Abstract
An efficient and convenient strategy for the enantioselective synthesis of enantiomerically enriched 10-ethyl-7,8-dihydro-γ-ionone isomers (R)-(+)-7, and (S)-(−)-7 are described utilizing a lipase mediated resolution protocol, and reductive elimination of the secondary allylic alcohol as the key step. The enantioselective and diastereoselective lipase kinetic acetylation of 4-hydroxy-γ-ionone derivatives 6a afforded the 4-acetyl-γ-ionone derivatives (−)-8, and the 4-hydrox-γ-ionone derivatives (+)-6a, which are suitable precursors of the desired products. Stereospecific palladium-mediated elimination of allylic acetate provides the target compounds with an excellent enantiomeric excess and yield. Additionally, the novel 4,5-didehydro-α-ionone 13 is obtained from readily prepared (2,6,6-trimethylcyclohexa-2,4-dien-1-yl) methanol 9. The structures of all newly synthesized compounds have been elucidated by 1H, 13C NMR, GC-MS, and IR spectrometry. These compounds represent a new class of odorants that may be of pivotal relevance in industrial perfumery.
1. Introduction
Timberol® and Ionones are among the most important fragrance constituents due to their distinctive fine woody, amber, violet and rose scents [1,2]. The industrial creation of new perfumes [3,4] needs two essential lines of research: the discovery of new odorous molecules and the reinvestigation or chemical modification of older commercial products. Due to the unpredictable relationship between chemical structure and odor [5], the latter approach is particularly interesting from a chemical point of view. Indeed, many fragrances are sold as a mixture of isomers whose specific contribution to the perceived odor may be very different. Moreover, olfactory evaluation shows that the regioisomeric purity and the absolute stereochemistry of these compounds dramatically determines the fragrance properties, sometimes with amazingly pronounced differences between the notes and the odor thresholds of the isomers. Taking advantage of processes based on the enzyme-mediated resolution, Fuganti et al. [6] have reported a synthetic approach to the olfactory active components of the woody odorant timberol® whereas Serra et al. [7,8] described the enantioselective synthesis of a number of natural odorants with the ionone skeleton. Furthermore, the exocyclic double bond confers particularly characteristic nuances to the fragrance, which can favorably complement other widely used compound from the same family [9–15].
In this context, we have focused our attention on the odorants in a combination of timberol and ionone framework (Figure 1) that are of pivotal relevance in industrial perfumery.
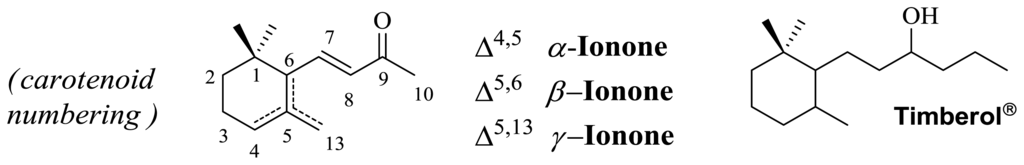
Figure 1.
Timberol and Ionone framework.
2. Results and Discussion
2.1. Preparation of 10-Ethyl-7,8-dihydro-γ-ionone
We prepared compound 7 in racemic and enantiomer-enriched form starting from commercially available racemic α-ionone. It was introduced by Dragoco [16] as a synthetic fragrance with fixative properties with the brand name timberol®. Funganti et al. have previously developed a stereoselective procedure that allows the conversion of α-ionone derivatives into γ-ionone derivatives [17,18]. The regioselective base-mediated isomerization of 4,5-epoxy-4,5-dihydro-α-ionone followed by reductive elimination of the obtained allylic alcohols were the key steps of our syntheses. Therefore, we decided to apply the latter synthetic pathway for the conversion of compounds 1 into 7. The synthesis began with the reaction of α-ionone 1 with Br2 and NaOH as base in a mixture of H2O and dioxane, the resulting intermediate acid was treated with MeOH in the presence of a catalytic amount of H2SO4 to afford the corresponding methyl ester. The later compound was reduced with LiAlH4 to produce the allylic alcohol 2. The latter allylic alcohol was oxidized and the obtained aldehyde was treated with propyl magnesium bromide. The resulting ionol was then converted into pure 3 by an oxidation reaction using MnO2 (Scheme 1).
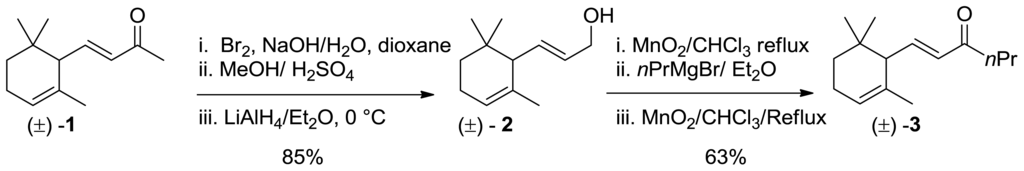
Scheme 1.
Synthesis of α-ionone isomer 3.
Following, we submitted α-ionone isomer 3 to epoxidation procedure with m-chloroperbenzoic acid to afford separable cis/trans mixtures of epoxides 4a/4b respectively. The latter compounds subsequently underwent regiospecific hydrogenation using H2/Raney Nickel affording 5a/5b. The latter compounds were added to an excess (2.5–3 equiv, −78 °C) of LDA in THF and then warmed at reflux. After quenching, we obtained the cis/trans mixtures of alcohol 6a/6b respectively showing the same diastereoisomeric ratio as the starting epoxides (cis/trans 4:1). The obtained allylic alcohols were acetylated and then the acetate group was reductively removed by treatment with triethylammonium formate and palladium catalyst to give 10-ethyl-7,8-dihydro-γ-ionone (±)-7 (Scheme 2).
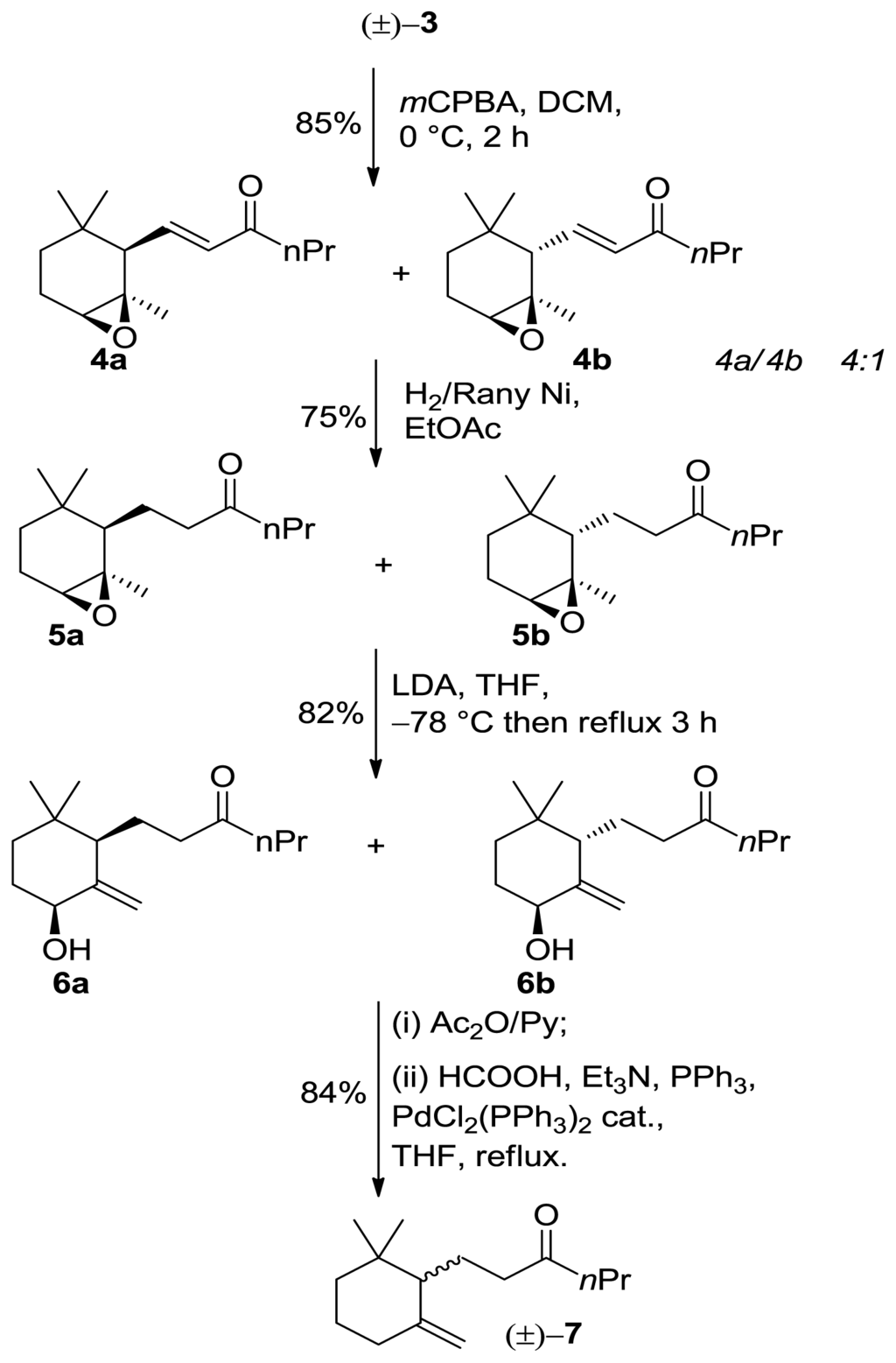
Scheme 2.
Synthesis of 10-ethyl-7,8-dihydro-γ-ionone (±)-7.
The above mentioned allylic alcohols 6a/6b are suitable starting materials for the preparation of enantio enriched isomers 7. Indeed, we have established that lipase-mediated acetylation of 4-hydroxy γ-ionone yield enantiopure (4R,6S)-4-acetoxy-γ-ionone [17]. The reaction proceeds with high enantioselectivity and with complete diastereoselectivity allowing the exclusive transformation of the cis isomers.
Therefore, we performed the enzyme mediated resolution of the above mentioned alcohols (Scheme 2). The described reductive elimination of the acetate group proceeds without racemization allowing the preparation of enantioenriched 10-ethyl-7,8-dihydro-γ-ionone isomers. Noteworthy, since the diastereoisomeric allylic alcohols 6a/6b are separable by chromatography, the resolution procedure gives the corresponding acetylated compounds with high ee and de leaving unreacted alcohols with low de. Accordingly, racemic alcohol 6a was acetylated with vinyl acetate in the presence of lipase PS as a catalyst. The reaction was interrupted at 50% of conversion to give unreacted alcohol (+)-6a (99% de, 92% ee) and acetate (−)-8 (99% de, 99% ee). The reductive removal of the acetoxy group converted the latter compounds into 10-ethyl-7,8-dihydro-γ-ionone (+)-7 (92% ee) and 10-ethyl-7,8-dihydro-γ-ionone (−)-7 (99% ee), respectively (Scheme 3).
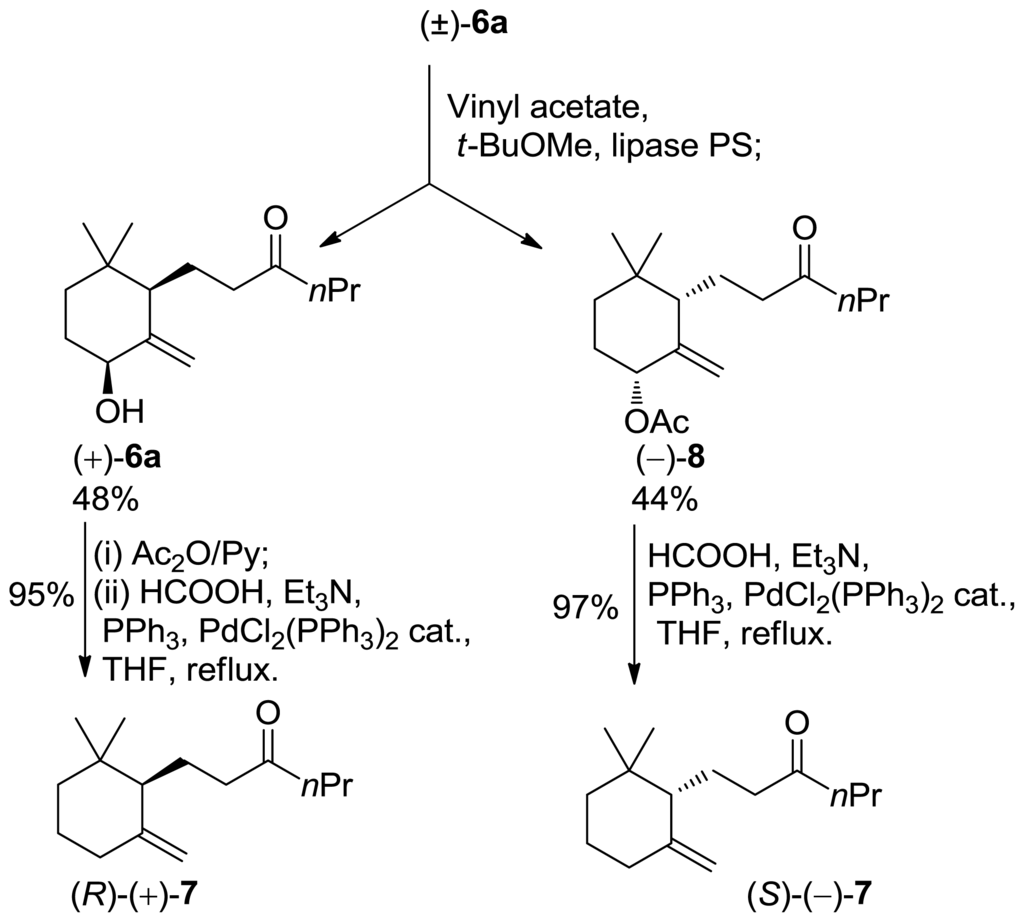
Scheme 3.
Synthesis of enantio enriched 10-ethyl-7,8-dihydro-γ-ionone isomer (+) and (−)-7.
2.2. Synthesis of 4,5-Didehydro-α-ionone
Aldol condensation of readily available aldehyde with acetone in the presence of base and Wittig reaction with phosphonate were not successful to reach the target compound because isomeric products are inseparable by the usual methodologies. It was found that the readily available alcohol 9 [19] is a good starting material for the synthesis. Tosylation of the free OH-group, followed by addition of thiophenol, deprotonated by NaH afforded the sulphide 10 in 95% yield. Oxidation of the latter compound with a mixture of (NH4)6.Mo7O24 and H2O2 gives the sulphone 11 in moderate yield where the disulfide occurred as a by-product. Sulphone 11 was deprotonated by nBuLi at −78 °C, then epoxide in DMPU were added, followed by the addition of BF3.OEt2 to the reaction mixture. The reaction mixture was stirred for a further 5 h at −78 °C and was left overnight to warm at RT to give 12 in 43% yield that was oxidized by Dess–Martin reagent to give the corresponding ketone in quantitative yield. Removal of PhSO2 group by DBU regenerates the target compound 13 (Scheme 4) [20].
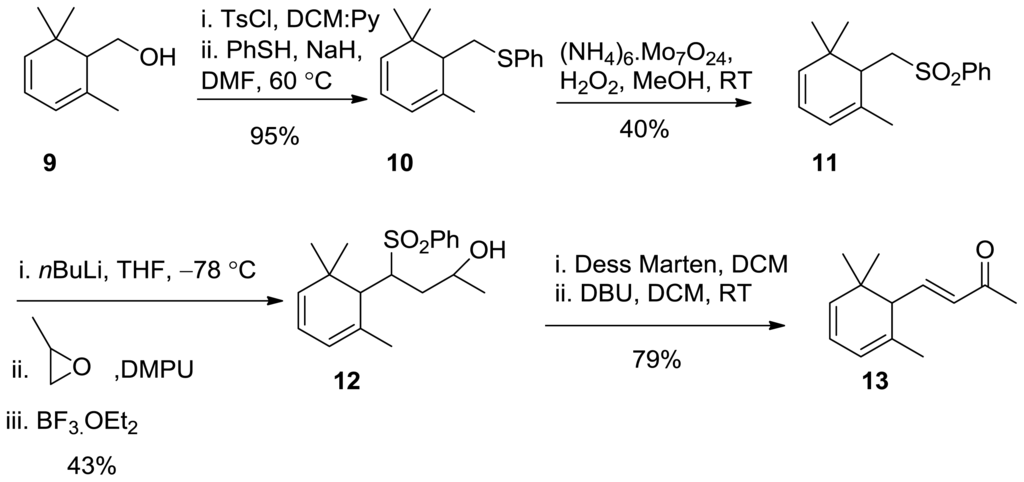
Scheme 4.
Synthesis of 4,5-didehydro-α-ionone 13.
3. Experimental Section
3.1. General Procedures and Instrumentation
All moisture-sensitive reactions were carried out under a static atmosphere of nitrogen. All reagents were of commercial quality. Lipase from Pseudomonas cepacia (PS), Amano Pharmaceuticals Co., Japan, 30 units/mg, was employed in this work. TLC: Merk silica gel 60 F254 plates. Column chromatography (CC): silica gel. GC-MS analyses: HP-6890 gas chromatograph equipped with a 5973 mass detector, using a HP-5MS column (30 m × 0.25 mm, 0.25 μm firm thickness; Hewlett Packard). Chiral GC analyses: DANI-HT-86.10 gas chromatograph; enantiomer excesses determined on a CHIRASIL DEX CB-Column. Optical rotations: Jasco-DIP-181 digital polarimeter. 1H and 13C Spectra: CDCl3 solution at room temperature; Bruker-AC-400 spectrometer at 400 and 100 MHz, respectively; chemical shifts in ppm relative to internal SiMe4 (=0 ppm), J values in Hz. IR spectra were recorded on a Perkin-Elmer 2000 FT-IR spectrometer; films; ν in cm−1. Melting points were measured on a Reichert apparatus, equipped with a Reichert microscope, and are uncorrected. Microanalyses were determined on an analyzer 1106 from Carlo Erba.
3.2. Synthesis of (E)-3-(2,6,6-Trimethylcyclohex-2-en-1-yl)prop-2-en-1-ol 2
2 was prepared according to reported literature [21] as a colourless oil; 1H NMR (400 MHz, CDCl3) δ 5.75 (d, J = 16.0 Hz, 1H), 5.64 (dt, J = 16.0, 6.0 Hz, 1H), 5.45 (d, J = 7.0 Hz, 1H), 4.35 (d, J = 14.0 Hz, 2H), 2.65 (d, J = 6.2 Hz, 1H), 1.75 (s, 3H), 1.65–1.53 (m, 2H), 1.49–1.41 (m, 2H), 1.31 (br s, 1H), 0.98 (s, 6H).
3.3. Synthesis of (E)-1-(2,6,6-Trimethylcyclohex-2-en-1-yl)hex-1-en-3-one (3)
To a mixture of 2 (25 g, 64.4 mmol) in CHCl3 (200 mL), MnO2 (30 g, 345 mmol) was added, and the mixture was stirred at reflux for 6 h. The mixture was then cooled, filtered (using filter paper) and the organic phase was concentrated under reduced pressure to afford an oil (13 g). The latter was dissolved in dry diethyl ether (150 mL) and treated under stirring with an excess of n-propylmagnesium bromide (100 mL of a 1 M solution in ether) keeping the reaction temperature under 5 °C by external cooling (ice bath). The usual work-up afforded crude carbinol that was dissolved in CHCl3 (200 mL) and treated with MnO2 (30 g, 345 mmol) stirring at reflux for 12 h. After filtration and concentration, the crude ketone was purified by chromatography (hexane/Et2O 95:5) and bulb to bulb distillation (oven temperature 105 °C, 0.4 mmHg) to afford pure 3 (8.4 g, 63% yield), 98% chemical purity (GC)) as a colorless oil;1H NMR (400 MHz, CDCl3) δ 6.64 (dd, J = 15.6, 9.6 Hz, 1H), 6.06 (d, J = 15.6 Hz, 1H), 5.48 (bs, 1H), 2.52 (t, J = 7.2 Hz, 2H), 2.27 (d, J = 10 Hz, 1H), 2.04 (m, 1H), 1.67 (ddd, J = 13.4, 6.5, 1.4 Hz, 1H), 1.55 (s, 3H), 1.41 (m, 2H), 1.23 (m, 2H), 0.94 (t, J = 1.7 Hz, 3H), 0.93 (s, 3H), 0.82 (s, 3H); 13C NMR (100 MHz) δ 212.8, 135.3, 132.6, 121.4, 73.2, 54.5, 40.0, 32.0, 27.9, 27.3, 19.0, 14.3; IR (nujol, cm−1) 1684, 1619, 1554. GC-MS m/z: 221.18 (M+, 1); Anal. Calcd for C15H24O: C, 81.76; H, 10.98.Found: C, 81.75; H, 10.90.
3.4. General Procedure for the Synthesis of Adducts 4a/4b
m-Chloroperbenzoic acid (7.4 g, of 75% wet acid, 32 mmol) was added to a solution of racemic α-ionone isomer 3 (7 g, 30 mmol) in methylene chloride (75 mL) at 0 °C. The reaction mixture was stirred at 0 °C for 2 h and then filtered in order to remove the m-clorobenzoic acid precipitate. The organic phase was washed with saturated Na2SO3 solution (50 mL) and saturated NaHCO3 solution (50 mL), respectively, dried over Na2SO4 and concentrated under reduced pressure. The residue was chromatographed on a silica gel column (hexane/Et2O 9:1) to give the corresponding α-epoxy-derivatives.
3.4.1. (E)-1-[(1R,2R,6S)-1,3,3-Trimethyl-7-oxabicyclo[4.1.0]heptan-2-yl]hex-1-en-3-one (4a)
1H NMR (400 MHz, CDCl3) δ 5.45-5.41 (m, 1H), 5.39 (bs, 1H), 5.48 (bs, 1H), 4.10 (bs, 1H), 2.10 (d, J = 6.4 Hz, 1H), 2.00 (m, 2H), 1.59 (dd, J = 4.4, 1.6 Hz, 1H), 1.54 (m, 2H), 1.40 (m, 2H), 1.37 (s, 3H), 0.94 (td, J = 7.2, 1.6 Hz, 3H), 0.93 (s, 3H), 0.81(s, 3H); 13C NMR (100 MHz) δ 212.8, 135.3, 132.6, 121.4, 73.2, 54.5, 40.0, 32.0, 27.9, 27.3, 19.0, 14.3; IR (nujol, cm−1) 1684; GC-MS m/z: 237.18 (M+, 1); Anal. Calcd for C15H24O2: C, 76.23; H, 10.24. Found: C, 76.34; H, 10.55.
3.4.2. (E)-1-[(1R,2S,6S)-1,3,3-Trimethyl-7-Oxabicyclo[4.1.0]heptan-2-yl]hex-1-en-3-One (4b)
1H NMR (400 MHz, CDCl3) δ 5.47-5.43 (m, 1H), 5.41 (bs, 1H), 5.52 (bs, 1H), 4.13 (bs, 1H), 2.14 (d, J = 6.4 Hz, 1H), 2.00 (m, 2H), 1.61 (dd, J = 4.4, 1.6 Hz, 1H), 1.54 (m, 2H), 1.45 (m, 2H), 1.37 (s, 3H), 0.94 (td, J = 7.2, 1.6 Hz, 3H), 0.93 (s, 3H), 0.81(s, 3H); 13C NMR (100 MHz) δ 212.4, 135.1, 132.3, 121.1, 73.3, 54.1, 40.2, 32.5, 27.9, 27.3, 19.1, 14.1; IR (nujol, cm−1) 1680; GC-MS m/z: 237.18 (M+, 1); Anal. Calcd for C15H24O2: C, 76.23; H, 10.24. Found: C, 76.34; H, 10.55.
3.5. General Procedure for the Synthesis of Adducts 5a/5b
H2 (360 mL, 1 equiv) was adsorbed of a solution of 4a or 4b (4.5 g, 19mmol, 1 equiv) in EtOAc (150 mL) and excess of Rany-Ni for 2 h. The reaction mixture was filtered off and concentrated under reduced pressure. The residue was chromatographed on a silica gel column (hexane/EtOAc 9:1) to give the corresponding product.
3.5.1. 1-[(1R,2R,6S)-1,3,3-Trimethyl-7-Oxabicyclo[4.1.0]heptan-2-yl]hexan-3-One (5a)
(3.4 g, 74% yield, 98% chemical purity (GC)) as a colorless oil; 1H NMR (400 MHz, CDCl3) δ 2.85 (bs, 1H), 2.67–2.59 (m, 2H), 2.43–2.31 (m, 2H), 1.86–1.74 (m, 3H), 1.68–1.58 (m, 2H), 1.56–1.43 (m, 2H), 1.34–1.27 (m, 2H), 1.24 (s, 3H), 0.86 (t, J = 14.8 Hz, 3H), 0.81 (s, 3H), 0.76 (s, 3H); 13C NMR (100 MHz) δ 2.11.4, 60.2, 46.5, 45.0, 42.3, 31.8, 28.4, 27.9, 27.5, 27.0, 26.9, 22.3, 21.8, 17.6, 14.1; IR (nujol, cm−1) 1684, 1109, 990. GC-MS m/z: 239.19 (M+, 1); Anal. Calcd for C15H26O2: C, 75.58; H, 10.99. Found: C, 75.55; H, 11.02.
3.5.2. 1-[(1R,2S,6S)-1,3,3-Trimethyl-7-Oxabicyclo[4.1.0]heptan-2-yl]hexan-3-One (5b)
1H NMR (400 MHz, CDCl3) δ 2.83 (bs, 1H), 2.64–2.57 (m, 2H), 2.45–2.33 (m, 2H), 1.83–1.75 (m, 3H), 1.67–1.55 (m, 2H), 1.52–1.45 (m, 2H), 1.32–1.25 (m, 2H), 1.25 (s, 3H), 0.87 (t, J = 14.8 Hz, 3H), 0.82 (s, 3H), 0.77 (s, 3H); 13C NMR (100 MHz) δ 211.2, 60.3, 46.4, 45.2, 42.2, 31.9, 28.5, 27.9, 27.3, 27.1, 26.9, 22.3, 21.8, 17.6, 14.1; IR (nujol, cm−1) 1683, 1109, 990. GC-MS m/z: 239.19 (M+, 1); 55 (15). Anal. Calcd for C15H26O2: C, 75.58; H, 10.99. Found: C, 75.55; H, 11.02.
3.6. General Procedure for the Synthesis of Adducts 6a/6b
nBuLi (3.0 mL of a 10 M solution in hexane) was added dropwise to a cooled (−78 °C) solution of iPr2NH (5.3 g, 50 mmol) in dry THF (90 mL) under nitrogen. The mixture was stirred at this temperature for 30 min. then a solution of the epoxide 5a or 5b (2.5 g, 10 mmol) in dry THF (20 mL) was added dropwise. The reaction was gradually warmed to r.t (1 h) and then was heated under reflux until no more starting epoxide was detected by TLC analysis (3 h). After cooling to room temperature, the mixture was poured into a mixture of crushed ice and 5% HCl solution. (80 mL) and extracted with Et2O (3 × 200 mL). The organic phase was successively washed with saturated aqueous NH4Cl solution (100 mL), brine, dried over Na2SO4 and concentrated under reduced pressure. The residue was purified by chromatography (eluting from hexane/AcOEt 9:1 to hexane/AcOEt 1:1) to give allylic alcohol.
3.6.1. 1-((1R,5S)-5-Hydroxy-2,2-Dimethyl-6-Methylenecyclohexyl)hexan-3-One (6a)
6a (1.95 g, 82% yield, 98% chemical purity (GC)) as a colorless oil; 1H NMR (400 MHz, CDCl3) δ 5.16 (bs, 1H), 4.64 (bs, 1H), 3.94 (bs, 1H), 2.56–2.48 (m, 2H), 2.34–2.26 (m, 2H), 1.91–1.81 (m, 2H), 1.75–1.67 (m, 2H), 1.64–1.54 (m, 2H), 1.51–1.39 (m, 2H), 1.29–1.24 (m, 2H), 0.97 (s, 3H), 0.89 (t, J = 14.8 Hz, 3H), 0.73 (s, 3H); 13C NMR (100 MHz) δ 2.11.4, 60.2, 46.5, 45.0, 42.3, 31.8, 28.4, 27.9, 27.5, 27.0, 26.9, 22.3, 21.8, 17.6, 14.1; IR (nujol, cm−1) 3334, 1684, 1619. GC-MS m/z: 239.19 (M+, 1); Anal. Calcd for C15H26O2: C, 75.58; H, 10.99. Found: C, 75.65; H, 10.99.
3.6.2. 1-((1S,5S)-5-Hydroxy-2,2-Dimethyl-6-Methylenecyclohexyl)hexan-3-One (6b)
1H NMR (400 MHz, CDCl3) δ 5.18 (bs, 1H), 4.66 (bs, 1H), 3.95 (bs, 1H), 2.58–2.47 (m, 2H), 2.33–2.28 (m, 2H), 1.92–1.83 (m, 2H), 1.75–1.67 (m, 2H), 1.64–1.54 (m, 2H), 1.53–1.38 (m, 2H), 1.29–1.24 (m, 2H), 0.98 (s, 3H), 0.90 (t, J = 14.8 Hz, 3H), 0.75 (s, 3H); 13C NMR (100 MHz) δ 2.11.4, 60.2, 46.5, 45.0, 42.3, 31.8, 28.4, 27.9, 27.5, 27.0, 26.9, 22.3, 21.8, 17.6, 14.1; IR (nujol, cm−1) 3335, 1681, 1620. GC-MS m/z: 239.19 (M+, 1); Anal. Calcd for C15H26O2: C, 75.58; H, 10.99. Found: C, 75.65; H, 10.99.
3.7. General Procedure for Reduction of Allylic Alcohol 6a to 10-Ethyl-7,8-dihydro-γ-ionone Isomers 7
A sample of compound 6a (500 mg, 2.0 mmol) was converted into the corresponding acetate by treatment with pyridine (5 mL) and Ac2O (5 mL) at room temperature for 24 h. The crude product was added to a solution of formic acid (180 mg, 4.0 mmol), Et3N (440 mg, 4.4 mmol), (PPh3)2PdCl2 (41 mg, 0.06 mmol) and triphenylphosphine (75 mg, 0.3 mmol) in dry THF (20 mL). The mixture was refluxed under a static nitrogen atmosphere until reduction was complete (2 h, TLC analysis). The reaction was then diluted with ether (30 mL) and washed with water (20 mL), 5% HCl solution (20 mL), saturated aqueous NaHCO3 solution (20 mL), and brine. The organic phase was dried (Na2SO4) and concentrated under reduced pressure. The residue was purified by chromatography (hexane/Et2O 95:5) and bulb-to-bulb distillation to give γ-ionone isomers 7 (84% yield, 97% isomeric purity).
10-Ethyl-7,8-dihydro-γ-ionone (±)-7 (99% chemical purity (GC)) as a colorless oil; 1H NMR (400 MHz, CDCl3) δ 4.75 (bs, 1H), 4.50 (d, J = 2.4 Hz,1H), 2.38–2.30 (m, 2H), 2.27–2.18(m, 2H), 2.05–1.94(m, 2H), 1.84–1.76(m, 3H), 1.70–1.55 (m, 2H), 1.54–1.46 (m, 2H), 1.30–1.17 (m, 2H), 0.91 (s, 3H), 0.89 (t, J = 7.2 Hz, 3H), 0.86 (s, 3H); 13C NMR (100 MHz) δ 211.8, 149.5, 109.7, 53.9, 45.3, 41.7, 36.2, 35.2, 32.5, 28.6, 26.8, 23.9, 20.7, 17.7, 14.4; IR (nujol, cm−1) 1684, 1619. GC-MS m/z (rel intensity) 223.20 (M+, 1); Anal. Calcd for C15H26O: C, 81.02; H, 11.79. Found: C, 81.14; H, 11.67.
3.8. Lipase-Mediated Resolution of Alcohols 6a
Diastereoisomerically pure alcohol 6a (obtained from epoxide 5a) was employed in the resolution procedure. A sample of the above-mentioned racemic material (5 g, 22.5 mmol), lipase PS (5 g), vinyl acetate (25 mL) and tBuOMe (100 mL) was stirred at room temperature, and the formation of the acetate was monitored by TLC analysis. The reaction was stopped at about 50% of conversion of 6a into (−)-8. The enzyme was then filtered, and the solvent was evaporated at reduced pressure after which the residue was purified by chromatography (eluting from hexane/AcOEt 9:1 to hexane/AcOEt 1:1). The first-eluted fractions afforded derivatives (−)-8 (45% yield). The last eluted fractions afforded derivatives (+)-6a (49% yield).
3.8.1. (1S,3R)-4,4-Dimethyl-2-Methylene-3-(3-Oxohexyl)cyclohexyl Acetate (−)-8
(Colorless oil; 99% chemical purity, 99% de (GC); 99% ee (chiral GC); [α]24D= −11.73° (c = 1.0 g/dL, CHCl3); 1H NMR (400 MHz, CDCl3) δ 5.08 (bs, 1H), 4.67 (bs, 1H), 2.47–2.39 (m, 2H), 2.34–2.26 (m, 2H), 2.02 (s, 3H), 1.88–1.80 (m, 3H), 1.75–1.64 (m, 2H), 1.61–1.54 (m, 2H), 1.37–1.30 (m, 2H), 1.26–1.23 (m, 2H), 0.97 (s, 3H), 0.89 (t, J = 14.8 Hz, 3H), 0.80 (s, 3H); 13C NMR (100 MHz) δ 2.11.4, 170.3, 75.2, 60.7, 52.0, 45.3, 41.6, 30.0, 29.8, 21.6, 20.4, 17.7, 14.5, 14.1; IR (nujol, cm−1) 1684, 1619. GC-MS m/z (rel intensity) 281.20 (M+, 1); Anal. Calcd for C17H28O3: C, 72.82; H, 10.06. Found: C, 72.85; H, 10.11.
3.8.2. (1S,3R)-4,4-Dimethyl-2-methylene-3-(3-oxohexyl)cyclohexyl Acetate (+)-8
(Colorless oil; 99% chemical purity, 99% de (GC); 92% ee (chiral GC); [α]24D= +8.47° (c = 1.0 g/dL, CHCl3); IR, 1H NMR, MS: in accordance with that of (−)-8.
3.9. Synthesis of Enantioenriched 10-Ethyl-7,8-dihydro-γ-ionone Isomers
The above obtained compounds (−)-8, (+)-8 were submitted to the reductive deoxygenation procedure described above in Section 3.7 to afford 10-ethyl-7,8-dihydro-γ-ionone isomers (−)-7, (+)-7 respectively. The latter compounds showed the following analytical data.
3.9.1. (S)-1-(2,2-Dimethyl-6-Methylenecyclohexyl)hexan-3-One (−)-7
(Colorless oil, 99% chemical purity, 97% regioisomeric purity (chiral GC); [α]24D = −20.2° (c = 1.0 g/dL, CHCl3); IR, 1H NMR, MS: in accordance with that of (±)-7.
3.9.2. (R)-1-(2,2-Dimethyl-6-methylenecyclohexyl)hexan-3-one (+)-7
(Colorless oil; 99% chemical purity, 94% regioisomeric purity (chiral GC); [α]24D= +17.3° (c = 1.0 g/dL, CHCl3); IR, 1H NMR, MS: in accordance with that of (±)-7.
3.10. Synthesis of Phenyl((2,6,6-Trimethylcyclohexa-2,4-Dien-1-yl)methyl)Sulfane 10
In a 100 mL round bottom flask, 9 (3.5 g, 2.3 mmol) was dissolved in a mixture of Py:DCM (1:1, 12 mL). TsCl (5.7 g, 3 mmol, 1.3 equiv) was added into reaction mixture and stirred at room temperature for 1.5 h. Water was added and extracted with Et2O (3 × 100 mL); the organic phase was washed with sat. NaHCO3, then 5% HCL, brine, dried over Na2SO4, and concentrated under reduced pressure, and used for the next step without further purification in quantitative yield.
In a round bottom flask, thiophenol (2.6 g, 23.54mmol, 1.2 equiv) was dissolved in DMF (30 mL), NaH (1.4 g 60%, 3 equiv) was added portion wise. After 15 min, TsCl (6.2 g, 19.62 mmol) was added. The reaction mixture was stirred at room temperature for 1 h, then at 60 °C for 2.5 h, then cooled to room temperature 0.1 N NaOH (30 mL) was added and extracted with Et2O, the organic phase was washed with water, brine, dried over Na2SO4, and concentrated under reduced pressure, and the crude was purified by CC using nhexane:EtOAc 100:2; 4.8 g, 95% yield.
1H NMR (400 MHz, CDCl3) δ 7.41–7.23 (m, 5H), 5.20–5.87 (m, 3H), 3.58–3.95 (m, 2H), 2.8 (t, J = 10.4Hz, 1H), 1.85 (s, 3H), 1.04 (s, 3H), 0.92 (s, 3H); 13C NMR (100 MHz) δ 155.8, 154.9, 153.5, 128.9, 125.4, 117.6, 114.7, 53.3, 38.7, 33.2, 24.4, 22.3 IR (nujol, cm−1) 1680, 1606, 1454, 1100, 990. GC-MS m/z: 245.13 (M+, 1); Anal. Calcd for C16H20S: C, 78.63; H, 8.25. Found: C, 78.65; H, 8.35.
3.11. Synthesis of (((2,6,6-Trimethylcyclohexa-2,4-dien-1-yl)Methyl)Sulfonyl)Benzene (11)
In a round bottom flask, 10 (4.5 g, 18.44 mmol) was dissolved in MeOH (30 mL) and cooled at 0 °C. H2O2 (4 mL of 30%), and (NH4)2MoO4 (5.4 g, 22.12 mmol, 1.2 equiv) were added. The reaction mixture was warmed to room temperature, and stirred overnight. Na2S2O5 (solid ~ calculated 5 g) was added in order to remove the excess oxidant. Extracted with DCM, dried over Na2SO4, and concentrated under reduced pressure, and the crude was purified by CC using nhexane:EtOAc 100:10; 1.15 g, 40% yield.1H NMR (400 MHz, CDCl3) δ 7.43–7.25 (m, 5H), 5.89–5.21 (m, 3H), 3.98–3.68 (m, 2H), 2.8 (t, J = 10.4Hz, 1H), 1.86 (s, 3H), 1.05 (s, 3H), 0.95 (s, 3H); 13C NMR (100 MHz) δ 155.8, 154.9, 153.5, 128.9, 125.4, 117.6, 114.7, 53.3, 38.7, 33.2, 24.4, 22.3 IR (nujol, cm−1) 1680, 1606, 1454, 1100, 990. GC-MS m/z: 277.12 (M+, 1); Anal. Calcd for C16H20O2S: C, 69.53; H, 11.58. Found: C, 69.54; H, 11.60.
3.12. Synthesis of 4-(Phenylsulfonyl)-4-(2,6,6-Trimethylcyclohexa-2,4-Dien-1-yl)butan-2-ol (12)
In a three neck 100 mL round bottom flask, nBuLi (0.4 mL of a 10 M solution in hexane) was added dropwise to a cooled (−78 °C) solution of 11 (1.15 g, 4.16 mmol) in dry THF (25 mL) under nitrogen, then stirred for 30 min. Subsequently, a mixture of propylene epoxide (1.6 mL) in DMPU [1,3-Dimethyl-3,4,5,6-tetrahydro-2(1H)-pyrimidinone] (5 mL) was added, then BF3OEt2 (3 mL) was added and the reaction mixture was stirred for 5 h at −78 °C. The reaction mixture was allowed to warm up at room temperature and stirred overnight. Water (100 mL) was added and extracted with DCM (3 × 100 mL); the organic phase was washed with brine, dried over Na2SO4, and concentrated under reduced pressure, and the crude was purified by CC using nhexane:EtOAc 10:3 to 5:5 to afford 12. (N.B: recovered starting material 500 mg)
(600 mg, 43% yield, 98% chemical purity (GC)) as a colorless oil; 1H NMR (400 MHz, CDCl3) δ: 7.84–7.82 (m, 2h, Ph), 7.58–7.48 (m, 3h, Ph), 5.81 (d, J = 15.8 Hz, 1H), 5.67 (d, J = 15.8 Hz, 1H), 5.35–5.25(m, 1H), 3.77 (m, 1H), 3.59 (bs, 1H), 3.11(m, 1H), 2.54(d, J = 15.8 Hz, 1H), 2.28(t, J = 4.8 Hz, 1H), 2.09(s, 3H), 1.31 (bs, 3H), 1.29 (bs, 3H), 0.95 (d, J = 6 Hz, 1H) 0.89 (s, 3H); 13C NMR (100 MHz) δ 153.9, 144.7, 137.7, 133.8, 129.9, 123.4, 117.9, 64.8, 57.9, 45.1, 36.7, 25.2, 23.5; IR (nujol, cm−1) 3360, 1684, 1619, 1454, 1109, 990. GC-MS m/z: 335.16 (M+, 1); Anal. Calcd for C19H26O3S: C, 68.23; H, 7.84; S, 9.59. Found: C, 68.35; H, 7.85; S, 9.62.
3.13. Synthesis of (E)-4-(2,6,6-Trimethylcyclohexa-2,4-Dien-1-yl)but-3-en-2-One (13)
In a round bottom flask, 12 (0.5 g, 0.15 mmol) was dissolved in DCM (10 mL). Dess Martin (1.3 equiv, 0.82 g, 0.19 mmol) was added portion wise. After the reaction was stirred at room temperature for 2 h, Na2S2O5 (solid~calculated 0.5 g) was added in order to remove the excess oxidant. Extracted with DCM. The organic phase was washed with water, brine, dried over Na2SO4, and concentrated under reduced pressure. The crude was dissolved in DCM (5 mL), and DBU (0.1 mL) was added. The reaction was stirred at room temperature for 30 min, and the reaction mixture was washed with water, brine, dried over Na2SO4, and concentrated under reduced pressure. The crude was purified by CC using EtOAc: nhexane 7:3.
(0.15 g, 79% yield, 98% chemical purity (GC)) as a colorless oil: 1H NMR (400 MHz, CDCl3) δ 6.65 (dd, J = 15.6, 9.6 Hz, 1H), 6.06 (d, J = 15.6 Hz, 1H), 5.89–5.21 (m, 3H), 2.8 (t, J = 10.4Hz, 1H), 2.27(s, 3H), 1.86 (s, 3H), 1.05 (s, 3H), 0.95 (s, 3H); 13C NMR (100 MHz) δ 206.9, 156.2, 144.7, 133.7, 119.7, 117.9, 60.5 30.6, 28.3, 26.2. IR (nujol, cm−1) 1684, 1619, 1454, 1109, 990. GC-MS m/z: 191.14 (M+, 1); Anal. Calcd for C13H18O: C, 82.06; H, 9.53. Found: C, 82.11; H, 9.54.
4. Conclusions
A new stereospecific approach to the 10-ethyl-7,8-dihydro-γ-ionone isomers and 4,5-didehydroionone isomers are described. The 10-ethyl-7,8-dihydro-γ-ionone isomers (R)-(+)-7, and (S)-(−)-7 were prepared starting from commercially available α-ionone 1 in a few regioselective steps. Enantiomer-enriched (R)-(+)-7, and (S)-(−)-7were prepared by means of diastereoselective and enantioselective lipase-mediated acetylation of the racemic intermediate 4-hydroxy-γ-ionone derivative 6a/6b followed by a number of chemoselective and regioselective reactions. Moreover, the resolution step confirms the utility of the enzymatic approach to the preparation of enantiomer-enriched norterpenoid odorants.
Acknowledgments
The authors extend their appreciation to the Deanship of Scientific Research at King Saud University for funding the work through the research group project No. RGP-VPP-007.
References
- Ohloff, G. Scent and Fragrances: The Fascination of Fragrances and Their Chemical Perspectives; Springer-Verlag: Berlin, Germany, 1994. [Google Scholar]
- Pybus, D.H.; Sell, C.S. The Chemistry of Fragrances; The Royal Society of Chemistry: London, UK, 1999. [Google Scholar]
- Kraft, P.; Bajgrowicz, J.A.; Denis, C.; Fráter, G. Odds and trends: Recent developments in the chemistry of odorants. Angew. Chem. Int. Ed 2000, 39, 2980–3010. [Google Scholar]
- Sell, C.S. On the unpredictability of odor. Angew. Chem. Int. Ed 2006, 45, 6254–6261. [Google Scholar]
- Brenna, E.; Fronza, G.; Fuganti, C.; Righetti, A.; Serra, S. Enzyme-mediated preparation of the single enantiomers of the olfactory active components of the woody odorant timberol®. Helv. Chim. Acta 1999, 82, 1762–1773. [Google Scholar]
- Brenna, E.; Fuganti, C.; Serra, S. Synthesis and olfactory evaluation of the enantiomerically enriched forms of 7,11-epoxymegastigma-5(6)-en-9-one and 7,11-epoxymegastigma-5(6)-en-9-ols isomers, identified in Passiflora edulis. Tetrahedron 2005, 16, 1699–1704. [Google Scholar]
- Serra, S.; Barakat, A.; Fuganti, C. Chemoenzymatic resolution of cis- and trans-3,6-dihydroxy-α-ionone. Synthesis of the enantiomeric forms of dehydrovomifoliol and 8,9-dehydrotheaspirone. Tetrahedron 2007, 18, 2573–2580. [Google Scholar]
- Fuganti, C.; Serra, S.; Zenoni, A. Synthesis and olfactory evaluation of (+)- and (−)-γ-ionone. Helv. Chim. Acta 2000, 83, 2761–2768. [Google Scholar]
- Fehr, C.; Galindo, J. Syntheses of the enantiomers of γ-cyclogeranic acid, γ-cyclocitral, and γ-damascone: Enantioselective protonation of enolates. Helv. Chim. Acta 1995, 78, 539–552. [Google Scholar]
- Brenna, E.; Fuganti, C.; Ronzani, S.; Serra, S. Enzyme-mediated syntheses of the enantiomers of γ-irones. Helv. Chim. Acta 2001, 84, 3650–3666. [Google Scholar]
- Serra, S.; Fuganti, C. Synthesis of the enantiomeric forms of α- and γ-damascone starting from commercial racemic α-ionone. Tetrahedron 2006, 17, 1573–1580. [Google Scholar]
- Serra, S.; Gatti, F.G.; Fuganti, C. Lipase-mediated resolution of the hydroxy-cyclogeraniol isomers: Application to the synthesis of the enantiomers of karahana lactone, karahana ether, crocusatin C and γ-cyclogeraniol. Tetrahedron 2009, 20, 1319–1329. [Google Scholar]
- Brenna, E.; Fuganti, C.; Serra, S. Enantioselective perception of chiral odorants. Tetrahedron 2003, 14, 1–42. [Google Scholar]
- Abate, A.; Brenna, E.; Fuganti, C.; Gatti, F.G.; Serra, S. Odor and (Bio) diversity: Single enantiomers of chiral fragrant substances. Chem. Biodiv 2004, 1, 1888–1898. [Google Scholar]
- Abate, A.; Brenna, E.; Fuganti, C.; Gatti, F.G.; Serra, S. Lipase-catalysed preparation of enantiomerically enriched odorants. J. Mol. Catal. B Enzym 2004, 32, 33–51. [Google Scholar]
- Klein, E.; Rojahn, W. Fixative for perfume compositions DE 2807584, 24 February 1981.
- Barakat, A.; Brenna, E.; Fuganti, C.; Serra, S. Synthesis, olfactory evaluation and determination of the absolute configuration of the β- and γ-Iralia® isomers. Tetrahedron 2008, 19, 2316–2322. [Google Scholar]
- Serra, S.; Fuganti, C.; Brenna, E. Synthesis, Olfactory Evaluation, and determination of the absolute configuration of the 3,4-didehydroionone stereoisomers. Helv. Chim. Acta 2006, 89, 1110–1122. [Google Scholar]
- Kametani, T.; Suzuki, K.; Kurobe, H.; Nemoto, H. A new selenium-assisted cyclization-A biogenetic-type synthesis of safranal. Chem. Pharm. Bull 1981, 29, 105–109. [Google Scholar]
- Schulte-Elte, K.-H.; Margot, C.; Chapuis, C.; Simmons, D.P.; Reichlin, D. Optically active aliphatic alcohols and their application as perfume ingredients EP 457022, 10 April 1991.
- Abate, A.; Brenna, E.; Fuganti, C.; Malpezzi, L.; Serra, S. The enantiomers of iralia®: Preparation and odour evaluation. Tetrahedron 2007, 18, 1145–1153. [Google Scholar]
© 2012 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).