Abstract
Substituted imidazolinones containing heterocyclic carboxylic acids as substituents and possessing herbicidal properties were synthesized by cyclization of 2-amino-2,3-dimethylbutyramide with heterocyclic ethyl dicarboxylates under mild conditions. The use of NaH as base leads to a significant increase in the reaction rate and allows the preparation of the target compounds in high yields.
Introduction
Substituted imidazolinones were found to be exceedingly effective herbicidal agents, useful for the control of an exceptionally wide variety of plants at the end of the seventies [1]. Their effect is based on inhibition of the enzyme acetohydroxyacid synthase by reducing levels of valine, leucine and isoleucine and leading to disruption of protein and DNA synthesis. It was found that bioactivity of these compounds is appreciably higher when a N-containing heterocyclic carboxylic acid is present as a substituent in the imidazolinone ring. The first of the heterocyclyl-substituted imidazolinones was synthesized by Los [2,3].
Most methods for the synthesis of these compounds involve multistep processes and result in low yields of the desired products. One of them is based on oxidative condensation of 2-methylpyridine with 2-amino-2,3-dimethylbutyramide (ADBA) in the presence of elemental sulfur. The carboxyl group was then introduced into the pyridine ring by successive treatment with BuLi and CO2 at low temperature [4], however the yield of the desired product was very low. Chlorination of ethyl 2-methyl-3-quinolinecarboxylate gave a 2-trichloromethyl derivative which was treated with ADBA in the presence of the phase transfer catalyst [Bu4N]+[HSO4]– to give a 19% yield of imidazolinone [5]. Another method in which diethyl pyridinedicarboxylate is converted into 2-(2’-imidazolin-2’-yl)- pyridine-3-carboxylic acid has been described [6]. This last synthesis was carried out in five steps with isolation of all intermediates and again, the final yield of imidazolinones was low. In the present work we have developed a novel simple method for obtaining imidazolinones using NaH as base.
Results and Discussion
As we have found, the reaction of diethyl esters of 5-ethylpyridine-2,3-dicarboxylic acid (3a) and quinoline-2,3-dicarboxylic acid (3b) with 2-amino-2,3-dimethylbutyramide (1) proceeds easily at ambient temperature. The process is exothermic and a rise in the reaction mixture temperature is observed. In the presence of sodium hydride, cyclization occurs at a much lower temperature than that observed with other bases (CH3ONa, t-C4H9OK), and the yields of imidazolinones are higher.
Previously, a cyclization mechanism for the reaction between 1 and 3 was reported for substituted pyridine-2,3-dicarboxylic anhydride [6]. According to the proposed scheme, in the first step, the amino group reacts with the anhydride to give 6-(1’,2’-dimethyl-1’-aminocarbonyl)propyl-5H-pyrrolo- [3,4-b]pyridine-5,7(6H)-dione with the structure shown in Figure 1, below.
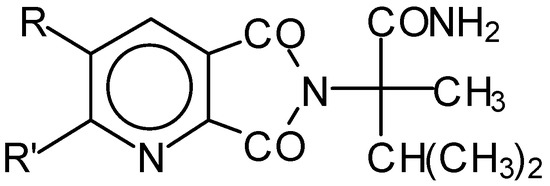
Figure 1.
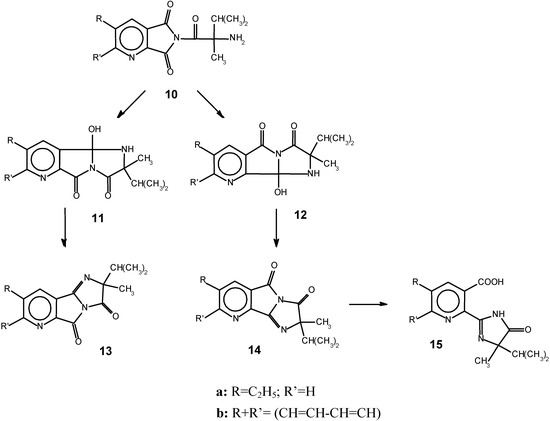
The amide group CONH2 is a weaker nucleophile and unable to attack the anhydride. The second step takes place in the presence of NaOH. The nucleophilic attack of the amide group in alkaline media on one of the carbonyl groups at pyridine ring of this compound gives a mixture of tricyclic imidazopyrrolopyridinediones 13 (15%) and 14 (85%). The second of them is unstable and hydrolizes easily to give a final product 15. It is interesting to note that only 2-(2’-imidazolin-2’-yl)-pyridine-3-carboxylic acid is formed and 3-(2’-imidazolin-2’-yl)-pyridine- 2-carboxylic acid was not found in the reaction mixture. In the process described here (Scheme 1), addition of NaH to the reaction mixture results in formation of sodium salt 2, and the amide anion becomes a stronger nucleophile than amine. We suggest that in this case the process begins with attack of CONH- on the carbonyl group of 3, and the amides 4 and 5 are formed as a result of this. 10. The next step is the formation of imidazopyrrolopyridinediones.
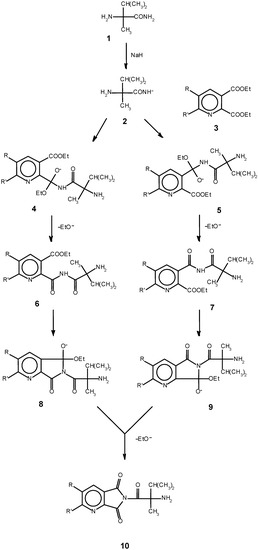
Scheme 1.
These possess an active hydrogen which reacts with the second molecule of NaH, and the CO-N–-CO anion obtained participates in an intramolecular nucleophilic attack forming the bicyclic structures 13-14. Only one isomer converts into final substance, so the resulting product is pure compound 15. The yield of 15 by this method was higher than reported in the literature data [5,6] where the products were obtained in 54.2-76.5% yields upon heating for 1-4 h at 80-110oC. By using NaH, the temperature of the reaction was decreased to 20oC and the reaction was complete in 15 min. The compounds obtained have a high degree of purity (98.5-99%).
Conclusions
The method presented allows preparation of substituted imidazolinones in high yields. The low temperature and short reaction times make the process much more effective compared to literature methods.
Acknowledgements
This research was supported by Tecnomyl SRL. We thank Prof. Eduardo R. Herrero for helpful discussions.
Experimental
General
IR spectra were recorded on a Jasco FT/IR-5300 spectrometer (KBr pellets). 1H-NMR spectra were recorded on a Bruker-200X (DMSO, 200.13 MHz). Mass spectra were obtained on a Finnigan 3300 F-100 at 70 eV. The purities of 15a-b were confirmed by HPLC analysis on a Jasco UV-975 chromatograph equipped with a C-18 column (flow, 1 mL/min; mobile phase: 80:20 methanol-water; λ=254 nm).
Preparation of 2-(4,5-dihydro-4-methyl-4-(1-methylethyl)-5-oxo-1H-imidazol-2-yl)-5-ethyl-3-pyridine-carboxylic acid (15a) and 2-(4,5-dihydro-4-methyl-4-(1-methylethyl)-5-oxo-1H-imidazol-2-yl)- 3-quinolinecarboxylic acid (15b).
NaH (0.02 mol, 60% suspension in oil) was added with stirring over 15 min. to a solution of 1 (0.01 mol) and 3a or 3b (0.01 mol) in dry toluene (10 mL), When the addition of NaH was complete, the reaction mixture was extracted with water (2 x 10 mL), and the aqueous phase was acidified to pH=2-3. The white precipitates formed were filtered, washed with water, and air dried overnight to give 15a (yield: 85.2 %, m.p. 173oC, recrystallized from toluene) and 15b (yield: 83.7 %, m.p. 224 oC, from i-propanol), respectively.
Spectral Data
15a: IR (cm-1): 3246, 1745, 1689, 1649, 613; 1H-NMR (ppm, δ): 0.82 (d, 3H, i-Pr-CH3); 0.86 (d, 3H, i-Pr-CH3); 1.25 (s, 3H, Me-CH3 ); 1.26 (t, 3H, Et-CH3 ); 1.93 (sp, 1H, CH); 2.78 (q, 2H, CH2); 3.31 (s, 1H, NH); 8,08 (d, 1H, Het 4-CH ); 8.69 (d, 1H, Het 6-CH); MS (m/e, %): 41 (23,3), 77 (16,8), 106 (23,3), 133 (100), 159 (32,9), 177 (81,9), 203 (81,2), 231 (20,2), 247 (83,8), 290 (18,4).
15b: IR (cm-1): 3231, 1736, 1703, 1643, 632; 1H-NMR (ppm, δ): 0.87 (d, 3H, i-Pr-CH3); 1.04 (d, 3H, i-Pr-CH3); 1.30 (s, 3H, Me-CH3); 1.98 (sp, 1H, CH); 3.34 (s, 1H, NH); 7.79 (d, 1H, Het 5-CH); 7.92 (d, 8-1H, Het CH); 8.19 (m, 2H, Het 6,7-CH); 8.91 (s, 1H, Het 4-CH ); MS (m/e, %): 41 (20,5), 101 (24,2), 128 (64,3), 155 (100), 181 (27,7), 199 (45,1), 225 (90,5), 253 (22,3), 268 (79,7), 312 (10,1).
References
- Wepplo, P.J. Imidazolinone herbicides: synthesis and novel chemistry. Pest. Sci. 1990, 29, 293–315. [Google Scholar] [CrossRef]
- Los, M. Ortho-(5-oxo-2-imidazolin-2-yl)arylcarboxylates – a new class of herbicides. In Pesticide Synthesis Through Rational Approaches, ACS Symp. Ser. No 225; American Chemical Society: Washington, D.C, 1984; pp. 29–44. [Google Scholar]
- Los, M. Synthesis and biology of the imidazolinone herbicides. In Proceedings of the 6th International Congress of Pesticide Chemistry (Ottawa, Aug. 10-15, 1986); Greenhaugh, R., Roberts, T.R., Eds.; Blackwell Scientific Publ.: Oxford, UK, 1987; pp. 35–42. [Google Scholar]
- Wepplo, P.J. Process for the preparation of pyridyl and quinolyl imidazolinones. US Pat. 4474962, 1984. [Google Scholar]
- Maulding, D.R.; Doehner, R.F. Process for the preparation of 2-(5-isopropyl-5-methyl-4-oxo-2-imidazolin-2-yl)-3-quinoline-carboxylic acid and alkyl 2-trichloromethyl-3-quinolinecarboxylate intermediates therefor. US Pat. 4459408, 1984. [Google Scholar]
- Los, M. 2-(2-imidazolin-2-yl)-pyridines and quinolines and use of said compounds as herbicide agents. US Pat. 4798619, 1989. [Google Scholar]
- Sample Availability: Available from MDPI.
© 2004 by MDPI (http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.