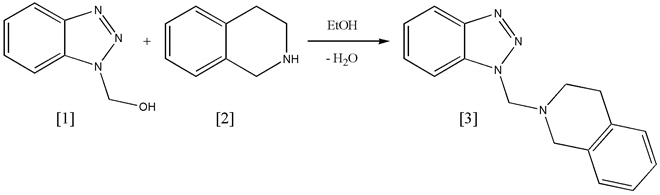
Benzotriazole-mediated heteroalkylations have been explored for many synthetic pathways. The principle of an aminoalkylation (or Mannich reaction) with primary or secondary amines was applied to the reaction of
1-hydroxymethylbenzotriazole [1] with 1,2,3,4-tetrahydroisoquinoline [2] which yielded very high quantities of crystalline N-benzotriazol-1-ylmethyl-1,2,3,4-tetrahydroisoquinoline [3].
1,2,3,4-Tetrahydroisoquinoline (19.9mmol, 2.64g) was added to 1-hydroxymethylbenzotriazole (2.96g, 19.9mmol) with stirring. Ethanol (5mL) was added and the mixture heated. The reaction was very fast and initial crystallisation of the product started already after a few seconds. More ethanol (15mL) was added and the solution refluxed with stirring for 30min. The mixture was kept at -5 ° C over night, the final product (5.14g, 98%) was collected under reduced pressure and washed with cold ethanol. It was recrystallised from ethanol and yielded N-benzotriazol-1-ylmethyl-1,2,3,4-tetrahydroisoquinoline [3] as white needles.
155-157 °C (EtOH, uncorrected).
UV lmax(nm; EtOH) / e (dm3mol-1cm-1) 206 / 27060, 253 / 6970 and 273 / 5808.
IR nmax(cm-1; Nujol) 1260, 1210, 1150, 1090, 1050, 955, 940, 740.
1H-NMR dH (200 MHz; CDCl3; Me4Si) 8.08 (1H, d, J 4), 7.68 (1H, d, J 4), 7.52 (1H, t, J 7), 7.38 (1H, t, J 7),
7.10-6.90 (4H, m), 5.64 (2H,s, NCH2N), 3.86 (2H, s, ArCH2N), 2.96-2.91 (4H, m, ArCH2CH2N).
13C-NMR dC (50 MHz; CDCl3) 29.0 (ArCH2CH2N), 48.4 (ArCH2N), 52.3 (ArCH2CH2N), 69.1 (NCH2N),
110.1, 118.4, 120.1, 124.1, 125.9, 126.4, 126.6, 127.7, 128.9, 133.6, 133.7, 134.0, 146.1.
Analysis Found (C16H16N4, 264.33, Calc.) C 72.6 (72.7), H 6.2 (6.1), N 21.2 (21.2).
Acknowledgments
One of the authors (C.L.) would like to thank the Northern Territory University for the receipt of a University Postgraduate Research Scholarship.
References
- Katritzky, A.R.; Lan, X.; Yang, J.Z.; Denisko, O.V. Properties and Synthetic Utility of N-Substituted Benzotriazoles. Chem. Rev. 1998, 98, 409. [Google Scholar] [CrossRef] [PubMed]
- Katritzky, A.R.; Rachwal, S.; Rachwal, B. The Chemistry of Benzotriazole. Part 3. The Aminoalkylation of Benzotriazole. J. Chem. Soc. Perkin Trans. I 1987, 799. [Google Scholar] [CrossRef]
- Viktor, M.; Rudolf, K. 1-Benzimidazolyl-1-benztriazolylmethane. Molecules 1997, 2, M12. [Google Scholar] [CrossRef]
Sample Availability: Available from the authors (C.L. or N.P.) and from MDPI. MDPI Reg. No. 15927. |
© 1999 MDPI. All rights reserved. Molecules website http://www.mdpi.org/molecules/.