Abstract
In this research, we investigate the interaction between the redox mediator ferrocene carboxylic acid (Fc-COOH) and glucose oxidase (GOD) in order to determine the thermodynamics parameters Kint, ΔGint, ΔHint, and ΔSint using simple UV–visible experiments at different temperatures. Positive values of ΔHint, ΔSint, together with a negative value of ΔGint indicate an entropy-driven hydrophobic interaction typical of spontaneous association processes. The homogeneous electron transfer rate constants between the oxidized organometallic mediator and the reduced enzyme (ks), along with their activation parameters (ΔGET≠, ΔHET≠ and ΔSET≠), were calculated using data obtained from foot of the wave analysis (FOWA) of cyclic voltammetry experiments performed at variable temperature. According to transition state theory, the obtained parameters indicate a low activation enthalpy that reflects minimal energetic requirements for electron transfer, while the large negative activation entropy suggests the formation of an ordered transition state. The positive activation free energy falls within the expected range for biological electron transfer processes. Variable temperature cyclic voltammetry experiments of ferrocene carboxylic acid (Fc-COOH) were also performed. The obtained ΔG°, ΔH°, and ΔS° parameters indicate strong stabilization of the redox pair, consistent with a small difference in solvation energy. Circular dichroism, UV–vis spectroscopy, and combined CD and UV–Vis Spectroelectrochemistry measurements performed during redox mediation demonstrate that no significant structural alterations occur in either the enzyme or the redox mediator before or during the electron transfer processes.
1. Introduction
During the last few decades, glucose oxidase (GOD) has played a central role in the development of electrochemical biosensors for the monitoring and diagnosis of diabetes mellitus (DM). Although numerous studies have addressed glucose quantification using GOD, this enzyme remains of considerable interest for the design of high-performance biofuel cells, artificial pancreas devices, and as a model system for analyzing electron transfer processes in redox proteins [1]. GOD is a flavoenzyme oxidoreductase with an approximate molecular mass of 160 KDa and contains flavin adenine dinucleotide (FAD) as a tightly bound cofactor. FAD is located within a hydrophobic cavity of the protein and mediates reversible redox transformations between its oxidized (FAD) and reduced (FADH2) forms. This cofactor acts as an acceptor of two electrons and two protons during the oxidation of , as illustrated by the following Equations (1) and (2) [2].
Because the active site of glucose oxidase (FAD) is deeply embedded within the protein matrix, direct electron transfer to an electrode is kinetically hindered, and no significant current is typically observed for biosensing or energy generation applications. Numerous strategies have been explored to enhance electron transfer, including the use of carbon nanotubes [3,4], platinum group nanoparticles [5,6], reconstructed or immobilized GOD constructs [7,8,9], coordination compounds, and organometallic redox mediators [10,11,12]. The latter approach offers distinct advantages, such as straightforward synthesis, efficient regeneration of reduced GOD, and precise control over the redox active species.
In redox mediated systems, the homogeneous electron transfer rate constant () between the oxidized mediator Med (ox) and the reduced GOD (FADH2) constitutes the key step governing current enhancement. It has been proposed that ks follows the Marcus cross relation (Equation (3), [13]), where k11 and k22 represent the self-exchange rate constants of the participating redox couples. The term f12 is a statistical factor relating k11 and k22 to the collision frequency of the reactants, typically close to unity. The equilibrium electron transfer constant, KET, is obtained by equating the Nerst equations of the couples Med(Red)/Med(Ox) and GOD(FAD)/GOD(FADH2) and depends on the difference in redox potentials for the exchange of n electrons, as shown in Equation (4).
However, the interaction between the enzyme and the redox mediator prior to the electron transfer is not accounted for in this model. Moreover, neither the activation parameters nor the molecular structural changes experienced for GOD during electron transfer have been explored. These factors may significantly influence glucose sensing performance and could contribute to the development of more accurate predictive models.
Ferrocene derivates are utilized as mediators in glucose sensors owing to their chemical stability, lower redox potential, biocompatibility, and reversible electrochemical characteristics. These properties facilitate the effective mediation of glucose oxidation [14,15]. Several ferrocene derivates have been extensively studied as a redox mediator for GOD, where their homogeneous electron transfer rate constants (ks) were determined using the Nicholson and Shain methods [16]. Notably, Fc-1, 1-CH3, Fc-H, Fc-CH=CH2, and Fc-COOH exhibited ks values of 7.76 × 104, 2.57 × 104, 3.01 × 104, and 2.01 × 105 L/(mol·s), respectively. It is evident that Fc-COOH achieved the highest ks among the tested ferrocene derivates where a strong interaction with GOD is proposed [17,18,19]. Additionally, Fc-COOH has shown reproducibility tests indicate that biosensors with this redox mediator sustain consistent response signals across multiple cycles with a deviation of less than 5% in peak current values [20]. The already presented properties make Fc-COOH a promising candidate, not only for biosensors, but also for other applications such as biofuel cells [21]. However, the interaction between Fc-COOH with GOD prior to electron transfer have not been demonstrated. Additionally, changes in molecular structural of GOD during electron transfer has not been explored. Both effects in combination should be considered in models that describe performance glucose biosensing and in other second-generation biosensors. Hence in this research, we investigate the molecular interaction between ferrocene carboxylic acid and glucose oxidase (GOD), using simple UV–visible experiments at different temperatures, to the obtain the thermodynamic parameter (Kint, ΔGint, ΔHint and ΔSint). Furthermore, the homogeneous electron transfer rate constants between the oxidized organometallic mediator and the reduced enzyme (ks) with their activation parameters (ΔGET≠, ΔHET≠ and ΔSET≠), were obtained using a foot of the wave analysis (FOWA) from cyclic voltammetry at variable temperature. Cyclic voltammetry of ferrocene carboxylic acid (Fc-COOH) at variable temperature was carried out to study the stability of the redox pair trough ΔG°, ΔH°, and ΔS° parameters. We conducted circular dichroism, UV–vis spectroscopy, as well as CD and UV–Vis spectroelectrochemistry measurements during the redox mediation. This research includes the application of CD and UV–Vis spectroelectrochemistry for the first time to evaluate potential structural modifications in the protein during the electron transfer process.
2. Results and Discussion
2.1. Calculation of the Equilibrium Constant and Thermodynamic Parameters for the Interaction Between GOD and Ferrocene Carboxylic Acid
Figure 1 shows the UV–Vis spectra of GOD (0.17 mM) recorded in the presence of increasing concentrations of Fc-COOH. The main electronic transition of the enzyme, initially observed at 275 nm (0.087 a.u.), progressively shifts to 260 nm accompanied by an increase in absorbance intensity (0.435 a.u.). This spectral behavior is consistent with the formation of an electron donor–acceptor complex mediated by weak noncovalent interactions. Similar responses have been previously reported for interactions between the tyrosine residues in GOD and various molecular probes; in the present case, the observed changes are attributed to the interaction between GOD and Fc-COOH [22,23].
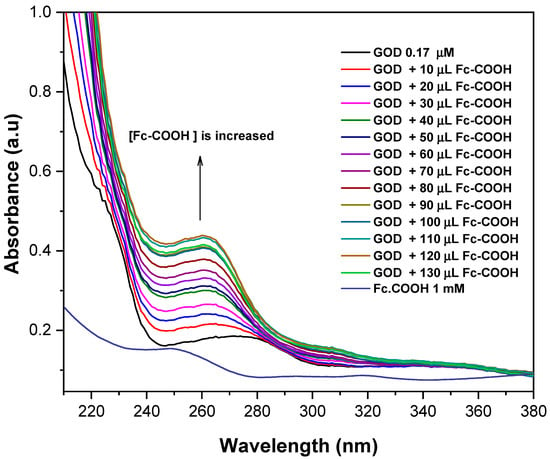
Figure 1.
UV–Vis spectra of 0.17 mM GOD in PBS 0.1M (pH = 7.2) in the presence of Fc-COOH 1 mM at 25 °C.
The interaction constant (Kint) was determined from the change in absorbance at 260 nm, where the formation of the electron donor–acceptor complex is most evident. The analysis was performed according to the Benesi–Hilderbrand model, (Equation (5)). In this equation, A0 represents the initial absorbance of GOD, A is the absorbance measured in the presence of Fc-COOH and Amax corresponds to the maximum absorbance associated with the formation of donor–acceptor complex under conditions of excess Fc-COOH [24,25].
A Benesi–Hilderbrand plot of at 260 nm yields a linear relationship, with an intercept of and a slope of (Figure 2). From the ratio of the slope to the interception, a Kint value of 125 ± 10 mol−1 was obtained. Control electronic spectra of Fc-COOH, (Figure S1), as well as experiments performed in the absence of GOD (Figure S2), confirm that no spectral interference affects the determination of the interaction constant (Kint).
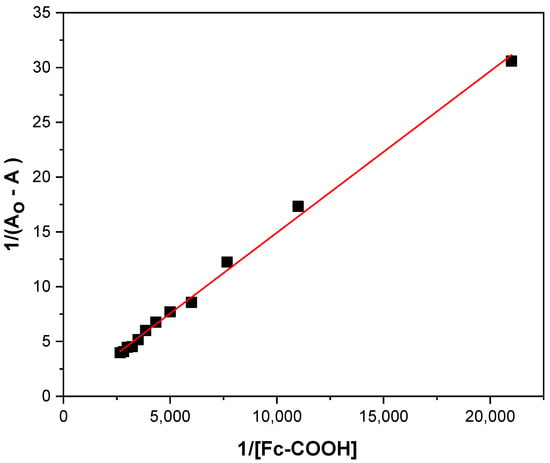
Figure 2.
Benesi–Hilderbrand equation at 260 nm for 0.17 mM GOD enzyme and additions of 1 × 10−3 M Fc-COOH in PBS 0.1 M (pH = 7.2) with temperature control at 25 °C.
To determine the thermodynamic parameters associated with the interaction between GOD and Fc-COOH, the equilibrium constant (Kint) was evaluated at different temperatures. Assuming that the enthalpy change (ΔH) remains approximately constant within the temperature range studied, the entropy (ΔSint) and free energy (ΔGint) values were obtained using the Van’t Hoof equation (Equation (6). The corresponding thermodynamic parameters were then calculated from the relationship ΔGint = ΔHint − TΔSint, (Table 1 and Figure S3).

Table 1.
Data of equilibrium constant (Kint), thermodynamic parameters (ΔHint, ΔSint and ΔGint) for the interaction of GOD with Fc-COOH, and rate constant (ks) with activation parameters (ΔHET‡, ΔSET‡ and ΔGET‡) for the redox mediation (electron transfer) of GOD with Fc-COOH.
According to the literature, the positive values of ΔHint and ΔSint obtained are indicative of a hydrophobic interaction. This behavior can be rationalized by considering that hydrophobic amino acids side chains, initially exposed to the solvent, become partially shielded upon binding of the mediator. This process releases ordered water molecules from the hydration shell into the bulk solution, thereby increasing the overall disorder or the system. The negative ΔGint values confirm the spontaneous nature of this entropy-driven interaction process [26].
Additionally, UV–Vis experiments performed with bovine serum albumin (BSA) under the same conditions used for GOD revealed no evidence of electron donor–acceptor complex formation with Fc-COOH. This result indicates that Fc-COOH has no measurable affinity for BSA (Figure S4).
The value of interaction constant Kint obtained (125 ± 10 L/mol) reveals a significant affinity between Fc-COOH and GOD, consistent with the level of pre-association required to facilitate fast electron-transfer processes without perturbing the protein structure. The positive ΔHint and ΔSint values clearly indicate a hydrophobic, entropy-driven interaction, while the negative free energy (ΔGint = −31 kJ/mol) confirms that the association is spontaneous and thermodynamically favorable, which is consistent with the behavior expected for efficient redox mediators approaching buried enzyme cofactors. Table 1 summarizes the equilibrium constant and thermodynamic parameters for the interaction between GOD and Fc-COOH. However, no similar data for GOD with other redox mediators are reported in the literature, opening a new door for research in this area. In future work we will study the molecular interaction between GOD and other ferrocene derivatives to propose a model that consider this effect in glucose biosensing performance.
2.2. Effect of Temperature on Redox Potential
Cyclic voltammograms recorded at 25 °C for 1 × 10−3 M ferrocene monocarboxylic acid (Fc-COOH) in PBS (pH = 7.2) at various scan rates are shown in Figure S5. The anodic and cathodic peak separation (59 mV to 60 mV) is consistent with a reversible one electron redox process, yielding a formal potential of 305 mV vs. Ag/AgCl. The ratio of cathodic to anodic peak currents (ipc/ipa) remains close to unity, confirming the reversibility of the Fc-COOH redox couple.
Temperature dependent measurements (25 °C to 50 °C) revealed the expected shift in E°, as shown in Figure S6. A plot of E° vs. temperature (T) exhibits a linear trend described by E°= −0.0003287T + 0.01181 (r = 0.98), presented in Figure S7. Using the slope (ΔE°/ΔT) and the formal redox potential (E°), the standard thermodynamic parameters ΔH° and ΔS° were calculated according to Equations (7) and (8). The Gibbs free energy, ΔG°, was subsequently obtained from ΔG° = ΔH − TΔS°.
The resulting thermodynamics parameters ΔG°= −28.7 KJ/mol, ΔH° = 1228 KJ/mol, and ΔS° = −9.6 KJ/mol have not been previously reported for this redox mediator. These values indicate a high stabilization for the redox pair due to small difference in solvation energy between the oxidized and reduced forms, accompanied by an increase in solvent orientation. Such behavior is consistent with an outer sphere electron transfer mechanism and agrees with the behavior reported [Fe(CN)6]4−/[Fe(CN)6]3− [27,28].
2.3. Calculation of the Homogeneous Electron Transfer Rate Constant GOD-Mediator
To evaluate the homogeneous electron transfer rate constant between GOD and Fc-COOH, cyclic voltammetry experiments were carried out at a scan rate of 1 mV/s for 1 × 10−3 M Fc-COOH in PBS (pH = 7.2) at 25 °C, both in the absence and in the presence of 0.05 M glucose and 1.8 mM GOD.
As shown in Figure 3 (a), in the absence of the enzyme, a reversible diffusion-controlled process is observed, characterized by a peak current (ip°). In contrast, upon addition of GOD (Figure 3, (b)), a catalytic current plateau (ik) emerges, which is indicative of an EiC’ mechanism, involving the following steps (Equations (9)–(11)):
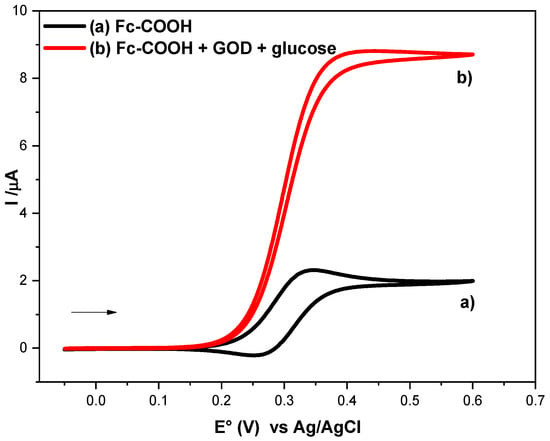
Figure 3.
Cyclic voltammograms of 1 × 10−3 M Fc-COOH in PBS 0.1M (pH = 7.2) in the presence of 0.05 M glucose in the absence (a) and in the presence (b) of 1.8 mM GOD enzyme at 1 mVs−1.
The rate constant (ks) in step II corresponds to the homogeneous electron transfer between ferricenium (Fc-COOH+) and the reduced form of the enzyme GOD (FADH2). This step constitutes the key process governing redox mediation. Although ks can, in principle, be calculated using the method reported by Nicholson and Shain [16], this approach presents several limitations, including the requirement of prior knowledge of diffusion coefficient and the assumption that no additional coupled chemical reactions occur. Therefore, the foot to the wave analysis (FOWA) method was employed in this study to determine ks according to Equation (12) [29,30,31].
A plot vs. has a slope equal to , which allowed us to calculate the rate constant. At 298.25 K, a ks value of 2 × 105 ± 5.0 × 103 L/(mol·s) was obtained.
2.4. Calculation of Kinetic Activation Parameters
Figure 4 shows the electrochemical responses of Fc-COOH in the presence of 0.17 mM GOD and 0.05 M D-glucose at different temperatures, recorded at a scan rate of 1 mV/s. An increase in the catalytic current is observed as the temperature rises, indicating an enhancement of the electron transfer kinetics. To determinate the homogeneous electron transfer rate constant (ks) at each temperature and to evaluate the corresponding activation parameters, FOWA was performed (Figure 5).
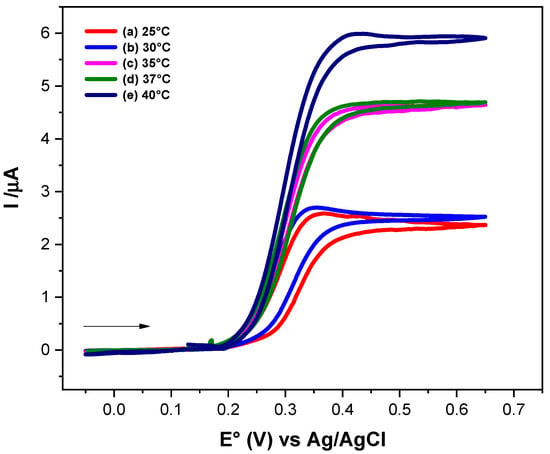
Figure 4.
Cyclic voltammograms of 1 × 10−3 M Fc-COOH, in PBS 0.1 M (pH = 7.2), in the presence of 0.05 M glucose and 0.17 mM GOD enzyme at scanning rate of 1 mV/s, at different temperatures.
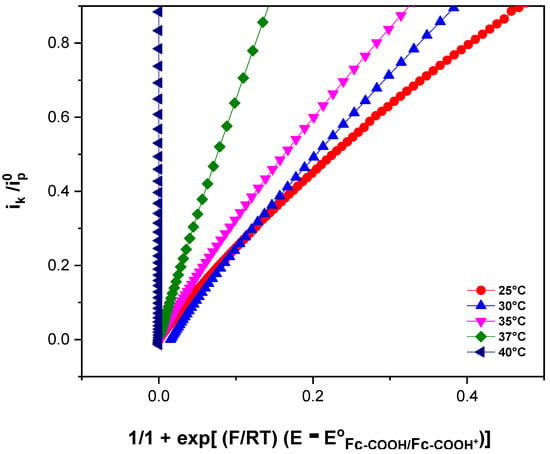
Figure 5.
FOWA of 1 × 10−3 M Fc-COOH in PBS 0.1 M (pH = 7.2) in the presence of 0.05 M glucose and 0.17 mM GOD enzyme at a scanning rate of 1 mV/s.
According to the transition state theory, the activation parameters can be obtained from a plot of ln(ks/T) vs. (1/T) (Figure 6) following Equation (13). Although this relationship was originally developed for gas phase reactions, it has been successfully applied to describe electron transfer processes in both metal complexes and proteins in solution [32,33,34,35]. The activation enthalpy and the activation entropy were derived from the slope and intercept of the linear fit, respectively.
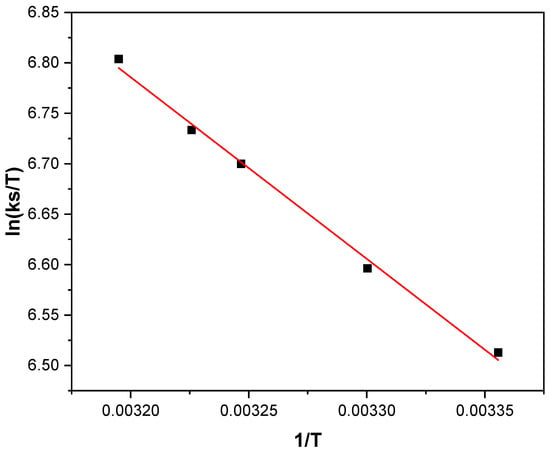
Figure 6.
ln(ks/T) vs. 1/T plot of 1 × 10−3 M Fc-COOH in phosphate buffer (pH = 7.2), ks values obtained from FOWA in the presence of 0.05 M glucose and 0.17 mM GOD enzyme using a scan rate 1mV/s at different temperatures.
The resulting and values indicate that the activated complex formed during the homogeneous electron transfer step exhibits a relatively low degree of disorder. This behavior suggests that the interaction between the redox mediator and the enzyme is sterically constrained and involves modes reorganization energy, which is consistent with a controlled molecular approach within the protein environment [36].
Table 1 summarizes the equilibrium constant and thermodynamic parameters for the interaction between GOD and Fc-COOH, as well as the rate constant and activation parameters associated with the redox mediation process between GOD and Fc-COOH. The obtained ks value (2.0 × 105 L·mol−1·s−1) places Fc-COOH among the fastest homogeneous redox mediators reported, comparable to the high performance behavior of [OsCl(Him)(bpy)2]+ (2.81 × 105 L/(mol·s) and [(η-MeC5H4)Mn(NO)(CN)2]Na (2.1 × 105 L/(mol·s), and confirm the value for Fc-COOH obtained by the Nicholson and Shain method previously described (2.01 × 105 L/(mol·s) [13,17,18,37]. This remarkable performance is noteworthy given the simplicity of the molecule, underscoring its efficiency as a homogeneous electron transfer mediator and supporting its suitability for bioelectrochemical applications.
Furthermore, the low activation enthalpy obtained ( = 14.9 kJ/mol) indicates minimal energetic requirements for electron transfer, whereas the strongly negative activation entropy reflects the formation of a highly ordered transition state. The resulting activation free energy ( = 42.7 kJ/mol) falls within the expected range for biological electron transfer processes, reinforcing the mechanistic validity of the proposed system [38,39]. Notably, a lack of comparable data for GOD interacting with other redox mediators has been reported in the literature. As mentioned previously in Section 2.1, future work will focus on investigating the molecular interaction between GOD and other ferrocene derivatives in order to develop a comprehensive model that accounts for these effects on glucose biosensing performance.
2.5. Study of the GOD–Mediator Interaction by Circular Dichroism Spectroscopy
After confirming the interaction between the enzyme and the mediator, circular dichroism (CD) spectroscopy was employed to evaluate whether GOD undergoes any conformational changes upon complex formation [40]. The CD spectrum of GOD shows two negative ellipticity bands at around 211 nm and 218 nm, which are characteristic of an a-helical secondary structure (Figure 7) [41]. These bands remained essentially unchanged after the addition of the redox mediator, indicating that the secondary structure of the enzyme is preserved. The minor variations detected are likely due to the intrinsic UV absorption of Fc-COOH, which can introduce slight interference in the CD measurements.
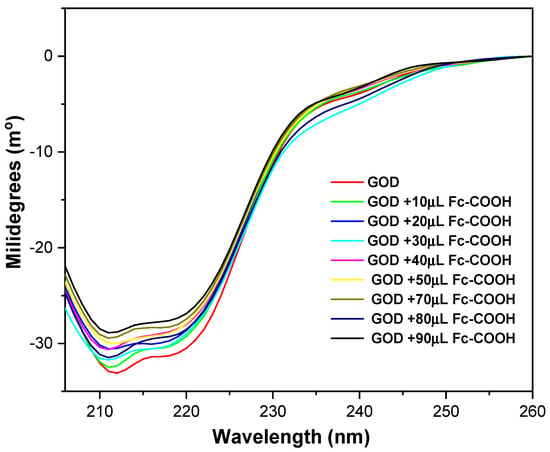
Figure 7.
Circular dichroism spectra of 0.17 mM GOD enzyme in phosphate buffer (pH = 7.2), with additions of Fc-COOH at 25 °C.
2.6. UV–Vis and Circular Dichroism Spectroelectrochemical Experiments
To assess potential structural or molecular changes occurring during the electron transfer process between GOD and Fc-COOH, UV–Vis and circular dichroism (CD) spectroelectrochemical measurements were performed. Figure S8 shows the UV–Vis spectra recorded using a platinum mesh as an optical transparent electrode (OTE). Upon application of a potential step to 400 mV, the Fe(II) transition 1A1g → 1E1g decreases in intensity, while a new absorption band at 625 nm, assigned to the Fe(III) transition 2Eg2 → 2E1g, grows during electrolysis.
Additional spectroelectrochemical experiments carried out for Fc-COOH in the presence of 0.05 M glucose and 0.51 mM GOD reveal a continuous increase in the Fe(III) bands transition, 2Eg2 → 2E1g, whereas the Fe(II) band remains nearly constant. This behavior confirms the occurrence of a homogeneous electron transfer between Fc-COOH+ and the reduced form of GOD.
Figure 8 shows the CD spectra of 0.17 mM GOD in phosphate buffer (pH = 7.2) recorded using a platinum OTE, while applying a potential of 400 mV vs. Ag/AgCl at one-minute intervals. Ellipticity changes at 218 nm provide insight into the α-helical content of the protein [42,43]. As illustrated in Figure 8 (a), before potential application the ellipticity, it is approximately 14.85 millidegrees. Upon applying a potential of 400 mV vs. Ag/AgCl, the a-helical content increases from roughly 5% (Figure 8, (b)) to nearly 10% over the course of the experiments (Figure 8, (b)–(h)). The ratio θ218nm/θ211nm, is used to evaluate the interaction between a-helices [40]; the value of 0.8–0.9 indicates the absence of interaction, whereas those near 1.0 ± 0.03 correspond to two-stranded coiled structure.
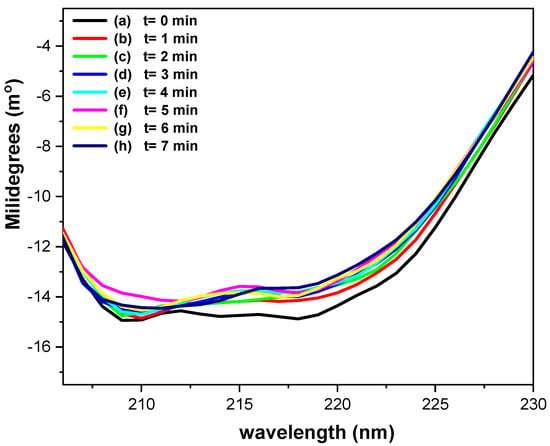
Figure 8.
Circular dichroism spectroelectrochemical spectra of 0.17 mM GOD in phosphate buffer (pH = 7.2), using a platinum OTE, applying a potential at 400 mV vs. Ag/AgCl, each spectrum was acquired every minute.
In the present CD spectroelectrochemical studies, the ratio values before (0.99) and during the electrolysis (≈0.95) remain within this range, suggesting no significant perturbation of a-helix interactions. These results suggest that the applied potential does not induce substantial alterations in the secondary structure of GOD during the electron transfer process.
Figure 9 presents the CD spectroelectrochemical results for 0.17 mM GOD in the presence of Fc-COOH and glucose in phosphate buffer (pH = 7.2), recorded under the same experimental conditions. Relative to the initial CD spectrum (Figure 9, (a)), a decrease of no more than 10% in the a-helical content is observed during electrolysis (Figure 9, (b)–(h)). The ellipticity ratio θ218nm/θ211nm decreases slightly from 0.94 (Figure 9, (a)) to 0.91 (Figure 9, (b)–(h)), which is a range consistent with non-interacting a-helices.
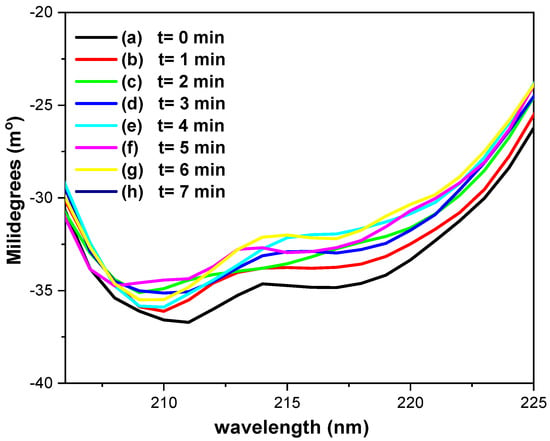
Figure 9.
Circular dichroism spectroelectrochemical spectra of 0.17 mM GOD in phosphate buffer (pH = 7.2), acquired using a platinum OTE in the presence of 0.05 M glucose and 1 × 10−3 M Fc-COOH, and with a potential at 400 mV vs. Ag/AgCl, each spectrum was acquired every minute.
These observations demonstrate that, both in the absence and presence of Fc-COOH and glucose, the secondary structure of GOD remains essentially preserved throughout the electrolysis process.
3. Materials and Methods
3.1. Materials
All chemicals and solvents were used as received from Aldrich Chemical Company (St. Louis, MO, USA), Acros Organics (Geel, Belgium) and J.T. Baker (Radnor, PA, USA). Glucose Oxidase (GOD Ec 1.1.3.4 type II) from Aspergillus niger (molecular weight of 186,000) was purchased from Sigma Aldrich (St. Louis, MO, USA). The enzyme concentration was determined spectrophotometrically following a method previously reported in the literature [44].
3.2. Methods
3.2.1. UV–Vis and Circular Dichroism Spectroscopy Studies
UV–Vis and circular dichroism (CD) measurements were carried out using a Biologic MOS-500 spectrophotometer over the 205–402 nm wavelength range, employing 0.1 cm optical path length quartz cuvette. Spectra of GOD (0.17 mM; 500 mL in PBS pH = 7.2) were recorded both in the absence and in the presence of successive additions (10mL) of a 1 mM ferrocene carboxylic acid (Fc-COOH) solution prepared in PBS (pH = 7.2). All measurements were conducted under temperature-controlled conditions using a Peltier unit. Spectra were corrected for dilution effects, and each measurement was carried out in triplicate. The interaction constant corresponds to the main value ± standard deviation.
3.2.2. Electrochemical Studies
Cyclic voltammetry measurements were performed using a Biological SP-300 potentiostat/galvanostat. Solutions of 1 × 10−3 M ferrocene carboxylic acid (Fc-COOH) in 0.1M PBS (pH = 7.2) employed as supporting electrolyte. A thermostated, classical three-electrode electrochemical cell was used, consisting of a glassy carbon disk electrode (F = 3mm) as working electrode, a platinum wire as the auxiliary electrodes, and a Basi Ag/AgCl (3 M NaCl) reference electrode equipped with a Luggin capillary. Prior to each measurement, the solutions were purged with nitrogen for 5 min. The working electrode was cleaned by manual polishing with 1mm diamond powder, followed by rinsing with distilled water and brief sonication. The Ru was around 100 W. All measurements were carried out by triplicate at 25, 30, 35, 40, 45, 50, 55 and 60 °C.
3.2.3. Determination of Homogeneous Electron Transfer Rate Constant (ks) for GOD Mediation
Cyclic voltammetry experiments were conducted using a Biologic SP-300 potentiostat/galvanostat. Measurements were performed in 1 × 10−3 M ferrocene carboxylic acid solutions prepared in 0.1 M phosphate buffer (pH = 7.2) containing 0.05 M glucose and 0.17 mM glucose oxidase (GOD), using a thermostated electrochemical cell. Prior to each experiment, the solutions were purged with nitrogen for 5 min. The working electrode was cleaned following the procedure detailed in the previous section. Measurements were recorded at 25, 30, 35, 40, 45, 50, 55, and 60 °C. All experiments were performed in triplicate, and the reported rate constant corresponded to average values with their associated standard deviation.
3.2.4. UV–Vis and Circular Dichroism Spectroelectrochemical Studies
Spectroelectrochemical experiments were carried out using a Biologic SP-50 potentiostat/galvanostat coupled either to UV–Vis diode array spectrophotometer (Thermo-Scientific evolution array) or to a circular dichroism spectropolarimeter, (MOS-500 Biologic, Paris, France). The optical transparent electrode (OTE) cell consisted of a 1mm optical path length quartz cuvette fitted with an optically transparent platinum mesh serving as the working electrode. A platinum wire was used as the auxiliary electrode, and a Basi Ag/AgCl (3 M NaCl) electrode served as the reference. Prior to each measurement, the solutions were purged with nitrogen, and electrochemical and spectroscopic data were recorded simultaneously. For UV–Vis experiments, 1 × 10−3 M solutions of the redox mediator in PBS (pH = 7.2) were used. For CD-coupled spectroelectrochemistry, a 0.17 mM GOD solution in PBS (pH = 7.2) was analyzed in the absence and presence of 1 × 10−3 M ferrocene carboxylic acid and 0.05 M glucose. Due to the use of a diode array UV–Vis spectrophotometer and a scanning CD spectropolarimeter, UV–Vis spectra were collected every 20 s, whereas CD spectra were acquired at 1 min intervals.
4. Conclusions
The thermodynamic parameters ΔHint, ΔSint, and ΔGint obtained indicate a significant affinity between Fc-COOH and GOD, revealing an entropy-driven, hydrophobic interaction and a thermodynamically favorable spontaneous association. The thermodynamic parameters determined for Fc-COOH (ΔG°, ΔH°, and ΔS°) represent new findings for this redox mediator and suggest strong stabilization of the redox pair, which can be attributed to the small difference in solvation energy between its oxidized and reduced forms, which is consistent with an outer sphere electron transfer mechanism. The activation parameters , , and indicate minimal energetic requirements for electron transfer, the formation of a highly ordered transition state, and an activation free energy within the expected range for biological electron transfer processes. However, to the best of our knowledge, neither molecular interaction parameters (ΔHint, ΔSint, ΔGint) nor electron transfer activation parameters (, , and ) for GOD interacting with other redox mediators have been reported in the literature, opening new avenues for research in this field. Future work will focus on investigating the molecular interaction between GOD and other ferrocene derivatives to propose a model that accounts for these effects on glucose biosensing performance. Moreover, using circular dichroism, UV–vis spectroscopy, and combined CD and UV–Vis spectroelectrochemical measurements, it was demonstrated for the first time that no significant structural changes occur in GOD during the electron transfer process, highlighting the value of coupling electrochemical and spectroscopic techniques.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/molecules31010102/s1, Figure S1: Electronic spectra of GOD in phosphate buffer (pH 7.2). Figure S2: Electronic spectra of Fc-COOH in phosphate buffer (pH 7.2). Figure S3: Van’t Hoff plot for the interaction between Fc-COOH and GOD in phosphate buffer (pH 7.2). Figure S4: UV–Vis spectra of 1.7 mM BSA in PBS 0.1 M (pH 7.2) in the presence of Fc-COOH 1 mM at 25 °C. Figure S5: Cyclic voltammogram of 1 × 10−3 M Fc-COOH, in PBS (pH 7.2), at scan rates values of 1, 2, 5, 10, 15, 20, 25, 50, 75, 100, 250, 500, and 750 mV/s. Figure S6: Cyclic voltammogram of 1 × 10−3 M Fc-COOH, in PBS (pH 7.2), at 100 mV/s, using a temperature range from 25 to 50 °C. Figure S7: E° vs. T plot for Fc-COOH, in PBS (pH 7.2), at 100 mV/s, using a temperature range from 25 to 50 °C. Figure S8: Spectro electrochemical response UV–Vis region of 1 × 10−3 M Fc-COOH in PBS (pH 7.2), using an OTTLE, applying a constant potential value of 400 mV vs. Ag/AgCl. Spectra were acquired every 20 min.
Author Contributions
Conceptualization, M.C.-R., L.G.T.-C., G.R.-O. and L.O.-F.; methodology, L.G.T.-C., G.R.-O. and J.O.-H.; validation, L.G.T.-C., J.O.-H. and J.P.F.R.-C.; formal analysis, L.G.T.-C., G.R.-O. and M.C.-R.; investigation, L.G.T.-C., G.R.-O. and J.O.-H.; resources, L.O.-F.; data curation, L.G.T.-C.; writing—original draft preparation, J.P.F.R.-C., M.C.-R., L.G.T.-C. and G.R.-O.; writing—review and editing, L.G.T.-C., G.R.-O. and L.O.-F.; visualization, L.G.T.-C.; supervision, G.R.-O. and L.O.-F.; project administration, L.O.-F.; funding acquisition, L.O.-F. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by “Secretaria de Ciencia Humanidades Tecnología e Innovación SECIHTI”, “Ciencia Básica y de Frontera 2023–2024”, Grant number CBF2023-2024-3108, by postdoctoral fellowship under the program “Estancias posdoctorales por México 2025”. CVU 689679, and by “Estancias posdoctorales por México 2024” for Grant no. 6364841.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in the article.
Acknowledgments
L.G.T.-C. and G.R.-O. acknowledge SECIHTI for the postdoctoral fellowship received.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| GOD | Glucose oxidase |
| FAD | Flavin Adenine Dinucleotide |
| FADH2 | Reduced Flavin Adenine Dinucleotide |
| Fc-COOH | Ferrocene carboxylic acid |
References
- Chen, D.; Wang, G.; Li, J. Interfacial Bioelectrochemistry: Fabrication, Properties and Applications of Functional Nanostructured Biointerfaces. J. Phys. Chem. C 2007, 111, 2351–2367. [Google Scholar] [CrossRef]
- Bauer, J.A.; Zámocka, M.; Majtan, J.; Bauerová-Hlinková, V. Glucose Oxidase, an Enzyme “Ferrari”: It’s Structure, Function, Production and Properties in the Light of Various Industrial and Biotechnological Applications. Biomolecules 2022, 12, 472. [Google Scholar] [CrossRef]
- De Poulpiquet, A.; Ciaccafava, A.; Lojou, E. New trends in enzyme immobilization at nanostructured interfaces for efficient electrocatalysis in biofuel cells. Electrochim. Acta 2014, 126, 104–114. [Google Scholar] [CrossRef]
- Le, P.G.; Wu, Q.; Kong, D.Y.; Ge, J.; Kim, M.I. Tailoring Nanotructured Supports to Achieve High Performance in Enzymatic Biofuel Cells. ACS Appl. Energy Matter. 2022, 5, 13113–13127. [Google Scholar] [CrossRef]
- Scherbahn, V.; Putze, M.T.; Dietzel, B.; Heinlein, T.; Schneider, J.J.; Lisdat, F. Biofuel cells based on direct enzyme–electrode contacts using PQQ-dependent glucose dehydrogenase/bilirubin oxidase and modified carbon nanotube materials. Biosens. Bioelectron. 2014, 61, 631–638. [Google Scholar] [CrossRef]
- Wang, X.; Kim, S.B.; Khang, D.; Kim, H.-H.; Kim, C.-J. Optimization characterization of covalent immobilization of glucose oxidase for bioelectronic devices. Biochem. Eng. J. 2016, 112, 20–31. [Google Scholar] [CrossRef]
- Campbell, A.S.; Jeong, Y.J.; Geier, S.M.; Koepsel, R.R.; Russell, A.J.; Islam, M.F. Membrane/Mediator-Free Rechargeable Enzymatic Biofuel Cell Utilizing Graphene/Single-Wall Carbon Nanotube Cogel Electrodes. ACS Appl. Mater. Interfaces 2015, 7, 4056–4065. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Joshi, P.P.; Hobbs, K.L.; Johnson, M.B.; Schmidtke, D.W. Nanostructured biosensors built by layer-by-layer electrostatic assembly of enzyme-coated single-walled carbon nanotubes and redox polymers. Langmuir 2006, 22, 9776–9783. [Google Scholar] [CrossRef]
- Sun, L.; Ma, Y.; Zhang, P.; Chao, L.; Huang, T.; Xie, Q.; Chen, C.; Yao, S. An amperometric enzyme electrode and its biofuel cell based on a glucose oxidase-poly(3-anilineboronic acid)-Pd nanoparticles bionanocomposite for glucose biosensing. Talanta 2015, 138, 100–107. [Google Scholar] [CrossRef]
- Reuillard, B.; Le Goff, A.; Cosnier, S. Polypyrrolic Bipyridine Bis(phenantrolinequinone) Ru(II) Complex/Carbon Nanotube Composites for NAD-Dependent Enzyme Immobilization and Wiring. Anal. Chem. 2014, 86, 4409–4415. [Google Scholar] [CrossRef]
- Jelliss, P.A.; Graham, S.S.; Josipovic, A.; Boyko, S.; Minteer, S.D.; Svoboda, V. Synthesis and characterization of ferracarborane–chitosan and ferracarborane–multiwalled carbon nanotube redox mediator conjugates for bioanode applications. Polyhedron 2013, 50, 36–44. [Google Scholar] [CrossRef]
- Ramírez-Delgado, V.; Cruz-Ramirez, M.; Hernández-Ayala, L.F.; Reyes-Vidal, Y.; Patakialvi, R.; García-Ramos, J.C.; Tenorio, F.J.; Ruiz-Azuara, L.; Ortiz-Frade, L. The Role of the π Acceptor Character of Polypyridine Ligands on the Electrochemical Response of Co (II) Complexes and its Effect on the Homogenous Electron Transfer Rate Constant with the Enzyme Glucose Oxidase. J. Mex. Chem. Soc. 2015, 59, 282–293. [Google Scholar] [CrossRef]
- Nakabayashi, Y.; Nakamura, K.; Kawachi, M.; Motoyama, T.; Yamauchi, O. Interactions of glucose oxidase with various metal polypyridine complexes as mediators of glucose oxidation. J. Biol. Inorg. Chem. 2003, 8, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Kavya, K.N.; Vinod, K.D.; Rakesh, K.P.; Siddharth, J.; Navakanta, B. An electrochemical continuous glucose monitoring sensor utilizing ferrocene-derivative as a mediator. Microchem. J. 2025, 213, 113795. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Hoshi, T.; Anzai, J. Glucose and lactate biosensors prepared by a layer-by-layer deposition of concanavalin A and mannose-labeled enzymes: Electrochemical response in the presence of electron mediators. Chem. Pharm. Bull. 2001, 49, 755–757. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, R.S.; Shain, I. Theory of Stationary Electrode Polarography. Single Scan and Cyclic Methods Applied to Reversible, Irreversible, and Kinetic Systems. Anal. Chem. 1964, 36, 706–723. [Google Scholar] [CrossRef]
- Cass, A.E.G.; Davis, G.; Francis, G.D.; Allen, H.; Aston, W.J.; John Higgins, I.; Plotkin, E.V.; Scott, L.D.L.; Turner, A.P.F. Ferrocene-mediated enzyme electrode for amperometric determination of glucose. Anal. Chem. 1984, 56, 667–671. [Google Scholar] [CrossRef]
- Hernández-Padilla, G.; Cruz-Ramírez, M.; Rebolledo-Chávez, J.P.F.; Ocampo-Hernández, J.; Mendoza, A.; Tenorio, F.J.; Domínguez Ramírez, L.; Ortiz-Frade, L. The role of molecular interaction between GOD and metal complexes on redox mediation processes. J. Mol. Struct. 2021, 1245, 131026. [Google Scholar] [CrossRef]
- Nagata, R.; Yokoyama, K.; Clark, S.A.; Karube, I. A glucose sensor fabricated by the screen printing technique. Biosens. Bioelectron. 1995, 10, 261–267. [Google Scholar] [CrossRef]
- Monkrathok, J.; Janphuang, P.; Suphachiaraphan, S.; Kampaengsri, S.; Kamkaew, A.; Chansaenpak, K.; Lisnund, S.; Blay, V.; Pinyou, P. Enhancing Glucose Biosensing with Graphene Oxide and Ferrocene-Modified Linear Poly(ethylenimine). Biosensors 2024, 14, 161. [Google Scholar] [CrossRef]
- Sahin, S.; Wongnateb, T.; Chaiyenb, P.; Yu, E.H. Glucose Oxidation Using Oxygen Resistant Pyranose-2-Oxidase for Biofuel Cell Applications. Chem. Eng. Trans. 2014, 41, 367–372. [Google Scholar] [CrossRef]
- Shao, Q.; Wu, P.; Xu, X.; Zhang, H.; Cai, C. Insight into the effects of graphene oxide sheets on the conformation and activity of glucose oxidase: Towards developing a nanomaterial-based protein conformation assay. Phys. Chem. Chem. Phys. 2012, 14, 9076. [Google Scholar] [CrossRef]
- Xu, X.; Wu, P.; Xu-, W.; Shao, Q.; An, L.; Zhang, H.; Cai, C.; Zhao, B. Effects of guanidinium ions on the conformational structure of glucose oxidase studied by electrochemistry, spectroscopy, and theoretical calculations: Towards developing a chemical-induced protein conformation assay. Phys. Chem. Chem. Phys. 2012, 14, 5824. [Google Scholar] [CrossRef]
- Kuntz, I.D.; Gasparro, F.P.; Johnston, M.D.; Taylor, R.P. Molecular Interactions and the Benesi-Hildebrand Equation. J. Am. Chem. Soc. 1968, 90, 4778–4781. [Google Scholar] [CrossRef]
- Exner, O. Calculating equilibrium constants from spectral data: Reliability of the Benesi-Hildebrand method and its modifications. Chemometr. Intell. Lab. 1997, 39, 85–93. [Google Scholar] [CrossRef]
- Ross, P.D.; Subramanian, S. Thermodynamics of protein association reactions: Forces contributing to stability. Biochemistry 1981, 20, 3096–3102. [Google Scholar] [CrossRef] [PubMed]
- Rodkey, F.L. The effect of temperature on the oxidation-reduction potential of the diphosphopyridine nucleotide system. J. Biol. Chem. 1959, 234, 188–190. [Google Scholar] [CrossRef]
- Reipa, V.; Holden, M.J.; Mayhew, M.P.; Vilker, V.L. Temperature dependence of the formal reduction potential of putidaredoxin. Biochim. Biophys. Acta-Bioenerg. 2000, 1459, 1–9. [Google Scholar] [CrossRef]
- Rebolledo-Chávez, J.P.; Cruz-Ramírez, M.; Patakfalvi, R.; Tenorio, F.J.; Ortiz-Frade, L. Insight on the mechanism of molecular catalysis of CO2 reduction with Fe(II)-polypyridine complex. Electrochim. Acta 2017, 247, 241–251. [Google Scholar] [CrossRef]
- Costentin, C.; Robert, M.; Savéant, J.-M. Current Issues in Molecular Catalysis Illustrated by Iron Porphyrins as Catalysts of the CO2-to-CO Electrochemical Conversion. Acc. Chem. Res. 2015, 48, 2996–3006. [Google Scholar] [CrossRef]
- Costentin, C.; Drouet, S.; Robert, M.; Savéant, J.-M. Turnover Numbers Turnover Frequencies Overpotential in Molecular Catalysis of Electrochemical Reactions Cyclic Voltammetry Preparative-Scale Electrolysis. J. Am. Chem. Soc. 2012, 134, 11235–11242. [Google Scholar] [CrossRef]
- Shlyk, O.; Samish, I.; Matěnová, M.; Dulebo, A.; Poláková, H.; Kaftan, D.; Scherz, A. A single residue controls electron transfer gating in photosynthetic reaction centers. Sci. Rep. 2017, 7, 44580. [Google Scholar] [CrossRef]
- Deepalakshmi, S.; Sivalingam, A.; Kannadasan, T.; Subramaniam, P.; Sivakumar, P.; Brahadeesh, S.T. Spectroscopic investigation on kinetics, thermodynamics and mechanism for electron transfer reaction of iron(III) complex with sulphur centered radical in stimulated biological system. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 124, 315–321. [Google Scholar] [CrossRef]
- Rocha-Ortiz, G.; Barrios-Velasco, A.; Monsalvo Zúñiga, O.; Cruz-Ramírez, M.; Mendoza, A.; Ramírez-Palma, L.G.; Rebolledo-Chávez, J.P.F.; Ortiz-Frade, L. The Role of Geometry in Cobalt–Polypyridine Complexes in the Electrochemical Reduction of CO2 Using UV–Vis Spectroelectrochemistry. Catalysts 2025, 15, 641. [Google Scholar] [CrossRef]
- Rocha-Ortiz, G.; Lara-Molineros, B.M.; Talavera-Contreras, L.G.; Cortés-Guzmán, F.; Rebolledo-Chávez, J.P.F.; Hernández-Padilla, G.; Ramírez-Palma, L.G.; Cruz-Ramírez, M.; Ortiz-Frade, L. Molecular Catalysis of CO2 Reduction with a Zn (II)–Bipyridine Complex. Processes 2025, 13, 3443. [Google Scholar] [CrossRef]
- Grove, T.Ž.; Ullmann, G.M.; Kostić, N.-M. Simultaneous true gated coupled electron-transfer reactions energetics of protein rearrangement. J. Inorg. Biochem. 2012, 106, 143–150. [Google Scholar] [CrossRef]
- Forrow, N.J.; Walters, S.J. Transition metal half-sandwich complexes as redox mediators to glucose oxidase. Biosens. Bioelectron. 2004, 19, 763–770. [Google Scholar] [CrossRef]
- Hianik, T. Biological membranes and Membrane mimics. In Bioelectrochemistry, Fundamentals, Expermiental Techniques and Applications; Wiley: Wiltshire, UK, 2008; pp. 87–148. [Google Scholar]
- Roat-Malone, R. Bioinorganic Chemistry; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2007; pp. 429–466. [Google Scholar]
- Kelly, S.M.; Jess, T.J.; Price, N.C. How to study proteins by circular dichroism. Biochim. Biophys. Acta-Proteins Proteom. 2005, 1751, 119–139. [Google Scholar] [CrossRef]
- Solomon, B.; Lotan, N.; Katchalski-Katzir, E. Enzymic activity and conformational properties of native and crosslinked glucose oxidase. Biopolymers 1977, 16, 1837–1851. [Google Scholar] [CrossRef]
- Ahmad, B.; Haq, S.K.; Varshney, A.; Moosavi-Movahedi, A.A.; Khan, R.H. Effect of trifluoroethanol on native and acid-induced states of glucose oxidase from Aspergillus niger. Biochemistry 2010, 75, 486–493. [Google Scholar] [CrossRef]
- Khatun Haq, S.; Faiz Ahmad, M.; Hasan Khan, R. The acid-induced state of glucose oxidase exists as a compact folded intermediate. Biochem. Biophys. Res. Commun. 2003, 303, 685–692. [Google Scholar] [CrossRef] [PubMed]
- Prévoteau, A.; Mano, N. Oxygen reduction on redox mediators may affect glucose biosensors based on “wired” enzymes. Electrochim. Acta 2012, 68, 128–133. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.