Abstract
The assembly mechanism for aggregation of amyloid fibril is important and fundamental for any quantitative and physical descriptions because it needs to have a deep understanding of both molecular and statistical physics. A theoretical model with three states including coil, helix and sheet is presented to describe the amyloid formation. The corresponding general mathematical expression of N molecule systems are derived, including the partition function and thermodynamic quantities. We study the equilibrium properties of the system in the solution and find that three molecules have the extreme value of free energy. The denaturant effect on molecular assemble is also discussed. Furthermore, we apply the kinetic theories to take account of the nucleation and growth of the amyloid in the solution. It has been shown that our theoretical results can be compared with experimental results.
1. Introduction
The aggregation of amyloid fibrils in biological processes is associated with neurodegenerative diseases such as Alzheimer’s, Parkinson’s and Huntington’s or prion diseases [1,2,3]. Despite the specificity of the proteins related with each individual neurodegenerative disease, these kinds of diseases are a nucleation process [4,5,6,7,8,9]. Under experimental conditions in vitro, the aggregation pathway can be obtained. However, it is not clear how to extrapolate these results to identify the dominant pathway and timescales under physiological conditions.
The current computation technique is unable to access even the accelerated timescales of the in vitro systems. Some computational techniques could be used to predict the assembly of amyloid in solution and their secondary structure changes [10,11]. However, these computational simulations are only feasible for millisecond time scale. The most simplistic physical description of proteins is analogy with colloidal particles. The random-coil like proteins exist in an unfolded state and the helix is very similar to folded state. These simple models have been used to explore the nucleation processes of amyloid fibrils. There are two classes of nucleation theories, one is the mass action theories, the other is nucleation models [12,13,14,15,16,17,18,19,20,21]. However, these methods missed the internal dynamics of the protein molecules. In this paper, a microscopic model of the assembly process is developed to explore the mechanism of amyloid fibrils and explain the transitions between the various assembly pathways as well as how side chain interactions determine the sheet structure in the aggregate phase. Based on the microscopic model, molecular equilibrium states and kinetic equations have been constructed to probe the physical properties of amyloid formation. The paper is organized as follow. Firstly, a single peptide molecule in the solution can be transited from a coil state to a helix state. Then two molecules will be formed by concentration in the solution that supplies a driving force. The combining force is resulted from the hydrogen bond. Based on the structure of two molecules, three molecules can be constructed with the aid of solution concentration. Furthermore, N molecule systems are able to be constructed, which is called -sheet. The corresponding general mathematical description of N molecule systems are derived, including the partition function and thermodynamic quantities. Then we study the equilibrium properties of the system in the solution. The phase diagrams of assembly structures are depicted. Furthermore, we employ the kinetic theories to study the amyloid formation. Free energy landscape and side chain effect can be illustrated. The theoretical predictions are in agreement with the experimental results.
2. Results
2.1. Single Molecule
The conformations in single molecule have sequences of helix and coil units [22]. The coil state is an ensemble of disordered conformations which often exists for higher temperature, otherwise, the helix state exists for lower temperature [23]. Therefore, the temperature determines the transition between coil and helix. The experimental temperature ranges from 10 to 60 °C [24]. This structural transition is a cooperative behavior [25,26].
In order to provide a valuable intuition into the nucleation process, we use a similar lattice model and suppose a single molecular chain has N units, each unit along the chain can be in either of the two states, H (helix) or C (coil). According to the ZB model [27,28], only the nearest neighbor interaction is considered, which is a modified one-dimensional Ising model. Therefore, there are four statistical weights, their matrix elements are . A propagation parameter s can be defined by the free energy change,
where s is slightly larger than 1, so the matrix element . The other parameter is as a nucleation parameter. In general, and takes from to , so is for helix next to coil, i.e., . On the other hand, one uses the statistical weight 1 for any coil, and . Then a matrix form of statistical weight can be defined as
Furthermore, the partition function can be obtained by summing over all the possible sequences in the one-dimensional chain with N monomers,
Let T be a transformation matrix to get a diagonalize matrix ,
where are the eigenvalues of M.
By employing , the partition function can be rewritten as
Because of , we have for large N, then
Based on , the average number of helix states can be computed by
and the fraction of helix states is defined as
The free energy for the single molecule can approximately be expressed as
We plot as a function of s for different as shown in Figure 1. There are two limitation cases for -value. For small , a sudden transition can occur for a narrow range of s, this is a helix-coil structural transition. For large , there is a non-cooperative behavior because is equal to for .
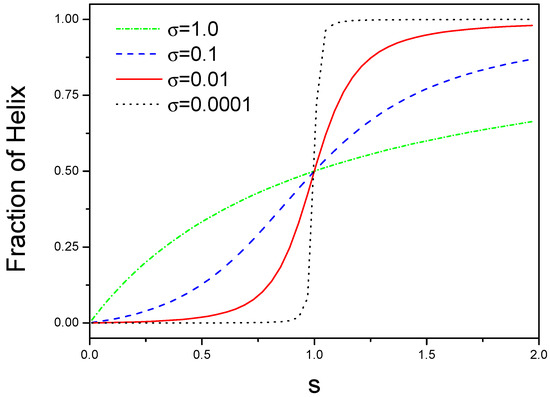
Figure 1.
Phase diagrams of structural transition from coil to helix for different interactions. The number of helical state in the block is plotted against s, where s is an equilibrium constant for a coil state converting into a helical state.
2.2. Two Molecules
There are two cases for two molecules, one is no interaction between two molecules, and the other case is the interaction between two molecules. The partition function can be written as
where is the degeneracy factor,
which represents that hydrogen bonds in the first molecular chain and hydrogen bonds in the second molecular chain. The other factor in the above is
where is the interaction parameter between the first molecule and the second molecule, which results from the hydrogen bond, and are the total number of hydrogen bond of molecule 1 and molecules 2 respectively. , , and are the partition functions for each segment as shown in Figure 2.
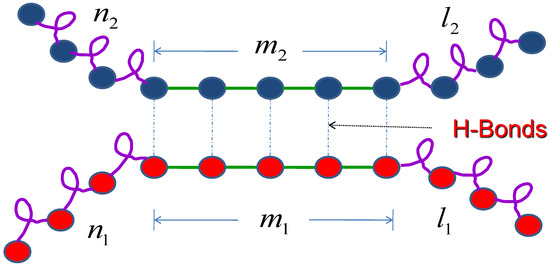
Figure 2.
Schematic illustration of the monomer attachment. Two molecules through intermolecular hydrogen bond form a simplistic helix-coil-sheet model. and are the total number of hydrogen bonds for molecule 1 and molecule 2 respectively.
By defining the parameters and to demonstrate the ratio of non-interaction,
then the partition function of the two molecules can be rewritten as
Actually, we are able to discuss how chain length influences the partition function. According to the derivation for the single molecule, the partition function can be simplified as
then is
Due to the same reason, is
thus we have
In terms of , we obtain
Therefore, the partition function of two molecules can be rewritten as
where M is the total number of the hydrogen bond, its maximum value is .
A function can be defined as
where . The other function can be defined as
The free energy of two molecules is obtained by
2.3. Three Molecules
Now we add the third molecule to the dimer to form a trimer. The partition function for this system can be represented as
where the degeneracy factor for three molecules
and
where and are the partition function for the segments of and respectively.
Furthermore, we can define . In a similar manner, , we have
We define a new function
where . Then the partition function of three molecules can be rewritten as
The free energy of three molecules can be obtained by
2.4. General Expression of n Molecules
Now we extend the expressions of n molecules with -strand, the first expression is the partition function of n molecules,
where the degeneracy factor of n molecules is
and
then function can be defined as
Let us define the function of as
where , and used , so the partition function of n molecules can be rewritten as
The expression of free energy is
As we mentioned, the reference state is the coil state which has a statistical weight of 1 (free energy ). The helix state is favorable and the nucleation parameter is unfavorable . A single -sheet is probably not stable in solution [29,30], so or slightly bigger than 1. However, a -sheet bilayer is more stable than a helix, thus we need to introduce a new parameter, z, to describe side chain interactions, then . The free energy of -sheet includes the conformation entropy (which supplies the repulsive force) and the interaction of hydrogen bond and the side-chain interactions (that is attractive force). Therefore, can be replaced by , the corresponding general expression of physics quantities for fibril structure with -sheet can be written as
where and
3. Discussion
3.1. Molecular Interaction with Denaturant
The influence of denaturant on amyloid fibril is investigated by the interaction parameter . When the denaturant urea is added in the system, the free energy is dependent on the denaturant concentration, and can be expanded as the first order approximation,
where .
The parameters s and are expressed as
where , . is for the helix state (folded state), and is for the coil state (unfolded state).
We make an approximation , the expression of is
When ,
If exp, it is that the strong folded state
If exp, it is that the strong unfolded state
3.2. Free Energy
As shown in Figure 3, is a function of that represents the effect of , and s. Here we choose , , s is from 0 to 2, is from to 1 and is from 0 to 2. When is zero, there is not any interaction between two molecular chains. Thus is chosen as a smaller quantity, the interaction is a repulsive force, otherwise, the attractive force for larger , so the range of is from to 2.
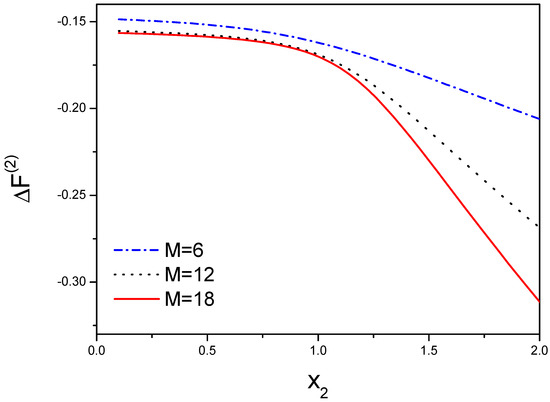
Figure 3.
The free energy is dependent on the interaction between the first molecule and the second molecule. , where the free energy for each bond is that illustrates the loss of conformational entropy from both chains, and indicates the contribution form the peptide tails not participating in the hydrogen bonds. accounts for the free energy difference from both chains. The free energy will be decreased when the number of the hydrogen bond increases from 6 to 18.
In Figure 4, we take and . This figure demonstrates the free energy and the function of are a function of and . Due to , we choose a few of values for that are resulted from the range of from 1 to 3.
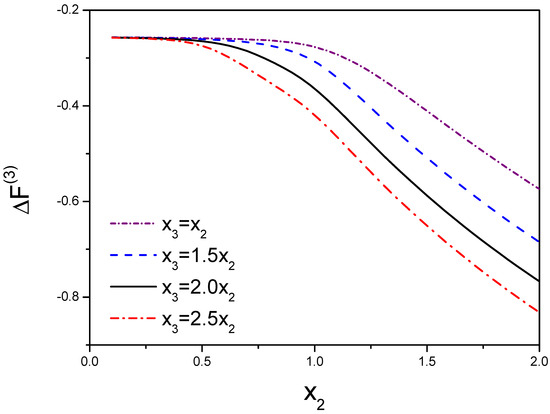
Figure 4.
Variation of three molecules’ free energy as a function of the molecular interactions. denotes the interactions between molecule 1 and molecule 2. is the interaction between the third molecule and dimer. is defined as . The competition between molecules results in the variation of the free energy.
By using the above equations, we can obtain the landscape of free energy. The parameters such as g, , s, even z stand for the different peptide states, and corresponding to , , -strand, -sheet and fibrils. is used to describe the number of hydrogen bond in the n-th chain. With the change of these parameters, the free energy will be a function as n. We have calculated the free energy for .
For counting the side chain effect, the interaction coefficients have been demonstrated in Figure 5,
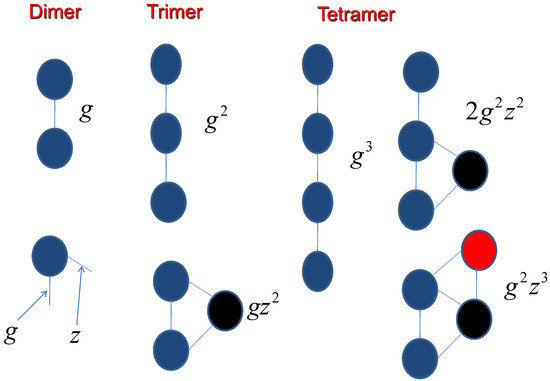
Figure 5.
The assembly process is dependent on the molecular interactions.
The energy landscape can be obtained as shown in Figure 6, where the parameters are , . The nucleation can occur because the condition is satisfied. We find that the free energy has a peak value when n is equal to 3. In other words, three molecules have higher free energy and four molecules have lower free energy. This is an important result about the assemble mechanism for aggregation of amyloid fibril. Based on this result, we develop the kinetic formulas to study the nucleation processes of amyloid fibrils.
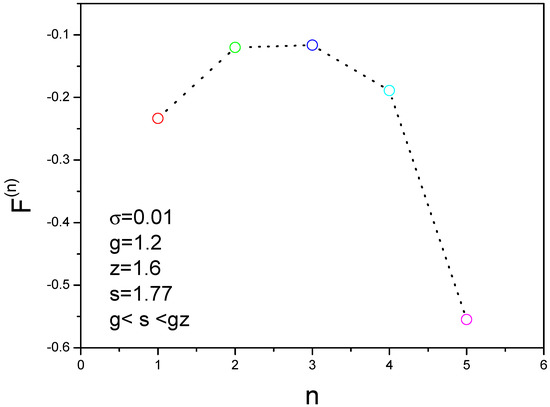
Figure 6.
Free energy landscape for different molecules gives a nonmonotonic function that has a peak at three molecules. is the free energy of an oligomer containing n molecules.
3.3. Peak Location in the 2D Nucleation Rate
A model of three molecules was presented for the dynamics of a nucleating trimer, the nucleation rate in 2D can be written as [31]
The analytic solution of is
where
The peak location is determined by the extremum of the nucleation rate,
then
By using the above equation,
where , , .
where .
Because denominator of is a constant,
where is a numerator of .
can be rewritten as
where , , and
We take the earlier terms including ,
By using , we have
By using , , , we get
The coefficients can be defined as
thus
When , the above expression can be written as
where
The expressions of and are employed by
where .
We will discuss three cases for taking different to calculate the coefficients of polynomial,
Case I:,
then inserting these coefficients and obtain the peak value of x,
which is in agreement with the numerical result [31].
Case II:,
then inserting these coefficients and obtain the peak value of x,
which is in agreement with the numerical result [31].
Case III:,
then inserting these coefficients and obtain the peak value of x,
which is in agreement with the numerical result [31].
It is reasonable to neglect the higher terms from the above data, i.e., and , so the polynomial is changed to
Due to , and consider x is a positive number, so the root of equation is simplified as
The nucleation flux is a nonmonotonic function of the number of the hydrogen bonds in dimer when the third molecule is added to form trimer. The peak values are a significant result of the deviation of the lowest free energy pathway.
3.4. Scaling Behavior of Nucleation Rate in 3D
The system of three molecules is unstable, so we have developed a stable 3D model. The nucleation rate in 3D model can be written as [32]
where
and
can be defined as
Inserting the above equation into , we have
furthermore,
With the help of
then
Based on the numerical result [32], we will discuss .
When , . In this case, the first approximation is
In the condition of
Due to , so the scaling behavior in this case is
This condition matches the numerical result, so the analytic result can be written as
where and is a constant including f and other terms.
In the experimental measurement of the nucleation process [33,34,35], kinetics were monitored by ThT fluorescence. The solution conditions for fibril formation were 100 mM KCl, 50 mM potassium phosphate, PH , 25 °C. Fiber sample were prepared as for transmission electron microscopy. The experimental result is . When we take in the analytic result, the theoretical result is in agreement with the experimental result.
4. Conclusions
In summary, a microscopic model with three states including coil, helix and sheet is constructed to explore the mechanism of amyloid formation. The partition function and thermodynamic quantities of many molecule systems are obtained by considering the repulsive and attractive interactions. The equilibrium properties of the system in the solution have been investigated. Free energy landscape and side chain effect are illustrated. It is found that the system of three molecules has higher free energy. The kinetic properties of molecules related with amyloid formation are also studied. By using the random walk model in 2D and 3D, the nucleation processes of amyloid fibrils are quantitatively demonstrated. The microscopic theoretical model and results can be in agreement with numerical and experimental results. These theoretical approaches of the microscopic model could be used to improve the computational simulations in new timescale.
Conflicts of Interest
The author declares no conflict of interest.
References
- Nelson, R.; Sawaya, M.R.; Balbirnie, M.; Madsen, A.Ø.; Riekel, C.; Grothe, R.; Eisenberg, D. Structure of the cross-β spine of amyloid-like fibrils. Nature 2005, 435, 773–778. [Google Scholar] [CrossRef] [PubMed]
- Knowles, T.P.J.; Waudby, C.A.; Devlin, G.L.; Cohen, S.I.A.; Aguzzi, A.; Vendruscolo, M.; Terentjev, E.M.; Welland, M.E.; Dobson, C.M. An Analytical Solution to the Kinetics of Breakable Filament Assembly. Science 2009, 326, 1533–1537. [Google Scholar] [CrossRef] [PubMed]
- Seeliger, J.; Estel, K.; Erwin, N.; Winter, R. Cosolvent effects on the fibrillation reaction of human IAPP. Phys. Chem. Chem. Phys. 2013, 15, 8902–8907. [Google Scholar] [CrossRef] [PubMed]
- Hardy, J.; Selkoe, D.J. The amyloid hypothesis of Alzheheimer’s disease: Progress and problems on the road to therapeutics. Science 2002, 297, 353–356. [Google Scholar] [CrossRef] [PubMed]
- Canchi, D.R.; Garcia, A.E. Cosolvent Effects on Protein Stability. Annu. Rev. Phys. Chem. 2013, 64, 273–293. [Google Scholar] [CrossRef] [PubMed]
- Miller, Y.; Ma, B.; Nussinov, R. Polymorphism in Alzheimer Aβ amyloid organization reflects conformational selection in a rugged energy landscape. Chem. Rev. 2010, 110, 4820–4838. [Google Scholar] [CrossRef] [PubMed]
- Dill, K.A. Theory for the folding and stability of globular proteins. Biochemistry 1985, 24, 1501–1509. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, K.; Dill, K.A. Theory for Protein Folding Cooperativity: Helix Bundles. J. Am. Chem. Soc. 2009, 131, 2306–2312. [Google Scholar] [CrossRef] [PubMed]
- Dobson, C.M. Protein folding and misfolding. Nature 2003, 426, 884–890. [Google Scholar] [CrossRef] [PubMed]
- Carballo-Pacheo, M.; Strodel, B. Advances in the Simulation of Protein Aggregation at the Atomistic Scale. J. Phys. Chem. B 2016, 120, 2991–2999. [Google Scholar] [CrossRef] [PubMed]
- Cheon, M.; Hall, C.K.; Chang, I. Structural Conversion of A β17C42 Peptides from Disordered Oligomers to U-Shape Protofilaments via Multiple Kinetic Pathways. PLoS Comput. Biol. 2015, 11, e1004258. [Google Scholar] [CrossRef] [PubMed]
- Kashchiev, D.; Auer, S. Nucleation of amyloid fibrils. J. Chem. Phys. 2010, 132, 215101. [Google Scholar] [CrossRef] [PubMed]
- Cabriolu, R.; Auer, S. Amyloid fibrillation kinetics: insight from atomistic nucleation theory. J. Mol. Biol. 2011, 411, 275–285. [Google Scholar] [CrossRef] [PubMed]
- Auer, S.; Meersman, F.; Dobson, C.M.; Vendruscolo, M. A Generic Mechanism of Emergence of Amyloid Protofilaments from Disordered Oligomeric Aggregates. PLoS Comput. Biol. 2008, 4, e1000222. [Google Scholar] [CrossRef] [PubMed]
- Schreck, J.S.; Yuan, J.M. Exactly solvable model for helix-coil-sheet transitions in protein systems. Phys. Rev. E 2010, 81, 061919. [Google Scholar] [CrossRef] [PubMed]
- Schmit, J.D. Kinetic theory of amyloid fibril templating. J. Chem. Phys. 2013, 138, 185102. [Google Scholar] [CrossRef] [PubMed]
- Knowles, T.P.J.; Simone, A.D.; Fitzpatrick, A.W.; Baldwin, A.; Meehan, S.; Rajah, L.; Vendruscolo, M.; Welland, M.E.; Dobson, C.M.; Terentjev, E.M. Twisting transition between crystalline and fibrillar phases of aggregated peptides. Phys. Rev. Lett. 2012, 109, 158101. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.F. Self-assembly of protein amyloids: A competition between amorphous and ordered aggregation. Phys. Rev. E 2009, 80, 031922. [Google Scholar] [CrossRef] [PubMed]
- Bellesia, G.; Shea, J.E. Diversity of kinetic pathways in amyloid fibril formation. J. Chem. Phys. 2009, 131, 111102. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.I.A.; Linse, S.; Luheshi, L.M.; Hellstrand, E.; White, D.A.; Rajah, L.; Otzen, D.E.; Vendruscolo, M.; Dobson, C.M.; Knowles, T.P.J. Proliferation of amyloid-β42 aggregates occurs through a secondary nucleation mechanism. Proc. Natl. Acad. Sci. USA 2013, 110, 9758–9763. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Muthukumar, M.J. Simulations of nucleation and elongation of amyloid fibrils. J. Chem. Phys. 2009, 130, 035102. [Google Scholar] [CrossRef] [PubMed]
- Poland, D.; Scheraga, H. Theory of Helix-Coil Transitions in Biopolymers; Academic Press: New York, NY, USA, 1970. [Google Scholar]
- Dill, K.; Bromberg, S. Molecular Driving Forces; Garland Science: New York, NY, USA, 2002. [Google Scholar]
- Smirnovas, V.; Winter, R.; Funk, T.; Dzwolak, W. Thermodynamic properties underlying the α-helix-to-β-sheet transition, aggregation, and amyloidogenesis of polylysine as probed by calorimetry, densimetry, and ultrasound velocimetry. J. Phys. Chem. B 2005, 109, 19043–19045. [Google Scholar] [CrossRef] [PubMed]
- Lifson, S.; Roig, A. On the Theory of Helix—Coil Transition in Polypeptides. J. Chem. Phys. 1961, 34, 1963–1974. [Google Scholar] [CrossRef]
- Van Gestel, J.; de Leeuw, S.W. A statistical mechanical theory of fibril formation in dilute protein solutions. Biophys. J. 2006, 90, 3134–3145. [Google Scholar] [CrossRef] [PubMed]
- Zimm, B.H.; Bragg, J.K. Theory of the Phase Transition between Helix and Random Coil in Polypeptide Chains. J. Chem. Phys. 1959, 31, 526–535. [Google Scholar] [CrossRef]
- Qian, H.; Schellman, J.A. Helix-coil studies: a comparative study for finite length polypeptides. J. Phys. Chem. 1992, 96, 3987–3994. [Google Scholar] [CrossRef]
- Bitan, G.; Kirkitadze, M.D.; Lomakin, A.; Vollers, S.S.; Benedek, G.B.; Teplow, D.B. Amyloid β-protein (Aβ) assembly: Aβ 40 and Aβ 42 oligomerize through distinct pathways. Proc. Natl. Acad. Sci. USA 2003, 100, 330–335. [Google Scholar] [CrossRef] [PubMed]
- Barghorn, S.; Nimmrich, V.; Striebinger, A.; Krantz, C.; Keller, P.; Janson, B.; Bahr, M.; Schmidt, M.; Bitner, R.; Harlan, J.; et al. Globular amyloid β-peptide oligomer—A homogenous and stable neuropathological protein in Alzheimer’s disease. J. Neurochem. 2005, 95, 834–847. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Schmit, J. Pseudo-one-dimensional nucleation in dilute polymer solutions. Phys. Rev. E 2016, 93, 060401(R). [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Schmit, J. Theory of amyloid fibril nucleation from folded proteins. Isr. J. Chem. 2017, 57, 738–749. [Google Scholar] [CrossRef] [PubMed]
- Ferrone, F.A. Assembly of Aβ Proceeds via Monomeric Nuclei. J. Mol. Biol. 2015, 427, 287–290. [Google Scholar] [CrossRef] [PubMed]
- Knowles, T.P.; White, D.A.; Abate, A.R.; Agresti, J.J.; Cohen, S.I.A.; Sperling, R.A.; De Genst, E.J.; Dobson, C.M.; Weitz, D.A. Observation of spatial propagation of amyloid assembly from single nuclei. Proc. Natl. Acad. Sci. USA 2011, 108, 14746–14751. [Google Scholar] [CrossRef] [PubMed]
- Ruschak, A.M.; Miranker, A.D. Fiber-dependent amyloid formation as catalysis of an existing reaction pathway. Proc. Natl. Acad. Sci. USA 2007, 104, 12341–12346. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).