Abstract
Multiple parallel hits, including genetic differences, insulin resistance and intestinal microbiota, account for the progression of non-alcoholic steatohepatitis (NASH). Multiple hits induce adipokine secretion, endoplasmic reticulum (ER) and oxidative stress at the cellular level that subsequently induce hepatic steatosis, inflammation and fibrosis, among which oxidative stress is considered a key contributor to progression from simple fatty liver to NASH. Although several clinical trials have shown that anti-oxidative therapy can effectively control hepatitis activities in the short term, the long-term effect remains obscure. Several trials of long-term anti-oxidant protocols aimed at treating cerebrovascular diseases or cancer development have failed to produce a benefit. This might be explained by the non-selective anti-oxidative properties of these drugs. Molecular hydrogen is an effective antioxidant that reduces only cytotoxic reactive oxygen species (ROS) and several diseases associated with oxidative stress are sensitive to hydrogen. The progress of NASH to hepatocellular carcinoma can be controlled using hydrogen-rich water. Thus, targeting mitochondrial oxidative stress might be a good candidate for NASH treatment. Long term clinical intervention is needed to control this complex lifestyle-related disease.
1. Introduction
Non-alcoholic fatty liver disease (NAFLD) is a common cause of chronic liver disease and a major indicator of metabolic syndrome that is becoming increasingly prevalent [1]. Non-alcoholic steatohepatitis (NASH) is a more severe form of NAFLD that is broadly defined by the presence of steatosis with inflammation and progressive fibrosis [2,3], ultimately leading to cirrhosis and hepatocellular carcinoma (HCC) [4–7]. A subset of patients with NAFLD develops NASH through poorly understood mechanisms.
The development of NASH has been considered a “two hit” process [8]. The first hit is the development of hepatic steatosis and the second hit includes cellular stresses such as oxidative stress, apoptosis and gut-derived lipopolysaccharide (LPS). However, a patatin-like phospholipase 3 (PNPLA3) gene polymorphism also plays a key role in the development of NASH. Although fatty liver is usually non-progressive, it can progress in patients harboring the risk allele of the PNPLA3 gene. Thus, the development of NASH including this genetic polymorphism is now regarded as a “multiple hit” process (Figure 1).
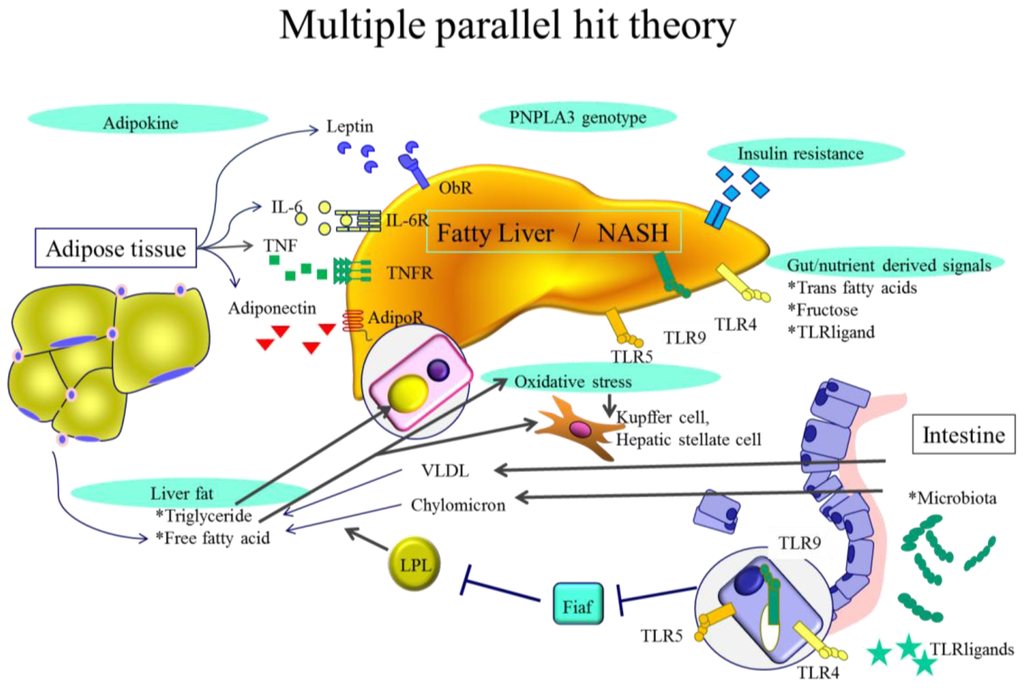
Figure 1.
Multiple parallel hit theory. Genome-wide association studies have confirmed importance of patatin-like phospholipase 3 (PNPLA3) gene polymorphism in NAFLD. This genetic polymorphism can differentiate simple steatosis with or without minimal inflammation and fibrosis progressing to NASH. In some instances, inflammation could precede steatosis and anti-tumor necrosis factor (TNF)-α antibody improves steatosis in ob/ob mice. Obesity and diabetes induce insulin resistance, adipocyte proliferation and changes in intestinal flora. Adipokines such as IL-6 and TNF-α produced by adipocytes affect hepatocyte fat content and liver inflammatory environment. Gut-derived signals could be affected by ingested trans fatty acids, fructose, or TLR ligands. Ingested free fatty acids and free cholesterol induce ER stress and oxidative stress resulting in hepatic inflammation and fibrogenesis.
Many sources of cellular stresses, including oxidative stress, apoptosis and gut-derived LPS, trigger an inflammatory response and progressive liver damage [9]. Oxidative stress appears to be responsible for initiating necroinflammation, and reactive oxygen species (ROS) that are generated during free fatty acid metabolism in microsomes, peroxisomes and mitochondria comprise an established source of oxidative stress [10]. Mitochondria are the most important cellular source of ROS, and mitochondrial dysfunction might therefore play a central role in the pathological mechanisms of NASH. Although the mechanisms of mitochondrial dysfunction are not clearly understood, emerging data suggest that ROS, lipid peroxidation products and tumor necrosis factor-α (TNF-α) are involved in the second hit, which induces the progression of simple steatosis to NASH. Furthermore, ROS induce the directional migration of resident hepatic pro-fibrogenic cells, resulting in liver fibrosis [11].
Several studies have suggested that antioxidants such as vitamin E along with 1-aminobenzotriazole (ABT) and thiazolidinediones (insulin sensitizers) confer benefits upon patients with NAFLD or NASH [12–15]. In particular, the thiazolidinedione pioglitazone helps to improve insulin sensitivity, steatosis and inflammation. However, the improvement is not histologically clear [12]. Furthermore, most clinical studies of atherosclerotic diseases with dietary antioxidants have not generated clear results, partly because of the non-selective effects of these anti-oxidative drugs and difficulties associated with cytosolic distribution [16].
Molecular hydrogen has therapeutic value as an antioxidant through its ability to reduce one of the cytotoxic ROS hydroxyl radicals, but not superoxide, hydrogen peroxide or nitric oxide. Hydrogen is distributed into cytosol without any specific receptors or hydrophilicity [17]. Inhaled hydrogen gas (~4% H2 in air) can reduce infarct size in rat models of focal cerebral and myocardial ischemia reperfusion injury [18]. Drinking water containing therapeutic doses of hydrogen (hydrogen-rich water; HW) represents an alternative model for delivering molecular hydrogen to treat ROS-induced pathologies [19]. We recently showed that HW can counter oxidative stress in mouse models of NASH.
Here, we review the importance of oxidative stress in the pathogenesis of NASH and the effect of anti-oxidants including HW.
2. NASH Pathogenesis—General Characteristics
The pathogenesis of NASH is unclear. The two-hit theory that was suggested 15 years ago indicated that the first hit is hepatic steatosis and the second is caused by gut-derived endotoxins, oxidative stress or proinflammatory cytokines [20]. The concept of multiple parallel hits has recently been considered [21]. Inflammation can occasionally precede steatosis and patients with NASH can present without much steatosis, suggesting that inflammation can occur first [22]. Anti-tumor necrosis (TNF)-α antibody improves steatosis in ob/ob mice [23]. These results suggest that primary inflammation can induce secondary steatosis. Additionally, simple steatosis and NASH are considered separate diseases, each with a different pathogenesis.
2.1. Genetic Background
Genome-wide association studies (GWAS) have uncovered many disease- or treatment-susceptible genes. Several GWAS of various races have confirmed the importance of PNPLA3 gene polymorphisms in NAFLD [24]. This genetic polymorphism differentiates between simple steatosis with or without minimal inflammation and fibrosis that progresses to NASH [25,26]. Patients with the NASH-sensitive single nucleotide polymorphism (SNP) rs738409 G/G genotype might progress not only to simple steatosis but also to NASH, probably under the same types of metabolic stimulation. The function of PNPLA3 is not well known since mice deficient in PNPLA3 develop neither fatty liver nor liver injury. However, the overexpression of sterol-regulated binding protein 1c (SREBP-1c) results in its binding to the transcription start site of the mouse PNPLA3 gene and additional PNPLA3 knockdown can decrease the intracellular triglyceride content in primary hepatocytes [27]. Thus, PNPLA3 might function as a downstream target gene of SREBP-1c to mediate SREBP-1c stimulation of lipid accumulation. This genetic characteristic might be the “first hit” and subsequent hits might affect disease progression.
2.2. Visceral Obesity
Obesity is a growing global epidemic among adults and children that is associated with many diseases such as hypertension, diabetes mellitus, hyperlipidemia and NAFLD. Obesity, hypertriglyceridemia, and hypertension are predictive risk factors for NAFLD [28]. Visceral fat accumulation in obesity correlates with various organ pathologies including cerebrovascular diseases, cancer and NASH. Visceral fat accumulation is regarded as a significant risk factor for the development of NAFLD and NASH. A study from Japan has found that the severity of hepatic steatosis determined by ultrasound positively correlates with visceral fat accumulation and insulin resistance in both obese and non-obese individuals, suggesting that hepatic steatosis is influenced by visceral fat accumulation regardless of obesity [29]. Visceral obesity has become quite common even among children world-wide over the past decade, thanks to a shift towards Western style diets that are high in calories, fat and fructose [30].
2.3. Insulin Resistance
Insulin resistance is an independent risk factor for NAFLD severity [29]. Adipose and hepatic insulin resistance progressively increases across NAFLD stages even in non-obese, non-diabetic and normolipidemic patients. The oral glucose tolerant test (OGTT) shows impaired pancreatic β-cell function in patients with NASH but not in those with simple steatosis [31]. Also, a more atherogenic postprandial lipoprotein profile indicates systemic insulin resistance and hepatic free fatty acid (FFA) accumulation.
2.4. Hepatic Steatosis
Fatty liver is the basic feature of NAFLD and NASH. Triglycerides are the main types of lipids stored in the liver of patients with NAFLD. The toxic lipids in NASH and the non-toxic lipids in simple steatosis might be different [32]. Diacylglycerol acyltransferase 2 (DGAT2) catalyzes the final step in hepatocyte triglyceride biosynthesis. Hepatic steatosis and the dietary triglyceride contents induced in a model of obese-simple fatty liver are reduced by DGAT2 antisense oligonucleotides in a manner that does not correlate with changes in body weight, adiposity or insulin sensitivity [33]. However, a DGAT2 antisense oligonucleotide increased levels of hepatic free fatty acids, lipid oxidant stress, lobular necroinflammation and fibrosis in mice fed with a methionine choline-deficient (MCD) diet that generates inflammation and fibrosis with hepatic steatosis, whereas hepatic triglyceride content decreased [32]. These results suggest that the pathogenesis and treatment of steatosis in simple fatty liver and in NASH are different. Human genetic variability analysis of a lifestyle intervention has shown that the DGAT2 gene polymorphism is related to a decrease in liver fat, while changes in insulin resistance do not correlate [34]. Since insulin resistance is the key marker for NASH, the DGAT2 gene polymorphism might only be associated with non-progressive fatty liver.
3. NASH Pathogenesis—Cellular Levels
Since the significance of apparently similar fat droplets in simple fatty liver and NASH hepatocytes differ in DGAT2 knockdown experiments, analyzing the molecular pathogenesis of NASH at the cellular level is quite important.
3.1. Adipokines
Adipokines are multifunctional secreted factors that are primarily considered to be derived from adipose tissue. Such tissue has been regarded simply as a means of storing energy, whereas it is now considered to be a complex organ that is involved in the control of many processes including metabolic, immunological and inflammatory responses. Adiponectin is the most abundant and adipose tissue-specific adipokine. Mature adipocytes mainly produce adiponectin in white adipose tissue and expression and secretion levels increase during adipocyte differentiation. Levels of adiponectin are significantly higher in females than in males, in whom serum androgens become more evident during puberty [35]. Adiponectin levels inversely correlate with visceral obesity and insulin resistance and weight loss is an inducer of adiponectin synthesis. Proinflammatory adipokines such as TNF-α or IL-6 suppress adiponectin, which has anti-inflammatory, anti-atherogenic and anti-diabetic properties [36]. Adipose tissue is also the main producer of the adipokine leptin and its levels directly correlate with body fat mass and adipocyte size [37]. Leptin production is mainly regulated by food intake, and hormones related to eating such as insulin increase leptin secretion and vice versa. Leptin is also regulated by sex hormones, because testosterone inhibits leptin production whereas ovarian sex steroids increase it, resulting in higher levels in females. Proinflammatory endotoxin, IL-1 and TNF-α increase the secretion of leptin, which has central and peripheral effects. Leptin acts on hypothalamic cells, inhibits anabolic pathways, activates catabolic pathways, inhibits appetite and stimulates energy expenditure. Peripheral leptin increases basal metabolism, regulates pancreatic cell functions and insulin secretion, and affects T cell generation and the differentiation of T helper 1 cells in lymph nodes. Leptin-deficient (ob/ob) mice and leptin receptor-deficient (db/db) mice are severely obese and have increased pituitary and adrenal hormone production, hyperglycemia, elevated insulin and decreased immune function.
Several groups have rather found that serum adiponectin levels are controversially lower in NAFLD than in NASH or are the same [38–41]. A meta-analysis of 27 studies of 698 controls and 1545 patients with NAFLD found that serum adiponectin levels are low in NAFLD and much lower in NASH [36]. Since adiponectin and leptin exert antagonistic effects on liver fibrogenesis and inflammation, the ratio of adiponectin to leptin might be a better marker with which to distinguish NASH from NAFLD. Levels of adiponectin receptor II are decreased in human liver biopsy specimens and in mouse models of NASH [42,43]. However, since contradictory results have suggested that lower serum adiponectin induce high expression of hepatic adiponectin receptor II as a compensatory response, the function of these novel adipokines and receptors requires further investigation [44,45].
3.2. Endotoxin and Gut Derived Signals
Gut microbiota play many roles in NAFLD and NASH, hepatocellular carcinoma, cardiac functions, vascular atherosclerosis, diabetes and other conditions. Endotoxin or LPS produced by gut microbiota could be delivered to the liver via the portal vein, which raises the question of why such toxic materials flow into the portal vein through the intestinal barrier. Patients with biopsy-proven NAFLD have increased intestinal permeability with disrupted intercellular tight junctions in the intestine [46]. These abnormalities are related to increased bacterial overgrowth in the small intestine. Murine NAFLD models of bacterial overgrowth develop compositional changes and increased intestinal permeability with a concurrent reduction in the expression of tight junction proteins [47]. Plasma endotoxin levels are significantly higher in patients with NAFLD and in murine NASH models [46,48]. A high-fat diet could increase LPS concentrations two- to three-fold [49]. Proinflammatory inflammasomes induce inflammation in the liver of patients with NAFLD, but an inflammasome-deficient model developed exacerbated hepatic steatosis and inflammation through the influx of TLR4 and TLR9 agonists into the portal vein [50]. The microbiota of these inflammasome-deficient mice differed from those of wild-type mice with NASH. Furthermore, co-housing inflammasome-deficient and wild-type mice resulted in intestinal inflammation and exacerbated hepatic steatosis in the wild-type mice. This finding suggested that altered microbiota in inflammasome-deficient mice could be transferred to healthy mice resulting in intestinal inflammation, increased permeability and NAFLD. The gut and oral periodontal status correlates with the progression of liver disease [51]. Treating periodontitis could improve transaminases in NAFLD and, in fact, several probiotics that control gut microbiota improve NAFLD [52–54]. Studies using models of hepatocarcinogenesis have found that a high-fat diet increases levels of deoxycholic acid, a gut bacterial metabolite that damages DNA and exacerbates hepatocarcinogenesis [55]. Antibiotics could abrogate these effects. Gut microbiota affect not only NAFLD, but also obesity-related hepatocarcinogenesis.
3.3. Toll-Like Receptors (TLRs)
Toll-like receptors are sensors of microbial and endogenous danger signals that are expressed and activated in innate immune cells and in liver parenchymal cells and they contribute to the progression of NASH. Gut microbiota might release pathogen- or damage-associated molecular patterns (PAMPs or DAMPs), which are TLR ligands following the activation of downstream signals. Among 10 TLRs (TLR1-10) identified in humans and 13 (TLR1-9, 11–13) determined in mammals, TLR2, TLR4, and TLR9 seem to be involved in the pathogenesis of NASH. Toll-like receptor 2 is a receptor for multiple glycolipids or lipoproteins in bacteria adhering to the cell surface of monocytes, myeloid dendritic cells or mast cells. Toll-like receptor 4 is a LPS ligand located on the surfaces of monocytes, myeloid dendritic cells, mast cells, B cells and intestinal epithelium. This receptor ligated with representative pathogens of gut microbiota has been studied in detail. Levels of free cholesterol (but not of cholesterol ester) are increased in hepatic stellate cells (HSC) in NAFLD resulting in increased TLR4 protein levels and fibrogenic HSC [56]. Kupffer cells are phagocytes of various cellular, viral, or bacterial components that are sources of hepatic pro-inflammatory and pro-fibrogenic cytokines. Cholesterol phagocytosed to Kupffer cells can induce their activation along with TLR4 upregulation [57]. Toll-like receptor 9 is located on endoplasmic reticulum (ER) or endosomes of plasmacytoid dendritic cells or B cells and is regarded as a ligand for unmethylated CpG DNA particles that might be released from bacteria. These molecules have been analyzed in several NAFLD and NASH models. Free cholesterol can accumulate in fibrogenic hepatic stellate cells, resulting in an increase in TLR4 through suppressing the endosomal-lysosomal degradation pathway of TLR4. The increased expression of TLR4 sensitizes cells to TGF-β induced activation [56]. The results of TLR studies in different NASH models notably vary. So far, mouse NASH models can represent only a portion of the pathogenesis. For example, the choline-deficient amino acid-defined (CDAA) diet model mouse develops steatosis with relatively mild hepatitis or fibrosis with obesity, whereas the methionine and choline deficient (MCD) diet model mouse develops steatosis with severe hepatitis and fibrosis without obesity. The CDAA diet improved NASH outcomes, whereas the MCDD diet worsened NASH outcomes in TLR2 knockout mice [58,59]. This discrepancy might be true since NASH itself is also heterogeneous and complex. TLR4 and TLR9 agonists might flow into the portal veins of inflammasome-deficient MCDD mouse models and thus exacerbate NASH [50]. Inflammasomes are multiprotein complexes composed of nucleotide-binding domain and leucine-rich repeat protein 3 (NLRP3), apoptosis-associated speck-like protein containing CARD (ASC) and procaspase 1 that are DAMP or PAMP sensors. Inflammasome activation leads to the processing and secretion of proinflammatory cytokines IL1β and IL-18, whereas knockdown results in MCD NASH exacerbation. These perplexing findings indicate that the intestinal DAMP or PAMP barrier function that is disrupted in inflammasome knockout mice overcomes the anti-inflammatory effect in the liver and TLR4 and TLR9 ligands outflow into the portal vein where they stimulate NASH progression. Toll-like receptor 5 is the ligand for microbial flagellin. Mice that are deficient inTLR-5 develop spontaneous colitis, profound metabolic syndrome including hyperphagia, hypertension and insulin resistance brought about through intestinal microbiota transport [60,61]. However, another study found no such pathologies in TLR5-deficient mice [62]. The authors concluded that this discrepancy might have been induced by their animal facilities, an indication that might be important to consider for all such molecular functions.
3.4. Endoplasmic Reticulum (ER) Stress
Toxic lipids such as free fatty acids, diacylglyceride, phospholipids and free cholesterol activate several cellular stress pathways [63]. One epicenter for these stress responses is the ER, a membranous network that functions in the synthesis and assembly of secretory and membrane proteins to achieve their proper conformation. Proteins that are related to lipid metabolism are enriched whereas protein synthesis and transport functions are downregulated in obese hepatic ER [64]. The maintenance of ER function requires high concentrations of intra-ER Ca2+, which is actively controlled by sarco (endo) plasmic reticulum Ca2+-ATPase (SERCA). Free cholesterol accumulation triggers ER stress by altering the critical free cholesterol-to-phospholipid ratio of the ER membrane, which is needed to maintain its fluidity. Among the ER enzymes, SERCA ATPase is particularly sensitive to ER membrane cholesterol contents that can inhibit SERCA conformational changes and activity. Such changes induce a decrease in physiologically high intra-ER Ca2+ concentrations that result in impaired ER function known as ER stress. Such stress is one of the most important factors for disease progression in NASH along with hepatocyte apoptosis and hepatic stellate cell (HSC) or Kupffer cell activation.
The chaperone response is blunted in obese mice as a result of dysfunctional X-Box binding protein 1 (XBP1), which is a master regulator of ER folding capacity [65]. Levels of liver-specific SERCA2b are reduced in obese mice with increased ER stress. Increasing SERCA2b levels reduces ER stress in the liver and increases glucose tolerance [66]. The SERCA inhibitor thapsigargin induces an increase in cytosolic calcium contents with a decrease in ER calcium resulting in mitochondrial cytochrome C release and the apoptosis of cultured primary rat hepatocytes [67]. Kupffer cells function as antigen presenting cells by displaying major histocompatibility complex (MHC) peptides on the cell surface. These peptides are processed by proteasomal degradation in the cytosol and then translocated to the ER, where they undergo N-terminal trimming and loading onto MHC for export to the cell surface. The ER also contains inflammatory cytokine-inducible factors such as stimulator of interferon genes (STING) that induce type I interferon genes upon release [68]. Endoplasmic reticulum stress induces the activation of such MHC-related and non-related pro-inflammatory responses. These results suggest that diseases could be treated with anti-ER stress agents as noted in the above model mice. However, ER stress also sensitizes activated, but not quiescent HSC to apoptosis, resulting in the resolution of fibrosis [69]. Non-selective anti-ER stress treatment might be ineffective and treatment targeted towards ER stress should be designed to focus on the specific status of ER conditions.
3.5. Oxidative Stress
3.5.1. Oxidative Stress in Hepatocytes
Oxidative stress is involved in the mechanisms of aging, carcinogenesis and atherosclerotic progression. Excessive oxidative stress induced by mitochondrial, peroxisomal and microsomal reactive oxygen species (ROS) in NASH results in apoptosis as well as damage to nuclear and mitochondrial DNA. Limited antioxidant defenses contribute to the processes of both NASH and hepatocarcinogenesis [70,71]. Physiologically low levels of ROS are involved in necessary vital cellular processes indicating that the balance of oxidative anti-oxidative responses is important [72]. An imbalance in the mitochondrial respiratory chain is the main source of ROS, superoxide, hydrogen peroxide, and hydroxyl radicals. Acute oxidative stress can be induced by several types of organ stress such as inflammation, organ infarction, shock and reperfusion injury. Under oxidative stress, oxidative reactions such as β-oxidation, tricarboxylic acid (TCA) cycle turn oxidized (NAD+ and FAD) into reduced (NADH and FADH2) cofactors (Figure 2). Subsequent oxidation of these reduced cofactors results in the delivery of electrons to the final acceptor molecular oxygen through the mitochondrial respiratory chain, namely the electron transport chain (ETC; complexes I, III, and IV). Under physiological conditions, most reactive, incompletely reduced forms of oxygen, such as superoxide, are detoxified into water by various anti-oxidant defenses and repair enzymes to maintain a relatively low steady state of oxidants.
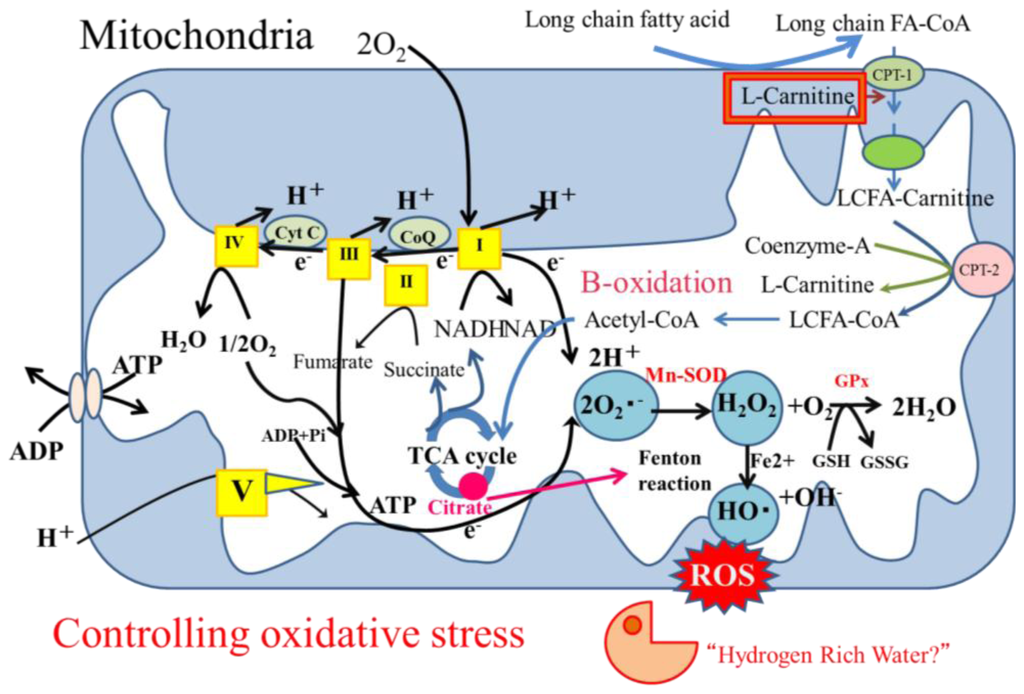
Figure 2.
Mitochondria as producers of oxidative stress. High levels of plasma free fatty acids allow upregulation of hepatic free fatty acids. Long-chain fatty acids taken up into mitochondria as complexes with l-carnitine are then transferred to β-oxidation pathway. Under oxidative stress, oxidative reactions convert oxidized cofactors (NAD+ and FAD) into reduced cofactors (NADH and FADH2) and deliver electrons to respiratory chain. Imbalance between increased delivery of electrons to respiratory chain and their decreased outflow from this chain causes electrons and ROS products to accumulate. Anti-oxidant defenses such as superoxide dismutase (SOD), glutathione peroxidase (GPx) or catalase can metabolize hydrogen peroxide to non-toxic H2O. However, the Fenton and/or Haber-Weiss reactions generate highly reactive toxic ROS, hydrogen peroxide. Hydrogen as selective cytotoxic ROS scavenger and l-carnitine as mitochondrial function supporting factor might be good candidates for controlling mitochondrial oxidative stress.
The mitochondrial capacity to control the oxidative balance will collapse under continuous oxidative stress. Excess superoxide could be generated within injured mitochondria through electron leakage and a resulting excess of superoxide would be converted to hydrogen peroxide by superoxide dismutase (SOD). Glutathione peroxidase (GPx) or catalase can metabolize hydrogen peroxide to non-toxic H2O, but the Fenton and/or Haber-Weiss reactions generate highly reactive toxic ROS, hydrogen peroxide. Such oxidative stress produced by Fenton reactions is mediated by iron. Although its role in NASH is not fully understood, levels of iron are elevated in NASH, which is an inducer of oxidative stress and reduced iron levels resulted in fair outcomes for patients with chronic liver diseases [73]. Elevated plasma citrate levels in NAFLD promotes iron mediated hydroxyl radical formation in vitro [74]. Excess fatty acid and glucose results in the elevation of pyruvate and acetyl-CoA that subsequently increases the formation of citrate, which induces iron related oxidative stress. The reactivity of ROS dictates toxicity and high reactivity results in a short life and limited diffusion (half life: superoxide and hydrogen peroxide 10−6 and 10−9 sec, respectively) [72]. However, ROS can attack polyunsaturated fatty acids (PUFA) and initiate lipid peroxidation resulting in the formation of aldehyde by-products such as 4-hydroxy-2-nonenal (4-HNE) or malondialdehyde (MDA) [75]. These 4-HNE and MDA have longer half-lives than ROS and they can diffuse into other sites and thus spread oxidative stress. Mitochondrial free fatty acid oxidation is not inhibited until respiration is severely impaired, resulting in accelerated ROS production until mitochondria are lost. Thus, mitochondria comprise the main source of oxidative stress, ER stress can induce superoxide and hydrogen peroxide with monooxygenases such as cytochrome P450 and peroxisomes can induce cytosolic hydrogen peroxide associated with fatty acid oxidation [76,77].
Mitochondrial antioxidant defenses are not powerful enough to control continuous oxidative stress such as that imposed by long chain free fatty acids and this results in oxidative damage to the ETC complexes and mitochondrial DNA [78,79]. The production of ROS in the inner membrane of mitochondria easily attacks proximal mitochondrial DNA. However, DNA repair mechanisms are thought to play a central role in preventing the accumulation of DNA mutations and in the maintenance of DNA stability [80]. Although both nuclear and mitochondrial DNA repair pathways exist, mitochondria are deficient in several of them [80]. Estrogen can recover one pathway of mitochondrial DNA repair, namely the base excision repair (BER) pathway that becomes impaired in aged female rats, indicating that estrogen confers benefits on DNA pathways in females [81]. Because of these mitochondrial DNA deficiencies in guarding against stress reactions, mitochondrial DNA could be easily broken or mutated by oxidative stress [82]. The accumulation of mitochondrial DNA mutations and damage results in a dysfunction of the ETC and leads to increased ROS as well as subsequent additional DNA damage. These oxidative stress chain reactions produce large amounts of ROS and target other mitochondria or organelles and induce cellular apoptosis.
The mitochondrial proliferation and differentiation program might be impaired in NASH. The most important regulator of mitochondrial biogenesis is the transcription coactivator peroxisome proliferator-activated receptor (PPAR)-γ-coactivator-1α (PGC-1α) [83] that coordinates the large number of genes required for mitochondrial biogenesis. The activity of PGC-1α is impaired in the fatty liver, which results in a decrease in mitochondrial biogenesis [84]. Decreases in mitochondrial DNA and mitochondrial DNA-encoded polypeptides are representative findings in NASH, whereas the mitochondrial DNA content is increased in simple fatty liver [85]. The complementary activation of mitochondrial DNA in simple fatty liver might help to protect the liver from inflammation and fibrosis, whereas a decrease in NASH induces progressive inflammation and fibrosis.
3.5.2. Oxidative Stress Affects outside Hepatocytes
Among the non-parenchymal cells that comprise almost 40% of all liver cells, Kupffer cells and HSC play significant roles in the progression of chronic liver inflammation and fibrosis progression [75]. Excess fatty acid accumulation in hepatocytes induces oxidative stress from not only mitochondria but also peroxisomes or microsomes. These cytotoxic ROS and lipid peroxidation products can diffuse into the extracellular space affecting Kupffer cells and HSC. These cellular oxidative stresses from hepatocytes and the direct uptake of free fatty acids or free cholesterol in Kupffer cells induce the activation of nuclear-factor κB, which induces the synthesis of TNF-α and several proinflammatory cytokines such as IL-6 or IL-8 [86]. Kupffer cells in patients with NASH produce TGF-β resulting in HSCs acquiring a fibrogenic myofibroblast-like phenotype.
Exposing primary HSC or HSC cell lines to hydrogen peroxide leads to an increase in the gene expression of ER chaperone BIP binding transmembrane proteins such as inositol requiring enzyme 1 (IRE1a), or activating transcription factor 4 (ATF4). These ER stresses in HSCs result in increased autophagy and HSC activation to fibrogenic status [87]. Among the most characteristic features of HSC activation is the loss of cytoplasmic lipid droplets, which are composed of retinyl esters and triglycerides [88]. Autophagy is present in all cell types and it is up-regulated as an adaptive response under cellular stress to generate intracellular nutrients and energy. Autophagy is up-regulated in activated HSC of mice with liver damage. However, cytoplasmic lipid droplets are maintained and stay quiescent in autophagy-defective HSCs, representing oxidative stress-induced ER stress and autophagy as a key event in HSC activation [89].
4. Treatment for NASH
4.1. General Aspects
Since NAFLD and NASH have emerged as lifestyle-associated diseases, lifestyle intervention is an important approach to their treatment. Healthy and Western-style diets differentiate the risk for NAFLD progression [90]. Even a one-year intensive lifestyle intervention that comprised dietary modifications and physical activity improved waist circumference, visceral abdominal fat, blood pressure, insulin resistance and hepatic fat content in obese patients [91]. Western-style diets, especially those that are rich in trans-fatty acids, are powerful inducers of obesity and NAFLD needs to be avoided [92]. However, to maintain compliance with such therapeutic measures as restrained food consumption is mentally challenging and the rebound rate is high [93]. Such patients require pharmacological therapies.
Because the general characteristics of NASH comprise obesity and insulin resistance, anti-insulin resistance treatment has played significant roles. From this viewpoint, the ability of insulin sensitizing anti-diabetic drugs to treat NASH has been analyzed. Among these, the PPAR-α agonist pioglitazone and metformin have clinically improved NASH. However, neither of these drugs could improve liver histology. Metformin induced weight loss, whereas pioglitazone induced weight gain. Metformin has brought about histological improvements in the non-diabetic NASH mouse model [94]. Human trials are usually too short to generate histological improvements. These anti-diabetic insulin sensitizing drugs also have anti-oxidative effects. Glucagon like peptide-1 (GLP-1) and gastric inhibitory polypeptide (GIP) are both incretins, which are a group of gastrointestinal hormones that cause increased insulin release from pancreatic beta cells, and they might be good targets for treating NASH. A GLP-1 receptor agonist analogue improved metabolic, biochemical and histopathological indices of NASH in mice via restoring hepatic lipid oxidation [95]. A GLP-1 receptor agonist in type 2 diabetic patients with NAFLD caused a reduction in intrahepatic lipid content that correlated with diabetic control [96]. Dipeptidylpeptidase-IV (DPP-IV) degrades GLP-1 and GIP and thus the inhibition of DPP-IV extends the half-life of endogenous GLP-1 and GIP, resulting in diabetic control. The long-term administration of a DPP-IV inhibitor has reduced liver fat content in animals with diet-induced hepatic steatosis and insulin resistance [97].
The representative antioxidant vitamin E improved the non-alcoholic fatty liver disease activity scores (NAS) for clinical and histological activity within two years but increased insulin resistance and plasma triglyceride levels [12]. However, the recovery of fibrosis progression was not proven [98]. Antioxidants had no effect on body weight, waist circumference and cholesterol metabolism. l-carnitine is a precursor of carnitine-palmitoyltransferase 1 (CPT-1), the rate-limiting enzyme for mitochondrial β-oxidation that affects mitochondrial function. Any deficiency in the mitochondrial carnitine-dependent transport system results in curtailed fatty acid oxidation. l-carnitine supplementation reduces TNF-α, liver function parameters, plasma glucose levels and histological scores [99]. Pentoxiphylline is a methylxanthine derivative that increases red blood cell flexibility, reduces blood viscosity and decreases platelet aggregation. In addition, pentoxiphylline suppresses TNF-α gene transcription and it is a hydroxyl and peroxyl radical scavenger that results in anti-oxidative effects. A randomized controlled trial (RCT) has proven that pentoxiphylline decreases free-radical-mediated lipid oxidation and improves clinical and histological NASH [100,101].
Lipid-lowering drugs such as statins can also improve ALT and radiological steatosis in hyperlipidemic patients with NAFLD, but histological improvements are not evident [102]. Ezetimibe is a Niemann-Pick C1-like protein inhibitor that can reduce the intestinal accumulation of free cholesterol. Ezetimibe has reduced histological NAS improvement in mice and in 10 patients with NASH, indicating a need for larger RCT [103,104].
Ursodeoxycholic acid (UDCA) is also reportedly effective in some instances. Several RCT have found improvements in ALT but not in liver histology even at high doses [105]. Combinations of UDCA and vitamin E have improved ALT and histological NAS scores [106].
Tumor necrosis factor (TNF)-α is one of the main cytokines involved in adipocyte-related inflammation including NASH. Powerful anti-TNF-α agents such as infliximab (a chimeric monoclonal antibody), adalimumab (a human monoclonal antibody) and etanercept (a fusion protein) have severe side effects such as tuberculosis that would render them unacceptable as therapy for NAFLD [107]. The anti-oxidative agent pentoxifylline described above also has an anti-TNF-α function that is partially involved in the favorable effects on NASH.
A pro-inflammatory intestinal microbiome has been identified in mice and in patients with NASH. Probiotics such as butyrate-producing agents reduce hepatic triglyceride contents and induce anti-oxidative enzymes that help to prevent the progression of NASH to hepatocellular carcinoma [53].
4.2. Hydrogen-Rich Water as a Candidate Treatment for NASH
4.2.1. Hydrogen as an Antioxidant Treatment
Controversy surrounds antioxidant therapies because ROS have essential functions in living organisms. Balancing oxidative stress is the key issue, and bioavailability and bioaccessibility are needed to elicit an effective response to drugs. Antioxidants have effective chemical activities in vitro, however, many failures have been shown in proving in vivo fair effects [108]. Many cerebrovascular studies have investigated the effects of the representative antioxidant vitamin E. A meta-analysis of the effect of vitamin E on stroke revealed a 10% reduction in ischemic stroke accompanied by a 22% increase in hemorrhagic stroke. Antioxidants are likely to cause the progression of cancer [109]. Stem cell like cancer cells have powerful antioxidative properties that protect them from oxidative stress and thus prevent their apoptosis [110]. Oxidative stress upon normal cells might induce them to become cancer cells that are highly resistant to further oxidative stress. Inducing oxidative stress under these conditions is an approach to treat cancers that several trials are investigating [111]. However, this approach is likely to kill normal cells and induce new cancer development from normal cells. Thus, oxidative stress must be controlled according to clinical circumstances. The clinical findings of antioxidant therapies have not always been favorable [112]. This might be because mitochondria do not effectively take up antioxidants that would then interfere with the essential mechanisms of oxidative stress that protects cells from infection or other invasive cellular injury [113]. Ohsawa et al. [18] have found that molecular hydrogen has powerful antioxidant effects with unique features. In cultured cells H2 scavenges hydroxyl radicals but not superoxide or hydrogen peroxide and nitric oxide (NO). Levels of intracellular superoxide increased in cells cultured with the mitochondrial respiratory complex III inhibitor, antimycin A and then rapidly converted to hydrogen peroxide and hydroxyl radicals. Culture with H2 decreased levels of hydroxyl radicals but not those of superoxide, hydrogen peroxide or steady-state levels of NO in cells. Even nuclear levels of hydroxyl radicals were notably decreased. As a result, H2 prevented a decline in the mitochondrial membrane potential and the decrease in cellular ATP synthesis suggestive of effective antioxidants.
To defend cells against bacterial invasion, hydrogen peroxide is converted to hypochlorous acid by myeloperoxidase, indicating that oxidative stress is important for survival [114]. Additionally, NO functions as a neurotransmitter that is essential for blood vessel dilation and it protects against endothelial cell activation, suggesting that oxidative stress is important for survival [115]. Treatment with H2 reduces levels of hydroxyl radicals but not those of superoxide or hydrogen peroxide, which have physiological roles in cell survival.
Most hydrophilic compounds are retained at membranes and never reach the cytoplasm, whereas hydrophobic compounds such as vitamin E cannot penetrate biomembranes in the absence of specific carriers or receptors. In contrast, H2 can diffuse into cytoplasm and intracellular organelles such as mitochondria, the ER and the nucleus.
High concentrations of H2 are not cytotoxic. Breathing high concentrations of H2 in gas has been used to treat decompression sickness and arterial gas thrombosis after deep diving [116].
Several disease models have been created to determine the effects of molecular hydrogen (Table 1) [19,70,117–129]. Hydrogen can be ingested mainly by gas inhalation or drinking hydrogen-rich water. Hydrogen can be inhaled via hydrogen gas delivered through a ventilator with a face mask. Arterial blood levels of H2 increase depending upon the concentration of inhaled H2 gas. The diffusion of H2 gas has been monitored in the rat myocardium [118], in which the H2 concentration was increased by two thirds in the ischemic compared with the non-ischemic myocardium. Ischemic volume was decreased one day after middle cerebral artery occlusion in a rat model of cerebral infarction that had inhaled H2, the wider volume difference one week after occlusion between these and control rats indicated improvements in chronic ischemic stress [18]. The inhalation of H2 reduces inflammatory responses associated with ventilator-induced lung injury at local and systemic levels via its antioxidant, anti-inflammatory and anti-apoptotic effects [130].

Table 1.
Effectiveness of hydrogen.
Drinking hydrogen-rich water is a straightforward method of daily administration at outpatient clinics because up to 0.8 mM H2 (1.6 ppm, w/v) can be dissolved in water under atmospheric pressure. Glass or plastic containers are unsuitable for conserving H2 since it can penetrate rapidly, whereas aluminum bags can retain H2 for long periods [113]. Hydrogen-rich water can be prepared by dissolving hydrogen gas in water under high pressure or by the reaction of magnesium metal with water. The detection of H2 at μM levels in the liver and the fact that the H2 concentration peaks at five minutes after hydrogen-rich water reaches the stomach suggests that the liver is a good H2 target [122].
4.2.2. Hydrogen as an Antioxidant Treatment Candidate for NASH
The effects of hydrogen on chemically induced liver damage have been studied in mouse models of liver damage induced by GalN/LPS, CCl4 and diethylnitrosamine (DEN) [125]. Hydrogen was given intraperitoneally every three hours after the administration of chemicals. Serum levels of TNF-α and IL-6 and transaminase levels decreased in mice with GalN/LPS-induced acute liver injury after H2 administration. Hepatic fibrogenesis markers such as collagen-α1 or α-smooth muscle actin (SMA) were reduced in model mice with CCl4-induced liver cirrhosis and proliferative activities of hepatocytes were reduced in mice with DEN induced hepatic tumorigenesis.
Kawai et al. have reported that drinking hydrogen-rich water has favorable effects in NASH models [70]. Plasma transaminase levels, histological NAS, hepatic TNF-α, IL-6 and fatty acid synthesis-related gene expression and the oxidative stress biomarker 8-OHdG were decreased in the livers of established MCD diet-induced NASH models administered with hydrogen-rich water or anti-oxidative pioglitazone. Although the decrease in hepatic cholesterol was smaller in the group given hydrogen-rich water, serum oxidative stress was reduced and antioxidant function was higher than that in the pioglitazone group. Another NASH mouse model was constructed to determine whether hydrogen affects hepatocarcinogenesis. The Stellic Research Institute created a mouse model of streptozotocin-induced NASH (STAM) that represents hepatocarcinogenesis within 16 weeks [131]. Hydrogen-rich water reduced the number and size of hepatocellular carcinoma lesions in this model compared with controls.
The consumption of hydrogen-rich water might effectively treat NASH by reducing hepatic oxidative stress, inflammation, and hepatocarcinogenesis. We emphasize that because the results of many investigations into the effects of antioxidants upon diseases associated with oxidative stress have been disappointing, hydrogen might be the same as those of previously discouraged agents. More basic and clinical understanding of this novel potential treatment option is required.
5. Conclusions
Multiple parallel hits derived from lifestyles are now believed to cause NASH. The pathogenesis of cellular damage is also heterogeneous, but oxidative stress seems to be one key factor that affects the progression of NASH. Several approaches to control this hidden phenomenon are often unsuccessful, because it is also essential for life. However, new approaches that target mitochondria such as l-carnitine, pentoxyphyllin, or molecular hydrogen have favorable effects. Optimal treatment protocols and combinations of these elements should be further investigated.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Pacifico, L.; Anania, C.; Martino, F.; Poggiogalle, E.; Chiarelli, F.; Arca, M.; Chiesa, C. Management of metabolic syndrome in children and adolescents. Nutr. Metab. Cardiovasc. Dis 2011, 21, 455–466. [Google Scholar]
- Matteoni, C.A.; Younossi, Z.M.; Gramlich, T.; Boparai, N.; Liu, Y.C.; McCullough, A.J. Nonalcoholic fatty liver disease: A spectrum of clinical and pathological severity. Gastroenterology 1999, 116, 1413–1419. [Google Scholar]
- Brunt, E.M.; Kleiner, D.E.; Wilson, L.A.; Unalp, A.; Behling, C.E.; Lavine, J.E.; Neuschwander-Tetri, B.A. Portal chronic inflammation in nonalcoholic fatty liver disease (NAFLD): A histologic marker of advanced NAFLD-Clinicopathologic correlations from the nonalcoholic steatohepatitis clinical research network. Hepatology 2009, 49, 809–820. [Google Scholar]
- Yatsuji, S.; Hashimoto, E.; Tobari, M.; Taniai, M.; Tokushige, K.; Shiratori, K. Clinical features and outcomes of cirrhosis due to non-alcoholic steatohepatitis compared with cirrhosis caused by chronic hepatitis C. J. Gastroenterol. Hepatol 2009, 24, 248–254. [Google Scholar]
- Hatanaka, K.; Kudo, M.; Fukunaga, T.; Ueshima, K.; Chung, H.; Minami, Y.; Sakaguchi, Y.; Hagiwara, S.; Orino, A.; Osaki, Y. Clinical characteristics of NonBNonC-HCC: Comparison with HBV and HCV related HCC. Intervirology 2007, 50, 24–31. [Google Scholar]
- Fassio, E.; Alvarez, E.; Dominguez, N.; Landeira, G.; Longo, C. Natural history of nonalcoholic steatohepatitis: A longitudinal study of repeat liver biopsies. Hepatology 2004, 40, 820–826. [Google Scholar]
- Ono, M.; Saibara, T. Clinical features of nonalcoholic steatohepatitis in Japan: Evidence from the literature. J. Gastroenterol 2006, 41, 725–732. [Google Scholar]
- Gentile, C.L.; Pagliassotti, M.J. The role of fatty acids in the development and progression of nonalcoholic fatty liver disease. J. Nutr. Biochem 2008, 19, 567–576. [Google Scholar]
- Csak, T.; Ganz, M.; Pespisa, J.; Kodys, K.; Dolganiuc, A.; Szabo, G. Fatty acids and endotoxin activate inflammasome in hepatocytes which release danger signals to activate immune cells in steatohepatitis. Hepatology 2011, 54, 133–144. [Google Scholar]
- Pessayre, D. Role of mitochondria in non-alcoholic fatty liver disease. J. Gastroenterol. Hepatol 2007, 22, S20–S27. [Google Scholar]
- Novo, E.; Busletta, C.; Bonzo, L.V.; Povero, D.; Paternostro, C.; Mareschi, K.; Ferrero, I.; David, E.; Bertolani, C.; Caligiuri, A.; et al. Intracellular reactive oxygen species are required for directional migration of resident and bone marrow-derived hepatic pro-fibrogenic cells. J. Hepatol 2011, 54, 964–974. [Google Scholar]
- Sanyal, A.J.; Chalasani, N.; Kowdley, K.V.; McCullough, A.; Diehl, A.M.; Bass, N.M.; Neuschwander-Tetri, B.A.; Lavine, J.E.; Tonascia, J.; Unalp, A.; et al. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N. Engl. J. Med 2010, 362, 1675–1685. [Google Scholar]
- Nan, Y.M.; Wu, W.J.; Fu, N.; Liang, B.L.; Wang, R.Q.; Li, L.X.; et al. Antioxidants vitamin E and 1-aminobenzotriazole prevent experimental non-alcoholic steatohepatitis in mice. Scand. J. Gastroenterol 2009, 44, 1121–1131. [Google Scholar]
- Bugianesi, E.; Gentilcore, E.; Manini, R.; Natale, S.; Vanni, E.; Villanova, N.; David, E.; Rizzetto, M.; Marchesini, G. A randomized controlled trial of metformin versus vitamin E or prescriptive diet in nonalcoholic fatty liver disease. Am. J. Gastroenterol 2005, 100, 1082–1090. [Google Scholar]
- Musso, G.; Gambino, R.; Cassader, M.; Pagano, G. A meta-analysis of randomized trials for the treatment of nonalcoholic fatty liver disease. Hepatology 2010, 52, 79–104. [Google Scholar]
- Steinhubl, S.R. Why have antioxidants failed in clinical trials? Am. J. Cardiol 2008, 101, S14–S19. [Google Scholar]
- Ohta, S. Molecular hydrogen is a novel antioxidant to efficiently reduce oxidative stress with potential for the improvement of mitochondrial diseases. Biochim. Biophys. Acta 2011, 1820, 586–594. [Google Scholar]
- Ohsawa, I.; Ishikawa, M.; Takahashi, K.; Watanabe, M.; Nishimaki, K.; Yamagata, K.; Katsura, K.; Katayama, Y.; Asoh, S.; Ohta, S. Hydrogen acts as a therapeutic antioxidant by selectively reducing cytotoxic oxygen radicals. Nat. Med 2007, 13, 688–694. [Google Scholar]
- Cardinal, J.S.; Zhan, J.; Wang, Y.; Sugimoto, R.; Tsung, A.; McCurry, K.R.; Billiar, T.R.; Nakao, A. Oral hydrogen water prevents chronic allograft nephropathy in rats. Kidney Int 2010, 77, 101–109. [Google Scholar]
- Day, C.P.; James, O.F. Steatohepatitis: A tale of two “hits”? Gastroenterology 1998, 114, 842–845. [Google Scholar]
- Tilg, H.; Moschen, A.R. Evolution of inflammation in nonalcoholic fatty liver disease: The multiple parallel hits hypothesis. Hepatology 2010, 52, 1836–1846. [Google Scholar]
- Tiniakos, D.G.; Vos, M.B.; Brunt, E.M. Nonalcoholic fatty liver disease: Pathology and pathogenesis. Annu. Rev. Pathol 2010, 5, 145–171. [Google Scholar]
- Li, Z.; Yang, S.; Lin, H.; Huang, J.; Watkins, P.A.; Moser, A.B.; Desimone, C.; Song, X.Y.; Diehl, A.M. Probiotics and antibodies to TNF inhibit inflammatory activity and improve nonalcoholic fatty liver disease. Hepatology 2003, 37, 343–350. [Google Scholar]
- Romeo, S.; Kozlitina, J.; Xing, C.; Pertsemlidis, A.; Cox, D.; Pennacchio, L.A.; Boerwinkle, E.; Cohen, J.C.; Hobbs, H.H. Genetic variation in PNPLA3 confers susceptibility to nonalcoholic fatty liver disease. Nat. Genet 2008, 40, 1461–1465. [Google Scholar]
- Valenti, L.; Alisi, A.; Galmozzi, E.; Bartuli, A.; Del Menico, B.; Alterio, A.; Dongiovanni, P.; Fargion, S.; Nobili, V. I148M patatin-like phospholipase domain-containing 3 gene variant and severity of pediatric nonalcoholic fatty liver disease. Hepatology 2010, 52, 1274–1280. [Google Scholar]
- Kawaguchi, T.; Sumida, Y.; Umemura, A.; Matsuo, K.; Takahashi, M.; Takamura, T.; Yasui, K.; Saibara, T.; Hashimoto, E.; Kawanaka, M.; et al. Genetic polymorphisms of the human PNPLA3 gene are strongly associated with severity of non-alcoholic fatty liver disease in Japanese. PloS One 2012, 7, e38322. [Google Scholar]
- Qiao, A.; Liang, J.; Ke, Y.; Li, C.; Cui, Y.; Shen, L.; Zhang, H.; Cui, A.; Liu, X.; Liu, C.; et al. Mouse patatin-like phospholipase domain-containing 3 influences systemic lipid and glucose homeostasis. Hepatology 2011, 54, 509–521. [Google Scholar]
- Tsuneto, A.; Hida, A.; Sera, N.; Imaizumi, M.; Ichimaru, S.; Nakashima, E.; Seto, S.; Maemura, K.; Akahoshi, M. Fatty liver incidence and predictive variables. Hypertens. Res 2010, 33, 638–643. [Google Scholar]
- Eguchi, Y.; Eguchi, T.; Mizuta, T.; Ide, Y.; Yasutake, T.; Iwakiri, R.; Hisatomi, A.; Ozaki, I.; Yamamoto, K.; Kitajima, Y.; et al. Visceral fat accumulation and insulin resistance are important factors in nonalcoholic fatty liver disease. J. Gastroenterol 2006, 41, 462–469. [Google Scholar]
- Nobili, V.; Svegliati-Baroni, G.; Alisi, A.; Miele, L.; Valenti, L.; Vajro, P. A 360-degree overview of paediatric NAFLD: Recent insights. J. Hepatol 2013, 58, 1218–1229. [Google Scholar]
- Musso, G.; Cassader, M.; de Michieli, F.; Rosina, F.; Orlandi, F.; Gambino, R. Nonalcoholic steatohepatitis versus steatosis: Adipose tissue insulin resistance and dysfunctional response to fat ingestion predict liver injury and altered glucose and lipoprotein metabolism. Hepatology 2012, 56, 933–942. [Google Scholar]
- Yamaguchi, K.; Yang, L.; McCall, S.; Huang, J.; Yu, X.X.; Pandey, S.K.; Bhanot, S.; Monia, B.P.; Li, Y.X.; Diehl, A.M. Inhibiting triglyceride synthesis improves hepatic steatosis but exacerbates liver damage and fibrosis in obese mice with nonalcoholic steatohepatitis. Hepatology 2007, 45, 1366–1374. [Google Scholar]
- Yu, X.X.; Murray, S.F.; Pandey, S.K.; Booten, S.L.; Bao, D.; Song, X.Z.; Kelly, S.; Chen, S.; McKay, R.; Monia, B.P.; et al. Antisense oligonucleotide reduction of DGAT2 expression improves hepatic steatosis and hyperlipidemia in obese mice. Hepatology 2005, 42, 362–371. [Google Scholar]
- Kantartzis, K.; Machicao, F.; Machann, J.; Schick, F.; Fritsche, A.; Haring, H.U.; Stefan, N. The DGAT2 gene is a candidate for the dissociation between fatty liver and insulin resistance in humans. Clin. Sci 2009, 116, 531–537. [Google Scholar]
- Corbetta, S.; Bulfamante, G.; Cortelazzi, D.; Barresi, V.; Cetin, I.; Mantovani, G.; Bondioni, S.; Beck-Peccoz, P.; Spada, A. Adiponectin expression in human fetal tissues during mid- and late gestation. J. Clin. Endocrinol. Metab 2005, 90, 2397–2402. [Google Scholar]
- Polyzos, S.A.; Toulis, K.A.; Goulis, D.G.; Zavos, C.; Kountouras, J. Serum total adiponectin in nonalcoholic fatty liver disease: A systematic review and meta-analysis. Metabolism 2011, 60, 313–326. [Google Scholar]
- Carbone, F.; la Rocca, C.; Matarese, G. Immunological functions of leptin and adiponectin. Biochimie 2012, 94, 2082–2088. [Google Scholar]
- Shimada, M.; Kawahara, H.; Ozaki, K.; Fukura, M.; Yano, H.; Tsuchishima, M.; Bondioni, S.; Beck-Peccoz, P.; Spada, A. Usefulness of a combined evaluation of the serum adiponectin level, HOMA-IR, and serum type IV collagen 7S level to predict the early stage of nonalcoholic steatohepatitis. Am. J. Gastroenterol 2007, 102, 1931–1938. [Google Scholar]
- Younossi, Z.M.; Jarrar, M.; Nugent, C.; Randhawa, M.; Afendy, M.; Stepanova, M.; Bondioni, S.; Beck-Peccoz, P.; Spada, A. A novel diagnostic biomarker panel for obesity-related nonalcoholic steatohepatitis (NASH). Obes. Surg 2008, 18, 1430–1437. [Google Scholar]
- Argentou, M.; Tiniakos, D.G.; Karanikolas, M.; Melachrinou, M.; Makri, M.G.; Kittas, C.; Kalfarentzos, F. Adipokine serum levels are related to liver histology in severely obese patients undergoing bariatric surgery. Obes. Surg 2009, 19, 1313–1323. [Google Scholar]
- Lemoine, M.; Ratziu, V.; Kim, M.; Maachi, M.; Wendum, D.; Paye, F.; Bastard, J.P.; Poupon, R.; Housset, C.; Capeau, J.; et al. Serum adipokine levels predictive of liver injury in non-alcoholic fatty liver disease. Liver Int 2009, 29, 1431–1438. [Google Scholar]
- Kaser, S.; Moschen, A.; Cayon, A.; Kaser, A.; Crespo, J.; Pons-Romero, F.; Ebenbichler, C.F.; Patsch, J.R.; Tilg, H. Adiponectin and its receptors in non-alcoholic steatohepatitis. Gut 2005, 54, 117–121. [Google Scholar]
- Matsunami, T.; Sato, Y.; Ariga, S.; Sato, T.; Shimomura, T.; Kashimura, H.; Hasegawa, Y.; Yukawa, M. Regulation of synthesis and oxidation of fatty acids by adiponectin receptors (AdipoR1/R2) and insulin receptor substrate isoforms (IRS-1/-2) of the liver in a nonalcoholic steatohepatitis animal model. Metabolism 2011, 60, 805–814. [Google Scholar]
- Nannipieri, M.; Cecchetti, F.; Anselmino, M.; Mancini, E.; Marchetti, G.; Bonotti, A.; Baldi, S.; Solito, B.; Giannetti, M.; Pinchera, A.; et al. Pattern of expression of adiponectin receptors in human liver and its relation to nonalcoholic steatohepatitis. Obes. Surg 2009, 19, 467–474. [Google Scholar]
- Ma, H.; Gomez, V.; Lu, L.; Yang, X.; Wu, X.; Xiao, S.Y. Expression of adiponectin and its receptors in livers of morbidly obese patients with non-alcoholic fatty liver disease. J. Gastroenterol. Hepatol 2009, 24, 233–237. [Google Scholar]
- Miele, L.; Valenza, V.; la Torre, G.; Montalto, M.; Cammarota, G.; Ricci, R.; Masciana, R.; Forgione, A.; Gabrieli, M.L.; Perotti, G.; et al. Increased intestinal permeability and tight junction alterations in nonalcoholic fatty liver disease. Hepatology 2009, 49, 1877–1887. [Google Scholar]
- Brun, P.; Castagliuolo, I.; di Leo, V.; Buda, A.; Pinzani, M.; Palu, G.; Martines, D. Increased intestinal permeability in obese mice: New evidence in the pathogenesis of nonalcoholic steatohepatitis. Am. J. Physiol. Gastrointest. Liver Physiol 2007, 292, G518–G525. [Google Scholar]
- Cani, P.D.; Bibiloni, R.; Knauf, C.; Waget, A.; Neyrinck, A.M.; Delzenne, N.M.; Burcelin, R. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes 2008, 57, 1470–1481. [Google Scholar]
- Cani, P.D.; Amar, J.; Iglesias, M.A.; Poggi, M.; Knauf, C.; Bastelica, D.; Neyrinck, A.M.; Fava, F.; Tuohy, K.M.; Chabo, C.; et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes 2007, 56, 1761–1772. [Google Scholar]
- Henao-Mejia, J.; Elinav, E.; Jin, C.; Hao, L.; Mehal, W.Z.; Strowig, T.; Thaiss, C.A.; Kau, A.L.; Eisenbarth, S.C.; Jurczak, M.J.; et al. Inflammasome-mediated dysbiosis regulates progression of NAFLD and obesity. Nature 2012, 482, 179–185. [Google Scholar]
- Tamaki, N.; Takaki, A.; Tomofuji, T.; Endo, Y.; Kasuyama, K.; Ekuni, D.; Yasunaka, T.; Yamamoto, K.; Morita, M. Stage of hepatocellular carcinoma is associated with periodontitis. J. Clin. Periodontol 2011, 38, 1015–1020. [Google Scholar]
- Yoneda, M.; Naka, S.; Nakano, K.; Wada, K.; Endo, H.; Mawatari, H.; Imajo, K.; Nomura, R.; Hokamura, K.; Ono, M.; et al. Involvement of a periodontal pathogen, Porphyromonas gingivalis on the pathogenesis of non-alcoholic fatty liver disease. BMC Gastroenterol 2012, 12. [Google Scholar] [CrossRef]
- Endo, H.; Niioka, M.; Kobayashi, N.; Tanaka, M.; Watanabe, T. Butyrate-producing probiotics reduce nonalcoholic fatty liver disease progression in rats: New insight into the probiotics for the gut-liver axis. PloS One 2013, 8, e63388. [Google Scholar]
- Xu, R.Y.; Wan, Y.P.; Fang, Q.Y.; Lu, W.; Cai, W. Supplementation with probiotics modifies gut flora and attenuates liver fat accumulation in rat nonalcoholic fatty liver disease model. J. Clin. Biochem. Nutr 2012, 50, 72–77. [Google Scholar]
- Yoshimoto, S.; Loo, T.M.; Atarashi, K.; Kanda, H.; Sato, S.; Oyadomari, S.; Iwakura, Y.; Oshima, K.; Morita, H.; Hattori, M.; et al. Obesity-induced gut microbial metabolite promotes liver cancer through senescence secretome. Nature 2013, 499, 97–101. [Google Scholar]
- Tomita, K.; Teratani, T.; Suzuki, T.; Shimizu, M.; Sato, H.; Narimatsu, K.; Okada, Y.; Kurihara, C.; Irie, R.; Yokoyama, H.; et al. Free cholesterol accumulation in hepatic stellate cells: Mechanism of liver fibrosis aggravation in nonalcoholic steatohepatitis in mice. Hepatology 2013. [Google Scholar] [CrossRef]
- Leroux, A.; Ferrere, G.; Godie, V.; Cailleux, F.; Renoud, M.L.; Gaudin, F.; Naveau, S.; Prevot, S.; Makhzami, S.; Perlemuter, G.; et al. Toxic lipids stored by Kupffer cells correlates with their pro-inflammatory phenotype at an early stage of steatohepatitis. J. Hepatol 2012, 57, 141–149. [Google Scholar]
- Miura, K.; Yang, L; van Rooijen, N.; Brenner, D.A.; Ohnishi, H.; Seki, E. Toll-like receptor 2 and palmitic acid cooperatively contribute to the development of nonalcoholic steatohepatitis through inflammasome activation in mice. Hepatology 2013, 57, 577–589. [Google Scholar]
- Rivera, C.A.; Gaskin, L.; Allman, M.; Pang, J.; Brady, K.; Adegboyega, P.; Pruitt, K. Toll-like receptor-2 deficiency enhances non-alcoholic steatohepatitis. BMC Gastroenterol 2010, 10. [Google Scholar] [CrossRef]
- Vijay-Kumar, M.; Sanders, C.J.; Taylor, R.T.; Kumar, A.; Aitken, J.D.; Sitaraman, S.V.; Neish, A.S.; Uematsu, S.; Akira, S.; Williams, I.R.; et al. Deletion of TLR5 results in spontaneous colitis in mice. J. Clin. Invest 2007, 117, 3909–3921. [Google Scholar]
- Vijay-Kumar, M.; Aitken, J.D.; Carvalho, F.A.; Cullender, T.C.; Mwangi, S.; Srinivasan, S.; Sitaraman, S.V.; Knight, R.; Ley, R.E.; Gewirtz, A.T. Metabolic syndrome and altered gut microbiota in mice lacking Toll-like receptor 5. Science 2010, 328, 228–231. [Google Scholar]
- Letran, S.E.; Lee, S.J.; Atif, S.M.; Flores-Langarica, A.; Uematsu, S.; Akira, S.; Cunningham, A.F.; McSorley, S.J. TLR5-deficient mice lack basal inflammatory and metabolic defects but exhibit impaired CD4 T cell responses to a flagellated pathogen. J. Immunol 2011, 186, 5406–5412. [Google Scholar]
- Musso, G.; Gambino, R.; Cassader, M. Cholesterol metabolism and the pathogenesis of non-alcoholic steatohepatitis. Prog. Lipid Res 2013, 52, 175–191. [Google Scholar]
- Fu, S.; Yang, L.; Li, P.; Hofmann, O.; Dicker, L.; Hide, W.; Lin, X.; Watkins, S.M.; Ivanov, A.R.; Hotamisligil, G.S. Aberrant lipid metabolism disrupts calcium homeostasis causing liver endoplasmic reticulum stress in obesity. Nature 2011, 473, 528–531. [Google Scholar] [Green Version]
- Park, S.W.; Zhou, Y.; Lee, J.; Lu, A.; Sun, C.; Chung, J.; Ueki, K.; Ozcan, U. The regulatory subunits of PI3K, p85α and p85β, interact with XBP-1 and increase its nuclear translocation. Nat. Med 2010, 16, 429–437. [Google Scholar]
- Park, S.W.; Zhou, Y.; Lee, J.; Ozcan, U. Sarco(endo)plasmic reticulum Ca2+-ATPase 2b is a major regulator of endoplasmic reticulum stress and glucose homeostasis in obesity. Proc. Natl. Acad. Sci. USA 2010, 107, 19320–19325. [Google Scholar]
- Canova, N.K.; Kmonickova, E.; Martinek, J.; Zidek, Z.; Farghali, H. Thapsigargin, a selective inhibitor of sarco-endoplasmic reticulum Ca2+-ATPases, modulates nitric oxide production and cell death of primary rat hepatocytes in culture. Cell Biol. Toxicol 2007, 23, 337–354. [Google Scholar]
- Barker, J.R.; Koestler, B.J.; Carpenter, V.K.; Burdette, D.L.; Waters, C.M.; Vance, R.E.; Valdivia, R.H. STING-dependent recognition of cyclic di-AMP mediates type I interferon responses during Chlamydia trachomatis infection. MBio 2013, 4. [Google Scholar] [CrossRef]
- De Minicis, S.; Candelaresi, C.; Agostinelli, L.; Taffetani, S.; Saccomanno, S.; Rychlicki, C.; Trozzi, L.; Marzioni, M.; Benedetti, A.; Svegliati-Baroni, G. Endoplasmic Reticulum stress induces hepatic stellate cell apoptosis and contributes to fibrosis resolution. Liver Int 2012, 32, 1574–1584. [Google Scholar]
- Kawai, D.; Takaki, A.; Nakatsuka, A.; Wada, J.; Tamaki, N.; Yasunaka, T.; Koike, K.; Tsuzaki, R.; Matsumoto, K.; Miyake, Y.; et al. Hydrogen-rich water prevents progression of nonalcoholic steatohepatitis and accompanying hepatocarcinogenesis in mice. Hepatology 2012, 56, 912–921. [Google Scholar]
- Bugianesi, E. Non-alcoholic steatohepatitis and cancer. Clin. Liver Dis 2007, 11, 191–207. [Google Scholar]
- Mittler, R.; Vanderauwera, S.; Suzuki, N.; Miller, G.; Tognetti, V.B.; Vandepoele, K.; Gollery, M.; Shulaev, V.; van Breusegem, F. ROS signaling: The new wave? Trends Plant Sci 2011, 16, 300–309. [Google Scholar]
- Nelson, J.E.; Wilson, L.; Brunt, E.M.; Yeh, M.M.; Kleiner, D.E.; Unalp-Arida, A.; Kowdley, K.V. Relationship between the pattern of hepatic iron deposition and histological severity in nonalcoholic fatty liver disease. Hepatology 2011, 53, 448–457. [Google Scholar]
- Van de Wier, B.; Balk, J.M.; Haenen, G.R.; Giamouridis, D.; Bakker, J.A.; Bast, B.C.; den Hartog, G.J.; Koek, G.H.; Bast, A. Elevated citrate levels in non-alcoholic fatty liver disease: The potential of citrate to promote radical production. FEBS Lett 2013, 587, 2461–2466. [Google Scholar]
- Rolo, A.P.; Teodoro, J.S.; Palmeira, C.M. Role of oxidative stress in the pathogenesis of nonalcoholic steatohepatitis. Free Radic. Biol. Med 2012, 52, 59–69. [Google Scholar]
- Fritz, R.; Bol, J.; Hebling, U.; Angermuller, S.; Volkl, A.; Fahimi, H.D.; Mueller, S. Compartment-dependent management of H2O2 by peroxisomes. Free Radic. Biol. Med 2007, 42, 1119–1129. [Google Scholar]
- Zangar, R.C.; Davydov, D.R.; Verma, S. Mechanisms that regulate production of reactive oxygen species by cytochrome P450. Toxicol. Appl. Pharmacol 2004, 199, 316–331. [Google Scholar]
- Rachek, L.I.; Yuzefovych, L.V.; Ledoux, S.P.; Julie, N.L.; Wilson, G.L. Troglitazone, but not rosiglitazone, damages mitochondrial DNA and induces mitochondrial dysfunction and cell death in human hepatocytes. Toxicol. Appl. Pharmacol 2009, 240, 348–354. [Google Scholar]
- Ricci, C.; Pastukh, V.; Leonard, J.; Turrens, J.; Wilson, G.; Schaffer, D.; Schaffer, S.W. Mitochondrial DNA damage triggers mitochondrial-superoxide generation and apoptosis. Am. J. Physiol. Cell Physiol 2008, 294, C413–C422. [Google Scholar]
- Gredilla, R.; Bohr, V.A.; Stevnsner, T. Mitochondrial DNA repair and association with aging—An update. Exp. Gerontol 2010, 45, 478–488. [Google Scholar]
- Leclere, R.; Torregrosa-Munumer, R.; Kireev, R.; Garcia, C.; Vara, E.; Tresguerres, J.A.; Gredilla, R. Effect of estrogens on base excision repair in brain and liver mitochondria of aged female rats. Biogerontology 2013, 14, 383–394. [Google Scholar]
- Murphy, M.P. How mitochondria produce reactive oxygen species. Biochem. J 2009, 417, 1–13. [Google Scholar]
- Scarpulla, R.C. Metabolic control of mitochondrial biogenesis through the PGC-1 family regulatory network. Biochim. Biophys. Acta 2011, 1813, 1269–1278. [Google Scholar]
- Aharoni-Simon, M.; Hann-Obercyger, M.; Pen, S.; Madar, Z.; Tirosh, O. Fatty liver is associated with impaired activity of PPARγ-coactivator 1alpha (PGC1α) and mitochondrial biogenesis in mice. Lab. Invest 2011, 91, 1018–1028. [Google Scholar]
- Chiappini, F.; Barrier, A.; Saffroy, R.; Domart, M.C.; Dagues, N.; Azoulay, D.; Sebagh, M.; Franc, B.; Chevalier, S.; Debuire, B.; et al. Exploration of global gene expression in human liver steatosis by high-density oligonucleotide microarray. Lab. Invest 2006, 86, 154–165. [Google Scholar]
- Hui, J.M.; Hodge, A.; Farrell, G.C.; Kench, J.G.; Kriketos, A.; George, J. Beyond insulin resistance in NASH: TNF-α or adiponectin? Hepatology 2004, 40, 46–54. [Google Scholar]
- Hernandez-Gea, V.; Hilscher, M.; Rozenfeld, R.; Lim, M.P.; Nieto, N.; Werner, S.; Devi, L.A.; Friedman, S.L. Endoplasmic reticulum stress induces fibrogenic activity in hepatic stellate cells through autophagy. J. Hepatol 2013, 59, 98–104. [Google Scholar]
- Blaner, W.S.; O’Byrne, S.M.; Wongsiriroj, N.; Kluwe, J.; D’Ambrosio, D.M.; Jiang, H.; Schwabe, R.F.; Hillman, E.M.; Piantedosi, R.; Libien, J. Hepatic stellate cell lipid droplets: A specialized lipid droplet for retinoid storage. Biochim. Biophys. Acta 2009, 1791, 467–473. [Google Scholar]
- Hernandez-Gea, V.; Ghiassi-Nejad, Z.; Rozenfeld, R.; Gordon, R.; Fiel, M.I.; Yue, Z.; Czaja, M.J.; Friedman, S.L. Autophagy releases lipid that promotes fibrogenesis by activated hepatic stellate cells in mice and in human tissues. Gastroenterology 2012, 142, 938–946. [Google Scholar]
- Oddy, W.H.; Herbison, C.E.; Jacoby, P.; Ambrosini, G.L.; O’Sullivan, T.A.; Ayonrinde, O.T.; Olynyk, J.K.; Black, L.J.; Beilin, L.J.; Mori, T.A.; et al. The Western dietary pattern is prospectively associated with nonalcoholic fatty liver disease in adolescence. Am. J. Gastroenterol 2013, 108, 778–785. [Google Scholar]
- Goodpaster, B.H.; Delany, J.P.; Otto, A.D.; Kuller, L.; Vockley, J.; South-Paul, J.E.; Thomas, S.B.; Brown, J.; McTigue, K.; Hames, K.C.; et al. Effects of diet and physical activity interventions on weight loss and cardiometabolic risk factors in severely obese adults: A randomized trial. JAMA 2010, 304, 1795–1802. [Google Scholar]
- Neuschwander-Tetri, B.A.; Ford, D.A.; Acharya, S.; Gilkey, G.; Basaranoglu, M.; Tetri, L.H.; Brunt, E.M. Dietary trans-fatty acid induced NASH is normalized following loss of trans-fatty acids from hepatic lipid pools. Lipids 2012, 47, 941–950. [Google Scholar]
- Soetens, B.; Braet, C.; Moens, E. Thought suppression in obese and non-obese restrained eaters: Piece of cake or forbidden fruit? Eur. Eat. Disord. Rev 2008, 16, 67–76. [Google Scholar]
- Musso, G.; Cassader, M.; Rosina, F.; Gambino, R. Impact of current treatments on liver disease, glucose metabolism and cardiovascular risk in non-alcoholic fatty liver disease (NAFLD): A systematic review and meta-analysis of randomised trials. Diabetologia 2012, 55, 885–904. [Google Scholar]
- Svegliati-Baroni, G.; Saccomanno, S.; Rychlicki, C.; Agostinelli, L.; de Minicis, S.; Candelaresi, C.; Faraci, G.; Pacetti, D.; Vivarelli, M.; Nicolini, D.; et al. Glucagon-like peptide-1 receptor activation stimulates hepatic lipid oxidation and restores hepatic signalling alteration induced by a high-fat diet in nonalcoholic steatohepatitis. Liver Int 2011, 31, 1285–1297. [Google Scholar] [Green Version]
- Cuthbertson, D.J.; Irwin, A.; Gardner, C.J.; Daousi, C.; Purewal, T.; Furlong, N.; Goenka, N.; Thomas, E.L.; Adams, V.L.; Pushpakom, S.P.; et al. Improved glycaemia correlates with liver fat reduction in obese, type 2 diabetes, patients given glucagon-like peptide-1 (GLP-1) receptor agonists. PloS One 2012, 7, e50117. [Google Scholar]
- Kern, M.; Kloting, N.; Niessen, H.G.; Thomas, L.; Stiller, D.; Mark, M.; Klein, T.; Bluher, M. Linagliptin improves insulin sensitivity and hepatic steatosis in diet-induced obesity. PLoS One 2012, 7, e38744. [Google Scholar]
- Hoofnagle, J.H.; van Natta, M.L.; Kleiner, D.E.; Clark, J.M.; Kowdley, K.V.; Loomba, R.; Neuschwander-Tetri, B.A.; Sanyal, A.J.; Tonascia, J. Vitamin E and changes in serum alanine aminotransferase levels in patients with non-alcoholic steatohepatitis. Aliment. Pharmacol. Ther 2013, 38, 134–143. [Google Scholar]
- Malaguarnera, M.; Gargante, M.P.; Russo, C.; Antic, T.; Vacante, M.; Avitabile, T.; li Volti, G.; Galvano, F. l-carnitine supplementation to diet: A new tool in treatment of nonalcoholic steatohepatitis—A randomized and controlled clinical trial. Am. J. Gastroenterol 2010, 105, 1338–1345. [Google Scholar]
- Zein, C.O.; Lopez, R.; Fu, X.; Kirwan, J.P.; Yerian, L.M.; McCullough, A.J.; Hazen, S.L.; Feldstein, A.E. Pentoxifylline decreases oxidized lipid products in nonalcoholic steatohepatitis: New evidence on the potential therapeutic mechanism. Hepatology 2012, 56, 1291–1299. [Google Scholar]
- Zein, C.O.; Yerian, L.M.; Gogate, P.; Lopez, R.; Kirwan, J.P.; Feldstein, A.E.; McCullough, A.J. Pentoxifylline improves nonalcoholic steatohepatitis: A randomized placebo-controlled trial. Hepatology 2011, 54, 1610–1619. [Google Scholar]
- Nelson, A.; Torres, D.M.; Morgan, A.E.; Fincke, C.; Harrison, S.A. A pilot study using simvastatin in the treatment of nonalcoholic steatohepatitis: A randomized placebo-controlled trial. J. Clin. Gastroenterol 2009, 43, 990–994. [Google Scholar]
- Deushi, M.; Nomura, M.; Kawakami, A.; Haraguchi, M.; Ito, M.; Okazaki, M.; Ishii, H.; Yoshida, M. Ezetimibe improves liver steatosis and insulin resistance in obese rat model of metabolic syndrome. FEBS Lett 2007, 581, 5664–5670. [Google Scholar]
- Yoneda, M.; Fujita, K.; Nozaki, Y.; Endo, H.; Takahashi, H.; Hosono, K.; Suzuki, K.; Mawatari, H.; Kirikoshi, H.; Inamori, M.; et al. Efficacy of ezetimibe for the treatment of non-alcoholic steatohepatitis: An open-label, pilot study. Hepatol. Res 2010, 40, 613–621. [Google Scholar]
- Leuschner, U.F.; Lindenthal, B.; Herrmann, G.; Arnold, J.C.; Rossle, M.; Cordes, H.J.; Zeuzem, S.; Hein, J.; Berg, T. High-dose ursodeoxycholic acid therapy for nonalcoholic steatohepatitis: A double-blind, randomized, placebo-controlled trial. Hepatology 2010, 52, 472–479. [Google Scholar]
- Pietu, F.; Guillaud, O.; Walter, T.; Vallin, M.; Hervieu, V.; Scoazec, J.Y.; Dumortier, J. Ursodeoxycholic acid with vitamin E in patients with nonalcoholic steatohepatitis: Long-term results. Clin. Res. Hepatol. Gastroenterol 2012, 36, 146–155. [Google Scholar]
- Ford, A.C.; Peyrin-Biroulet, L. Opportunistic Infections With Anti-Tumor Necrosis Factor-α Therapy in Inflammatory Bowel Disease: Meta-Analysis of Randomized Controlled Trials. Am. J. Gastroenterol 2013, 108, 1268–1276. [Google Scholar]
- Bast, A.; Haenen, G.R. Ten misconceptions about antioxidants. Trends Pharmacol. Sci 2013, 34, 430–436. [Google Scholar]
- Watson, J. Oxidants, antioxidants and the current incurability of metastatic cancers. Open Biol 2013, 3. [Google Scholar] [CrossRef]
- Yae, T.; Tsuchihashi, K.; Ishimoto, T.; Motohara, T.; Yoshikawa, M.; Yoshida, G.J.; Wada, T.; Masuko, T.; Mogushi, K.; Tanaka, H.; et al. Alternative splicing of CD44 mRNA by ESRP1 enhances lung colonization of metastatic cancer cell. Nat. Commun 2012, 3. [Google Scholar] [CrossRef]
- Trachootham, D.; Alexandre, J.; Huang, P. Targeting cancer cells by ROS-mediated mechanisms: A radical therapeutic approach? Nat. Rev. Drug Discov 2009, 8, 579–591. [Google Scholar]
- Hackam, D.G. Review: Antioxidant supplements for primary and secondary prevention do not decrease mortality. ACP J. Club. 2007, 147, p. 4. Available online: http://acpjc.acponline.org/Content/147/1/Issue/ACPJC-2007-147-1-004.htm (accessed on 11 October 2013).
- Ohta, S. Recent progress toward hydrogen medicine: Potential of molecular hydrogen for preventive and therapeutic applications. In Curr. Pharm. Des; 2011; Volume 17, pp. 2241–2252. [Google Scholar]
- Winterbourn, C.C. Biological reactivity and biomarkers of the neutrophil oxidant, hypochlorous acid. Toxicology 2002, 181–182, 223–227. [Google Scholar]
- Liao, J.K. Linking endothelial dysfunction with endothelial cell activation. J. Clin. Invest 2013, 123, 540–541. [Google Scholar]
- Fontanari, P.; Badier, M.; Guillot, C.; Tomei, C.; Burnet, H.; Gardette, B.; Jammes, Y. Changes in maximal performance of inspiratory and skeletal muscles during and after the 7.1-MPa Hydra 10 record human dive. Eur. J. Appl. Physiol 2000, 81, 325–328. [Google Scholar]
- Fukuda, K.I.; Asoh, S.; Ishikawa, M.; Yamamoto, Y.; Ohsawa, I.; Ohta, S. Inhalation of hydrogen gas suppresses hepatic injury caused by ischemia/reperfusion through reducing oxidative stress. Biochem. Bioph. Res. Co 2007, 361, 670–674. [Google Scholar]
- Hayashida, K.; Sano, M.; Ohsawa, I.; Shinmura, K.; Tamaki, K.; Kimura, K.; Endo, J.; Katayama, T.; Kawamura, A.; Kohsaka, S.; et al. Inhalation of hydrogen gas reduces infarct size in the rat model of myocardial ischemia-reperfusion injury. Biochem. Biophys. Res. Commun 2008, 373, 30–35. [Google Scholar]
- Kawamura, T.; Huang, C.S.; Tochigi, N.; Lee, S.; Shigemura, N.; Billiar, T.R.; Okumura, M.; Nakao, A.; Toyoda, Y. Inhaled hydrogen gas therapy for prevention of lung transplant-induced ischemia/reperfusion injury in rats. Transplantation 2010, 90, 1344–1351. [Google Scholar]
- Fu, Y.; Ito, M.; Fujita, Y.; Ichihara, M.; Masuda, A.; Suzuki, Y.; Maesawa, S.; Kajita, Y.; Hirayama, M.; Ohsawa, I.; et al. Molecular hydrogen is protective against 6-hydroxydopamine-induced nigrostriatal degeneration in a rat model of Parkinson’s disease. Neurosci. Lett 2009, 453, 81–85. [Google Scholar]
- Ohsawa, I.; Nishimaki, K.; Yamagata, K.; Ishikawa, M.; Ohta, S. Consumption of hydrogen water prevents atherosclerosis in apolipoprotein E knockout mice. Biochem. Biophys. Res. Commun 2008, 377, 1195–1198. [Google Scholar]
- Kamimura, N.; Nishimaki, K.; Ohsawa, I.; Ohta, S. Molecular hydrogen improves obesity and diabetes by inducing hepatic FGF21 and stimulating energy metabolism in db/db mice. Obesity 2011, 19, 1396–1403. [Google Scholar]
- Amitani, H.; Asakawa, A.; Cheng, K.; Amitani, M.; Kaimoto, K.; Nakano, M.; Ushikai, M.; Li, Y.; Tsai, M.; Li, J.B.; et al. Hydrogen improves glycemic control in type1 diabetic animal model by promoting glucose uptake into skeletal muscle. PloS One 2013, 8, e53913. [Google Scholar]
- Feng, Y.; Wang, R.; Xu, J.; Sun, J.; Xu, T.; Gu, Q.; Wu, X. Hydrogen-rich saline prevents early neurovascular dysfunction resulting from inhibition of oxidative stress in STZ-diabetic rats. Curr. Eye Res 2013, 38, 396–404. [Google Scholar]
- Sun, H.; Chen, L.; Zhou, W.; Hu, L.; Li, L.; Tu, Q.; Chang, Y.; Liu, Q.; Sun, X.; Wu, M.; et al. The protective role of hydrogen-rich saline in experimental liver injury in mice. J. Hepatol 2011, 54, 471–480. [Google Scholar]
- Nakashima-Kamimura, N.; Mori, T.; Ohsawa, I.; Asoh, S.; Ohta, S. Molecular hydrogen alleviates nephrotoxicity induced by an anti-cancer drug cisplatin without compromising anti-tumor activity in mice. Cancer Chemother. Pharmacol 2009, 64, 753–761. [Google Scholar]
- Buchholz, B.M.; Kaczorowski, D.J.; Sugimoto, R.; Yang, R.; Wang, Y.; Billiar, T.R.; McCurry, K.R.; Bauer, A.J.; Nakao, A. Hydrogen inhalation ameliorates oxidative stress in transplantation induced intestinal graft injury. Am. J. Transplant 2008, 8, 2015–2024. [Google Scholar]
- Kajiyama, S.; Hasegawa, G.; Asano, M.; Hosoda, H.; Fukui, M.; Nakamura, N.; Kitawaki, J.; Imai, S.; Nakano, K.; Ohta, M.; et al. Supplementation of hydrogen-rich water improves lipid and glucose metabolism in patients with type 2 diabetes or impaired glucose tolerance. Nutr. Res 2008, 28, 137–143. [Google Scholar]
- Nakao, A.; Toyoda, Y.; Sharma, P.; Evans, M.; Guthrie, N. Effectiveness of hydrogen rich water on antioxidant status of subjects with potential metabolic syndrome—An open label pilot study. J. Clin. Biochem. Nutr 2010, 46, 140–149. [Google Scholar]
- Huang, C.S.; Kawamura, T.; Lee, S.; Tochigi, N.; Shigemura, N.; Buchholz, B.M.; Kloke, J.D.; Billiar, T.R.; Toyoda, Y.; Nakao, A. Hydrogen inhalation ameliorates ventilator-induced lung injury. Crit. Care 2010, 14, R234:1–R234:15. [Google Scholar]
- Fujii, M.; Shibazaki, Y.; Wakamatsu, K.; Honda, Y.; Kawauchi, Y.; Suzuki, K.; Arumugam, S.; Watanabe, K.; Ichida, T.; Asakura, H.; et al. A murine model for non-alcoholic steatohepatitis showing evidence of association between diabetes and hepatocellular carcinoma. Med. Mol. Morphol 2013, 46, 141–152. [Google Scholar]
© 2013 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).