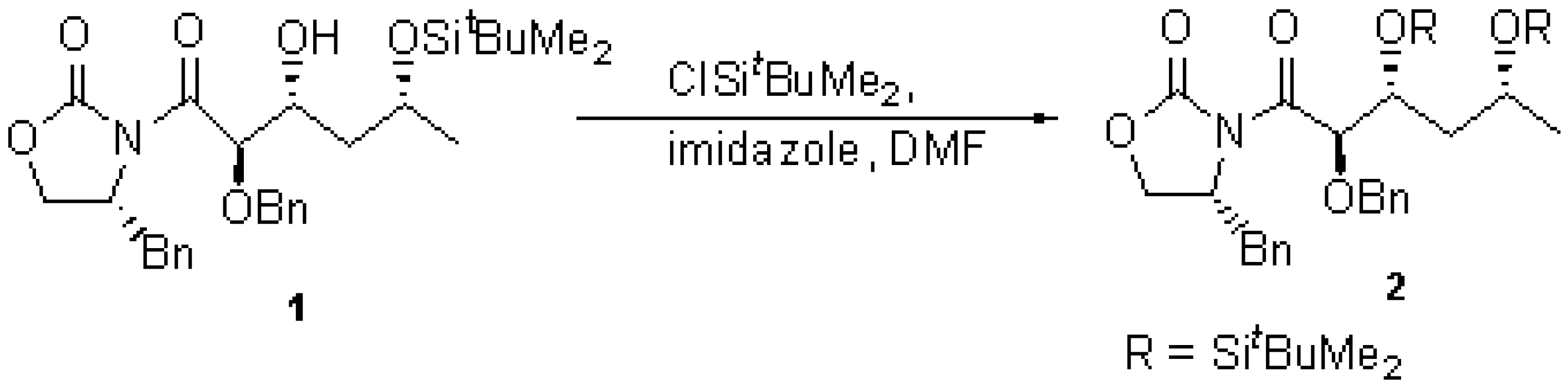
To a solution of alcohol 1 (225 mg, 0.43 mmol) [1] in dry N,N-dimethylformamide (0.9 ml) at 0°C under an atmosphere of nitrogen was added imidazole (72 mg, 1.1 mmol) and tert-butyldimethylsilyl chloride (67 mg, 0.45 mmol). The resultant solution was allowed to reach room temperature and stirred overnight. The reaction mixture was poured into ether (17 ml), washed with water (3 x 4 ml), brine (4 ml) then dried over sodium sulfate. Removal of the solvent at reduced pressure afforded a pale yellow oil that was purified by flash chromatography using light petroleum-ethyl acetate (4:1) as eluent to give the title compound 2 (246 mg, 89%) as a colourless oil.
[a]D -41.37 (c 0.614, CHCl3).
IR (cm-1, neat): 2956-2856s, 1789s, 1709s, 1386, 1110.
1H NMR (400 MHz, CDCl3): 0.01, 0.02, 0.06, 0.09 (12H, s, 2 x SiMe2), 0.84, 0.88 (18H, s, 2 x But), 1.13
(3H, d, J6′,5′ 6.0 Hz, H6′), 1.71-1.77 (1H, m, H4′A), 1.87-2.04 (1H, m, H4′B), 2.55 (1H, dd, Jgem 13.4 and J
9.9 Hz, CHCHAPh), 3.15 (1H, dd, Jgem 13.4 and J 3.2 Hz, CHCHBPh), 4.06-4.15 (4H, m, H5, H3′, H5′),
4.55-4.60 (1H, m, H4), 4.58 (1H, d, Jgem 11.5 Hz, OCHAPh), 4.64 (1H, d, Jgem 11.5 Hz, OCHBPh), 5.83
(1H, d, J2′,3′ 6.6 Hz, H2′), 7.19-7.41 (10H, m, Ph).
13C NMR (100 MHz, CDCl3): -4.8, -4.7, -4.6, -4.5 (CH3, 2 x SiMe2), 17.8, 18.0 (quat., 2 x CMe3), 24.3
(CH3, C6′), 25.7, 25.9 (CH3, 2 x CMe3), 37.7 (CH2, CHCH2Ph), 45.5 (CH2, C4′), 55.6 (CH, C4), 65.6
(CH, C5′), 66.1 (CH2, C5), 70.8 (CH, C3′), 73.2 (CH2, OCH2Ph), 80.7 (CH, C2′), 127.3, 127.9, 128.3,
128.4, 128.9, 129.4 [CH, 2 x Ph (last 4 peaks coincidental)], 135.2 (quat., CHCH2Ph), 137.5 (quat., OCH2Ph), 153.0 (quat., C2), 172.1 (quat., C1′).
CI-MS (LSIMS, NBA matrix): 642 (MH+, 3%), 584 (M-C4H10, 13), 510 (M-C6H16OSi, 9), 418 (10), 286
(14), 215 (10) and 159 (C8H19OSi, 100).
Anal. calc. for C35H55NO6Si2 M+ (EI), 641.3568; found M+, 641.3561.
References
- Brimble, M.A.; Park, J.S.O. J. Chem. Soc. Perkin Trans. I 2000, 697–709. [CrossRef]
Sample Availability: Available from the authors. |
© 2000 MDPI. All rights reserved. Molecules website www.mdpi.org/molecules/.