Abstract
The α-keto methylene group in 3,5-diaryl-2-cyclohexenones 2 and 3,5-diarylcyclohexanones 8 have been used to obtain fused pyrazoles and isoxazoles. The new compounds were characterized by IR and 1H-NMR spectral data.
Introduction
The exploitation of a simple molecule with different functionalities for the synthesis of heterocycles is a worthwhile contribution in the chemistry of heterocycles. In fact, the 6-carbethoxy-3,5-diarylcyclohexenone has been used as an effective synthon in some projected syntheses of benzoselenadiazoles/thiadiazoles [1], spirocyclohexanones [2], carbazole derivatives [3], fused isoxazoles and pyrazoles [4]. In the present communication the former has been chosen as a promising starting material to develop pyrazole and isoxazole rings. Earlier we have reported various fused and spiro isoxazoles and pyrazoles by 1,3-dipolar cycloaddition of nitrile oxides and nitrile imines to activated olefins [5]. The formation of isoxazoles from open chain α-hydroxymethylene ketones and hydroxylamine is well known [6]. The generality of this reaction is extended to cyclohexanone derivatives also [7]. In this communication preparations of a new class of fused pyrazoles and isoxazoles from 3,5-diarylcyclohexenones are reported.
Results and Discussion
The 3,5-diaryl-2-cyclohexenones 2 were prepared by the decarboxylation of the corresponding 6-carbethoxy-3,5-diarylcyclohexenones (1). The latter were obtained by Knoevenagel condensation of ethyl acetoacetate and 1,3-diaryl-2-propen-1-ones in the presence of sodium ethoxide. The Claisen like condensation of 2 with ethyl formate in the presence of sodium ethoxide gave 6-hydroxymethylene-3,5-diaryl-2-cyclohexenones 3. The formation of these products was confirmed by IR and 1H-NMR spectral studies.
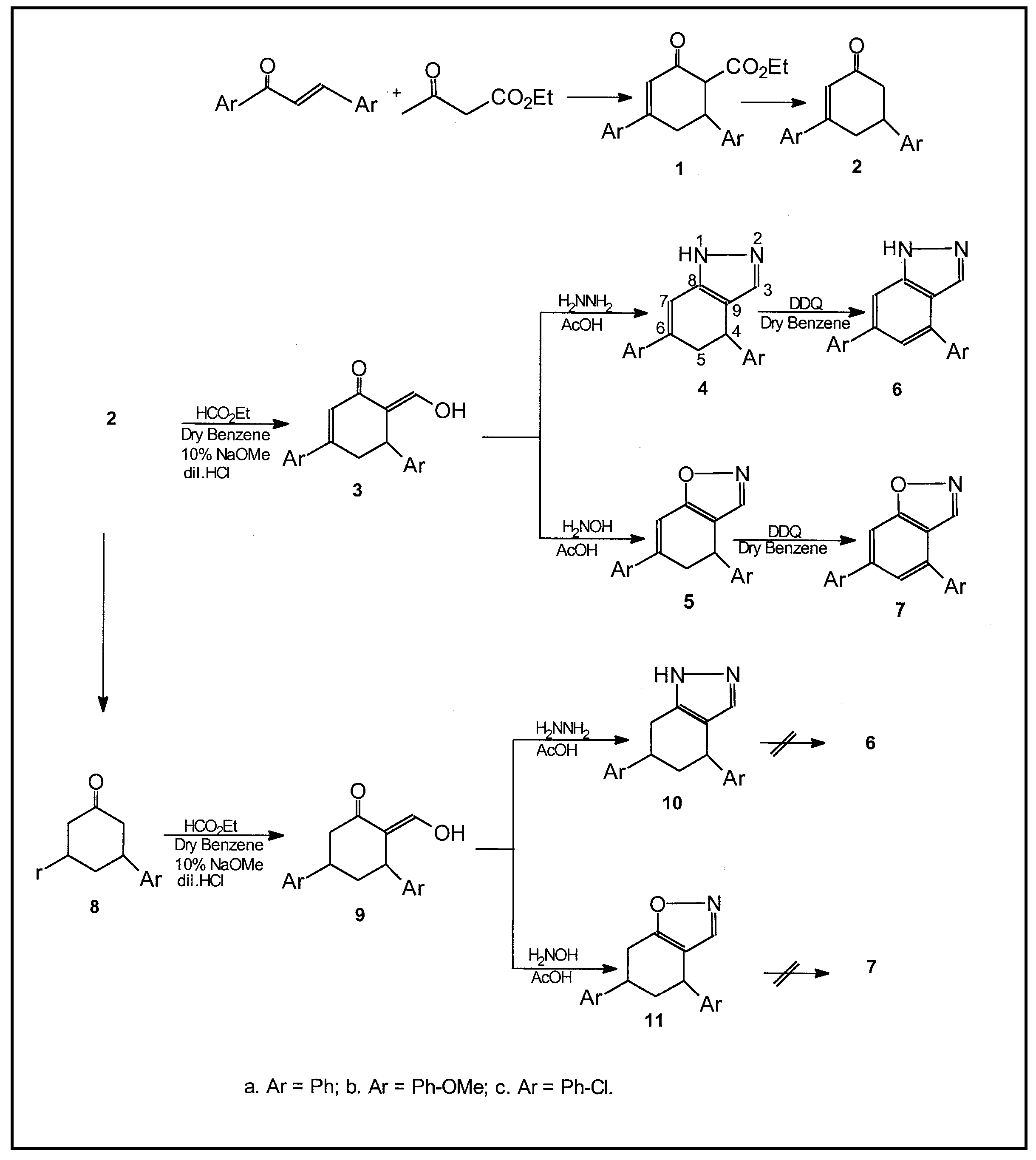
The IR spectra of 3 showed bands around 1645-1660 (C=O), 1590-1624 (C=C and C=CHOH) and 3310-3415 (OH). The 1H-NMR spectra showed two singlets, a triplet and a doublet at 5.38-5.42, 5.32-5.34, 4.37-4.38 and 3.22-3.26 for =CH-OH, H-2, H-5 and H-4, respectively. The cyclocondensation of 3 with hydrazine hydrate/hydroxylamine hydrochloride in AcOH afforded 4,5-dihydrobenzo[3,4-d]pyrazoles 4 / isoxazoles 5 (Table 1). The IR spectra of these compounds showed a band in the region 1560-1585 (C=N), whereas 4 displayed a band around 3390-3410 (NH). The 1H-NMR spectra of 4 and 5 exhibited a singlet, a triplet and a doublet at 5.30-5.38, 4.50-4.56 and 3.20-3.25 for H-7, H-4 and H-5, respectively. Aromatization of 4 and 5 with DDQ gave fused benzopyrazoles 6/isoxazoles 7. The absence of signals corresponding to methine and methylene protons of cyclohexadiene ring indicates that aromatization has indeed taken place. The compounds 4 -7 exhibited a multiplet in the region 7.12- 8.14 for Ar-H and H-3 [8]. However 4 and 6 displayed a broad singlet at 8.84-8.87 for NH which disappeared upon deuteration (Table 2). The IR spectra of 6 and 7 are replicas of those of 4 and 5.

Table 1.
Physical data of compounds 3-7 and 9-11.

Table 2.
1H-NMR Spectral Data of 3-7 and 9-11.
Compounds 2 on reduction with H2/Pd at 40 psi gave 3,5-diarylcyclohexanones 8. The 2-hydroxymethylene-3,5-diaryl-2-cyclohexanone 9 was prepared by the reaction of latter with ethyl formate. Cyclocondensation of 9 with hydrazine hydrate / hydroxylamine hydrochloride resulted 4,6-diaryl-4,5,6,7-tetrahydrobenzo[3,4-d]pyrazoles 10 / isoxazoles 11 (Table 1). The IR spectra of 9 are similar to those of 3 except for the C=C whereas their 1H-NMR spectra showed a triplet, two multiplets and a doublet at 3.64-3.69, 2.08-2.19, 2.98-3.05 and 2.89-2.94. The hydroxymethylene proton displayed a singlet at 5.32-5.40. However 10 and 11 exhibited a triplet, two multiplets and a doublet at 3.52-3.60, 2.10-2.21, 3.10-3.18 and 2.80-2.87 (Table 2). A multiplet at 7.14-8.01 accounts for Ar-H and H-3. In fact the δC values observed for C-3 in 4a, 5a, 10a and 11a around 136-138 ppm account for such an assignment (Table 3).The compounds 10 and 11 could not be oxidized with DDQ. Thus the versatality of a-keto methylene group in 2 and 8 indicates that they are potential intermediates for fused pyrazoles and isoxazoles.

Table 3.
13C-NMR Spectral Data of 4a, 5a, 10a and 11a.
Conclusions
The α-keto methylene group in substituted cyclohexenones has been utilized to get pyrazoles and isoxazoles via Claisen like condensation with ethyl formate followed by cyclocondensation with hydrazine hydrate and hydroxylamine hydrochloride.
Experimental
General
Melting points were determined on a Mel-Temp apparatus and are uncorrected. The purity of compounds was checked by TLC (silica gel H, BDH, ethyl acetate/hexane, 1:3). The IR spectra (KBr pellets) were recorded on a Perkin-Elmer grating infrared spectrophotometer model-337. The 1H-NMR spectra were recorded in CDCl3 on a Bruker Spectrospin Varian EM-360 spectrophotometer (90 MHz) with TMS as an internal standard. The elemental analyses were performed at Dr. Reddy’s Research Foundation, Hyderabad, India. The 6-carbethoxy-3,5-diaryl-2-cyclohexenones 1, 3,5-diaryl-2- cyclohexenones 2 and 3,5-diarylcyclohexanones 8 were prepared according to literature procedures [9].
6-Hydroxymethylene-3,5-diaryl-2-cyclohexenones(3)/2-hydroxymethylene-3,5-diarylcyclo-hexanones(9)
To a solution of 10% sodium methoxide (10 mL) in benzene (50 mL) at 00C, a solution of ethyl formate (10 mmol) in dry benzene (25 mL) was added. To this mixture 2 / 8 (10 mmol) in benzene (25 mL) was added. The mixture was stirred for 4 hours at room temperature and allowed to stand overnight. It was then diluted with cold water, acidified with dil. HCl and extracted with ether. The solvent was evaporated and the resultant compound was recrystallised from ethanol to get pure 3/9.
4,6-Diaryl-4,5-dihydrobenzo[3,4-d] pyrazoles 4/ 4,6-diaryl-4,5,6,7-tetrahydrobenzo[3,4-d] pyrazoles (10)
A solution of 3/9 (10 mmol) in glacial acetic acid (25 mL) was stirred with hydrazine hydrate (15 mmol) for 6-8 hours at 70-80o C. The solvent was removed under reduced pressure, and the residue was diluted with water. It was extracted with ether, washed with saturated NaHCO3 solution, water, brine solution and dried (Na2SO4). The solvent was removed and the crude product was purified by recrystallization from ethanol.
4,6-Diaryl-4,5-dihydrobenzo[3,4-d]isoxazoles(5)/4,6-diaryl-4,5,6,7-tetrahydrobenzo[3,4-d] isoxazoles (11)
A solution of 3/9 (10 mmol) in glacial acetic acid (25 mL) was stirred with hydroxylamine hydrochloride (12 mmol) for 6-8 hours at 70-80oC. The solvent was removed under reduced pressure. The viscous mass was diluted with water and extracted with ether. The organic layer was washed successively with saturated NaHCO3 solution, water and brine solution. It was dried (Na2SO4) and the solvent was removed. Recrystallization of the crude substance from ethanol afforded pure 5/11.
Oxidation of 4 and 5
A 1:1.5 molar mixture of 4/5 and DDQ in dry benzene (20 mL) was heated at reflux temperature for 2 hours under nitrogen atmosphere. The precipitated DDQ-H2 was filtered off and the filtrate was subjected to column chromatography on silica gel (60-120 mesh), BDH to obtain 6/7.
Acknowledgments
We are thankful to Council of Scientific and Industrial Research (CSIR), New Delhi for financial support under Major Research Project.
References
- Bhaskar Reddy, D.; Somasekhar Reddy, A.; Padmavathi, V. J. Chem. Research (S) 1998, 784.
- Padmavathi, V.; Sharmila, K.; Somasekhar Reddy, A.; Bhaskar Reddy, D. Indian J. Chem. (in press).
- Padmavathi, V.; Sharmila, K.; Padmaja, A.; Bhaskar Reddy, D. Heterocycl Commun 1999, 5, 451.
- Padmavathi, V.; Sharmila, K.; Balaiah, A.; Somasekhar Reddy, A.; Bhaskar Reddy, D. Synth. Commun. (in press).
- (a) Padmavathi, V.; Sumathi, R P.; Chandra Sekhar Babu, N.; Bhaskar Reddy, D. J Chem. Research (S) 1999, 610. ; and references cited therein; (b) Padmavathi, V.; Sumathi, R.P.; Venugopal Reddy, K.; Somasekhar Reddy, A.; Bhaskar Reddy, D. Synth. Commun. 2000, 30, 4007. ; (c) Bhaskar Reddy, D.; Somasekhar Reddy, A.; Padmaja, A. Synth. Commun. 1999, 29, 4433.
- (a) Claisen. Ber 1903, 36, 3664. ; (b) Claisen. Ber 1909, 42, 59.
- Johnson, W S.; Shelberg, W E. J. Am. Chem. Soc. 1945, 67, 1745. ; and references cited therein.
- Mohareb, R M.; Shams, H Z.; Aziz, S I. Sulfur Lett. 1991, 13, 101.
- (a) Balasubramanian, M.; D’souja, A. Tetrahedron 1968, 24, 5399. ; (b) Meyer, A Y.; Schelesinger, J. Israel J. Chem. 1970, 8, 671.
- Samples Availability: Samples are available from the authors.
© 2000 by MDPI (http://www.mdpi.org).