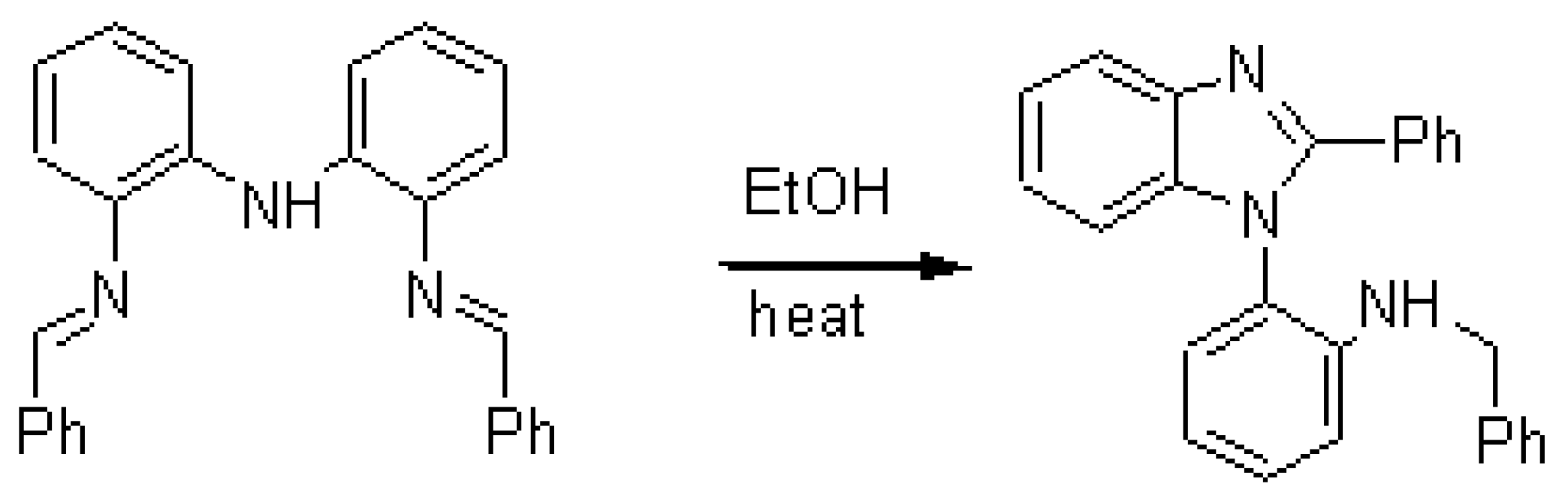
When the imine-amine compound, bis[(2-benzylideneimino)phenyl]amine [1], is heated in presence of a transition metal salt (for example FeCl3), a reaction occur leading to a new compound. After separation and characterization, we find it is a benzimidazole derivative, with a structure pattern frequently appearing in pharmaceutical molecules [1]. 0.5g red crystal of bis[(2-benzylideneimino)phenyl]amine is heated in ethanol for 30 minutes and changed into colorless. After cooling, 0.45g (90%) of white crystal product is collected by filtration.
M. p. 235 °C (ethanol).
1H NMR (400 MHz, CDCl3): 4.03 (t, JHH=5.58Hz, 1H, NH), 4.24 (d, JHH=5.58Hz, NCH2), 6.6-8.0 (m, 18H, Ph).
13C NMR (75 MHz, CDCl3): 47.0, 110.6, 112.1, 117.4, 119.8, 121.8, 123.1, 123.3, 126.7, 127.1, 128.4, 128.5, 128.6, 129.0, 129.6, 129.7, 130.4, 136.7, 138.4, 143.2, 143.9, 152.5.
IR (KBr): 3284, 3038, 2846, 1605, 1579.
Anal. Calc. for C26H21N3 (375.50 ): C 83.16, H 5.65, N 11.19; Found: C 83.25, H 5.67, N 11.07.
References
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2000 by the author. All rights reserved. Molecules website www.mdpi.org/molecules/.