A key intermediate in the synthesis of extended bipyridine ligands [1] is 4-hydroxymethylene-4-methyl-2,2-bipyridine (3), because the hydroxy group is readily converted into a variety of other chemical moieties, such as leaving or carbonyl groups. Two syntheses [2] for the preparation of 3 have been reported, both yielding 3 in two steps from 1 in 34% yield. We report here a more convenient one pot procedure giving an improved higher yield.
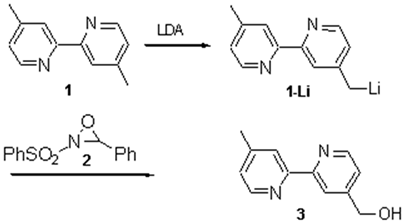
To a solution of one equivalent of 4,4-dimethyl-2,2-bipyridene (1) in dry THF were added at -78°C 1.02 equivalents of freshly prepared LDA in THF to form the deeply red anion. After stirring for 30 minutes one equivalent of 2-phenylsulfonyl-3-phenyloxaziridine 2 [3] in THF was slowly added whereby the solution turned yellow. The mixture was allowed to warm up to room temperature, quenched with aqueous sat. NH4Cl, washed with brine and the organic phase was evaporated to dryness. Column chromatography of the crude product on silica (CH2Cl2/CH3OH/NH3; 100/5/0.5) yielded 52 % of 4-hydroxymethylene-4-methyl-2,2-bipyridine 3. All spectroscopic data are identical to reported values in the literature.[2a].
1H NMR (300 MHz, CDCl3): d = 2.34 (s, 3H, CH3), 4.66 (s, 2H, CH2), 5.25 (br s, 1H OH), 7.05-7.18 (m, 2H), 8.07-8.19 (m, 2H), 8.39-8.46 (m, 2H).
13C NMR (75 MHz, CDCl3) d = 21.4 (CH3), 63.1 (CH2), 119.0 (CH), 121.4 (CH), 122.6 (CH), 125.0 (CH), 148.7 (C quart), 148.9 (CH), 149.2 (CH), 152.2 (C quart), 155.9 (C quart), 156.0 (C quart).
IR (KBr): 3200, 1596, 1456, 819.
References
- Lehn, J.-M.; Rigault, A.; Siegel, J.; Harrowfield, J.; Chevier, B.; Moras, D. Proc. Natl. Acad. Sci. U.S.A. 1987, 84, 2565. [Google Scholar] [CrossRef] [PubMed]
- a) Ciana, L. D.; Hamachi, I.; Meyer, T. J. J. Org. Chem. 1989, 54, 1731-1735; b) Chin, T.; Gao, Z.; Lelouche, I.; Shin, Y.-G.; Purandare, A.; Knapp, S.; Isied, S. S. J. Am. Chem. Soc. 1997, 119, 12849-12858; c) Polin, J.; Schmohel, E.; Balzani, V. Synthesis 1998, 3, 321-324; d) Veriot, G.; Dutasta, J.-P.; Matouzenko, G.; Collet, A. Tetrahedron1995,51, 389-400; e) Furue, M.; Kuroda, N.; Nozakura, S.-I. Chem. Lett. 1986, 1209-1212; f) Furue, M.; Yoshidzumi, T.; Kinoshita, S.; Kushida, T.; Nozakura, S.-I.; Kamachi, M. Bull. Chem. Soc. Jpn.1991, 64, 1632-1640.
- This reagent was synthesized within few hours from commercially available N-benzylidenebenzenesulfonamide according to a known procedure by Davis, F. A.; Chattopadhyay, S.; Towson, J. C.; Lal, S.; Reddy, T J. Org. Chem. 1988, 53, 2087-2089.
Sample Availability: available form the authors and MDPI. |
© 2001 MDPI. All rights reserved. Molecules website www.mdpi.org/molecules/