Synthesis and Computational Simulation of New Phosphorilated Sulfoximines with Insecticidal Activity
Abstract
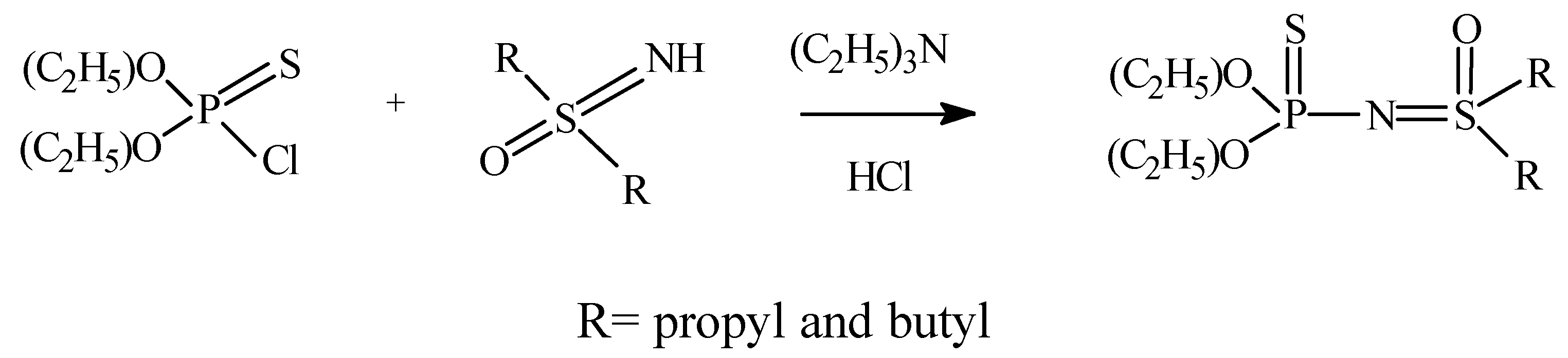
:Introduction
Experimental Methodology
Results and Discussion

References and Notes
- Wieczorkowski, J.; Jakobsen, P.; Treppendahl, S. Synthesis of N-Phosphorylated Dimethylsul-foximides. Acta Chemica Scandinavica 1983, 27. [Google Scholar] [CrossRef]
- Licastro, S.A.; Picollo, M.I.; Wood, E.; Melgar, F.; Zerba, E. Comp. Biochem. Physiol. 1986, 84C(1), 131.
- Licastro, S.A.; Zerba, E.; Wood, E.; Picollo, M.I. Pest. Science 1982, 13, 505.
- Ellman, G.L.; Courtney, K.D.; Andres, J.R.; Featherstone, M. Biochem. Pharmacol. 1961, 7, 88.
- Picollo, M.I.; Wood, E.; Zerba, E.; Licastro, S.A.; Rúveda, M. Acta Biochim. Clin. Latinoameric. 1976, 10, 67.
- Litchfield, J.T.; Wilcoxon, F. J. Pharmacol. Exp. Ther. 1949, 96, 99.
| Compounds | LD50 µg/insect |
| DPSNHPS (diethylphosphorothio propylsulfoximine) | 0.2786 |
| DBSNHPS (diethylphosphorothio butylsulfoximine) | 0.5572 |
| Compounds | I50 mol L-1 | |
| Housefly | Bovine erythrocyte | |
| DPSNHPO diethylphosphoropropylsulfoximine | 1.1 10-8 | 1.1 10-6 |
| DBSNHPO diethylphosphorobutylsulfoximine | 1.8 10-8 | 1.8 10-6 |
| Paraoxon | 2.9 10-8 | 8.9 10-7 |
Share and Cite
Bellozas Reinhard, M.E.; Licastrode, S.A. Synthesis and Computational Simulation of New Phosphorilated Sulfoximines with Insecticidal Activity. Molecules 2000, 5, 602-604. https://doi.org/10.3390/50300602
Bellozas Reinhard ME, Licastrode SA. Synthesis and Computational Simulation of New Phosphorilated Sulfoximines with Insecticidal Activity. Molecules. 2000; 5(3):602-604. https://doi.org/10.3390/50300602
Chicago/Turabian StyleBellozas Reinhard, M. E., and S. A. Licastrode. 2000. "Synthesis and Computational Simulation of New Phosphorilated Sulfoximines with Insecticidal Activity" Molecules 5, no. 3: 602-604. https://doi.org/10.3390/50300602
APA StyleBellozas Reinhard, M. E., & Licastrode, S. A. (2000). Synthesis and Computational Simulation of New Phosphorilated Sulfoximines with Insecticidal Activity. Molecules, 5(3), 602-604. https://doi.org/10.3390/50300602




