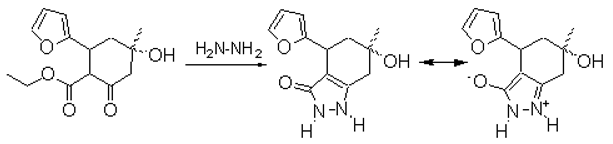
It is known that indazoles have varied biological activity [1,2]. 4-Furyl-6-hydroxy-6-methyl-1,2,4,5,6,7hexahydro-3H-indazole-3-one has been obtained for investigation of its biological activity. To a solution of 3-furyl-5-hydroxy-5-methyl-2-ethoxycarbonylcyclohexanone (1.24 g, 5 mmol) in ethanol (10 ml) were added an aqueous solution of hydrazine hydrate (52 %, 0.5 ml, 5 mmol) and acetic acid (0.5 ml), and the mixture heated to reflux until the reaction was complete (~1.5 h, TLC). The reaction mixture was then cooled to 5-10 °C, the crystalline product was collected, washed with cold ethanol and recrystallized from ethanol to yield 0.97 g (83 %) of hexahydroindazole-3-one as a white crystals.
M.p. 268-269 °C (ethanol).
1H-NMR (CF3COOD, 250 MHz): 7.48 (d, J1=2.0 Hz, 1H, H-5 furan), 6.33 (dd, J1=2.0 Hz, J2=3.6 Hz, 1H, H-4 furan), 6.00 (d, J2=3.6 Hz, 1H, H-3 furan), 4.60 (d, J=3.6 Hz, 1H, 4-H), 2.82 (broad s, 2H, 7-H, 7-H), 2.20 (dd, 1H, J1=6 Hz, J2=14 Hz, 5-H), 1.75 (dd, 1H, J2=14 Hz, J3=11 Hz, 5-H), 1.36 (c, 3H, CH3).
IR (cm-1, nujol): 3270, 3150, 1605, 1600.
UV [lmax(nm), log e (dm3mol-1cm-1)] (ethanol): 208(4,24), 253(3.63).
Anal. calc. for C12H14N2O3 (234,25): C 61.52, H 6.02, N 11.96. Found: C 61.24, H 6.29, N 11.68.
Supplementary Materials
References and Notes
- Aran, V.J.; Flores, M.; Munoz, P.; Paez, J.A.; Sanchez-Verdu, P.; Stud, M. Liebigs. Ann. 1996, 683.
- Morie, T.; Harada, H.; Kato, S. Synth. Commun. 1997, 27, 559.
Sample Availability: Available from the authors. |
© 1999 MDPI. All rights reserved. Molecules website www.mdpi.org/molecules/.