Mechanisms that Link Chronological Aging to Cellular Quiescence in Budding Yeast
Abstract
1. Introduction
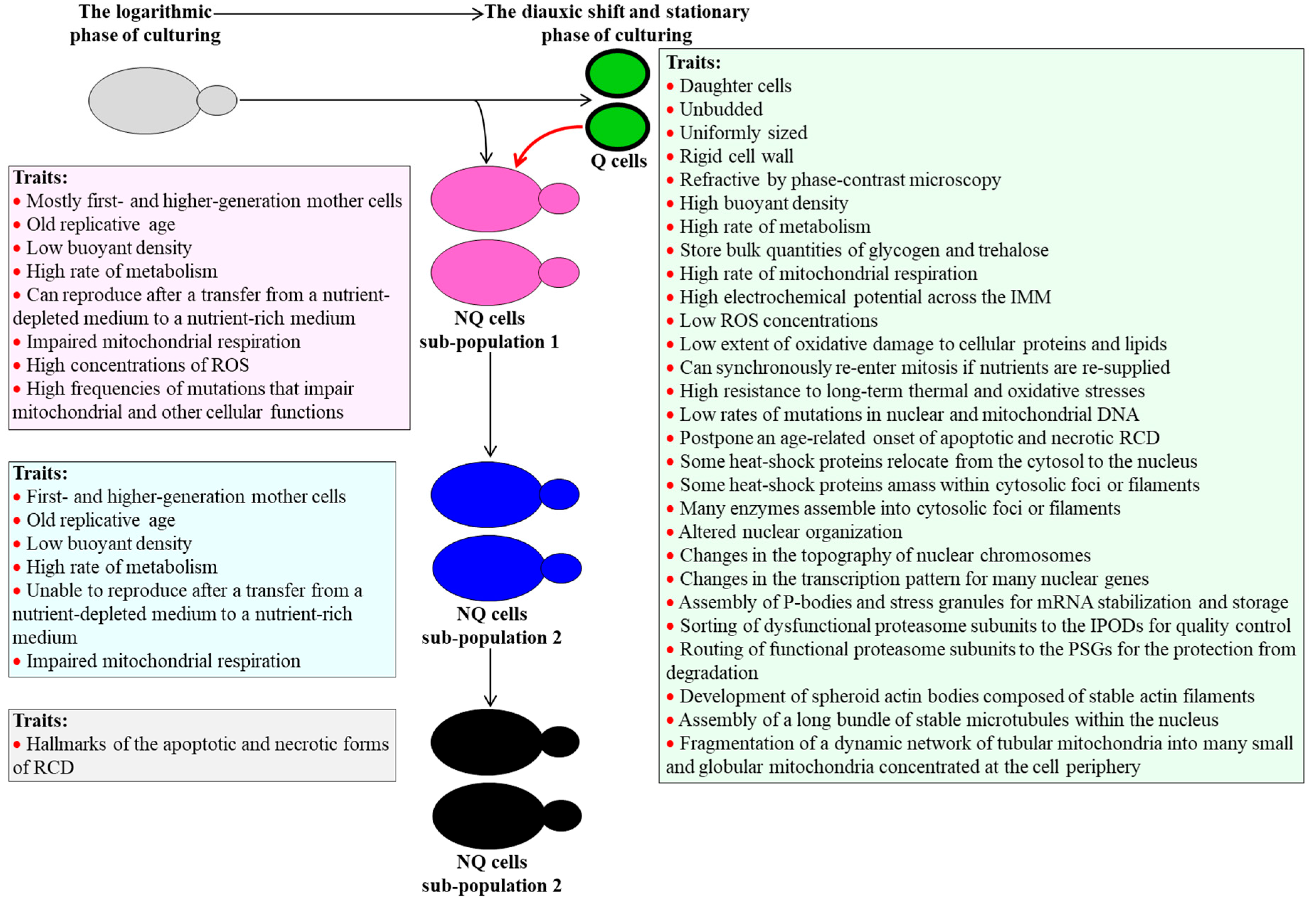
2. Traits of Q and NQ Cells Found in Yeast Populations Cultured Under Non-CR Conditions
2.1. Traits of Q Cells under Non-CR Conditions
2.2. Traits of NQ Cells under Non-CR Conditions
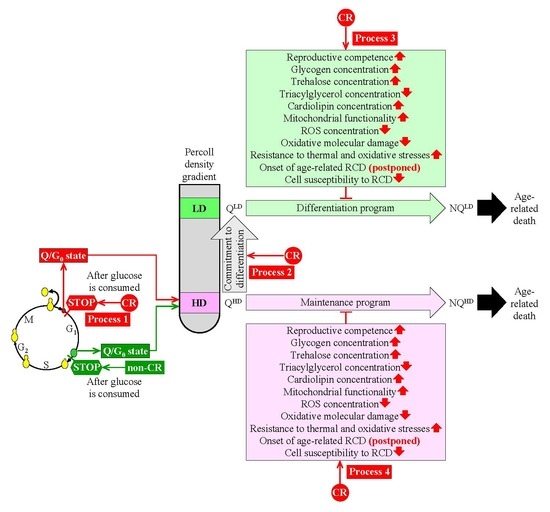
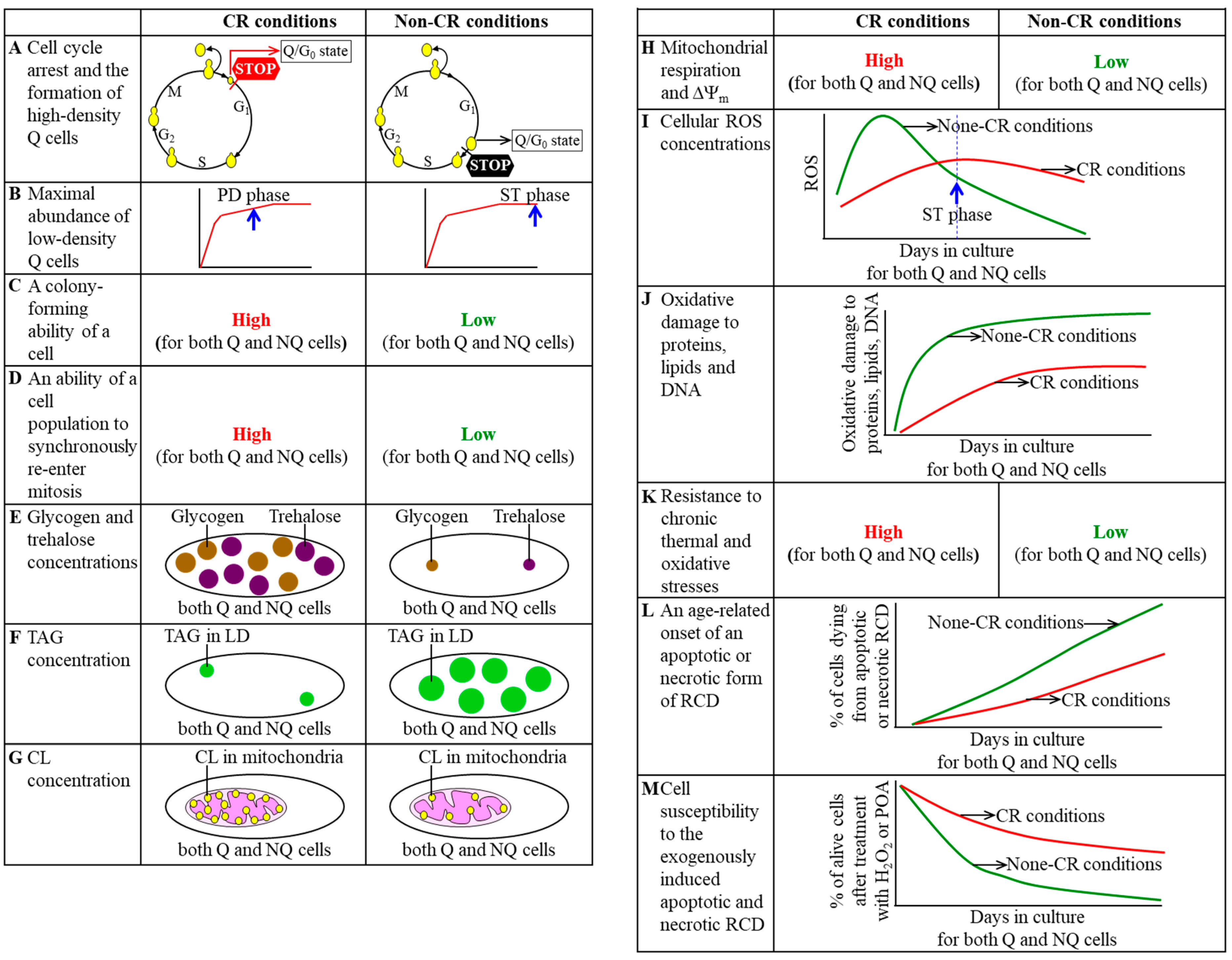
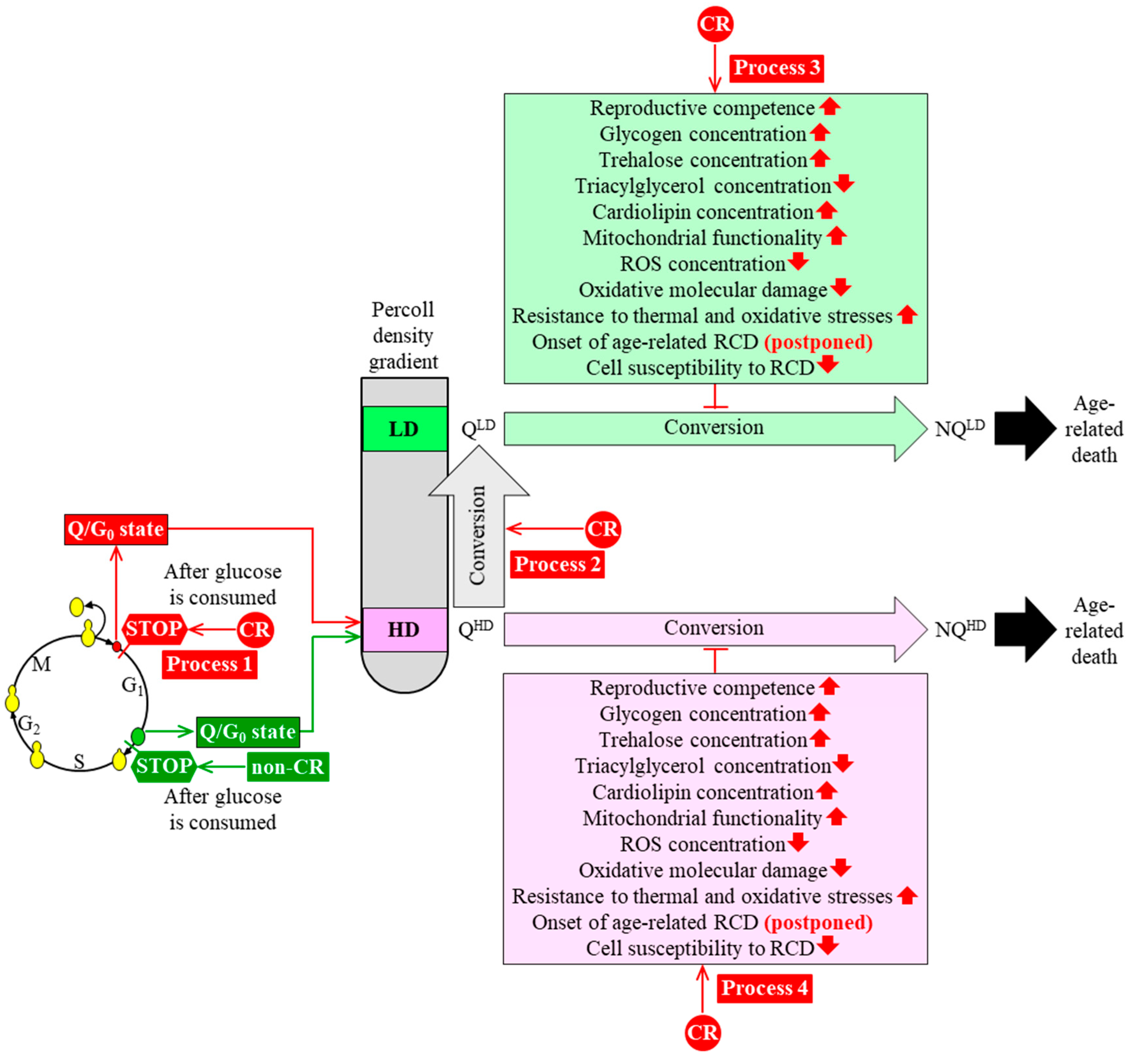
3. CR Diet Alters an Age-Related Chronology and Properties of Q and NQ Cells Found in Yeast Populations
4. A Hypothesis: The CR Diet Slows Yeast Chronological Aging Because It Alters an Age-Related Chronology and Certain Properties of Q Cells
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sinclair, D.A. Toward a unified theory of caloric restriction and longevity regulation. Mech. Ageing Dev. 2005, 126, 987–1002. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, A.A.; Bourque, S.D.; Kyryakov, P.; Gregg, C.; Boukh-Viner, T.; Beach, A.; Burstein, M.T.; Machkalyan, G.; Richard, V.; Rampersad, S.; et al. Effect of calorie restriction on the metabolic history of chronologically aging yeast. Exp. Gerontol. 2009, 44, 555–571. [Google Scholar] [CrossRef] [PubMed]
- Fraenkel, D.G. Yeast Intermediary Metabolism; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 2011; ISBN 978-0-87969-797-6. [Google Scholar]
- Broach, J.R. Nutritional control of growth and development in yeast. Genetics 2012, 192, 73–105. [Google Scholar] [CrossRef] [PubMed]
- Longo, V.D.; Shadel, G.S.; Kaeberlein, M.; Kennedy, B. Replicative and chronological aging in Saccharomyces cerevisiae. Cell Metab. 2012, 16, 18–31. [Google Scholar] [CrossRef]
- Arlia-Ciommo, A.; Leonov, A.; Piano, A.; Svistkova, V.; Titorenko, V.I. Cell-autonomous mechanisms of chronological aging in the yeast Saccharomyces cerevisiae. Microb. Cell 2014, 1, 163–178. [Google Scholar] [CrossRef]
- Allen, C.; Büttner, S.; Aragon, A.D.; Thomas, J.A.; Meirelles, O.; Jaetao, J.E.; Benn, D.; Ruby, S.W.; Veenhuis, M.; Madeo, F.; et al. Isolation of quiescent and nonquiescent cells from yeast stationary-phase cultures. J. Cell Biol. 2006, 174, 89–100. [Google Scholar] [CrossRef]
- Davidson, G.S.; Joe, R.M.; Roy, S.; Meirelles, O.; Allen, C.P.; Wilson, M.R.; Tapia, P.H.; Manzanilla, E.E.; Dodson, A.E.; Chakraborty, S.; et al. The proteomics of quiescent and nonquiescent cell differentiation in yeast stationary-phase cultures. Mol. Biol. Cell 2011, 22, 988–998. [Google Scholar] [CrossRef]
- De Virgilio, C. The essence of yeast quiescence. FEMS Microbiol. Rev. 2012, 36, 306–339. [Google Scholar] [CrossRef]
- Werner-Washburne, M.; Roy, S.; Davidson, G.S. Aging and the survival of quiescent and non-quiescent cells in yeast stationary-phase cultures. Subcell. Biochem. 2012, 57, 123–143. [Google Scholar]
- Miles, S.; Li, L.; Davison, J.; Breeden, L.L. Xbp1 directs global repression of budding yeast transcription during the transition to quiescence and is important for the longevity and reversibility of the quiescent state. PLoS Genet. 2013, 9, e1003854. [Google Scholar] [CrossRef]
- Aragon, A.D.; Rodriguez, A.L.; Meirelles, O.; Roy, S.; Davidson, G.S.; Tapia, P.H.; Allen, C.; Joe, R.; Benn, D.; Werner-Washburne, M. Characterization of differentiated quiescent and nonquiescent cells in yeast stationary-phase cultures. Mol. Biol. Cell 2008, 19, 1271–1280. [Google Scholar] [CrossRef] [PubMed]
- Smets, B.; Ghillebert, R.; De Snijder, P.; Binda, M.; Swinnen, E.; De Virgilio, C.; Winderickx, J. Life in the midst of scarcity: Adaptations to nutrient availability in Saccharomyces cerevisiae. Curr. Genet. 2010, 56, 1–32. [Google Scholar] [CrossRef] [PubMed]
- Peters, T.W.; Rardin, M.J.; Czerwieniec, G.; Evani, U.S.; Reis-Rodrigues, P.; Lithgow, G.J.; Mooney, S.D.; Gibson, B.W.; Hughes, R.E. Tor1 regulates protein solubility in Saccharomyces cerevisiae. Mol. Biol. Cell 2012, 23, 4679–4688. [Google Scholar] [CrossRef] [PubMed]
- Conrad, M.; Schothorst, J.; Kankipati, H.N.; Van Zeebroeck, G.; Rubio-Texeira, M.; Thevelein, J.M. Nutrient sensing and signaling in the yeast Saccharomyces cerevisiae. FEMS Microbiol. Rev. 2014, 38, 254–299. [Google Scholar] [CrossRef]
- Swinnen, E.; Ghillebert, R.; Wilms, T.; Winderickx, J. Molecular mechanisms linking the evolutionary conserved TORC1-Sch9 nutrient signalling branch to lifespan regulation in Saccharomyces cerevisiae. FEMS Yeast Res. 2014, 14, 17–32. [Google Scholar] [CrossRef]
- Sagot, I.; Laporte, D. The cell biology of quiescent yeast—A diversity of individual scenarios. J. Cell Sci. 2019, 132, jcs213025. [Google Scholar] [CrossRef]
- Fontana, L.; Partridge, L.; Longo, V.D. Extending healthy life span—From yeast to humans. Science 2010, 328, 321–326. [Google Scholar] [CrossRef]
- De Cabo, R.; Carmona-Gutierrez, D.; Bernier, M.; Hall, M.N.; Madeo, F. The search for antiaging interventions: From elixirs to fasting regimens. Cell 2014, 157, 1515–1526. [Google Scholar] [CrossRef]
- Madeo, F.; Carmona-Gutierrez, D.; Hofer, S.J.; Kroemer, G. Caloric restriction mimetics against age-associated disease: Targets, mechanisms, and therapeutic potential. Cell Metab. 2019, 29, 592–610. [Google Scholar] [CrossRef]
- Weissman, J.; Guthrie, C.; Fink, G.R. Guide to Yeast Genetics: Functional Genomics, Proteomics, and Other Systems Analysis; Academic Press: Burlington, NJ, USA, 2010; ISBN 9780123751713. [Google Scholar]
- Botstein, D.; Fink, G.R. Yeast: An experimental organism for 21st Century biology. Genetics 2011, 189, 695–704. [Google Scholar] [CrossRef] [PubMed]
- Feldmann, H. (Ed.) Yeast: Molecular and Cell Biology; Wiley-Blackwell: Weinheim, Germany, 2012; ISBN 978-3-527-33252-6. [Google Scholar]
- Leonov, A.; Feldman, R.; Piano, A.; Arlia-Ciommo, A.; Lutchman, V.; Ahmadi, M.; Elsaser, S.; Fakim, H.; Heshmati-Moghaddam, M.; Hussain, A.; et al. Caloric restriction extends yeast chronological lifespan via a mechanism linking cellular aging to cell cycle regulation, maintenance of a quiescent state, entry into a non-quiescent state and survival in the non-quiescent state. Oncotarget 2017, 8, 69328–69350. [Google Scholar] [CrossRef] [PubMed]
- Mohammad, K.; Titorenko, V.I. Yeast chronological aging is linked to cell cycle regulation. Cell Cycle 2018, 17, 1035–1036. [Google Scholar] [CrossRef] [PubMed]
- Chughtai, Z.S.; Rassadi, R.; Matusiewicz, N.; Stochaj, U. Starvation promotes nuclear accumulation of the hsp70 Ssa4p in yeast cells. J. Biol. Chem. 2001, 276, 20261–20266. [Google Scholar] [CrossRef] [PubMed]
- Narayanaswamy, R.; Levy, M.; Tsechansky, M.; Stovall, G.M.; O’Connell, J.D.; Mirrielees, J.; Ellington, A.D.; Marcotte, E.M. Widespread reorganization of metabolic enzymes into reversible assemblies upon nutrient starvation. Proc. Natl. Acad. Sci. USA 2009, 106, 10147–10152. [Google Scholar] [CrossRef]
- Noree, C.; Sato, B.K.; Broyer, R.M.; Wilhelm, J.E. Identification of novel filament-forming proteins in Saccharomyces cerevisiae and Drosophila melanogaster. J. Cell Biol. 2010, 190, 541–551. [Google Scholar] [CrossRef] [PubMed]
- Tapia, H.; Morano, K.A. Hsp90 nuclear accumulation in quiescence is linked to chaperone function and spore development in yeast. Mol. Biol. Cell 2010, 21, 63–72. [Google Scholar] [CrossRef]
- Liu, I.C.; Chiu, S.W.; Lee, H.Y.; Leu, J.Y. The histone deacetylase Hos2 forms an Hsp42-dependent cytoplasmic granule in quiescent yeast cells. Mol. Biol. Cell 2012, 23, 1231–1242. [Google Scholar] [CrossRef]
- Noree, C.; Monfort, E.; Shiau, A.K.; Wilhelm, J.E. Common regulatory control of CTP synthase enzyme activity and filament formation. Mol. Biol. Cell 2014, 25, 2282–2290. [Google Scholar] [CrossRef]
- Shah, K.H.; Nostramo, R.; Zhang, B.; Varia, S.N.; Klett, B.M.; Herman, P.K. Protein kinases are associated with multiple, distinct cytoplasmic granules in quiescent yeast cells. Genetics 2014, 198, 1495–1512. [Google Scholar] [CrossRef]
- Taddei, A.; Schober, H.; Gasser, S.M. The budding yeast nucleus. Cold Spring Harb. Perspect. Biol. 2010, 2, a000612. [Google Scholar] [CrossRef]
- Laporte, D.; Courtout, F.; Salin, B.; Ceschin, J.; Sagot, I. An array of nuclear microtubules reorganizes the budding yeast nucleus during quiescence. J. Cell Biol. 2013, 203, 585–594. [Google Scholar] [CrossRef]
- Laporte, D.; Sagot, I. Microtubules move the nucleus to quiescence. Nucl. Austin Tex. 2014, 5, 113–118. [Google Scholar] [CrossRef]
- Guidi, M.; Ruault, M.; Marbouty, M.; Loïodice, I.; Cournac, A.; Billaudeau, C.; Hocher, A.; Mozziconacci, J.; Koszul, R.; Taddei, A. Spatial reorganization of telomeres in long-lived quiescent cells. Genome Biol. 2015, 16, 206. [Google Scholar] [CrossRef]
- McKnight, J.N.; Boerma, J.W.; Breeden, L.L.; Tsukiyama, T. Global promoter targeting of a conserved lysine deacetylase for transcriptional shutoff during quiescence entry. Mol. Cell 2015, 59, 732–743. [Google Scholar] [CrossRef] [PubMed]
- Rutledge, M.T.; Russo, M.; Belton, J.M.; Dekker, J.; Broach, J.R. The yeast genome undergoes significant topological reorganization in quiescence. Nucleic Acids Res. 2015, 43, 8299–8313. [Google Scholar] [CrossRef]
- Laporte, D.; Courtout, F.; Tollis, S.; Sagot, I. Quiescent Saccharomyces cerevisiae forms telomere hyperclusters at the nuclear membrane vicinity through a multifaceted mechanism involving Esc1, the Sir complex, and chromatin condensation. Mol. Biol. Cell 2016, 27, 1875–1884. [Google Scholar] [CrossRef]
- Miles, S.; Breeden, L. A common strategy for initiating the transition from proliferation to quiescence. Curr. Genet. 2017, 63, 179–186. [Google Scholar] [CrossRef]
- Roche, B.; Arcangioli, B.; Martienssen, R. Transcriptional reprogramming in cellular quiescence. RNA Biol. 2017, 14, 843–853. [Google Scholar] [CrossRef]
- Ramachandran, V.; Shah, K.H.; Herman, P.K. The cAMP-dependent protein kinase signaling pathway is a key regulator of P body foci formation. Mol. Cell 2011, 43, 973–981. [Google Scholar] [CrossRef]
- Shah, K.H.; Zhang, B.; Ramachandran, V.; Herman, P.K. Processing body and stress granule assembly occur by independent and differentially regulated pathways in Saccharomyces cerevisiae. Genetics 2013, 193, 109–123. [Google Scholar] [CrossRef]
- Riback, J.A.; Katanski, C.D.; Kear-Scott, J.L.; Pilipenko, E.V.; Rojek, A.E.; Sosnick, T.R.; Drummond, D.A. Stress-triggered phase separation is an adaptive, evolutionarily tuned response. Cell 2017, 168, 1028–1040. [Google Scholar] [CrossRef] [PubMed]
- Peters, L.Z.; Karmon, O.; Miodownik, S.; Ben-Aroya, S. Proteasome storage granules are transiently associated with the insoluble protein deposit in Saccharomyces cerevisiae. J. Cell Sci. 2016, 129, 1190–1197. [Google Scholar] [CrossRef] [PubMed]
- Laporte, D.; Salin, B.; Daignan-Fornier, B.; Sagot, I. Reversible cytoplasmic localization of the proteasome in quiescent yeast cells. J. Cell Biol. 2008, 181, 737–745. [Google Scholar] [CrossRef] [PubMed]
- Weberruss, M.H.; Savulescu, A.F.; Jando, J.; Bissinger, T.; Harel, A.; Glickman, M.H.; Enenkel, C. Blm10 facilitates nuclear import of proteasome core particles. EMBO J. 2013, 32, 2697–2707. [Google Scholar] [CrossRef]
- Gu, Z.C.; Wu, E.; Sailer, C.; Jando, J.; Styles, E.; Eisenkolb, I.; Kuschel, M.; Bitschar, K.; Wang, X.; Huang, L.; et al. Ubiquitin orchestrates proteasome dynamics between proliferation and quiescence in yeast. Mol. Biol. Cell 2017, 28, 2479–2491. [Google Scholar] [CrossRef]
- Marshall, R.S.; Vierstra, R.D. Proteasome storage granules protect proteasomes from autophagic degradation upon carbon starvation. eLife 2018, 7, e34532. [Google Scholar] [CrossRef]
- Sagot, I.; Pinson, B.; Salin, B.; Daignan-Fornier, B. Actin bodies in yeast quiescent cells: An immediately available actin reserve? Mol. Biol. Cell 2006, 17, 4645–4655. [Google Scholar] [CrossRef]
- Laporte, D.; Gouleme, L.; Jimenez, L.; Khemiri, I.; Sagot, I. Mitochondria reorganization upon proliferation arrest predicts individual yeast cell fate. eLife 2018, 7, e35685. [Google Scholar] [CrossRef]
- Hartwell, L.H.; Unger, M.W. Unequal division in Saccharomyces cerevisiae and its implications for the control of cell division. J. Cell Biol. 1977, 75, 422–435. [Google Scholar] [CrossRef]
- Powell, C.D.; Quain, D.E.; Smart, K.A. Chitin scar breaks in aged Saccharomyces cerevisiae. Microbiology 2003, 149, 3129–3137. [Google Scholar] [CrossRef]
- Cabib, E.; Arroyo, J. How carbohydrates sculpt cells: Chemical control of morphogenesis in the yeast cell wall. Nat. Rev. Microbiol. 2013, 11, 648–655. [Google Scholar] [CrossRef]
- Barton, A.A. Some aspects of cell division in Saccharomyces cerevisiae. J. Gen. Microbiol. 1950, 4, 84–86. [Google Scholar] [CrossRef] [PubMed]
- Egilmez, N.K.; Chen, J.B.; Jazwinski, S.M. Preparation and partial characterization of old yeast cells. J. Gerontol. 1990, 45, B9–B17. [Google Scholar] [CrossRef] [PubMed]
- Sinclair, D.; Mills, K.; Guarente, L. Aging in Saccharomyces cerevisiae. Annu. Rev. Microbiol. 1998, 52, 533–560. [Google Scholar] [CrossRef] [PubMed]
- François, J.; Parrou, J.L. Reserve carbohydrates metabolism in the yeast Saccharomyces cerevisiae. FEMS Microbiol. Rev. 2001, 25, 125–145. [Google Scholar] [CrossRef]
- Singer, M.A.; Lindquist, S. Multiple effects of trehalose on protein folding in vitro and in vivo. Mol. Cell 1998, 1, 639–648. [Google Scholar] [CrossRef]
- Singer, M.A.; Lindquist, S. Thermotolerance in Saccharomyces cerevisiae: The Yin and Yang of trehalose. Trends Biotechnol. 1998, 16, 460–468. [Google Scholar] [CrossRef]
- Benaroudj, N.; Lee, D.H.; Goldberg, A.L. Trehalose accumulation during cellular stress protects cells and cellular proteins from damage by oxygen radicals. J. Biol. Chem. 2001, 276, 24261–24267. [Google Scholar] [CrossRef]
- Gancedo, C.; Flores, C.L. The importance of a functional trehalose biosynthetic pathway for the life of yeasts and fungi. FEMS Yeast Res. 2004, 4, 351–359. [Google Scholar] [CrossRef]
- Shi, L.; Sutter, B.M.; Ye, X.; Tu, B.P. Trehalose is a key determinant of the quiescent metabolic state that fuels cell cycle progression upon return to growth. Mol. Biol. Cell 2010, 21, 1982–1990. [Google Scholar] [CrossRef]
- Trevisol, E.T.; Panek, A.D.; Mannarino, S.C.; Eleutherio, E.C. The effect of trehalose on the fermentation performance of aged cells of Saccharomyces cerevisiae. Appl. Microbiol. Biotechnol. 2011, 90, 697–704. [Google Scholar] [CrossRef] [PubMed]
- Kyryakov, P.; Beach, A.; Richard, V.R.; Burstein, M.T.; Leonov, A.; Levy, S.; Titorenko, V.I. Caloric restriction extends yeast chronological lifespan by altering a pattern of age-related changes in trehalose concentration. Front. Physiol. 2012, 3, 256. [Google Scholar] [CrossRef] [PubMed]
- Eleutherio, E.; Panek, A.; De Mesquita, J.F.; Trevisol, E.; Magalhães, R. Revisiting yeast trehalose metabolism. Curr. Genet. 2015, 61, 263–274. [Google Scholar] [CrossRef]
- Babazadeh, R.; Lahtvee, P.J.; Adiels, C.B.; Goksör, M.; Nielsen, J.B.; Hohmann, S. The yeast osmostress response is carbon source dependent. Sci. Rep. 2017, 7, 990. [Google Scholar] [CrossRef] [PubMed]
- Mitrofanova, D.; Dakik, P.; McAuley, M.; Medkour, Y.; Mohammad, K.; Titorenko, V.I. Lipid metabolism and transport define the longevity of the yeast Saccharomyces cerevisiae. Front. Biosci. (Landmark Ed.) 2018, 23, 1166–1194. [Google Scholar] [PubMed]
- Walther, T.C.; Farese, R.V., Jr. Lipid droplets and cellular lipid metabolism. Annu. Rev. Biochem. 2012, 81, 687–714. [Google Scholar] [CrossRef]
- Kohlwein, S.D.; Veenhuis, M.; van der Klei, I.J. Lipid droplets and peroxisomes: Key players in cellular lipid homeostasis or a matter of fat—Store ‘em up or burn ‘em down. Genetics 2013, 193, 1–50. [Google Scholar] [CrossRef]
- Pol, A.; Gross, S.P.; Parton, R.G. Review: Biogenesis of the multifunctional lipid droplet: Lipids, proteins, and sites. J. Cell Biol. 2014, 204, 635–646. [Google Scholar] [CrossRef]
- Gao, Q.; Goodman, J.M. The lipid droplet—A well-connected organelle. Front. Cell. Dev. Biol. 2015, 3, 49. [Google Scholar] [CrossRef]
- Walther, T.C.; Chung, J.; Farese, R.V., Jr. Lipid droplet biogenesis. Annu. Rev. Cell. Dev. Biol. 2017, 33, 491–510. [Google Scholar] [CrossRef]
- Jackson, C.L. Lipid droplet biogenesis. Curr. Opin. Cell Biol. 2019, 59, 88–96. [Google Scholar] [CrossRef]
- Horvath, S.E.; Daum, G. Lipids of mitochondria. Prog. Lipid Res. 2013, 52, 590–614. [Google Scholar] [CrossRef]
- Baile, M.G.; Lu, Y.W.; Claypool, S.M. The topology and regulation of cardiolipin biosynthesis and remodeling in yeast. Chem. Phys. Lipids 2014, 179, 25–31. [Google Scholar] [CrossRef][Green Version]
- Mårtensson, C.U.; Doan, K.N.; Becker, T. Effects of lipids on mitochondrial functions. Biochim. Biophys. Acta 2017, 1862, 102–113. [Google Scholar] [CrossRef]
- Schlame, M.; Greenberg, M.L. Biosynthesis, remodeling and turnover of mitochondrial cardiolipin. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2017, 1862, 3–7. [Google Scholar] [CrossRef]
- Tatsuta, T.; Langer, T. Intramitochondrial phospholipid trafficking. Biochim. Biophys. Acta 2017, 1862, 81–89. [Google Scholar] [CrossRef]
- Bonawitz, N.D.; Chatenay-Lapointe, M.; Pan, Y.; Shadel, G.S. Reduced TOR signaling extends chronological life span via increased respiration and upregulation of mitochondrial gene expression. Cell Metab. 2007, 5, 265–277. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Shadel, G.S. Extension of chronological life span by reduced TOR signaling requires down-regulation of Sch9p and involves increased mitochondrial OXPHOS complex density. Aging (Albany N. Y.) 2009, 1, 131–145. [Google Scholar] [CrossRef] [PubMed]
- Breitenbach, M.; Laun, P.; Dickinson, J.R.; Klocker, A.; Rinnerthaler, M.; Dawes, I.W.; Aung-Htut, M.T.; Breitenbach-Koller, L.; Caballero, A.; Nyström, T.; et al. The role of mitochondria in the aging processes of yeast. Subcell. Biochem. 2012, 57, 55–78. [Google Scholar]
- Ocampo, A.; Liu, J.; Schroeder, E.A.; Shadel, G.S.; Barrientos, A. Mitochondrial respiratory thresholds regulate yeast chronological life span and its extension by caloric restriction. Cell Metab. 2012, 16, 55–67. [Google Scholar] [CrossRef]
- Leonov, A.; Titorenko, V.I. A network of interorganellar communications underlies cellular aging. IUBMB Life 2013, 65, 665–674. [Google Scholar] [CrossRef] [PubMed]
- Beach, A.; Leonov, A.; Arlia-Ciommo, A.; Svistkova, V.; Lutchman, V.; Titorenko, V.I. Mechanisms by which different functional states of mitochondria define yeast longevity. Int. J. Mol. Sci. 2015, 16, 5528–5554. [Google Scholar] [CrossRef]
- Ruetenik, A.; Barrientos, A. Dietary restriction, mitochondrial function and aging: From yeast to humans. Biochim. Biophys. Acta 2015, 1847, 1434–1447. [Google Scholar] [CrossRef]
- Giorgio, M.; Trinei, M.; Migliaccio, E.; Pelicci, P.G. Hydrogen peroxide: A metabolic by-product or a common mediator of ageing signals? Nat. Rev. Mol. Cell Biol. 2007, 8, 722–728. [Google Scholar] [CrossRef] [PubMed]
- Dakik, P.; Medkour, Y.; Mohammad, K.; Titorenko, V.I. Mechanisms through which some mitochondria-generated metabolites act as second messengers that are essential contributors to the aging process in eukaryotes across phyla. Front. Physiol. 2019, 10, 461. [Google Scholar] [CrossRef]
- Pan, Y. Mitochondria, reactive oxygen species, and chronological aging: A message from yeast. Exp. Gerontol. 2011, 46, 847–852. [Google Scholar] [CrossRef]
- Pan, Y.; Schroeder, E.A.; Ocampo, A.; Barrientos, A.; Shadel, G.S. Regulation of yeast chronological life span by TORC1 via adaptive mitochondrial ROS signaling. Cell Metab. 2011, 13, 668–678. [Google Scholar] [CrossRef]
- Shadel, G.S. Live longer on MARS: A yeast paradigm of mitochondrial adaptive ROS signaling in aging. Microb. Cell 2014, 1, 140–144. [Google Scholar] [CrossRef]
- Guaragnella, N.; Coyne, L.P.; Chen, X.J.; Giannattasio, S. Mitochondria-cytosol-nucleus crosstalk: Learning from Saccharomyces cerevisiae. FEMS Yeast Res. 2018, 18, foy088. [Google Scholar] [CrossRef]
- Ruetenik, A.; Barrientos, A. Exploiting Post-Mitotic Yeast Cultures to Model Neurodegeneration. Front. Mol. Neurosci. 2018, 11, 400. [Google Scholar] [CrossRef]
- Gladyshev, V.N. The origin of aging: Imperfectness-driven non-random damage defines the aging process and control of lifespan. Trends Genet. 2013, 29, 506–512. [Google Scholar] [CrossRef]
- Ogrodnik, M.; Salmonowicz, H.; Gladyshev, V.N. Integrating cellular senescence with the concept of damage accumulation in aging: Relevance for clearance of senescent cells. Aging Cell 2019, 18, e12841. [Google Scholar] [CrossRef]
- Calabrese, V.; Cornelius, C.; Cuzzocrea, S.; Iavicoli, I.; Rizzarelli, E.; Calabrese, E.J. Hormesis, cellular stress response and vitagenes as critical determinants in aging and longevity. Mol. Asp. Med. 2011, 32, 279–304. [Google Scholar] [CrossRef]
- Calabrese, V.; Cornelius, C.; Dinkova-Kostova, A.T.; Iavicoli, I.; Di Paola, R.; Koverech, A.; Cuzzocrea, S.; Rizzarelli, E.; Calabrese, E.J. Cellular stress responses, hormetic phytochemicals and vitagenes in aging and longevity. Biochim. Biophys. Acta 2012, 1822, 753–783. [Google Scholar] [CrossRef]
- Gladyshev, V.N. The free radical theory of aging is dead. Long live the damage theory! Antioxid. Redox Signal. 2014, 20, 727–731. [Google Scholar] [CrossRef]
- Dakik, P.; Titorenko, V.I. Communications between Mitochondria, the Nucleus, Vacuoles, Peroxisomes, the Endoplasmic Reticulum, the Plasma Membrane, Lipid Droplets, and the Cytosol during Yeast Chronological Aging. Front. Genet. 2016, 7, 177. [Google Scholar] [CrossRef]
- Gomez-Perez, A.; Kyryakov, P.; Burstein, M.T.; Asbah, N.; Noohi, F.; Iouk, T.; Titorenko, V.I. Empirical validation of a hypothesis of the hormetic selective forces driving the evolution of longevity regulation mechanisms. Front. Genet. 2016, 7, 216. [Google Scholar] [CrossRef]
- Herker, E.; Jungwirth, H.; Lehmann, K.A.; Maldener, C.; Fröhlich, K.U.; Wissing, S.; Büttner, S.; Fehr, M.; Sigrist, S.; Madeo, F. Chronological aging leads to apoptosis in yeast. J. Cell Biol. 2004, 164, 501–507. [Google Scholar] [CrossRef] [PubMed]
- Büttner, S.; Eisenberg, T.; Herker, E.; Carmona-Gutierrez, D.; Kroemer, G.; Madeo, F. Why yeast cells can undergo apoptosis: Death in times of peace, love, and war. J. Cell Biol. 2006, 175, 521–525. [Google Scholar] [CrossRef]
- Fabrizio, P.; Longo, V.D. Chronological aging-induced apoptosis in yeast. Biochim. Biophys. Acta 2008, 1783, 1280–1285. [Google Scholar] [CrossRef]
- Eisenberg, T.; Knauer, H.; Schauer, A.; Büttner, S.; Ruckenstuhl, C.; Carmona-Gutierrez, D.; Ring, J.; Schroeder, S.; Magnes, C.; Antonacci, L.; et al. Induction of autophagy by spermidine promotes longevity. Nat. Cell Biol. 2009, 11, 1305–1314. [Google Scholar] [CrossRef]
- Carmona-Gutierrez, D.; Eisenberg, T.; Büttner, S.; Meisinger, C.; Kroemer, G.; Madeo, F. Apoptosis in yeast: Triggers, pathways, subroutines. Cell Death Differ. 2010, 17, 763–773. [Google Scholar] [CrossRef]
- Eisenberg, T.; Carmona-Gutierrez, D.; Büttner, S.; Tavernarakis, N.; Madeo, F. Necrosis in yeast. Apoptosis 2010, 15, 257–268. [Google Scholar] [CrossRef]
- Laun, P.; Büttner, S.; Rinnerthaler, M.; Burhans, W.C.; Breitenbach, M. Yeast aging and apoptosis. Subcell. Biochem. 2012, 57, 207–232. [Google Scholar]
- Eisenberg, T.; Büttner, S. Lipids and cell death in yeast. FEMS Yeast Res. 2014, 14, 179–197. [Google Scholar] [CrossRef]
- Richard, V.R.; Beach, A.; Piano, A.; Leonov, A.; Feldman, R.; Burstein, M.T.; Kyryakov, P.; Gomez-Perez, A.; Arlia-Ciommo, A.; Baptista, S.; et al. Mechanism of liponecrosis, a distinct mode of programmed cell death. Cell Cycle 2014, 13, 3707–3726. [Google Scholar] [CrossRef]
- Sheibani, S.; Richard, V.R.; Beach, A.; Leonov, A.; Feldman, R.; Mattie, S.; Khelghatybana, L.; Piano, A.; Greenwood, M.; Vali, H.; et al. Macromitophagy, neutral lipids synthesis and peroxisomal fatty acid oxidation protect yeast from “liponecrosis,” a previously unknown form of programmed cell death. Cell Cycle 2014, 13, 138–147. [Google Scholar] [CrossRef]
- Arlia-Ciommo, A.; Svistkova, V.; Mohtashami, S.; Titorenko, V.I. A novel approach to the discovery of anti-tumor pharmaceuticals: Searching for activators of liponecrosis. Oncotarget 2016, 7, 5204–5225. [Google Scholar] [CrossRef]
- Falcone, C.; Mazzoni, C. External and internal triggers of cell death in yeast. Cell. Mol. Life Sci. 2016, 73, 2237–2250. [Google Scholar] [CrossRef]
- Fannjiang, Y.; Cheng, W.C.; Lee, S.J.; Qi, B.; Pevsner, J.; McCaffery, J.M.; Hill, R.B.; Basañez, G.; Hardwick, J.M. Mitochondrial fission proteins regulate programmed cell death in yeast. Genes Dev. 2004, 18, 2785–2797. [Google Scholar] [CrossRef]
- Pereira, C.; Silva, R.D.; Saraiva, L.; Johansson, B.; Sousa, M.J.; Côrte-Real, M. Mitochondria-dependent apoptosis in yeast. Biochim. Biophys. Acta 2008, 1783, 1286–1302. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohammad, K.; Baratang Junio, J.A.; Tafakori, T.; Orfanos, E.; Titorenko, V.I. Mechanisms that Link Chronological Aging to Cellular Quiescence in Budding Yeast. Int. J. Mol. Sci. 2020, 21, 4717. https://doi.org/10.3390/ijms21134717
Mohammad K, Baratang Junio JA, Tafakori T, Orfanos E, Titorenko VI. Mechanisms that Link Chronological Aging to Cellular Quiescence in Budding Yeast. International Journal of Molecular Sciences. 2020; 21(13):4717. https://doi.org/10.3390/ijms21134717
Chicago/Turabian StyleMohammad, Karamat, Jennifer Anne Baratang Junio, Tala Tafakori, Emmanuel Orfanos, and Vladimir I. Titorenko. 2020. "Mechanisms that Link Chronological Aging to Cellular Quiescence in Budding Yeast" International Journal of Molecular Sciences 21, no. 13: 4717. https://doi.org/10.3390/ijms21134717
APA StyleMohammad, K., Baratang Junio, J. A., Tafakori, T., Orfanos, E., & Titorenko, V. I. (2020). Mechanisms that Link Chronological Aging to Cellular Quiescence in Budding Yeast. International Journal of Molecular Sciences, 21(13), 4717. https://doi.org/10.3390/ijms21134717