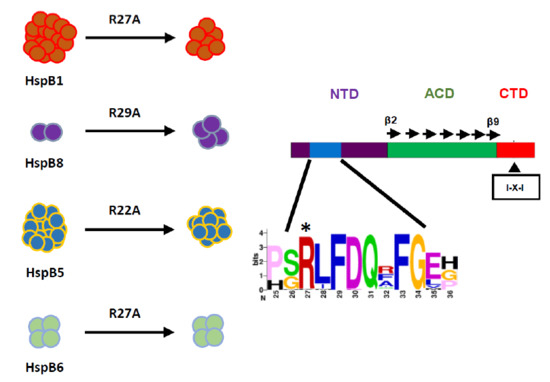
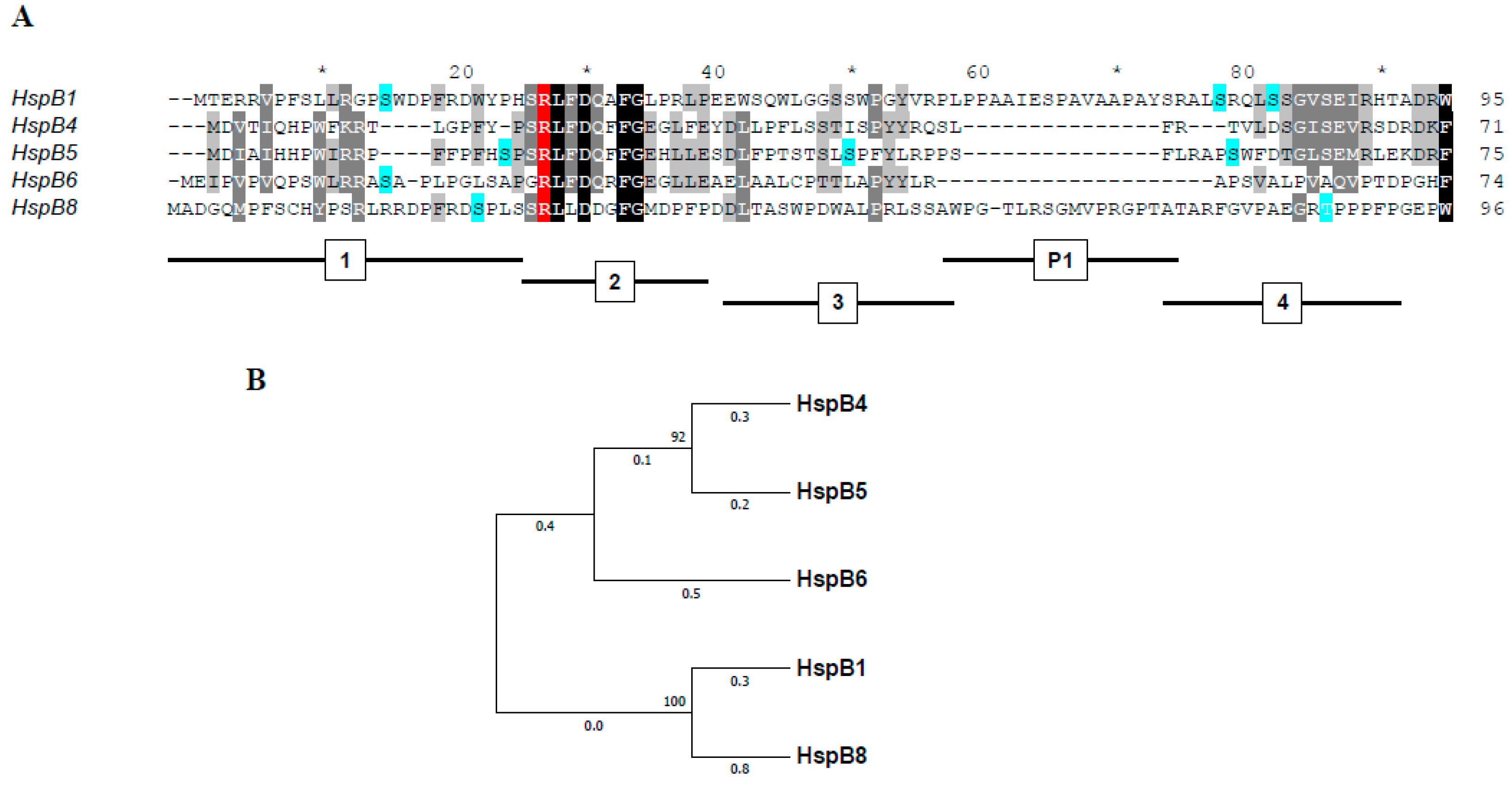
The Role of the Arginine in the Conserved N-Terminal Domain RLFDQxFG Motif of Human Small Heat Shock Proteins HspB1, HspB4, HspB5, HspB6, and HspB8
Abstract
1. Introduction
2. Results
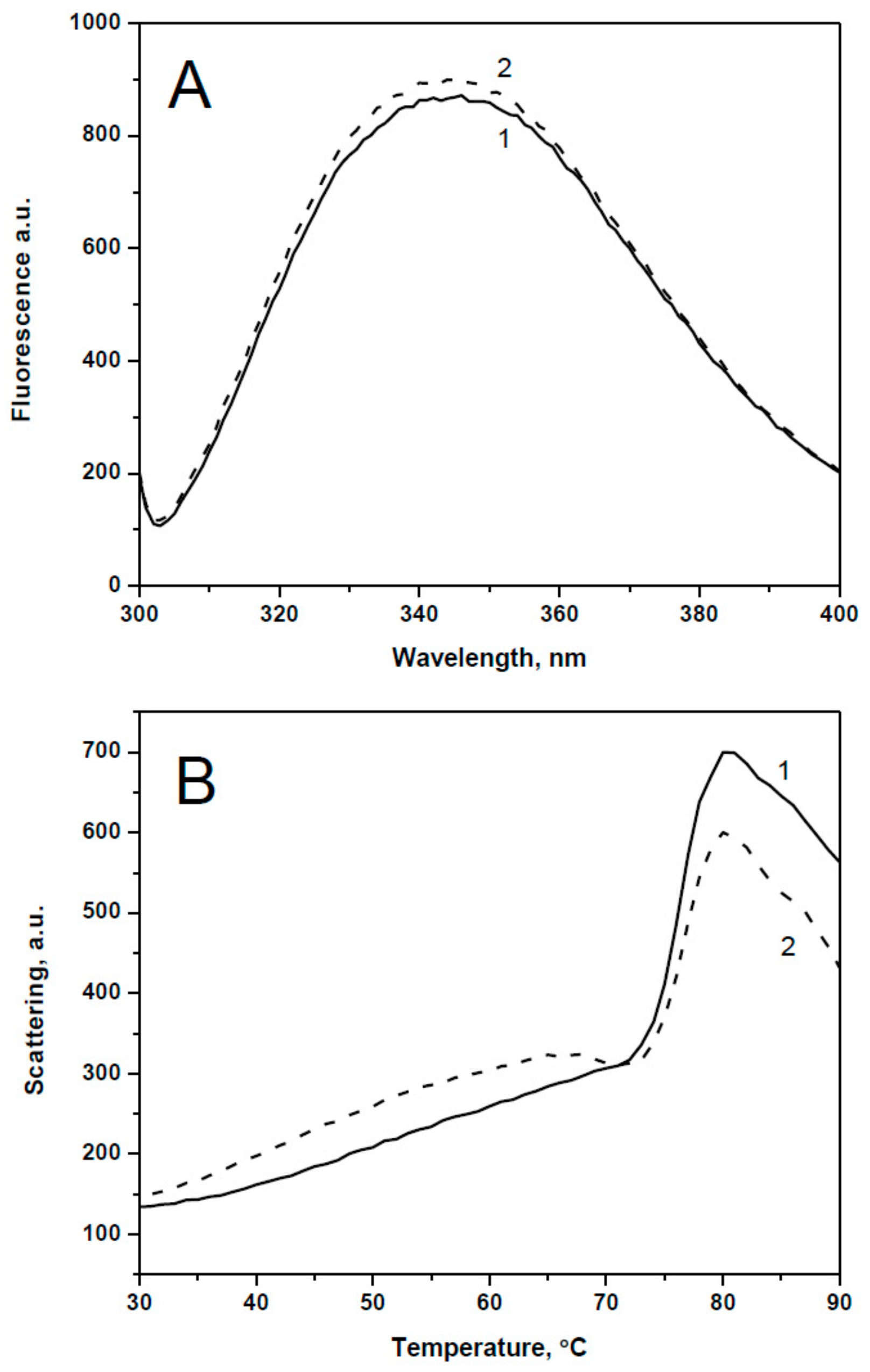
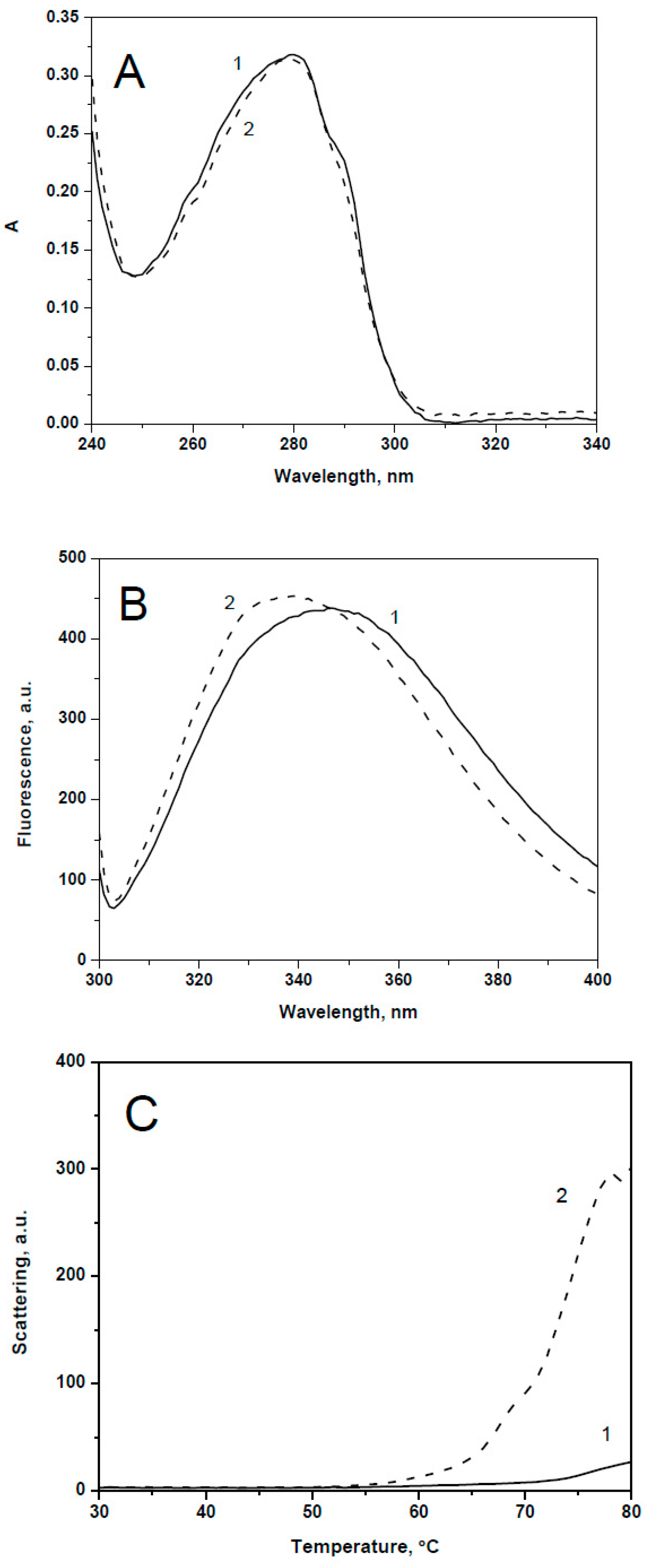
2.1. Spectral Properties and Thermal Stability of sHsps and Their Point Mutants
2.2. Effect of Arg/Ala Replacement on sHsp Quaternary Structure
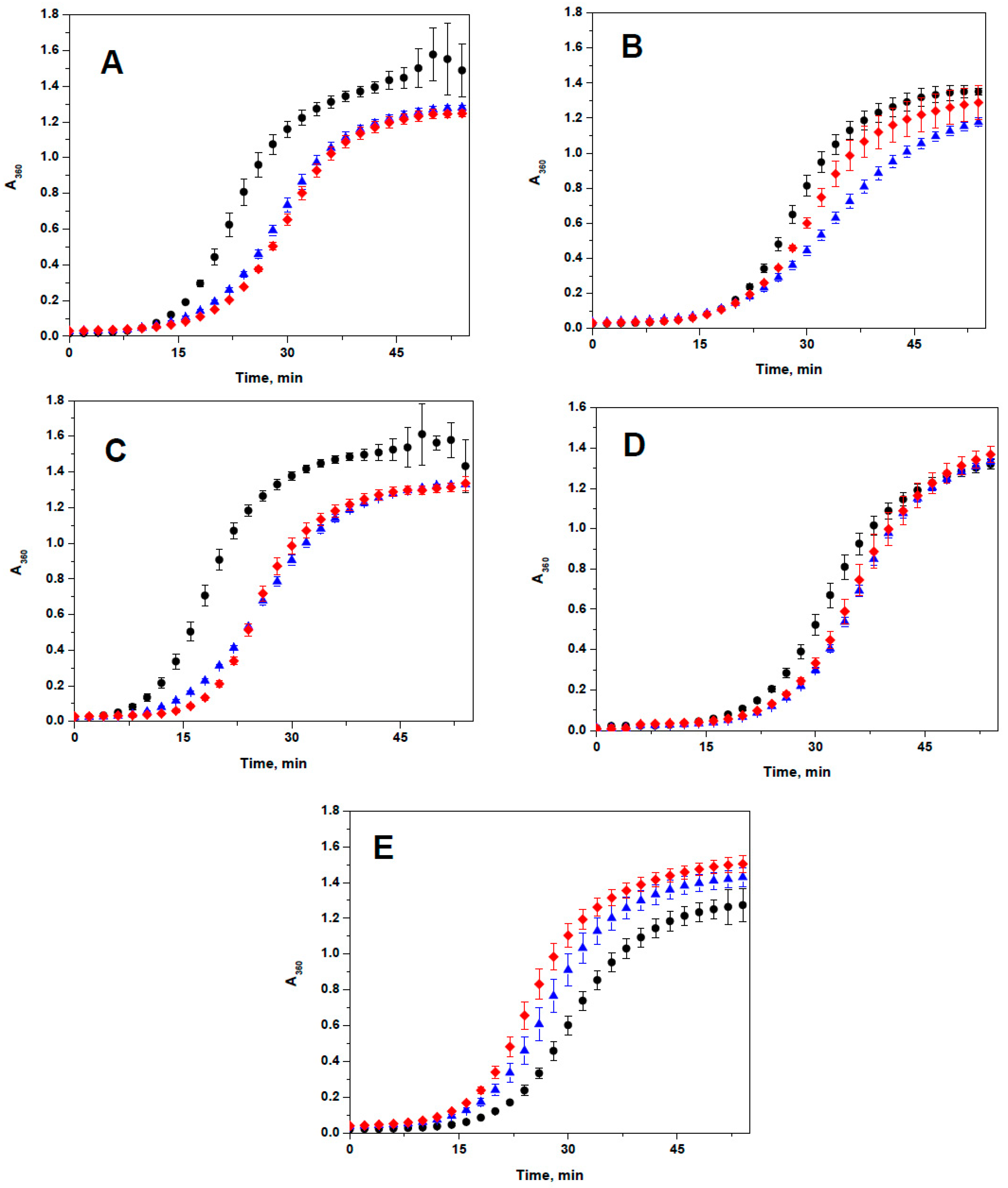
2.3. Lack of the Effect of Arg/Ala Replacement on Chaperone-Like Activity
3. Discussion
4. Materials and Methods
4.1. Proteins
4.2. Fluorescence Spectroscopy and Light Scattering
4.3. Size-Exclusion Chromatography
4.4. Analytical Ultracentrifugation
4.5. SEC-SAXS Studies
4.6. Crosslinking of HspB8
4.7. Chaperone-Like Activity
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Kriehuber, T.; Rattei, T.; Weinmaier, T.; Bepperling, A.; Haslbeck, M.; Buchner, J. Independent evolution of the core domain and its flanking sequences in small heat shock proteins. FASEB J. 2010, 24, 3633–3642. [Google Scholar] [CrossRef] [PubMed]
- Maaroufi, H.; Tanguay, R.M. Analysis and phylogeny of small heat shock proteins from marine viruses and their cyanobacteria host. PLoS ONE 2013, 8, e81207. [Google Scholar] [CrossRef] [PubMed]
- Slingsby, C.; Wistow, G.J. Functions of crystallins in and out of lens: Roles in elongated and post-mitotic cells. Prog. Biophys. Mol. Biol. 2014, 115, 52–67. [Google Scholar] [CrossRef] [PubMed]
- Bakthisaran, R.; Tangirala, R.; Rao, C.M. Small heat shock proteins: Role in cellular functions and pathology. Biochim. Biophys. Acta 2015, 1854, 291–319. [Google Scholar] [CrossRef] [PubMed]
- Treweek, T.M.; Meehan, S.; Ecroyd, H.; Carver, J.A. Small heat-shock proteins: Important players in regulating cellular proteostasis. Cell. Mol. Life Sci. 2015, 72, 429–451. [Google Scholar] [CrossRef] [PubMed]
- Poulain, P.; Gelly, J.C.; Flatters, D. Detection and architecture of small heat shock protein monomers. PLoS ONE 2010, 5, e9990. [Google Scholar] [CrossRef] [PubMed]
- Hochberg, G.K.; Benesch, J.L. Dynamical structure of αb-crystallin. Prog. Biophys. Mol. Biol. 2014, 115, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Delbecq, S.P.; Rosenbaum, J.C.; Klevit, R.E. A mechanism of subunit recruitment in human small heat shock protein oligomers. Biochemistry 2015, 54, 4276–4284. [Google Scholar] [CrossRef] [PubMed]
- Haslbeck, M.; Peschek, J.; Buchner, J.; Weinkauf, S. Structure and function of α-crystallins: Traversing from in vitro to in vivo. Biochim. Biophys. Acta 2016, 1860, 149–166. [Google Scholar] [CrossRef] [PubMed]
- Sobott, F.; Benesch, J.L.; Vierling, E.; Robinson, C.V. Subunit exchange of multimeric protein complexes. Real-time monitoring of subunit exchange between small heat shock proteins by using electrospray mass spectrometry. J. Biol. Chem. 2002, 277, 38921–38929. [Google Scholar] [CrossRef] [PubMed]
- Benesch, J.L.; Ayoub, M.; Robinson, C.V.; Aquilina, J.A. Small heat shock protein activity is regulated by variable oligomeric substructure. J. Biol. Chem. 2008, 283, 28513–28517. [Google Scholar] [CrossRef] [PubMed]
- Jovcevski, B.; Kelly, M.A.; Rote, A.P.; Berg, T.; Gastall, H.Y.; Benesch, J.L.; Aquilina, J.A.; Ecroyd, H. Phosphomimics destabilize hsp27 oligomeric assemblies and enhance chaperone activity. Chem. Biol. 2015, 22, 186–195. [Google Scholar] [CrossRef] [PubMed]
- Benndorf, R.; Hayess, K.; Ryazantsev, S.; Wieske, M.; Behlke, J.; Lutsch, G. Phosphorylation and supramolecular organization of murine small heat shock protein hsp25 abolish its actin polymerization-inhibiting activity. J. Biol. Chem. 1994, 269, 20780–20784. [Google Scholar] [PubMed]
- Aquilina, J.A.; Watt, S.J. The N-terminal domain of αb-crystallin is protected from proteolysis by bound substrate. Biochem. Biophys. Res. Commun. 2007, 353, 1115–1120. [Google Scholar] [CrossRef] [PubMed]
- Mymrikov, E.V.; Seit-Nebi, A.S.; Gusev, N.B. Large potentials of small heat shock proteins. Physiol. Rev. 2011, 91, 1123–1159. [Google Scholar] [CrossRef] [PubMed]
- Peschek, J.; Braun, N.; Rohrberg, J.; Back, K.C.; Kriehuber, T.; Kastenmuller, A.; Weinkauf, S.; Buchner, J. Regulated structural transitions unleash the chaperone activity of αb-crystallin. Proc. Natl. Acad. Sci. USA 2013, 110, E3780–E3789. [Google Scholar] [CrossRef] [PubMed]
- Dephoure, N.; Zhou, C.; Villen, J.; Beausoleil, S.A.; Bakalarski, C.E.; Elledge, S.J.; Gygi, S.P. A quantitative atlas of mitotic phosphorylation. Proc. Natl. Acad. Sci. USA 2008, 105, 10762–10767. [Google Scholar] [CrossRef] [PubMed]
- Heirbaut, M.; Strelkov, S.V.; Weeks, S.D. Everything but the ACD, Functional Conservation of the Non-conserved Terminal Regions in sHSPs. In The Big Book on Small Heat Shock Proteins; Tanguay, R.M., Hightower, L.E., Eds.; Springer International Publishing: Basel, Switzerland, 2015; pp. 197–227. [Google Scholar]
- Sudnitsyna, M.V.; Mymrikov, E.V.; Seit-Nebi, A.S.; Gusev, N.B. The role of intrinsically disordered regions in the structure and functioning of small heat shock proteins. Curr. Protein Pept. Sci. 2012, 13, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Hanazono, Y.; Takeda, K.; Oka, T.; Abe, T.; Tomonari, T.; Akiyama, N.; Aikawa, Y.; Yohda, M.; Miki, K. Nonequivalence observed for the 16-meric structure of a small heat shock protein, sphsp16.0, from schizosaccharomyces pombe. Structure 2013, 21, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Van Montfort, R.L.; Basha, E.; Friedrich, K.L.; Slingsby, C.; Vierling, E. Crystal structure and assembly of a eukaryotic small heat shock protein. Nat. Struct. Biol. 2001, 8, 1025–1030. [Google Scholar] [CrossRef] [PubMed]
- Merck, K.B.; De Haard-Hoekman, W.A.; Oude Essink, B.B.; Bloemendal, H.; De Jong, W.W. Expression and aggregation of recombinant α a-crystallin and its two domains. Biochim. Biophys. Acta 1992, 1130, 267–276. [Google Scholar] [CrossRef]
- Bova, M.P.; McHaourab, H.S.; Han, Y.; Fung, B.K. Subunit exchange of small heat shock proteins. Analysis of oligomer formation of αa-crystallin and hsp27 by fluorescence resonance energy transfer and site-directed truncations. J. Biol. Chem. 2000, 275, 1035–1042. [Google Scholar] [CrossRef] [PubMed]
- Heirbaut, M.; Beelen, S.; Strelkov, S.V.; Weeks, S.D. Dissecting the functional role of the N-terminal domain of the human small heat shock protein hspb6. PLoS ONE 2014, 9, e105892. [Google Scholar] [CrossRef] [PubMed]
- Pasta, S.Y.; Raman, B.; Ramakrishna, T.; Rao Ch, M. Role of the conserved srlfdqffg region of α-crystallin, a small heat shock protein. Effect on oligomeric size, subunit exchange, and chaperone-like activity. J. Biol. Chem. 2003, 278, 51159–51166. [Google Scholar] [CrossRef] [PubMed]
- Kumar, L.V.; Rao, C.M. Domain swapping in human α a and α b crystallins affects oligomerization and enhances chaperone-like activity. J. Biol. Chem. 2000, 275, 22009–22013. [Google Scholar] [CrossRef] [PubMed]
- Heirbaut, M.; Lermyte, F.; Martin, E.M.; Beelen, S.; Sobott, F.; Strelkov, S.V.; Weeks, S.D. Specific sequences in the n-terminal domain of human small heat-shock protein hspb6 dictate preferential hetero-oligomerization with the orthologue hspb1. J. Biol. Chem. 2017, 292, 9944–9957. [Google Scholar] [CrossRef] [PubMed]
- Santhoshkumar, P.; Sharma, K.K. Phe71 is essential for chaperone-like function in α a-crystallin. J. Biol. Chem. 2001, 276, 47094–47099. [Google Scholar] [CrossRef] [PubMed]
- Muranova, L.K.; Weeks, S.D.; Strelkov, S.V.; Gusev, N.B. Characterization of mutants of human small heat shock protein hspb1 carrying replacements in the n-terminal domain and associated with hereditary motor neuron diseases. PLoS ONE 2015, 10, e0126248. [Google Scholar] [CrossRef] [PubMed]
- Irobi, J.; Van Impe, K.; Seeman, P.; Jordanova, A.; Dierick, I.; Verpoorten, N.; Michalik, A.; De Vriendt, E.; Jacobs, A.; Van Gerwen, V.; et al. Hot-spot residue in small heat-shock protein 22 causes distal motor neuropathy. Nat. Genet. 2004, 36, 597–601. [Google Scholar] [CrossRef] [PubMed]
- Ghaoui, R.; Palmio, J.; Brewer, J.; Lek, M.; Needham, M.; Evila, A.; Hackman, P.; Jonson, P.H.; Penttila, S.; Vihola, A.; et al. Mutations in hspb8 causing a new phenotype of distal myopathy and motor neuropathy. Neurology 2016, 86, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Xiao, H.; Hu, Z.; Zhou, F.; Yang, B. Exploring the multifaceted roles of heat shock protein b8 (hspb8) in diseases. Eur. J. Cell Biol. 2018, 97, 216–229. [Google Scholar] [CrossRef] [PubMed]
- Weeks, S.D.; Baranova, E.V.; Heirbaut, M.; Beelen, S.; Shkumatov, A.V.; Gusev, N.B.; Strelkov, S.V. Molecular structure and dynamics of the dimeric human small heat shock protein hspb6. J. Struct. Biol. 2014, 185, 342–354. [Google Scholar] [CrossRef] [PubMed]
- Heirbaut, M.; Lermyte, F.; Martin, E.M.; Beelen, S.; Verschueren, T.; Sobott, F.; Strelkov, S.V.; Weeks, S.D. The preferential heterodimerization of human small heat shock proteins hspb1 and hspb6 is dictated by the n-terminal domain. Arch. Biochem. Biophys. 2016, 610, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.V.; Seit-Nebi, A.S.; Marston, S.B.; Gusev, N.B. Some properties of human small heat shock protein hsp22 (h11 or hspb8). Biochem. Biophys. Res. Commun. 2004, 315, 796–801. [Google Scholar] [CrossRef] [PubMed]
- Rambo, R.P.; Tainer, J.A. Accurate assessment of mass, models and resolution by small-angle scattering. Nature 2013, 496, 477–481. [Google Scholar] [CrossRef] [PubMed]
- Delbecq, S.P.; Jehle, S.; Klevit, R. Binding determinants of the small heat shock protein, αb-crystallin: Recognition of the ixi motif. EMBO J. 2012, 31, 4587–4594. [Google Scholar] [CrossRef] [PubMed]
- Koteiche, H.A.; Chiu, S.; Majdoch, R.L.; Stewart, P.L.; McHaourab, H.S. Atomic models by cryo-em and site-directed spin labeling: Application to the n-terminal region of hsp16.5. Structure 2005, 13, 1165–1171. [Google Scholar] [CrossRef] [PubMed]
- Jehle, S.; Vollmar, B.S.; Bardiaux, B.; Dove, K.K.; Rajagopal, P.; Gonen, T.; Oschkinat, H.; Klevit, R.E. N-terminal domain of alphaB-crystallin provides a conformational switch for multimerization and structural heterogeneity. Proc. Natl. Acad. Sci. USA 2011, 108, 6409–6414. [Google Scholar] [CrossRef] [PubMed]
- McDonald, E.T.; Bortolus, M.; Koteiche, H.A.; McHaourab, H.S. Sequence, structure, and dynamic determinants of hsp27 (hspb1) equilibrium dissociation are encoded by the n-terminal domain. Biochemistry 2012, 51, 1257–1268. [Google Scholar] [CrossRef] [PubMed]
- Rogalla, T.; Ehrnsperger, M.; Preville, X.; Kotlyarov, A.; Lutsch, G.; Ducasse, C.; Paul, C.; Wieske, M.; Arrigo, A.P.; Buchner, J.; et al. Regulation of hsp27 oligomerization, chaperone function, and protective activity against oxidative stress/tumor necrosis factor α by phosphorylation. J. Biol. Chem. 1999, 274, 18947–18956. [Google Scholar] [CrossRef] [PubMed]
- Hansen, L.; Yao, W.; Eiberg, H.; Kjaer, K.W.; Baggesen, K.; Hejtmancik, J.F.; Rosenberg, T. Genetic heterogeneity in microcornea-cataract: Five novel mutations in cryaa, crygd, and GJA8. Investig. Ophthalmol. Vis. Sci. 2007, 48, 3937–3944. [Google Scholar] [CrossRef] [PubMed]
- Devi, R.R.; Yao, W.; Vijayalakshmi, P.; Sergeev, Y.V.; Sundaresan, P.; Hejtmancik, J.F. Crystallin gene mutations in indian families with inherited pediatric cataract. Mol. Vis. 2008, 14, 1157–1170. [Google Scholar] [PubMed]
- Graw, J.; Klopp, N.; Illig, T.; Preising, M.N.; Lorenz, B. Congenital cataract and macular hypoplasia in humans associated with a de novo mutation in cryaa and compound heterozygous mutations in P. Graefes Arch. Clin. Exp. Ophthalmol. 2006, 244, 912–919. [Google Scholar] [CrossRef] [PubMed]
- Laurie, K.J.; Dave, A.; Straga, T.; Souzeau, E.; Chataway, T.; Sykes, M.J.; Casey, T.; Teo, T.; Pater, J.; Craig, J.E.; et al. Identification of a novel oligomerization disrupting mutation in cryαa associated with congenital cataract in a south australian family. Hum. Mutat. 2013, 34, 435–438. [Google Scholar] [CrossRef] [PubMed]
- Javadiyan, S.; Craig, J.E.; Souzeau, E.; Sharma, S.; Lower, K.M.; Pater, J.; Casey, T.; Hodson, T.; Burdon, K.P. Recurrent mutation in the crystallin α a gene associated with inherited paediatric cataract. BMC Res. Notes 2016, 9, 83. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.V.; Kasakov, A.S.; Seit-Nebi, A.S.; Marston, S.B.; Gusev, N.B. Structure and properties of k141e mutant of small heat shock protein hsp22 (hspb8, h11) that is expressed in human neuromuscular disorders. Arch. Biochem. Biophys. 2006, 454, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Sluchanko, N.N.; Chebotareva, N.A.; Gusev, N.B. Quaternary structure of human small heat shock protein hspb6 (hsp20) in crowded media modeled by trimethylamine n-oxide (tmao): Effect of protein phosphorylation. Biochimie 2015, 108, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Bukach, O.V.; Seit-Nebi, A.S.; Marston, S.B.; Gusev, N.B. Some properties of human small heat shock protein hsp20 (hspb6). Eur. J. Biochem. 2004, 271, 291–302. [Google Scholar] [CrossRef] [PubMed]
- Chowdary, T.K.; Raman, B.; Ramakrishna, T.; Rao, C.M. Mammalian hsp22 is a heat-inducible small heat-shock protein with chaperone-like activity. Biochem. J. 2004, 381, 379–387. [Google Scholar] [CrossRef] [PubMed]
- Villen, J.; Beausoleil, S.A.; Gerber, S.A.; Gygi, S.P. Large-scale phosphorylation analysis of mouse liver. Proc. Natl. Acad. Sci. USA 2007, 104, 1488–1493. [Google Scholar] [CrossRef] [PubMed]
- Shemetov, A.A.; Seit-Nebi, A.S.; Gusev, N.B. Phosphorylation of human small heat shock protein hspb8 (hsp22) by erk1 protein kinase. Mol. Cell. Biochem. 2011, 355, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Mymrikov, E.V.; Daake, M.; Richter, B.; Haslbeck, M.; Buchner, J. The chaperone activity and substrate spectrum of human small heat shock proteins. J. Biol. Chem. 2017, 292, 672–684. [Google Scholar] [CrossRef] [PubMed]
- Vos, M.J.; Kanon, B.; Kampinga, H.H. Hspb7 is a sc35 speckle resident small heat shock protein. Biochim. Biophys. Acta 2009, 1793, 1343–1353. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Koteiche, H.A.; McHaourab, H.S.; Stewart, P.L. Cryoelectron microscopy and epr analysis of engineered symmetric and polydisperse hsp16.5 assemblies reveals determinants of polydispersity and substrate binding. J. Biol. Chem. 2006, 281, 40420–40428. [Google Scholar] [CrossRef] [PubMed]
- Van de Klundert, F.A.; Smulders, R.H.; Gijsen, M.L.; Lindner, R.A.; Jaenicke, R.; Carver, J.A.; de Jong, W.W. The mammalian small heat-shock protein hsp20 forms dimers and is a poor chaperone. Eur. J. Biochem. 1998, 258, 1014–1021. [Google Scholar] [CrossRef] [PubMed]
- Studier, F.W. Protein production by auto-induction in high density shaking cultures. Protein Expr. Purif. 2005, 41, 207–234. [Google Scholar] [CrossRef] [PubMed]
- Mymrikov, E.V.; Seit-Nebi, A.S.; Gusev, N.B. Heterooligomeric complexes of human small heat shock proteins. Cell Stress Chaperones 2012, 17, 157–169. [Google Scholar] [CrossRef] [PubMed]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Lebowitz, J.; Lewis, M.S.; Schuck, P. Modern analytical ultracentrifugation in protein science: A tutorial review. Protein Sci. 2002, 11, 2067–2079. [Google Scholar] [CrossRef] [PubMed]
- David, G.; Perez, J. Combined sampler robot and high-performance liquid chromatography: A fully automated system for biological small-angle x-ray scattering experiments at the synchrotron soleil swing beamline. J. Appl. Crystallogr. 2009, 42, 892–900. [Google Scholar] [CrossRef]
- Alshammari, E.M.; Khan, S.; Jawed, A.; Adnan, M.; Khan, M.; Nabi, G.; Lohani, M.; Haque, S. Optimization of extraction parameters for enhanced production of ovotransferrin from egg white for antimicrobial applications. BioMed Res. Int. 2015, 2015, 934512. [Google Scholar] [CrossRef] [PubMed]
- Evans, R.W.; Holbrook, J.J. Differences in the protein fluorecence of the two iron(iii)-binding sites of ovotransferrin. Biochem. J. 1975, 145, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Weeks, S.D.; Muranova, L.K.; Heirbaut, M.; Beelen, S.; Strelkov, S.V.; Gusev, N.B. Characterization of human small heat shock protein hspb1 α-crystallin domain localized mutants associated with hereditary motor neuron diseases. Sci. Rep. 2018, 8, 688. [Google Scholar] [CrossRef] [PubMed]

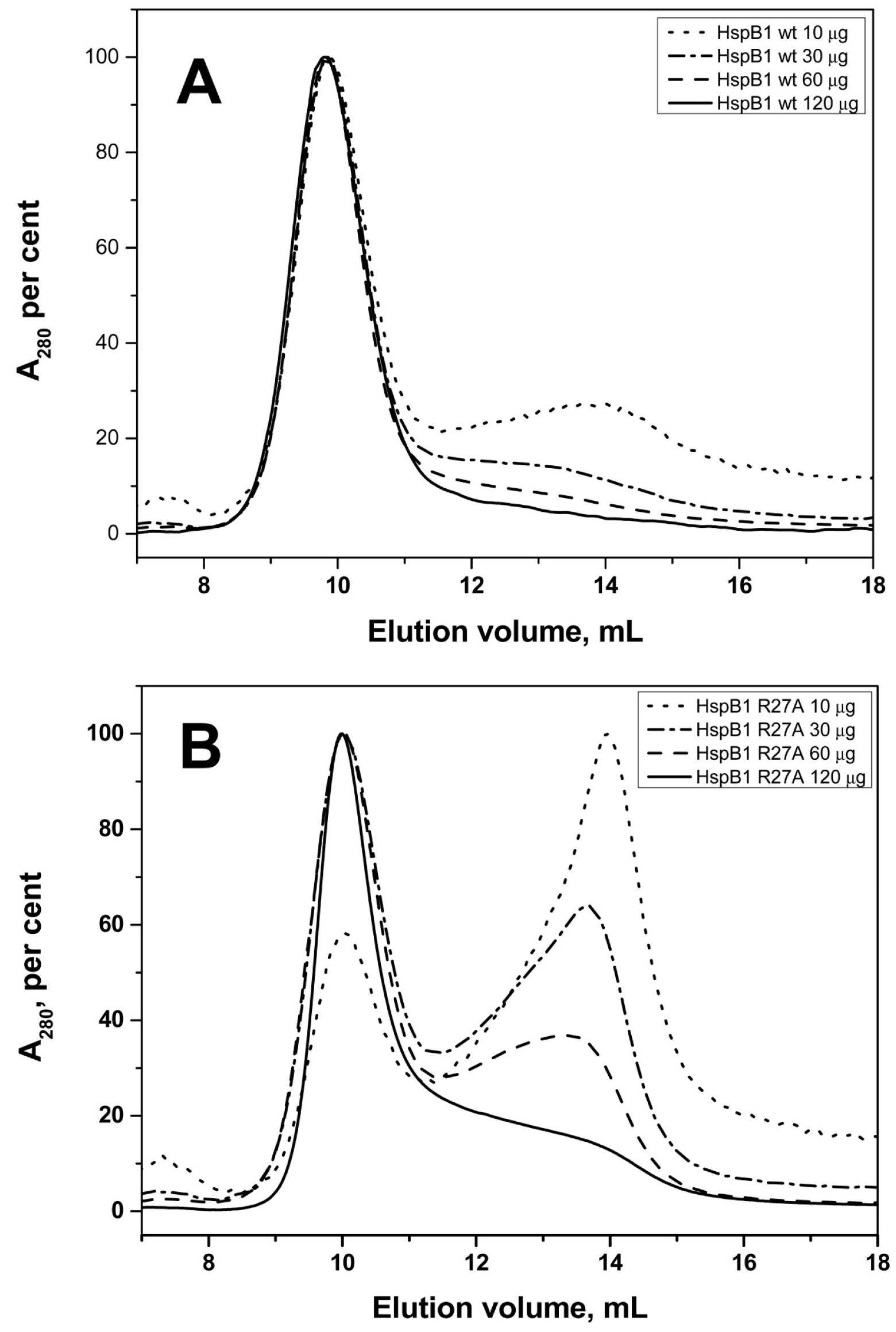
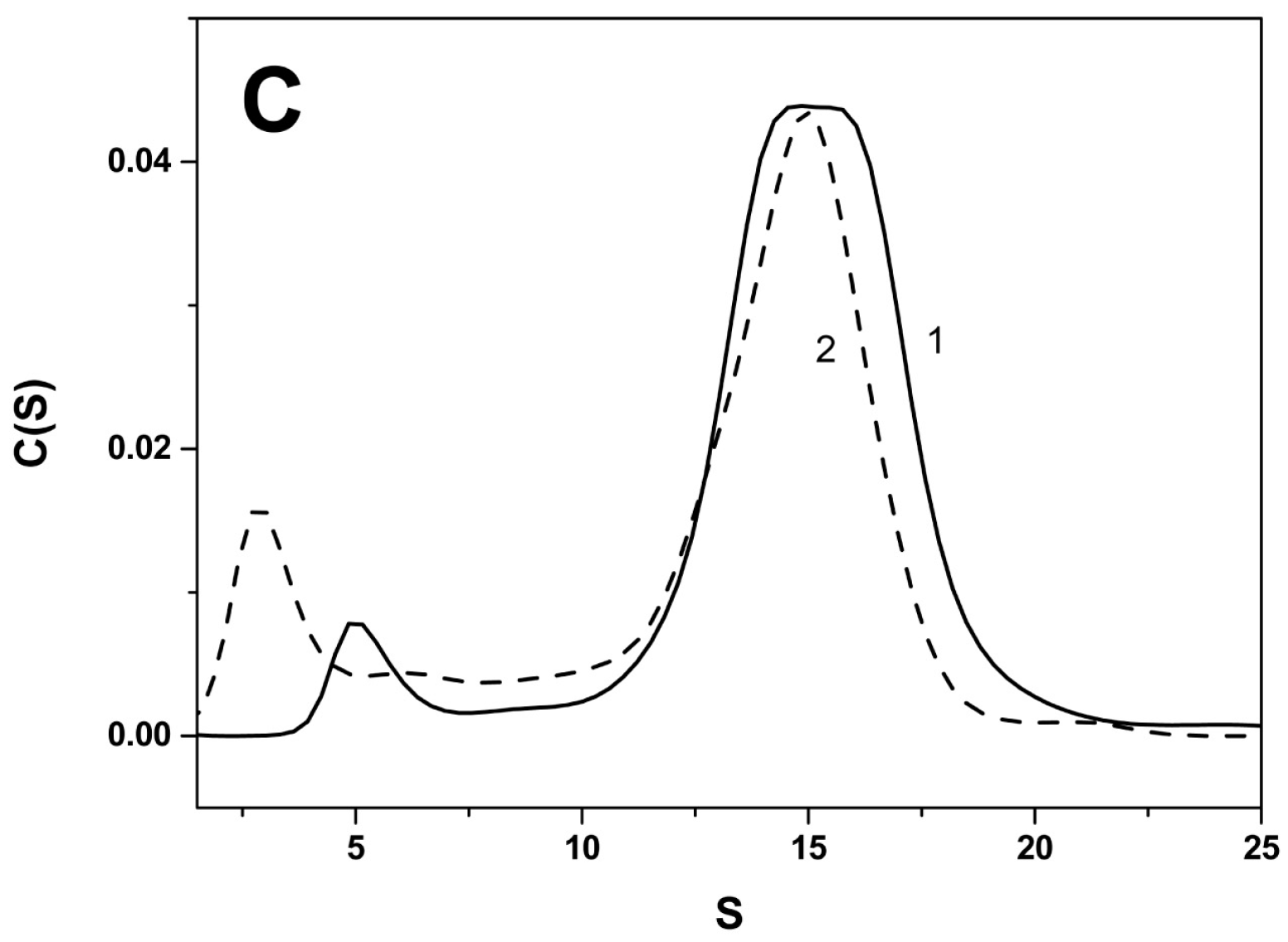
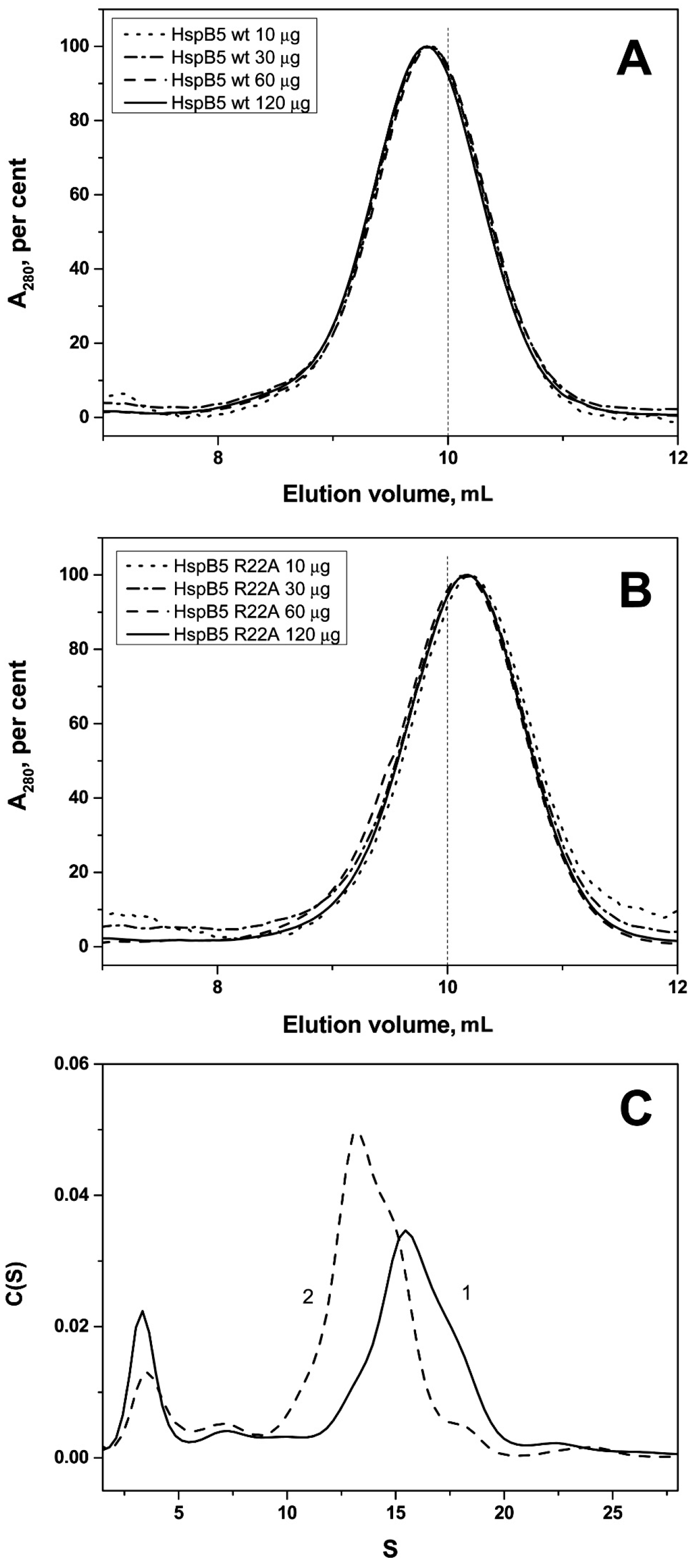
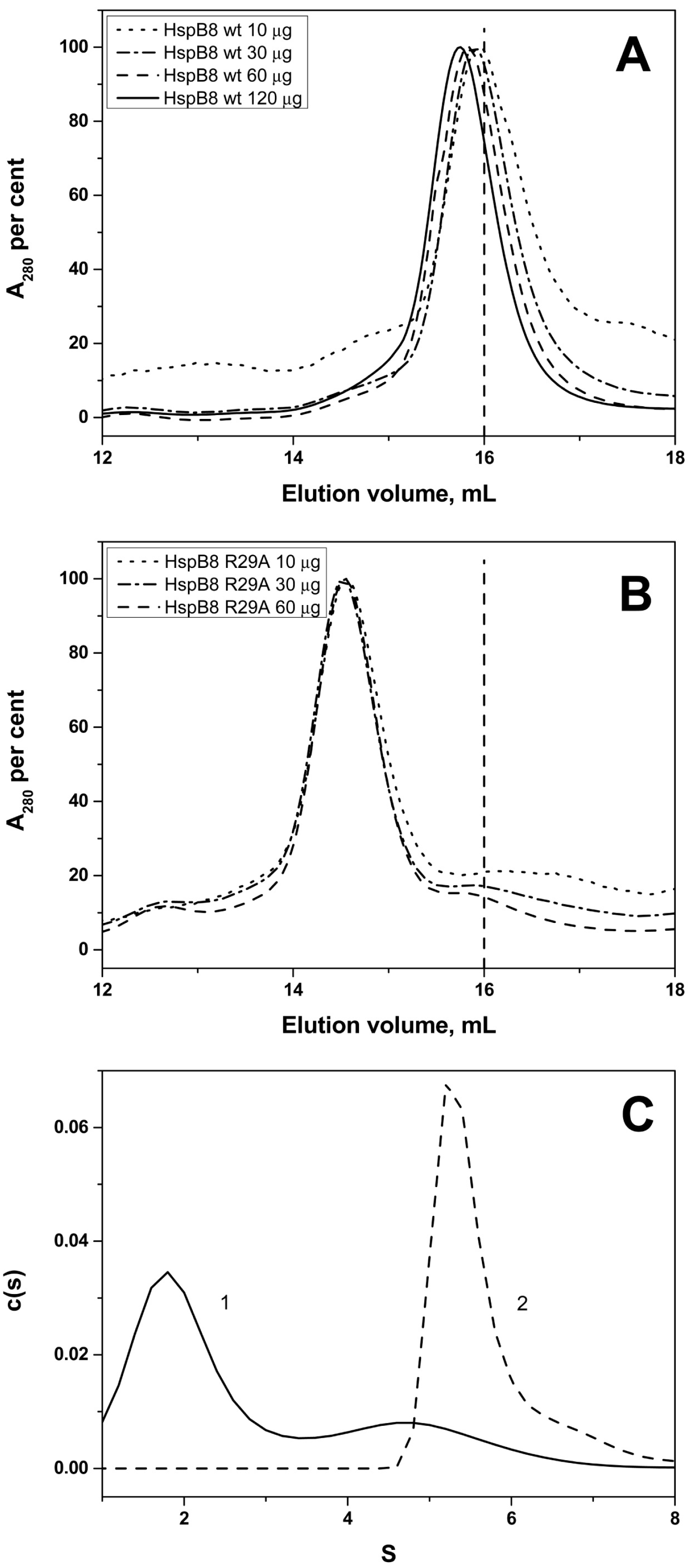

| Protein | Monomeric Mass, kDa | Apparent Mass/No. of Monomers WT | Apparent Mass/No. of Monomers Mutant |
|---|---|---|---|
| HspB1 | 22.8 | 540/~24 | 500/~22 + 80/~4 * |
| HspB4 | 19.9 | 590/~30 | 590/~30 |
| HspB5 | 20.2 | 540/~27 | 460/~23 |
| HspB6 | 17.1 | 50/~3 ** | 50/~3 |
| HspB8 | 21.6 | 33/~2 *** | 60/~3 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shatov, V.M.; Weeks, S.D.; Strelkov, S.V.; Gusev, N.B. The Role of the Arginine in the Conserved N-Terminal Domain RLFDQxFG Motif of Human Small Heat Shock Proteins HspB1, HspB4, HspB5, HspB6, and HspB8. Int. J. Mol. Sci. 2018, 19, 2112. https://doi.org/10.3390/ijms19072112
Shatov VM, Weeks SD, Strelkov SV, Gusev NB. The Role of the Arginine in the Conserved N-Terminal Domain RLFDQxFG Motif of Human Small Heat Shock Proteins HspB1, HspB4, HspB5, HspB6, and HspB8. International Journal of Molecular Sciences. 2018; 19(7):2112. https://doi.org/10.3390/ijms19072112
Chicago/Turabian StyleShatov, Vladislav M., Stephen D. Weeks, Sergei V. Strelkov, and Nikolai B. Gusev. 2018. "The Role of the Arginine in the Conserved N-Terminal Domain RLFDQxFG Motif of Human Small Heat Shock Proteins HspB1, HspB4, HspB5, HspB6, and HspB8" International Journal of Molecular Sciences 19, no. 7: 2112. https://doi.org/10.3390/ijms19072112
APA StyleShatov, V. M., Weeks, S. D., Strelkov, S. V., & Gusev, N. B. (2018). The Role of the Arginine in the Conserved N-Terminal Domain RLFDQxFG Motif of Human Small Heat Shock Proteins HspB1, HspB4, HspB5, HspB6, and HspB8. International Journal of Molecular Sciences, 19(7), 2112. https://doi.org/10.3390/ijms19072112