M. Long-term spermatogonial survival in cryopreserved and xenografted immature human testicular tissue. Hum. Reprod. 2008, 23, 2402–2414. [Google Scholar]
- Geens, M.; De Block, G.; Goossens, E.; Frederickx, V.; Van Steirteghem, A.; Tournaye, H. Spermatogonial survival after grafting human testicular tissue to immunodeficient mice. Hum. Reprod. 2006, 21, 390–396. [Google Scholar]
- Schlatt, S.; Honaramooz, A.; Ehmcke, J.; Goebell, P.J.; Rübben, H.; Dhir, R.; Dobrinski, I.; Patrizio, P. Limited survival of adult human testicular tissue as ectopic xenograft. Hum. Reprod. 2006, 21, 384–389. [Google Scholar]
- Goossens, E.; Geens, M.; De Block, G.; Tournaye, H. Spermatogonial survival in long-term human prepubertal xenografts. Fertil. Steril. 2008, 90, 2019–2022. [Google Scholar]
- Honaramooz, A.; Megee, S.O.; Rathi, R.; Dobrinski, I. Building a testis: Formation of functional testis tissue after transplantation of isolated porcine (Sus scrofa) testis cells. Biol. Reprod. 2007, 76, 43–47. [Google Scholar]
- Kita, K.; Watanabe, T.; Ohsaka, K.; Hayashi, H.; Kubota, Y.; Nagashima, Y.; Aoki, I.; Taniguchi, H.; Noce, T.; Inoue, K.; et al. Production of functional spermatids from mouse germline stem cells in ectopically reconstituted seminiferous tubules. Biol. Reprod. 2007, 76, 211–217. [Google Scholar]
- Arregui, L.; Rathi, R.; Megee, S.O.; Honaramooz, A.; Gomendio, M.; Roldan, E.R.; Dobrinski, I. Xenografting of sheep testis tissue and isolated cells as a model for preservation of genetic material from endangered ungulates. Reproduction 2008, 136, 85–93. [Google Scholar]
- Shetty, G.; Mitchell, J.M.; Lam, T.N.A.; Wu, Z.; Zhang, J.; Hill, L.; Tailor, R.C.; Peters, K.A.; Penedo, M.C.; Orwig, K.E.; et al. Donor spermatogenesis in de novo formed seminiferous tubules from transplanted testicular cells in rhesus monkey testis. Hum. Reprod. 2018, 33, 2249–2255. [Google Scholar]
- Mahmoud, H. Spermatogenesis in artificial three-dimensional system. Stem. Cells 2012, 30, 2355–2360. [Google Scholar]
- Steinberger, A.; Steinberger, E.; Perloff, W.H. Mammalian testes in organ culture. Exp. Cell Res. 1964, 36, 19–27. [Google Scholar]
- Steinberger, A.; Steinberger, E. Factors affecting spermatogenesis in organ cultures of mammalian testes. J. Reprod. Fertil. 1967, 2, 117–124. [Google Scholar]
- Ogawa, T. In vitro spermatogenesis: The dawn of a new era in the study of male infertility. Int. J. Urol. 2012, 19, 282–283. [Google Scholar]
- Sato, T.; Katagiri, K.; Gohbara, A.; Inoue, K.; Ogonuki, N.; Ogura, A.; Kubota, Y. and Ogawa, T. In vitro production of functional sperm in cultured neonatal mouse testes. Nature 2011, 471, 504–508. [Google Scholar]
- Dumont, L.; Arkoun, B.; Jumeau, F.; Milazzo, J.P.; Bironneau, A.; Liot, D.; Wils, J.; Rondanino, C.; Rives, N. Assessment of the optimal vitrification protocol for pre-pubertal mice testes leading to successful in vitro production of flagellated spermatozoa. Andrology 2015, 3, 611–625. [Google Scholar]
- Medrano, J.V.; Vilanova-Perez, T.; Fornes-Ferrer, V.; Navarro-Gomezlechon, A.; Martínez-Triguero, M.L.; García, S.; Gómez-Chacón, J.; Povo, I.; Pellicer, A.; Andrés, M.M.; et al. Influence of temperature, serum, and gonadotropin supplementation in short- and long-term organotypic culture of human immature testicular tissue. Fertil. Steril. 2018, 110, 1045–1057.e3. [Google Scholar]
- Yang, S.; Ping, P.; Ma, M.; Li, P.; Tian, R.; Yang, H.; Liu, Y.; Gong, Y.; Zhang, Z.; Li, Z.; et al. Generation of haploid spermatids with fertilization and development capacity from human spermatogonial stem cells of cryptorchid patients. Stem Cell Rep. 2014, 3, 663–675. [Google Scholar]
- De Michele, F.; Poels, J.; Weerens, L.; Petit, C.; Evrard, Z.; Ambroise, J.; Gruson, D.; Wyns, C. Preserved seminiferous tubule integrity with spermatogonial survival and induction of Sertoli and Leydig cell maturation after long-term organotypic culture of prepubertal human testicular tissue. Hum. Reprod. 2017, 32, 32–45. [Google Scholar]
- Lee, J.H.; Kim, H.J.; Kim, H.; Lee, S.J.; Gye, M.C. In vitro spermatogenesis by threedimensional culture of rat testicular cells in collagen gel matrix. Biomaterials 2006, 27, 2845–2853. [Google Scholar]
- Stukenborg, J.B.; Wistuba, J.; Luetjens, C.M.; Elhija, M.A.; Huleihel, M.; Lunenfeld, E.; Gromoll, J.; Nieschlag, E.; Schlatt, S. Coculture of spermatogonia with somatic cells in a novel three dimensional soft-agar-culture-system. J. Androl. 2008, 29, 312–329. [Google Scholar]
- Sadri-Ardekani, H.; Mizrak, S.C.; van Daalen, S.K.; Korver, C.M.; Roepers-Gajadien, H.L.; Koruji, M.; Hovingh, S.; de Reijke, T.M.; de la Rosette, J.J.; van der Veen, F.; et al. Propagation of human spermatogonial stem cells in vitro. JAMA 2009, 302, 2127–2134. [Google Scholar]
- Sadri-Ardekani, H.; Akhondi, M.A.; van der Veen, F.; Repping, S.; van Pelt, A.M. In Vitro Propagation of Human Prepubertal Spermatogonial Stem. Cells. JAMA 2011, 305, 2416–2418. [Google Scholar]
- Stukenborg, J.B.; Schlatt, S.; Simoni, M.; Yeung, C.H.; Elhija, M.A.; Luetjens, C.M.; Huleihel, M.; Wistuba, J. New horizons for in vitro spermatogenesis? An update on novel three-dimensional culture systems as tools for meiotic and postmeiotic differentiation of testicular germ cells. Mol. Hum. Reprod. 2009, 15, 971–980. [Google Scholar]
- Huleihel, M.; Nourashrafeddin, S.; Plant, T.M. Application of three dimensional culture systems to study mammalian spermatogenesis, with an emphasis on the rhesus monkey (Macaca mulatta). Asian J. Androl. 2015, 17, 972–980. [Google Scholar]
- Abu Elhija, M.; Lunenfeld, E.; Schlatt, S.; Huleihel, M. Differentiation of murine male germ cells to spermatozoa in a soft agar culture system. Asian J. Androl. 2012, 14, 285–293. [Google Scholar]
- AbuMadighem, A.; Solomon, R.; Stepanovsky, A.; Kapelushnik, J.; Shi, Q.; Meese, E.; Lunenfeld, E.; Huleihel, M. Development of spermatogenesis in vitro in three-dimensional culture from spermatogonial cells of busulfan-treated immature mice. Int. J. Mol. Sci. 2018, 19, 3804–3820. [Google Scholar]
- Abofoul-Azab, M.; Lunenfeld, E.; Levitas, E.; Zeadna, A.; Younis, J.; Bar-Ami, S.; Huleihel, M. Identification of Premeiotic, Meiotic, and Postmeiotic Cells in Testicular Biopsies Without Sperm from Sertoli Cell-Only Syndrome Patients. Int. J. Mol. Sci. 2019, 20, 470. [Google Scholar] [CrossRef]
- Huleihel, N.; Azab, M.; Levitas, E.; Fisch, B.; Pinkas, H.; Stein, A.; Younis, J.S.; Bar-Ami, S.; Orvieto, R.; Ziadna, A.; et al. The capacity to induce generation of premeiotic and meiotic cells from biopsies without sperm from non-obstructive azoospermic and Klinefelter syndrome patients were different under in vitro culture conditions. Fertil. Steril. 2015, 104, e91. [Google Scholar]
- Azab, M.; Lunenfeld, E.; Kapelushnik, J.; Huleihel, M. Fertility reservation of pre-pubertal cancer patient boys before aggressive chemotherapy. Preliminary results from in vitro cultures of fresh testicular tissue from three pre-pubertal patients. Fertil. Steril. 2013, 100, S63. [Google Scholar]
- Valli-Pulaski, H.; Peters, K.A.; Gassei, K.; Steime, S.R.; Sukhwani, M.; Hermann, B.P.; Dwomor, L.; David, S.; Fayomi, A.P.; Munyoki, S.K.; et al. Testicular tissue cryopreservation: 8 years of experience from a coordinated network of academic centers. Hum. Reprod. 2019, 2019, 1–12. [Google Scholar] [CrossRef]
- Baert, Y.; Dvorakova-Hortova, K.; Margaryan, H.; Goossens, E. Mouse in Vitro Spermatogenesis on Alginate-Based 3D Bioprinted Scaffolds. Biofabrication 2019, 11, 035011. [Google Scholar] [CrossRef]
- Alves-Lopes, J.P.; Stukenborg, J.B. Testicular organoids: A new model to study the testicular microenvironment in vitro? Hum. Reprod. Update 2018, 24, 176–191. [Google Scholar]
- Lancaster, M.A.; Knoblich, J.A. Organogenesis in a dish: Modeling development and disease using organoid technologies. Science 2014, 345, 1247125. [Google Scholar]
- Clevers, H. Modeling development and disease with organoids. Cell 2016, 165, 1586–1597. [Google Scholar]
- Huch, M.; Knoblich, J.A.; Lutolf, M.P.; Martinez-Arias, A. The hope and the hype of organoid research. Development 2017, 144, 938–941. [Google Scholar]
- Pendergraft, S.S.; Sadri-Ardekani, H.; Atala, A.; Bishop, C.E. Three-dimensional testicular organoid: A novel tool for the study of human spermatogenesis and gonadotoxicity in vitro dagger. Biol. Reprod. 2017, 96, 720–732. [Google Scholar]
- Baert, Y.; De Kock, J.; Alves-Lopes, J.P.; Söder, O.; Stukenborg, J.-B.; Goossens, E. Primary human testicular cells self-organize into organoids with testicular properties. Stem Cell Rep. 2017, 1, 30–38. [Google Scholar]
- Alves-Lopes, J.P.; Soder, O.; Stukenborg, J.B. Testicular organoid generation by a novel in vitro three-layer gradient system. Biomaterials 2017, 130, 76–89. [Google Scholar]
- Topraggaleh, T.R.; Valojerdi, M.R.; Montazeri, L.; Baharvand, H. A testis-derived macroporous 3D scaffold as a platform for the generation of mouse testicular organoids. Biomater. Sci. 2019, 7, 1422–1436. [Google Scholar]
- Komeya, M.; Kimura, H.; Nakamura, H.; Yokonishi, T.; Sato, T.; Kojima, K.; Hayashi, K.; Katagiri, K.; Yamanaka, H.; Sanjo, H.; et al. Long-term ex vivo maintenance of testis tissues producing fertile sperm in a microfluidic device. Sci. Rep. 2016, 6, 21472. [Google Scholar]
- Komeya, M.; Hayashi, K.; Nakamura, H.; Yamanaka, H.; Sanjo, H.; Kojima, K.; Sato, T.; Yao, M.; Kimura, H.; Fujii, T.; et al. Pumpless Microfluidic System Driven by Hydrostatic Pressure Induces and Maintains Mouse Spermatogenesis in Vitro. Sci. Rep. 2017, 7, 15459. [Google Scholar] [CrossRef]
- Yamanaka, H.; Komeya, M.; Nakamura, H.; Sanjo, H.; Sato, T.; Yao, M.; Kimura, H.; Fujii, T.; Ogawa, T. A monolayer microfluidic device supporting mouse spermatogenesis with improved visibility. Biochem. Biophys. Res. Commun. 2018, 500, 885–891. [Google Scholar]
- Takahashi, K.; Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006, 126, 663–676. [Google Scholar]
- Takahashi, K.; Tanabe, K.; Ohnuki, M.; Narita, M.; Ichisaka, T.; Tomoda, K.; Yamanaka, S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 2007, 131, 861–872. [Google Scholar]
- Hou, J.; Yang, S.; Yang, H.; Liu, Y.; Hai, Y.; Chen, Z.; Guo, Y.; Gong, Y.; Gao, W.-Q.; Li, Z.; et al. Generation of male differentiated germ cells from various types of stem cells. Reproduction 2014, 147, R179–R188. [Google Scholar]
- Gauthier-Fisher, A.; Kauffman, A.; Librach, C.L. Potential use of stem cells for fertility preservation. Andrology 2020, 8, 862–878. [Google Scholar]
- Mishra, S.; Kacin, E.; Stamatiadis, P.; Franck, S.; Van der Jeught, M.; Mertes, H.; Pennings, G.; De Sutter, P.; Sermon, K.; Heindryckx, B.; et al. The role of the reprogramming method and pluripotency state in gamete differentiation from patient-specific human pluripotent stem cells. Mol. Hum. Reprod. 2018, 24, 173–184. [Google Scholar]
- Nagamatsu, G.; Hayashi, K. Stem cells, in vitro gametogenesis and male fertility. Reproduction 2017, 154, F79–F91. [Google Scholar]
- Rombaut, C.; Mertes, H.; Heindryckx, B.; Goossens, E. Human in vitro spermatogenesis from pluripotent stem cells: In need of a stepwise differentiation protocol? Mol. Hum. Reprod. 2018, 24, 47–54. [Google Scholar]
- Shimizu, T.; Shiohara, M.; Tai, T.; Nagao, K.; Nakajima, K.; Kobayashi, H. Derivation of integration-free iPSCs from a Klinefelter syndrome patient. Reprod. Med. Biol. 2016, 15, 35–43. [Google Scholar]
- Wang, X.; Liao, T.; Wan, C.; Yang, X.; Zhao, J.; Fu, R.; Yao, Z.; Huang, Y.; Shi, Y.; Chang, G.; et al. Efficient generation of human primordial germ cell-like cells from pluripotent stem cells in a methylcellulose-based 3D system at large scale. PeerJ 2019, 6, e6143. [Google Scholar]
- Kee, K.; Angeles, V.T.; Flores, M.; Nguyen, H.N.; Pera, R.A.R. Human dazl, daz and boule genes modulate primordial germ-cell and haploid gamete formation. Nature 2009, 462, 222–225. [Google Scholar]
- Easley, C.A., IV; Phillips, B.T.; McGuire, M.M.; Barringer, J.M.; Valli, H.; Hermann, B.P.; Simerly, C.R.; Rajkovic, A.; Miki, T.; Orwig, K.E.; et al. Direct differentiation of human pluripotent stem cells into haploid spermatogenic cells. Cell Rep. 2012, 2, 440–446. [Google Scholar]
- Kee, K.; Gonsalves, J.M.; Clark, A.T.; Pera, R.A. Bone morphogenetic proteins induce germ cell differentiation from human embryonic stem cells. Stem. Cells Dev. 2006, 15, 831–837. [Google Scholar]
- Teramura, T.; Takehara, T.; Kawata, N.; Fujinami, N.; Mitani, T.; Takenoshita, M.; Matsumoto, K.; Saeki, K.; Iritani, A.; Sagawa, N.; et al. Primate embryonic stem cells proceed to early gametogenesis in vitro. Cloning Stem. Cells 2007, 9, 144–156. [Google Scholar]
- Park, T.S.; Galic, Z.; Conway, A.E.; Lindgren, A.; Van Handel, B.J.; Magnusson, M.; Richter, L.; Teitell, M.A.; Mikkola, H.K.; Lowry, W.E.; et al. Derivation of primordial germ cells from human embryonic and induced pluripotent stem cells is significantly improved by coculture with human fetal gonadal cells. Stem. Cells 2009, 27, 783–795. [Google Scholar]
- Yamauchi, K.; Hasegawa, K.; Chuma, S.; Nakatsuji, N.; Suemori, H. In vitro germ cell differentiation from cynomolgus monkey embryonic stem cells. PLoS ONE 2009, 4, e5338. [Google Scholar]
- Hayashi, K.; Ohta, H.; Kurimoto, K.; Aramaki, S.; Saitou, M. Reconstitution of the mouse germ cell specification pathway in culture by pluripotent stem cells. Cell 2011, 146, 519–532. [Google Scholar]
- Yuan, Y.; Zhou, Q.; Wan, H.; Shen, B.; Wang, X.; Wang, M.; Feng, C.; Xie, M.; Gu, T.; Zhou, T.; et al. Generation of fertile offspring from Kit(w)/Kit(w/v) mice through differentiation of gene corrected nuclear transfer embryonic stem cells. Cell Res. 2015, 25, 851–863. [Google Scholar]
- Sosa, E.; Chen, D.; Rojas, E.J.; Hennebold, J.D.; Peters, K.A.; Wu, Z.; Lam, T.N.; Mitchell, J.M.; Sukhwani, M.; Tailor, R.C.; et al. Differentiation of primate primordial germ cell-like cells following transplantation into the adult gonadal niche. Nat. Commun. 2018, 9, 5339. [Google Scholar]
- Irie, N.; Surani, M.A. Efficient induction and isolation of human primordial germ cell-like cells from competent human pluripotent stem cells. Methods Mol. Biol. 2017, 1463, 217–226. [Google Scholar]
- Panula, S.; Medrano, J.V.; Kee, K.; Bergström, R.; Nguyen, H.N.; Byers, B.; Wilson, K.D.; Wu, J.C.; Simon, C.; Hovatta, O.; et al. Human germ cell differentiation from fetal- and adult-derived induced pluripotent stem cells. Hum. Mol. Genet. 2011, 20, 752–762. [Google Scholar]
- Medrano, J.V.; Ramathal, C.; Nguyen, H.N.; Simon, C.; Reijo Pera, R.A. Divergent RNA-binding proteins, DAZL and VASA, induce meiotic progression in human germ cells derived in vitro. Stem. Cells 2012, 30, 441–451. [Google Scholar]
- Eguizabal, C.; Montserrat, N.; Vassena, R.; Barragan, M.; Garreta, E.; Garcia-Quevedo, L.; Vidal, F.; Giorgetti, A.; Veiga, A. and Belmonte, J.I. Complete meiosis from human induced pluripotent stem cells. Stem. Cells 2011, 29, 1186–1195. [Google Scholar]
- Shetty, G.; Uthamanthil, R.K.; Zhou, W.; Shao, S.H.; Weng, C.C.; Tailor, R.C.; Hermann, B.P.; Orwig, K.E.; Meistrich, M.L. Hormone Suppression with GnRH Antagonist Promotes Spermatogenic Recovery from Transplanted Spermatogonial Stem Cells in Irradiated Cynomolgus Monkeys. Andrology 2013, 1, 886–898. [Google Scholar]
- Masala, A.; Faedda, R.; Alagna, S.; Satta, A.; Chiarelli, G.; Rovasio, P.P.; Ivaldi, R.; Taras, M.S.; Lai, E.; Bartoli, E. Use of testosterone to prevent cyclophosphamide-induced azoospermia. Ann. Intern. Med. 1997, 126, 292–295. [Google Scholar]
- Thomson, A.B.; Critchley, H.O.; Kelnar, C.J.; Wallace, W.H. Late reproductive sequelae following treatment of childhood cancer and options for fertility preservation. Best Pract. Res. Clin. Endocrinol. Metab. 2002, 16, 311–334. [Google Scholar]
- Ogawa, T.; Dobrinski, I.; Brinster, R.L. Recipient preparation is critical for spermatogonial transplantation in the rat. Tissue Cell 1999, 31, 461–472. [Google Scholar]
- Ogawa, T.; Dobrinski, I.; Avarbock, M.R.; Brinster, R.L. Leuprolide, a gonadotropin-releasing hormone agonist, enhances colonization after spermatogonial transplantation into mouse testes. Tissue Cell 1998, 30, 583–588. [Google Scholar]
- Dobrinski, I.; Ogawa, T.; Avarbock, M.R.; Brinster, R.L. Effect of the GnRH-agonist leuprolide on colonization of recipient testes by donor spermatogonial stem cells after transplantation in mice. Tissue Cell 2001, 33, 200–207. [Google Scholar]
- Ohmura, M.; Ogawa, T.; Ono, M.; Dezawa, M.; Hosaka, M.; Kubota, Y.; Sawada, H. Increment of murine spermatogonial cell number by gonadotropin-releasing hormone analogue is independent of stem cell factor c-kit signal. Biol. Reprod. 2003, 68, 2304–2313. [Google Scholar]
- Zhang, Z.; Renfree, M.B.; Short, R.V. Successful intra- and interspecific male germ cell transplantation in the rat. Biol. Reprod. 2003, 68, 961–967. [Google Scholar]
- Wang, G.; Shao, S.H.; Weng, C.C.; Wei, C.; Meistrich, M.L. Hormonal suppression restores fertility in irradiated mice from both endogenous and donor-derived stem spermatogonia. Toxicol. Sci. 2010, 117, 225–237. [Google Scholar]
- Meng, J.; Holdcraft, R.W.; Shima, J.E.; Griswold, M.D.; Braun, R.E. Androgens regulate the permeability of the blood-testis barrier. Proc. Natl. Acad. Sci. USA 2005, 102, 16696–16700. [Google Scholar]
- Shetty, G.; Wu, Z.; Lam, T.N.A.; Phan, T.T.; Orwig, K.E.; Meistrich, M.L. Effect of hormone modulations on donor-derived spermatogenesis or colonization after syngeneic and xenotransplantation in mice. Andrology 2019, 7, 257–265. [Google Scholar]
- Sredni, B.; Caspi, R.R.; Klein, A.; Kalechman, Y.; Danziger, Y.; Ben, Y.M.; Tamari, T.; Shalit, F.; Albeck, M. A new immunomodulating compound (AS-101) with potential therapeutic application. Nature 1987, 330, 173–176. [Google Scholar]
- Sredni, B. Immunomodulating tellurium compounds as anti-cancer agents. Semin. Cancer Biol. 2012, 22, 60–69. [Google Scholar]
- Sredni, B.; Weil, M.; Khomenok, G.; Lebenthal, I.; Teitz, S.; Mardor, Y.; Ram, Z.; Orenstein, A.; Kershenovich, A.; Michowiz, S.; et al. Ammonium trichloro(dioxoethylene-o,o’)tellurate (AS101) sensitizes tumors to chemotherapy by inhibiting the tumor interleukin 10 autocrine loop. Cancer Res. 2004, 64, 1843–1852. [Google Scholar]
- Sredni, B.; Tichler, T.; Shani, A.; Catane, R.; Kaufman, B.; Strassmann, G.; Albeck, M.; Kalechman, Y. Predominance of TH1 response in tumor-bearing mice and cancer patients treated with AS101. J. Natl. Cancer Inst. 1996, 88, 1276–1284. [Google Scholar]
- Kalechman, Y.; Zuloff, A.; Albeck, M.; Strassmann, G.; Sredni, B. Role of endogenous cytokines secretion in radioprotection conferred by the immunomodulator ammonium trichloro(dioxyethylene-O-O0 )tellurate. Blood 1995, 85, 1555–1561. [Google Scholar]
- Kalechman, Y.; Sredni, B.; Weinstein, T.; Freidkin, I.; Tobar, A.; Albeck, M.; Gafter, U. Production of the novel mesangial autocrine growth factors GDNF and IL-10 is regulated by the immunomodulator AS101. J. Am. Soc. Nephrol. 2003, 14, 620–630. [Google Scholar]
- Carmely, A.; Meirow, D.; Peretz, A.; Albeck, M.; Bartoov, B.; Sredni, B. Protective effect of the immunomodulator AS101 against cyclophosphamide-induced testicular damage in mice. Hum. Reprod. 2009, 24, 1322–1329. [Google Scholar]
- Demetri, G.D.; Griffin, J.D. Granulocyte colony-stimulating factor and its receptor. Blood 1991, 78, 2791–2808. [Google Scholar]
- Shimamura, M.; Kobayashi, Y.; Yuo, A.; Urabe, A.; Okabe, T.; Komatsu, Y.; Itoh, S.; Takaku, F. Effect of human recombinant granulocyte colony stimulating factor on hematopoietic injury in mice induced by 5-fluorouracil. Blood 1987, 69, 353–355. [Google Scholar]
- Uckun, F.M.; Souza, L.; Waddick, K.G.; Wick, M.; Song, C.W. In vivo radioprotective effects of recombinant human granulocyte colony-stimulating factor in lethally irradiated mice. Blood 1990, 75, 638–645. [Google Scholar]
- Kim, J.; Lee, S.; Jeon, B.; Jang, W.; Moon, C.; Kim, S. Protection of spermatogenesis against gamma ray-induced damage by granulocyte colony-stimulating factor in mice. Andrologia 2010, 43, 87–93. [Google Scholar]
- Kotzur, T.; Benavides-Garcia, R.; Mecklenburg, J.; Sanchez, J.R.; Reilly, M.; Hermann, B.P. Granulocyte Colony-Stimulating Factor (G-CSF) Promotes Spermatogenic Regeneration From Surviving Spermatogonia After High-Dose Alkylating Chemotherapy. Reprod. Biol. Endocrinol. 2017, 15, 7. [Google Scholar] [CrossRef]
- Khanlarkhani, N.; Pasbakhsh, P.; Mortezaee, K.; Naji, M.; Amidi, F.; Najafi, A.; Sobhani, A.; Zendedel, A. Effect of Human Recombinant Granulocyte Colony-Stimulating Factor on Rat Busulfan-Induced Testis Injury. J. Mol. Histol. 2016, 47, 59–67. [Google Scholar] [CrossRef]
- Benavides-Garcia, R.; Joachim, R.; Pina, N.A.; Mutoji, K.N.; Reilly, M.A.; Hermann, B.P. Granulocyte colony-stimulating factor prevents loss of spermatogenesis after sterilizing busulfan chemotherapy. Fertil. Steril. 2015, 103, 270–280. [Google Scholar]
- Zhu, Y.; Yin, Q.; Wei, D.; Yang, Z.; Du, Y.; Ma, Y. Autophagy in male reproduction. Sys Biol. Reprod. Med. 2019, 65, 265–272. [Google Scholar]
- Noguchi, M.; Hirata, N.; Tanaka, T.; Suizu, F.; Nakajima, H.; Chiorini, J.A. Autophagy as a modulator of cell death machinery. Cell Death Dis. 2020, 11, 517. [Google Scholar]
- Zhang, M.; Liu, F.; Zhou, P.; Wang, Q.; Xu, C.; Li, Y.; Bian, L.; Liu, Y.; Zhou, J.; Wang, F.; et al. The MTOR signaling pathway regulates macrophage differentiation from mouse myeloid progenitors by inhibiting autophagy. Autophagy 2019, 15, 1150–1162. [Google Scholar]
- Meng, D.; Frank, A.R.; Jewell, J.L. mTOR signaling in stem and progenitor cells. Development 2018, 145. [Google Scholar] [CrossRef]
- Bento, C.F.; Renna, M.; Ghislat, G.; Puri, C.; Ashkenazi, A.; Vicinanza, M.; Menzies, F.M.; Rubinsztein, D.C. Mammalian autophagy: How does it work? Annu. Rev. Biochem. 2016, 85, 685–713. [Google Scholar]
- Phadwal, K.; Watson, A.S.; Simon, A.K. Tightrope act: Autophagy in stem cell renewal, differentiation, proliferation, and aging. Cell Mol. Life Sci. 2013, 70, 89–103. [Google Scholar]
- Wullschleger, S.; Loewith, R.; Hall, M.N. TOR signaling in growth and metabolism. Cell 2006, 124, 471–484. [Google Scholar]
- Liu, S.; Huang, L.; Geng, Y.; He, J.; Chen, X.; Xu, H.; Li, R.; Wang, Y.; Ding, Y.; Liu, X. Rapamycin inhibits spermatogenesis by changing the autophagy status through suppressing mechanistic target of rapamycin-p70S6 kinase in male rats. Mol. Med. Rep. 2017, 16, 4029–4037. [Google Scholar]
- Shemesh, I.I.; Rozen-Zvi, B.; Kalechman, Y.; Gafter, U.; Sredni, B. Prevents Diabetic Nephropathy Progression and Mesangial Cell Dysfunction: Regulation of the AKT Downstream Pathway. PLoS ONE 2014, 9, e114287. [Google Scholar] [CrossRef]
- Li, D.; Yang, H.; Nan, H.; Liu, P.; Pang, S.; Zhao, Q.; Karni, R.; Kamps, M.P.; Xu, Y.; Zhou, J.; et al. Identification of key regulatory pathways of myeloid differentiation using an mESC-based karyotypically normal cell model. Blood 2012, 120, 4712–4719. [Google Scholar]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).