Abstract
Nα-Urocanylhistamine and two related compounds were synthesized by using PyBOP coupling protocols. These compounds represent naturally occurring histamine derivatives.
Introduction
Nα-Urocanylhistamine (1) was reported in 1973 as being naturally occurring in neogastropod mollusks [1]. It is one of several natural histamine derivatives known [2], although little information exists about the possible role of this compound in the living systems where it is found. Moreover, there are no literature reports describing the synthesis of this compound or of closely related analogs. Herein we report the synthesis of 1-3, work that was initially undertaken as part of a project to discover new classes of histamine H2 receptor agonists. These compounds are structurally related to compounds that have proven to be useful in metal complexation [3] and non-enzymatic catalysis [4].
Results and Discussion
The coupling reactions were straightforward with benzotriazol-1-yl-oxytripyrrolidinophosphonium hexafluorophosphate (PyBOP) [5], although the reactions were unsuccessful when attempted with either dicyclohexylcarbodiimide (DCC) or 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (EDC.HCl). In the case of DCC, a reagent that has been previously used for the synthesis of amide derivatives of histamine [3], only an O→N-acyl rearrangement product and starting materials were present after 24 h. The product yields following purification by chromatography on SiO2 for 1-3 were good (68–86%).

Scheme 1.
Scheme 1.
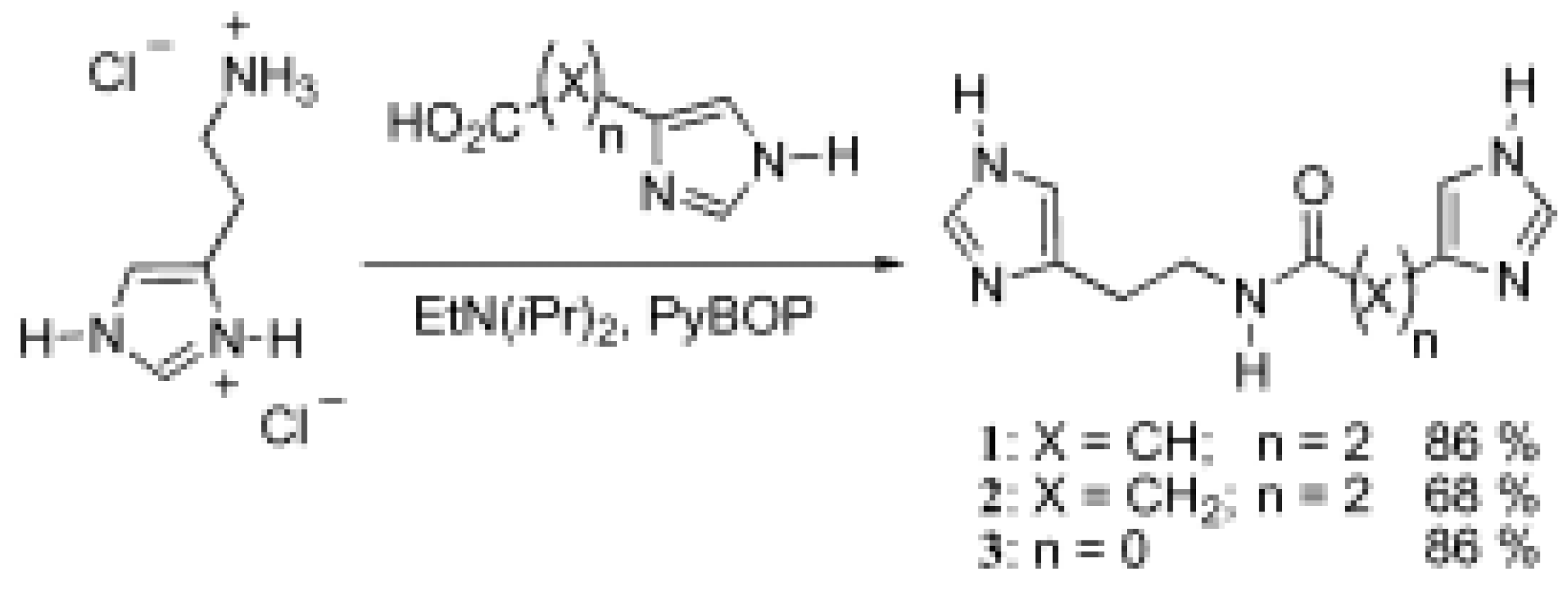
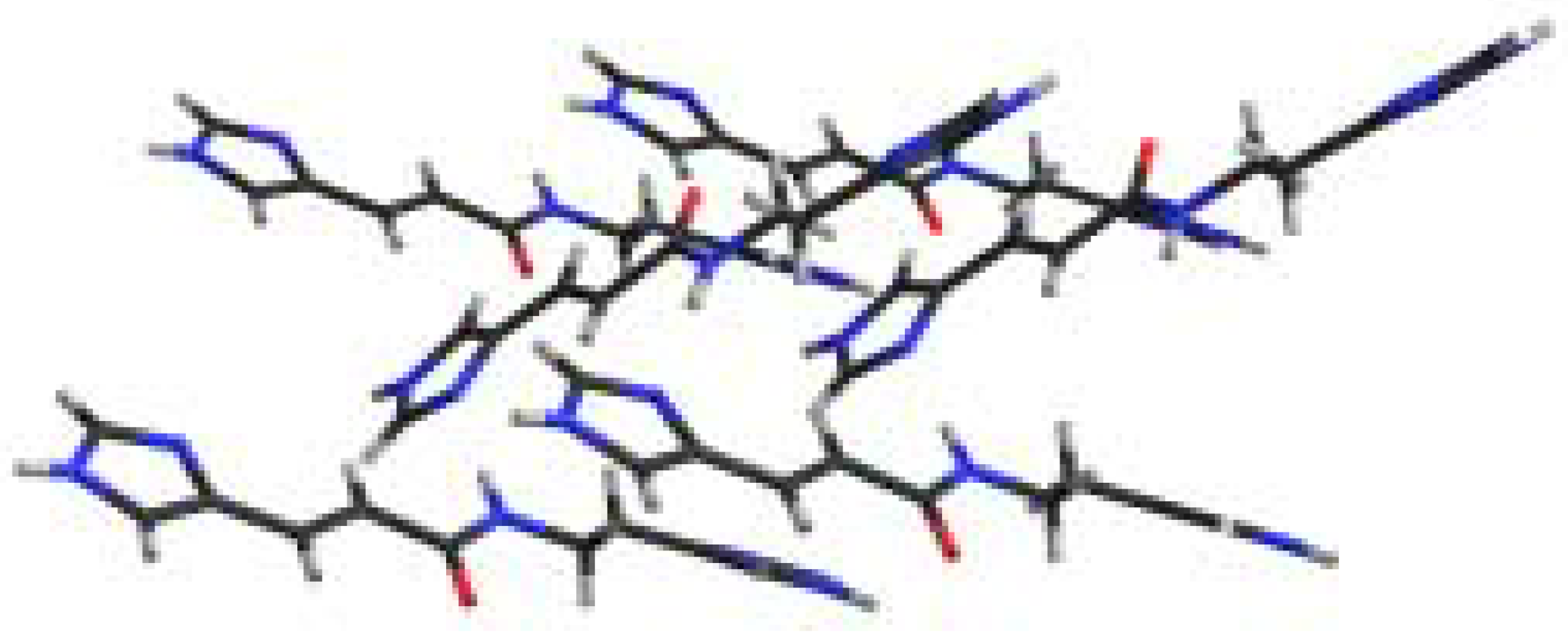
The X-ray crystal structure of 1 was determined by using a crystalline block obtained from methanol. In the solid-state, 1 forms intermolecular hydrogen bonded dimers [NH…O, 2.76 Å, ∠ = 165°] and hydrogen bonded chains from amide to imidazole [NH…N, 2.94 Å, ∠ = 174°] as well as between imidazole partners [NH…N, 2.77 Å, ∠ = 175°] (Figure 1 and Figure 2) [6].

Figure 1.
X-ray crystal structure of 1 illustrating the intermolecular NH…O hydrogen bonded dimer
Figure 1.
X-ray crystal structure of 1 illustrating the intermolecular NH…O hydrogen bonded dimer
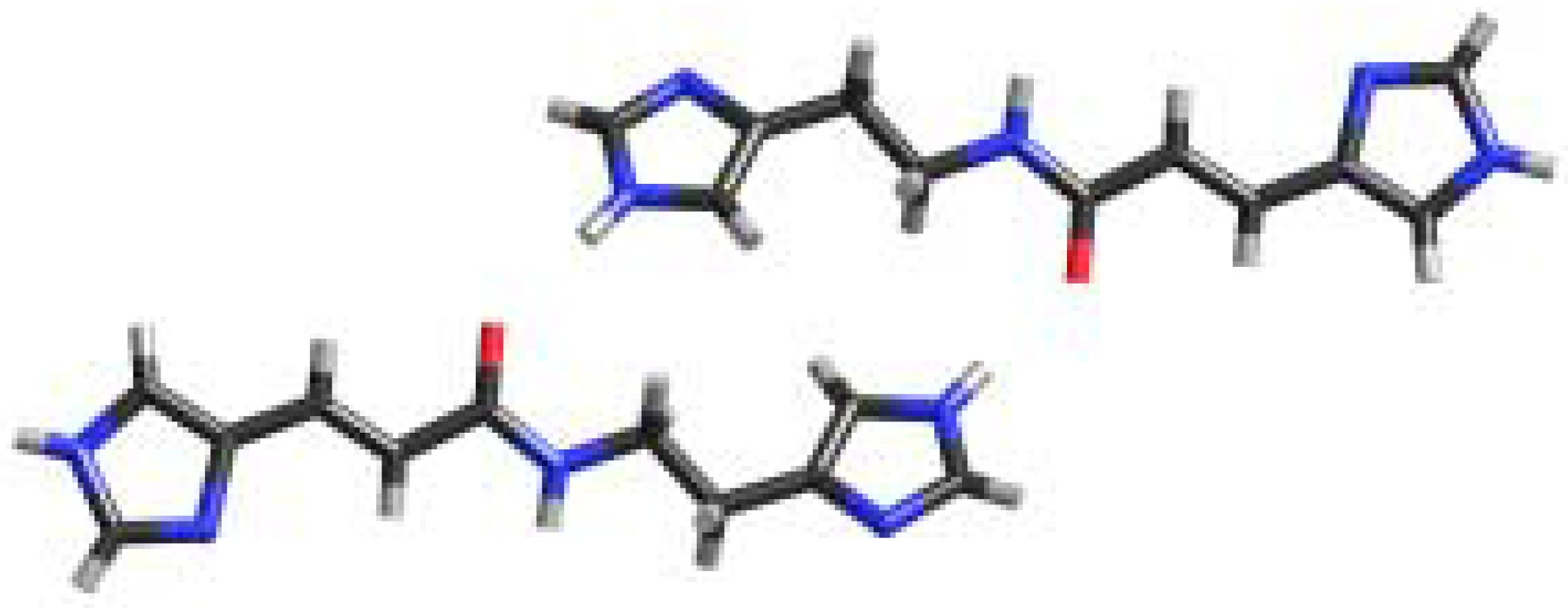

Figure 2.
X-ray crystal structure of 1 illustrating association of the dimer via NH…N amide to imidazole hydrogen bonding.
Figure 2.
X-ray crystal structure of 1 illustrating association of the dimer via NH…N amide to imidazole hydrogen bonding.
Conclusions
The coupling reagent PyBOP proves to be useful for the synthesis of amide derivatives of histamine providing good yields to purified products.
Experimental
General
All apparatus were oven-dried and cooled in a dessicator. Reagent grade CH2Cl2 was distilled from CaH2 before use. With the exception of dihydrourocanic acid, all reagents were purchased commercially and used as received. Dihydrourocanic acid was obtained by catalytic hydrogenation (5% Pd/C) of urocanic acid in methanol followed by crystallization from methanol. Thin-layer chromatography was done on Analtech 250 μm silica gel HLF Uniplates and the plates were visualized by using UV and I2 as well as ninhydrin spray for amines. Melting points were obtained on a Fisher-Johns melting point apparatus and are uncorrected. Elemental analysis of 1 was done by Desert Analytics in Tucson, Arizona. 1H and 13C NMR spectra were measured in CD3OD at 400 MHz and 50.0 MHz, respectively, and are referenced to CH3OH [1H (δ 3.31) and 13C (δ 49.1)]. X-ray data for 1 was collected at –70 °C on a Siemens P4 four-circle diffractometer with a Bruker SMART 1000 CCD to provide 2147 reflections [Fo > 2sig(Fo)] out of 2674 total reflections for final values of R = 0.035 and Rw = 0.094.
General Procedure for the Preparation of 1-3.
Histamine dihydrochloride was added to MeOH and two equiv. of diisopropylethylamine (DIEA) was added to yield a clear solution. The MeOH was removed under reduced pressure and the residue dissolved in anhydrous dimethylformamide. To this solution at 0°C was added the carboxylic acid (1 equiv), additional DIEA (3 equiv), and PyBOP (1 equiv) all dissolved in CH2Cl2. The resulting slurry was stirred 24 h at room temperature before removing the solvent under reduced pressure. The product was purified by column chromatography on SiO2 with CH2Cl2/MeOH (9:1) as the eluant.
3-(3H-Imidazol-4-yl)-N-[2-(1H-imidazol-4-yl)-ethyl]-acrylamide (1). mp 240-241°C; 1H-NMR δ 7.61 (s, 1 H), 7.52 (s, 1 H), 7.31 (d, J = 15.6 Hz, 1 H), 7.20 (s, 1 H), 6.76 (s, 1 H), 6.35 (d, J = 15.6 Hz, 1 H), 3.43 (t, J = 7.2 Hz, 2 H), 2.73 (t, J = 7.2 Hz, 2 H) (the alkene carbons were observed only as broad signals and are reported to the nearest ppm); 13C‑NMR δ 167.2, 136.5, 135, 134.1, 133.8, 130.3, 121, 117.5, 116.2, 38.7, 26.0; FAB MS m/z 232 [M+H]+; Anal. Calcd for C11H15N5O: C, 57.13; H, 5.66; N, 30.28. Found: C, 57.27; H, 5.76; N, 30.04. X-ray Data: C11H13N5O, Mr = 231.26, monoclinic, P21/n, a = 10.865(3) Å, b = 7.540(2) Å, c = 14.256(3) Å, β = 98.98(4), V = 1153.5(5) Å3, Z = 4.
3-(3H-Imidazol-4-yl)-N-[2-(1H-imidazol-4-yl)-ethyl]-propionamide (2). mp 163-164°C; 1H-NMR δ 8.23 (s, 1 H), 8.21 (s, 1 H), 7.02 (s, 1 H), 6.99 (s, 1 H), 3.38 (t, J = 6.4 Hz, 2 H), 2.86 (t, J = 7.2 Hz, 2 H), 2.78 (t, J = 6.4 Hz, 2 H), 2.47 (t, J = 7.2 Hz, 2 H); 13C-NMR δ 174.0, 135.5, 135.0, 134.8, 133.9, 117.4, 116.8, 39.5, 35.6, 26.4, 22.2; FAB MS m/z 234 [M+H]+.
3H-Imidazole-4-carboxylic acid [2-(1H-imidazol-4-yl)-ethyl]-amide (3). mp 175-177°C; 1H-NMR δ 8.48 (s, 1 H), 7.53 (s, 1 H), 7.45 (s, 1 H), 7.12 (s, 1 H), 3.46 (t, J = 6.8 Hz, 2 H), 2.81 (t, J = 6.8 Hz, 2 H); 13C-NMR δ 165.1, 137.4, 135.9, 135.0, 133.2, 121.7, 117.7, 39.0, 26.2; FAB MS m/z 206 [M+H]+.
References and Notes
- Roseghini, M.; Alcala, A. C.; Vitali, T. Experientia 1973, 29, 940.Roseghini, M.; Alcala, A. C. Biochem. Pharmacol. 1974, 23, 1431.Roseghini, M.; Severini, C.; Erspamer, G. F.; Erspamer, V. Toxicon 1996, 34. 33.
- Roseghini, M. Gen. Pharmacol. 1976, 7, 221.
- Schehmann, E.; Altman, J.; Karaghiosoff, K.; Beck, W. Inorg. Chem. 1995, 34, 2316.
- Podyminogin, M. A.; Vlassov, V. V.; Giege, R. Nucl. Acids Res. 1993, 21, 5950. [CrossRef]
- Coste, J.; Le-Nguyen, D.; Castro, B. Tetrahedron Lett. 1990, 31, 205.
- CCDC 196068 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge via www.ccdc.cam.ac.uk/conts/retrieving.html (or from the CCDC, 12 Union Road, Cambridge CB2 1EZ, UK; fax: (+44) 1223 336033; e-mail: deposit@ccdc.cam.ac.uk)
- Sample Availability: Available from MDPI (http://www.mdpi.org)
© 2002 by MDPI (http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
