Abstract
A computational study was performed in order to rationalize the high exo stereoselectivity in the cycloaddition reactions of sugar-derived dienophiles with cyclopentadiene.
Introduction
The sequence of Diels-Alder reactions for the synthesis of pentalenolactones showed a marked preference toward the formation of cycloadducts exo-β. The aldehyde α,β-insaturated 1, in particular, rendered the cycloadduct exo-β 2, [1,2] showing complete stereoselectivity control.
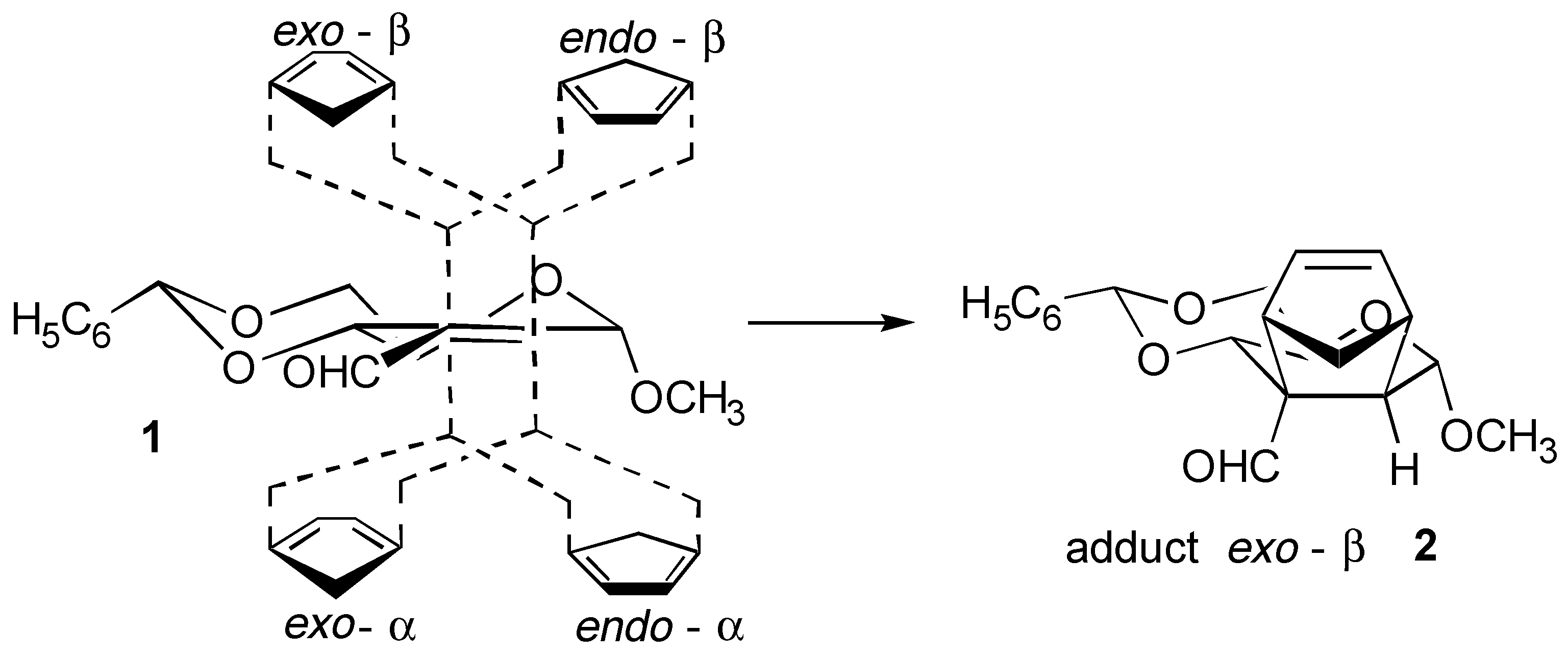
The formation of this reaction product is not predicted on the basis of the Alder rules that postulate the cycloadduct endo as the most favoured one.
Experimental
The heats of formation were calculated for the different reaction products using the semiempirical AM1 [3] method as implemented in the AMPAC 2.1 package. The stationary points obtained were characterized by force constants calculations. The reaction paths were calculated by the reaction coordinate method. The calculations provided the localizations of the transition states for such cycloaddition reactions.
Results and Discussion
Theoretical calculations were carried out to examine the thermodynamic of the formation of adduct exo-β 2. Therefore, the heats of formation of four possible stereoisomers were calculated, indicating higher stability for β adducts than for α adducts (4-5 Kcal/mol). Nevertheless, the energy difference between the endo and exo adducts (0.02 Kcal/mol) was too small to account for the exo selectivity of the cycloaddition process.
When the reaction pathways were studied, we found transition states that would support the observed endo/exo stereoselectivity.
Acknowledgements:
CONICET, International Foundation for Science, Universidad Nacional de Rosario, “Agencia Nacional de Promoción Científica y Tecnológica”, CONICOR, SECyT-UNC.
References and Notes
© 2000 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).