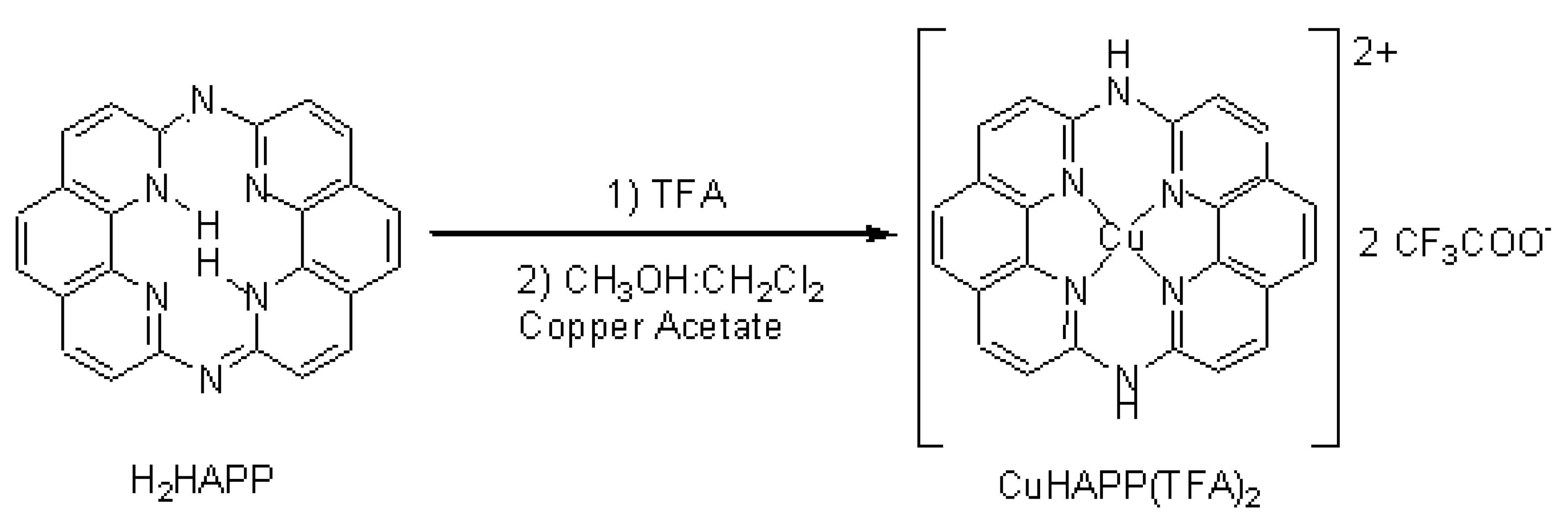
1,14:7,8-Diethenotetrapyrido-[2,1,6-de:2,1,6-gh:2,1,6-na][1,3,5,8,10,12] hexaazacyclotetradecine (H2HAPP) was synthesized according to Ogawas method[1]. 20 mg H2HAPP was dissolved in 0.5 mL trifluoroacetic acid, then 2mL ether was added, and the white precipitate [H4HAPP(TFA)2] was filtered off and washed with diethyl ether for several times. The mixture of this white precipitate and 50 mg copper acetate was refluxed in CH3OH:CH2Cl2 (V:V, 1:1) for 1 hour, the solvent evaporated, and the orange precipitate was washed with methanol. The product was purified by diffusing diethyl ether into a TFA solution at 4°C. Brown-yellow needle crystals of CuHAPP(TFA)2 were obtained, 23 mg. Yield: 65.7%.
NMR : no NMR signal could be observed because Cu++ ion is paramagnetic.
IR (KBr): 2716 (br), 1666 (s), 1642 (s), 1619 (m), 1597 (s), 1565 (s), 1505 (m), 1467 (s), 1421 (m), 1382 (m), 1369 (m), 1360 (m), 1291 (s), 1256 (m), 1198 (s), 1169 (s), 1151 (s), 1129 (s), 1106 (m), 866 (m), 828 (m), 800 (m), 749 (w), 717 (m), 672 (m), 642 (w), 606 (m), 576 (m), 519 (w), 486 (m).
UV-Vis ( in CH3OH:CH2Cl2, V:V, 1:1): 329.5, 281.0, 224.5 (spectra is pH sensitive).
FAB-MS ([M-1]+): 448.
Anal. Calc. for C28H14N6O4F6Cu (675.5): C 49.74, H 2.07, N 12.43 %; Found: C 49.70, H 2.32, N 12.15%.
Supplementary Materials
Acknowledgments
Support for this research provided by the National Science Council, Taiwan is gratefully acknowledged.
References
- Ogawa, S.; Yamaguchi, T.; Gotoh, N. J. Chem. Soc., Perkin Trans. I 1974, 976.
Sample Availability: Available from the authors and from MDPI. |
© 2000 MDPI. All rights reserved. Molecules website www.mdpi.org/molecules/.