Effect of Substituents on the O-O Bond Rupture of Different Organic Peroxides in Toluene Solution
Abstract
:Introduction
Experimental
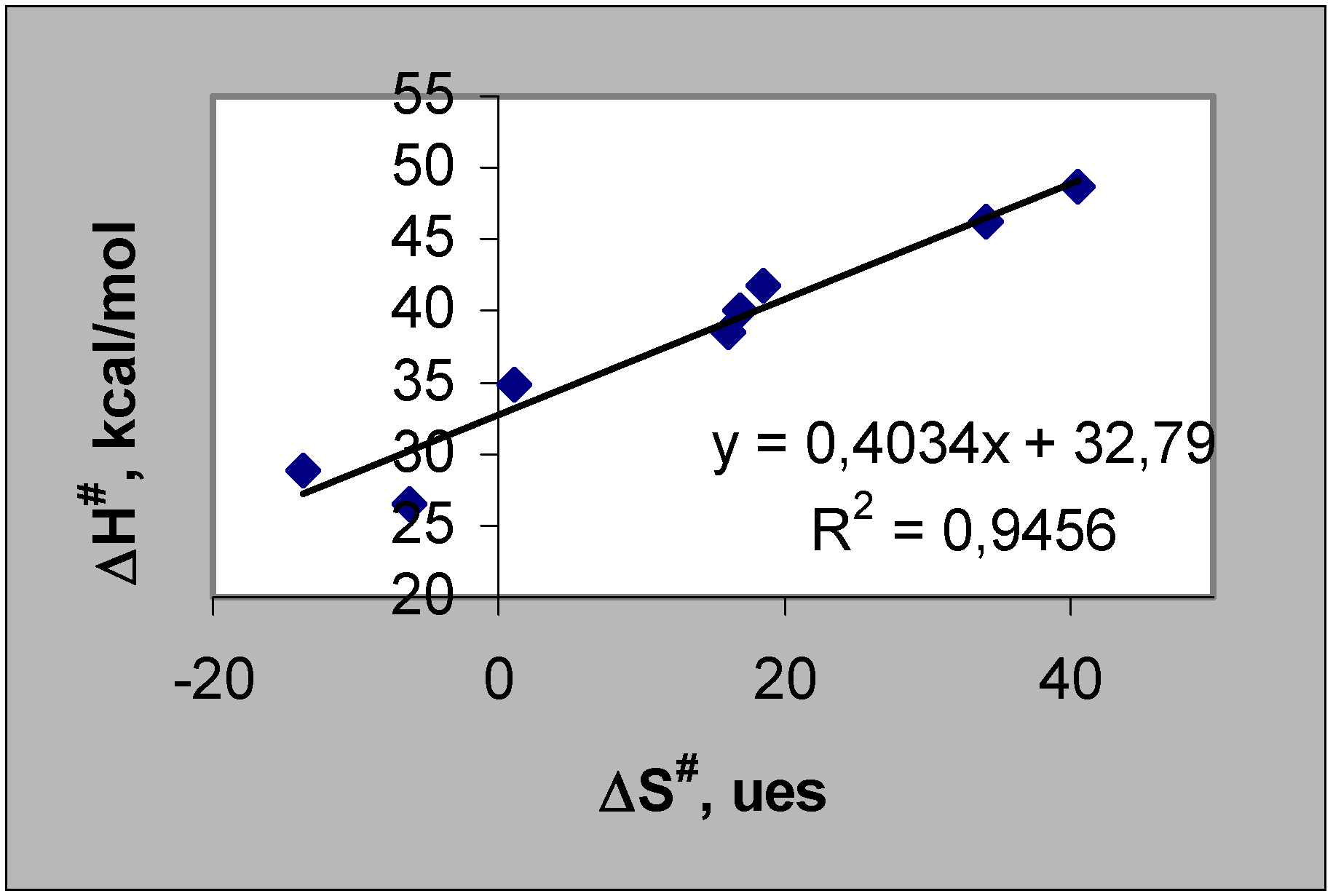
Results and Discussion
Acknowledgements
References
- Cafferata, L. F. R.; Eyler, G. N.; Cañizo, A. I.; Alvarez, E. E. J. Org. Chem. 1991, 56, 411.
- Mc Cullough, K. J.; Morgan, A. R.; Nonhebel, D. C.; Pauson, P. L.; White, G. J. J. Chem. Res. Synop. 1980, 34, M 0601.
- Leffler, J. E. J. Org. Chem. 1955, 20, 1202.
© 2000 MDPI. All rights reserved.
Share and Cite
Eyler, G.N.; Cañizo, A.I.; Mateo, C.M.; Alvarez, E.E.; Nesprías, R.K. Effect of Substituents on the O-O Bond Rupture of Different Organic Peroxides in Toluene Solution. Molecules 2000, 5, 360-361. https://doi.org/10.3390/50300360
Eyler GN, Cañizo AI, Mateo CM, Alvarez EE, Nesprías RK. Effect of Substituents on the O-O Bond Rupture of Different Organic Peroxides in Toluene Solution. Molecules. 2000; 5(3):360-361. https://doi.org/10.3390/50300360
Chicago/Turabian StyleEyler, G. N., A. I. Cañizo, C. M. Mateo, E. E. Alvarez, and R. K. Nesprías. 2000. "Effect of Substituents on the O-O Bond Rupture of Different Organic Peroxides in Toluene Solution" Molecules 5, no. 3: 360-361. https://doi.org/10.3390/50300360
APA StyleEyler, G. N., Cañizo, A. I., Mateo, C. M., Alvarez, E. E., & Nesprías, R. K. (2000). Effect of Substituents on the O-O Bond Rupture of Different Organic Peroxides in Toluene Solution. Molecules, 5(3), 360-361. https://doi.org/10.3390/50300360



