Abstract
Four brassinosteroids analogs of homoteasterone and homotyphasterol bearing 5α-OH and 5α-F groups have been synthesized and their bioactivities evaluated.
Introduction
Brassinosteroides are a new class of phytohormones with properties of enhancing plant growth and plant cell division. Since the discovery of brassinolide, in 1979 –first compound of this series— a wide variety of research programs arose concerning biosynthesis, mechanisms of action [1] and possible applications in agriculture [2].
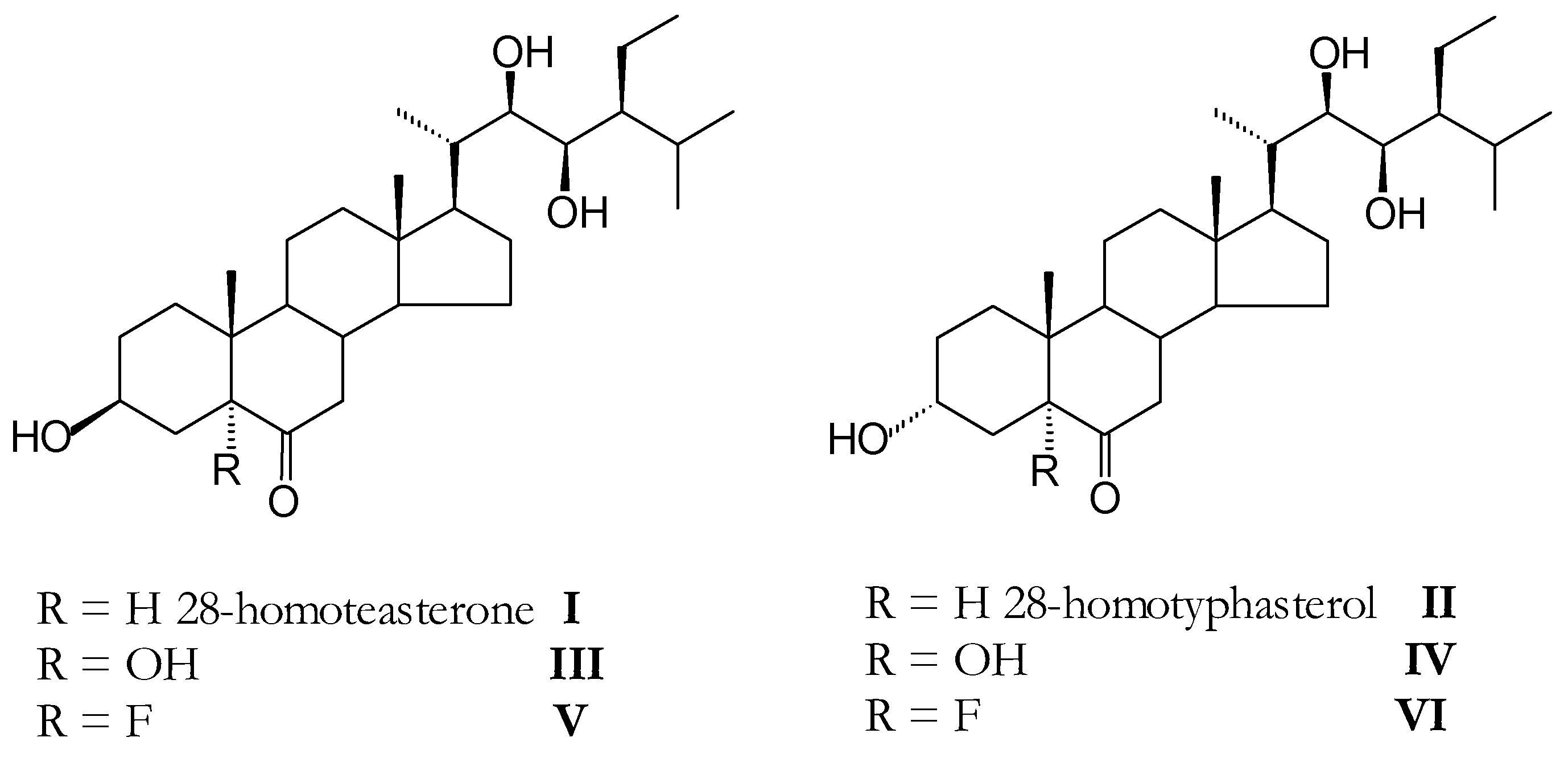
It has been already stablished that the presence of a substituent at C-5 with the ability to form an hydrogen bonding with the substituent of C-3 may change the bioactivity response of these compounds [3]. In our laboratory we have already synthesized two natural brassinosteroids homoteasterone (I) and homotyphasterol [4] (II) and in this work we introduce the synthesis of four new analogs in which the 5α-H group of compounds I and II has been replaced by a 5α-OH group (compounds III and IV) or a 5α-F group (compounds V and VI), respectively.
Bioactivities of new compounds have been evaluated with the rice lamina inclination bioassay [5] using the mentioned natural brassinosteroids as standards.
Results and Discussion
New compounds have been synthesized as shown in the following scheme.
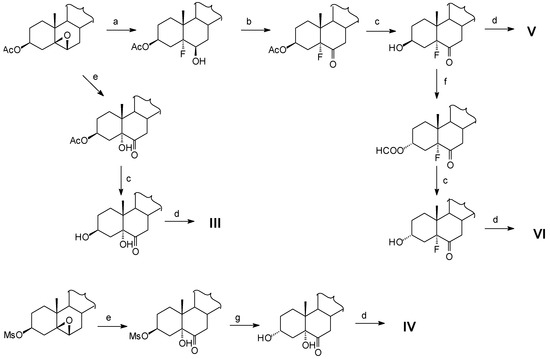
a) BF3·Et2O / Et2O / t.a. b) PCC / CH2Cl2 / t.a. c) K2CO3 / MeOH / THF / t.a. d) K2OsO4. 2H2O / (DHQD)2Phal / methansulfonamide / K3Fe(CN)6 / K2CO3 / t-BuOH / H2O / t.a. / e) Jones / t.a. f) DEAD / PPh3 / HCOOH / benzene / t.a. g) Li2CO3 / DMF / water / reflux.
All the compounds have been characterized by 1H, 13C and 19F NMR spectroscopy. First data concerning bioactivities of the analogs show the same decreasing effect when an OH or a F group were introduced at C-5. This result may induce some interesting information concerning biochemical action of these compounds at a molecular level.
Acknowledgements:
This work has been done with grants from UBACyT and CONICET. We wish to thank UMYMFOR for spectroscopic determinations.
References and Notes
- Clouse, S.; Sasse, J. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1998, 49, 427. [PubMed]
- Kamuro, Y.; Takatsuto, S. Brassinosteroids: Steroidal Plant Hormones; Sakurai, A., Yokota, T., Clouse, S., Eds.; Springer-Verlag: Tokyo, 1999; pp. 223–241. [Google Scholar]
- Brosa, C.; Zamora, I.; Terricabras, E.; Soca, L.; Peracaula, R.; Rodríguez, C. Lipids 1997, 32, 12, 1341.
- Takatsuto, S.; Ikekewa, N. J. Chem. Soc.Perkin Trans I: 1984, 439.
- Wada, K.; Marumo, S.; Abe, H.; Morishita, T.; Nakamura, K.; Uchishama, M.; Mori, K. Agric. Biol. Chem. 1984, 48(3), 719.