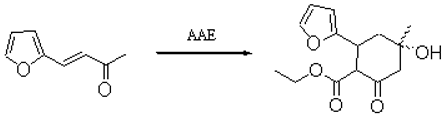
Furylcyclohexanones can be prepared by the reaction of acetoacetic ester (AAE) with b-furyl-a,b-unsaturated ketones [1,2]. To a mixture of a solution of sodium (0.5 g, 0.002 mol) in propanol (10 ml) and AAE (30 g, 0.23 mol) was added gradually with stirring furfurylidenacetone (13.6 g, 0.1 mol) over 15 min. During this time the mixture became homogeneous. It was left to stand at room temperature overnight before hexane (30 ml) and acetic acid (1 ml) were added. The white precipitate was filtered off, washed with hexane and recrystallized from ethanol to give 10.0g (40.3 %) of desired product as white crystals.
M.p. 114-115 °C (ethanol).
1H-NMR (60 MHz, CDCl3): 7.38 (d, J1=2.0 Hz, 1H, 5-H furan), 6.28 (dd, J1=2.0 Hz, J2=3.6 Hz, 1H, 4-H furan), 6.08 (d, J2=3.6 Hz, 1H, 3-H furan), 4.07 (q, J=7.0 Hz, 2H, CH3CH2O), 3.28-3.70 (m, 2H, 2-H, 3-Hcyclohexanone), 2.73 (broad s, 1H, OH), 1.92-2.50 (m, 4H, 4-H, 4-H, 6-H, 6-H cyclohexanone), 1.30 (s, 3H, CH3), 1.08 (t, J = 7.0 Hz, 3H, CH3CH2O).
IR (cm-1, nujol): 3420, 3150, 1725, 1695.
UV [lmax (nm), log e (dm3mol-1cm-1)] (ethanol): 217 (4.02).
Anal. calc. for C14H18O5 (266.28): C 63.14, H 6.81; found: C 63.52, H 6.52.
Supplementary Materials
The following supporting information can be downloaded at: www.mdpi.com/xxx/s1.
References
- Orlova, N.A.; Gerasimova, T.N.; Kudinova, M.A.; Tolmachev, N.A. Zhurnal Organicheskoy Khimii (Journal of Organic Chemistry, Russia. 1990, 20, 1313.
- Usova, E.B.; Lysenko, L.I.; Krapivin, G.D.; Kul’nevich, V.G. Khimiya Geterotsiklicheskikh Soedineniy (Chemistry of Heterocyclic Compounds, Russia) 1996, 5, 639.
Sample Availability: Available from the authors and from MDPI. |
© 2000 MDPI. All rights reserved. Molecules website www.mdpi.org/molecules/.