Abstract
The analysis of fluorescence spectra of a set of structurally related AMFQ let to identify the effects of structural changes and the presence of electric charge generated by acid-base reaction on the emission spectra.
Introduction
The fluorescence produced by quinolone ring has been extensively used in analytical determination of AMFQs in biological fluids and bacterial uptake studies.
It is well known the effect of polarity and pH on both intensity and wavelength of the emission of some AMFQs like norfloxacin (I) and ciprofloxacin (II). Variation of the emission of I and II as a consequence of pH changes is related to the variation in the proportions of the species (+0), (00), (+-), and (0-).
| Compound | R1 | R′4 | 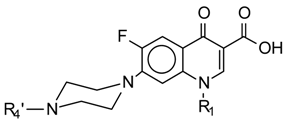 |
| I | -C2H5 | H | |
| II | c-C3H5 | H | |
| III | -C2H5 | -I-(CH3)2 | |
| IV | -C2H5 | -CO-CH3 | |
| V | -C2H5 | SO2-C6H4-NH2 | |
| VI | -C2H5 | SO2-C6H4-NH-CH3 | |
| VII | -C2H5 | SO2-C6H4-N-(CH3)2 | |
| VIII | c-C3H5 | SO2-C6H4-NH2 | |
| IX | c-C3H5 | SO2-C6H4-NH-CH3 | |
| X | -C2H5 | SO2-C6H4- CH3 | |
| XI | c-C3H5 | SO2-C6H4- CH3 |
In order to identify the main factors that affect light emission in aqueous solution, a set of 11 structurally related compounds was used (table I). Compounds V-XI are new active AMFQs synthesized in our laboratory.
Emission spectra were recorded at two pHs (4.8 and 8) which were selected taken into account the pKa of the ionizables groups.
Results and Discussions
The analysis of such results let to relate the emission parameters with both presence and type of electric charge in the molecules.
| Compound | Excitation | Emission | U.V. Absortion Coeficients | |||
| λmax (pH= 4,8 -8) | Intensity (pH =4,8) | λ Max (pH = 4,8) | Intensity (pH = 8,0) | λ max (pH =8,0) | ξ (L.mol-1.cm-1) | |
| I | 272 nm | 5040 | 444 nm | 2402 | 415 nm | 32400 |
| II | 270 nm | 5885 | 447 nm | 3074 | 417 nm | 28800 |
| III | 278 nm | 6968 | 440 nm | 2634 | 409 nm | 33846 |
| IV | 272 nm | 600.0 | 443 nm | 3085 | 435 nm | 35733 |
| V | 272 nm | 540.3 | 445 nm | 2178 | 427 nm | 54900 |
| VI | 274 nm | 664.0 | 440 nm | 833.5 | 424 nm | 53430 |
| VII | 272 nm | 589.7 | 443 nm | 625.2 | 420 nm | 49252 |
| VIII | 272 nm | 659.8 | 442 nm | 1788 | 431 nm | 41000 |
| IX | 270 nm | 874.9 | 444 nm | 763.8 | 426 nm | 48700 |
| X | 274 nm | -------- | --------- | 3388 | 428 nm | 33900 |
| XI | 276 nm | -------- | --------- | 3950 | 431 nm | 42200 |
Emission at pH 4.8. In this condition +HBH is the prevalent species of I and II, their spectra exhibit a higher intensity and a emission λmax shifted to the red with respect to that recorded at pH 8. A similar behavior is observed with III, in which the prevalent species is +BH and exhibits the highest intensity registered. On the other hand, compounds IV-IX exhibit emission λmax which are not significatively different from those of their zwitterionic analogs I-III, however, their quantum yields are 8 to 10 times lower.
Emission at pH 8. The ionization of 3-COOH yields zwitterionic and/or anionic species. Thus, at pH 8 the proportion of prevalent species of I or II are in the order +HB- > B- ≥ BHoo; the resulting λem are shifted 30 nm to the blue and quantic yields lowered with respect to pH 4.8. A similar change occurs with III also, which is essentially as +B- in this condition and it λem is 409 nm.
Compounds IV-XI are essentially as B-, their λem lie in the range 420-435 nm, that is, at higher wavelengths than I-III. Therefore, it seems that the emission of fluorescence of zwitterionic species +HB- and +B- occurs at lower wavelengths than that of anionic species B-.
In summary: a) cationic species +HBH exhibit the higher fluorescence intensity; b) the emission of zwitterionic species +HB- and +B- is about a half of that of the formers; c) the emission of anionic species B- is highly variable, ranging from ones even higher than that of zwitterions to others sensible lower; d) neutral species BH exhibit the lower emission.
References and Notes
- Huang, Zuyun; Huang, Houping; Takashi, Zhinin Lin; Zeng, Korenaga Yu-e. Analytical Science 1997, 13 (supple), 77.
- Schimer, Roger E. Fluorometric Analysis. In Orden Methods of Pharmaceutical Analysisde CRC. Press; Vol I, 2nd ed; pp. 213–271.
- Asuquio, L.J.; Piddok. J. Antimicob. Chemother. 1993, 31, 865.