Abstract
The widespread use of β-lactam antibiotics in veterinary medicine and food production has contributed to the rise of antibiotic-resistant bacteria, posing significant health risks to humans. This issue is recognized by various regulatory agencies, which settled maximum residue limits (MRLs) for antibiotics in animal-derived foods. To adhere to these regulations, sensitive and selective methods are required for monitoring antibiotic residues. Due to the critical importance of sample preparation in the analysis, numerous extraction techniques have been developed. This review focuses on various methodologies for extracting β-lactam antibiotics from different food matrices. The paper summarizes the procedures for the extraction of β-lactam antibiotics identified in the literature, indicating their detailed methodology. The summary may be useful for any laboratories preparing new applications for the determination of antibiotics in food. Research studies analyzed in the paper were collected from databases, such as Google Scholar, PubMed, and Scopus. After a close evaluation of about 200 articles (published between 2010 and 2024), 35 of them, which met the criteria, were included in the analysis.
1. Introduction
The term “antibiotics” is used to refer to drugs of natural, semisynthetic, or synthetic origin [1]. These drugs are widely used in clinical practice, since the discovery of penicillin. The purpose of their use on bodies is to kill or stop the growth of particular microorganisms. Antibiotics are commonly used in human as well as in veterinary medicine to treat infectious diseases. As international trade continues to expand, including the trade of meat and other animal-based foods, antibiotics are increasingly used in animal feed to enhance growth and support health [2].
However, the extensive use of antibiotics in veterinary medicine and meat production may be a cause of concern [3]. The frequent use of the antimicrobial is seen as the main cause of antibiotic residue in animals’ meat. Arguably, food is the main source of antibiotics that are stored in human bodies. The consumption of meat with excess antibiotics can have an impact on humans’ health, especially on creating antibiotic resistance. In addition to promoting antimicrobial resistance, the presence of antibiotic residues in food can cause alterations in the human gut microbiota, leading to dysbiosis, weakened immunity, and increased susceptibility to infections. Furthermore, antibiotic residues may trigger allergic reactions, particularly in sensitive individuals. Some antibiotic residues are also associated with immunopathological effects, nephrotoxicity, hepatotoxicity, reproductive disorders, and, in rare cases, mutagenic or carcinogenic effects. Therefore, the presence of antibiotic residues in food represents a serious public health risk [4]. This resistance may be transmitted to the general population, creating antibiotic-resistant diseases [5]. What is more, the effects of using extensive amounts of drugs in animal-derived food production may be very far-reaching, as their residues might be transferred into communal wastewater through urine [6].
The risk of creating antibiotic-resistant diseases, as well as the increasing consumers’ awareness of food safety, led to developing food and nutrition safety regulations. Professionals in the field are obliged to control and ensure access to good quality and nutritious food [7]. The main purpose of those regulations is to supply humans with food that is free from any contamination that could be harmful to their health.
For enforcement at the national level and in the international food trade, residues of antibiotics and other veterinary drugs are now monitored around the world by many governments and private laboratories. However, differences exist in the regulation of antibiotics for food-producing animals between countries. For example, in the United States of America, the Federal Food, Drug, and Cosmetic Act was amended in 1996 by the Food and Drug Administration Center for Veterinary Medicine to strengthen control over veterinary drugs. Instead of banning all antibiotics, the United States establishes maximum residue limits (referred to as “tolerances”) for many substances to ensure food safety, which are published in 21 CFR Part 556. In the European Union, the 27 Member States amended Directive 2001/82/EC and Regulation 726/2004 of the European Parliament and the Council. In the EU, the use of antibiotics is more strictly regulated, and maximum residue limits (MRLs) for veterinary drugs in food are clearly defined under Regulation (EU) No 37/2010. This means that while both the United States and the European Union aim to protect public health, their approaches to antibiotic regulation differ, particularly in how they set and enforce limits for residues in food products [8].
Although the use of antibiotics in meat production is almost inevitable, the European Union formulated standards that determine the maximum residue limits (MRLs) of veterinary drugs in food of animal origin. The limits for antibiotic residues have been established by Commission Regulation (EU) 37/2010 and apply to various products of animal origin [2,9]. This regulation sets the maximum allowable levels of veterinary drug residues in animal tissues following the administration of veterinary medicines. It is important to note that MRLs differ depending on the type of antibiotic, the type of animal, and even the type of tissue. Each antibiotic is assigned specific MRLs, indicating the maximum residue level that is considered safe in a particular animal product for consumers. Adhering to MRLs is crucial to minimize health risks and to control antibiotic resistance. International and national organizations monitor compliance with these standards through food control programs to ensure the safety of animal-origin food products on the market.
Considering these regulations and the need for reliable monitoring of β-lactam antibiotic residues in food, this review examines various extraction and detection methods. The first section provides a general overview of β-lactam antibiotics, followed by a discussion of extraction methods used in the analysis of antibiotic residues. Earlier studies used traditional extraction techniques for β-lactam antibiotics, whereas in recent years (2010–2024) there has been significant progress in both extraction methods, such as QuEChERS, and detection methods, including the widespread use of high-performance liquid chromatography coupled with tandem mass spectrometry (LC-MS/MS), allowing for the simultaneous determination of multiple β-lactams in complex food matrices. In the final section, specific examples of extraction methods described in the literature are presented in chronological order, from the oldest to the most recent developments.
2. Methodology
Research studies focusing on the detection of antibiotic residues were collected from the Google Scholar, PubMed, and Scopus databases. The research concentrates on publications addressing the topic of extraction of β-lactam antibiotic residues from animal-derived food and their chromatographic analysis from 2010 to 2024. There are many methods for antibiotic analysis, so a selection of publications was made to ensure a variety of extraction and analytical techniques. The following keywords were used in the search: “antibiotic”, “β-lactam”, “antibiotic residues”, “β-lactam antibiotic residues”, “extraction of antibiotics from food”, and “drug residues in animal products”. Over 600 records were identified, and after removing duplicates and irrelevant entries, approximately 200 articles were selected for further evaluation. Publications addressing the extraction of β-lactam antibiotic residues from animal-derived food and their chromatographic analysis were included. Only peer-reviewed studies published in international journals, using chromatographic techniques (e.g., HPLC, LC-MS/MS, and UHPLC), with full-text availability were selected. Based on title and abstract screening, studies focusing on microbiological and immunochemical methods, those without chromatographic detection, and those using unsuitable matrices (e.g., blood, water, and drugs) were excluded. Additionally, review articles, studies not related to animal-derived food matrices, and purely in vitro experiments without food matrices were also excluded. Some full texts were unavailable, and additional studies were removed due to missing data or being published in languages other than English. Ultimately, 35 studies meeting the criteria were included in the review, providing insights into β-lactam antibiotic extraction methods from animal-derived food and their chromatographic analysis.
3. β-Lactam Antibiotics
There are a few types of antibiotics certified by license to be used in animals’ treatment. Antibiotics used in veterinary medicine can be categorized into certain groups depending on their chemical structure or action mechanism [10]. The amount of each of the drugs must be controlled individually but all of the antibiotics should be dosed under veterinary supervision.
The authors decided to focus on β-lactam antibiotics due to their extensive use in animal-derived food, as well as their efficacy and consequently their relevance in food safety. β-Lactams are among the most widely used classes of antibiotics in veterinary medicine because of their broad-spectrum activity, relatively low toxicity, and cost-effectiveness. However, their frequent application has contributed significantly to the emergence of antimicrobial resistance, posing a serious threat to public health. Monitoring β-lactam residues in food is, therefore, essential to ensure consumer safety and to prevent the spread of resistant bacteria through the food chain.
The main negative effect of β-lactam use is acute allergic reaction, which is rare and mainly associated with penicillins. However, adverse effects, such as urticaria or angioneurotic edema, were observed more commonly. There were instances of lethargy, pyrexia, vomiting, and other reactions in pigs and horses. Sensitization as well as hypersensitivity to penicillin are quite common in humans during treatment [10].
The review examines the antibiotics’ usage, extraction methods, and detection techniques to ensure food safety and regulatory compliance. β-Lactams create a group of natural or semisynthetic antibiotics that contain a β-lactam ring in their molecular structure (Figure 1 and Figure 2) [11]. Their action mechanism is based on restraining the synthesis of the bacterial cell wall, causing their death. This group contains drugs such as penicillin derivatives, cephalosporins, monobactams, carbapenems, and β-lactamase inhibitors [12]. They are the biggest as well as the most widely utilized antibiotics in clinical practice. In veterinary medicine, β-lactam antibiotics are used to treat numerous bacterial infections in various animals, with pets, such as dogs or cats, and livestock, such as cattle, pigs, and poultry, as examples [13].
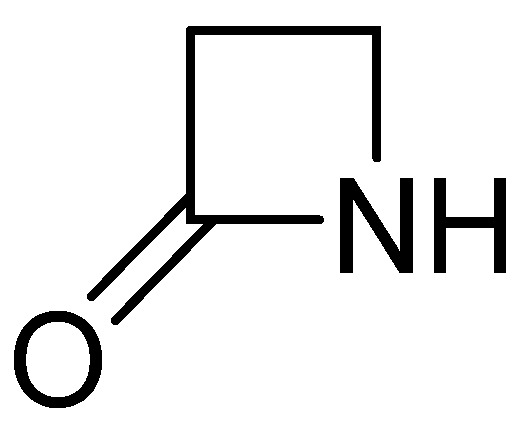
Figure 1.
The β-lactam ring.
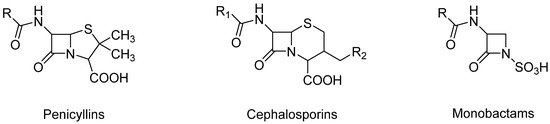
Figure 2.
Chemical structures of β-lactams.
Penicillins, a subgroup of beta-lactam antibiotics, are derived from both semisynthetic and natural sources and share a feature concerning their structure: the aminopenicillanic acid nucleus. This nucleus consists of a beta-lactam ring attached to a thiazolidine ring (Figure 2). Natural penicillins are produced by various species of the Penicillium fungi. The diversity in the penicillin class is due to alterations at position 6 of the aminopenicillanic acid ring, with variations in the side chain leading to differences in antibacterial activity and pharmacokinetic properties [14]. These modifications allow for the classification of penicillins into several types: natural penicillins (penicillins G and V), penicillins resistant to staphylococcal penicillinase (oxacillin, dicloxacillin, and nafcillin), aminopenicillin (amoxicillin and ampicillin), carboxypenicillin (carbenicillin), and ureidopenicillin (piperacillin) [6]. Examples of antibiotics from the penicillin group are shown in Figure 3. Toxicological data, including molecular formulas, CAS numbers, LD50 values, and PubChem references for the antibiotics shown, are summarized in Table 1 [15,16,17,18,19,20,21,22,23,24].
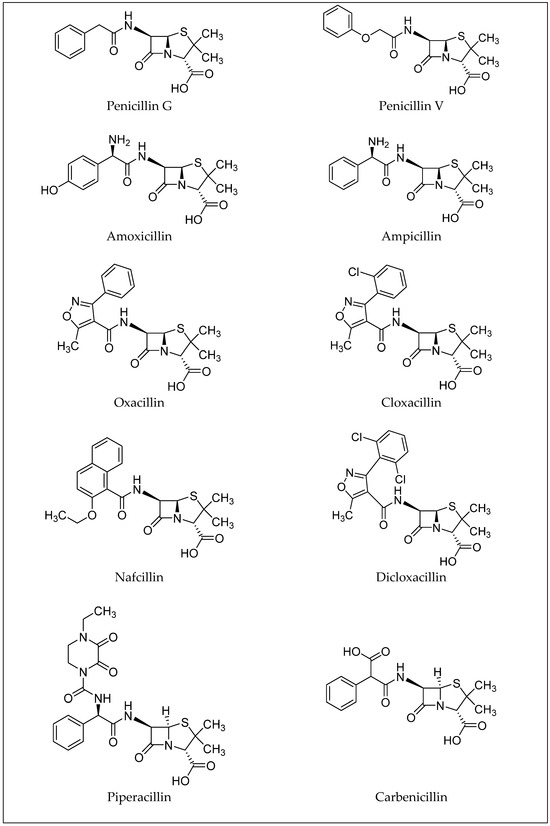
Figure 3.
Examples of antibiotics from the penicillin group.

Table 1.
Data for penicillins, including molecular formulas, CAS numbers, LD50 values (oral, rat), and PubChem references.
Cephalosporins constitute a significant group of antibiotics that are structurally and functionally related to penicillins but with a broader spectrum of activity. Cephalosporins are categorized by generations, which generally correspond to their order of development and spectrum of antibacterial activity [25]. First-generation cephalosporins, like cephalexin and cefazolin, are most effective against Gram-positive bacteria. Later generations (up to the fifth generation) have increased activity against Gram-negative bacteria and improved resistance to beta-lactamase enzymes [10,25,26]. Examples of antibiotics from the cephalosporin group are shown in Figure 4. Toxicological data, including molecular formulas, CAS numbers, LD50 values, and PubChem references for the antibiotics shown, are summarized in Table 2 [27,28,29,30,31,32,33].
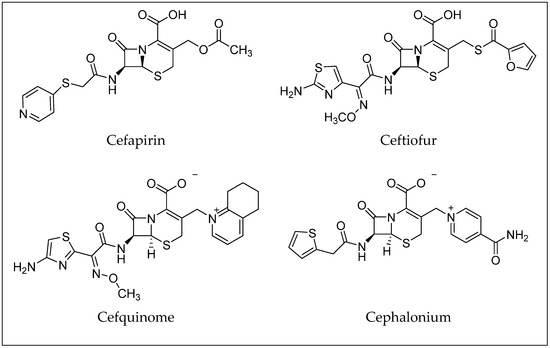
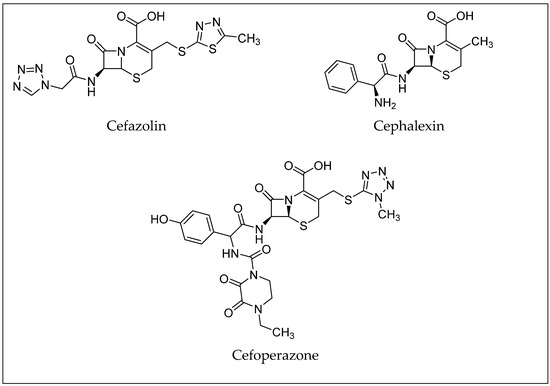
Figure 4.
Examples of antibiotics from the cephalosporin group.

Table 2.
Data for cephalosporins, including molecular formulas, CAS numbers, LD50 values (rat), and PubChem references.
Monobactams contain a single antibiotic, Aztreonam, which is structurally different from other beta-lactams due to its monocyclic beta-lactam ring (Figure 2). Monobactams are primarily active against aerobic Gram-negative bacteria, including Pseudomonas aeruginosa, and are useful for patients with penicillin allergies [6].
4. Extraction Methods
Sample preparation plays a critical role in the analysis of antibiotics in various matrices. Matrices often contain compounds, such as protein, fat, sugar, and inorganic salts, that can hinder the detection of antibiotics, leading to inaccurate results. Impurities can influence the separation of target compounds, cause matrix effects, and lower the sensitivity of detection. To meet these challenges, several pretreatment methods are commonly used: liquid–liquid extraction (LLE), solid-phase extraction (SPE), ion-paired extraction (IPE), fast, easy, cheap, effective, robust, and safe (QuEChERS), matrix solid-phase dispersion (MSPD), dispersive-solid phase extraction (d-SPE), molecularly imprinted polymer extraction (MIP), magnetic molecularly imprinted polymer extraction (MMIP), and various microextraction methods [34]. The choice of technique depends on the matrix being tested, the properties of the antibiotics of interest, and the detection method used. Each of these techniques has its specific advantages and disadvantages.
Liquid–liquid extraction (LLE) is one of the earliest developed sample preparation techniques, which is still widely used, particularly in the analysis of biological samples. It involves transferring the analyte from one liquid phase, usually aqueous, to another liquid phase, typically an immiscible organic solvent, based on the differences in solubility of the substance between the two phases. However, this method has several drawbacks. It can lead to the formation of emulsions and requires large sample volumes and toxic organic solvents, which result in significant waste and environmental pollution. Additionally, LLE is costly, time-consuming, and unsuitable for hydrophilic compounds [35,36].
Common solvents used in extraction include acetonitrile, methanol, and ethyl acetate. Acetonitrile allows for high recovery rates while minimizing the co-extraction of matrix components, and it also denatures proteins and inactivates enzymes. In contrast, methanol and ethyl acetate tend to extract a greater amount of matrix components. Therefore, in multicomponent analyses, it is crucial to strike a balance between recovery and extract purity [37,38].
Over the years, various methods for extracting antibiotics using different solvents have been developed. Acetonitrile has been used, among other solvents, to detect different groups of antibiotics, including β-lactams [39,40]. However, in the case of β-lactams, the use of methanol may lead to their degradation, and acetonitrile is often not an efficient solvent for their extraction [41]. Rocco and colleagues [41] proposed an effective extraction method using a mixture of water, acetonitrile, and dimethyl sulfoxide (DMSO). LLE with trichloroacetic acid has also been used for the extraction of β-lactams and other antibiotics from fish samples [42].
In the ion-paired extraction (IPE) technique, a variant of liquid–liquid extraction (LLE), the extraction of ionic compounds is achieved by converting them into neutral forms. Positively charged ions react with appropriate organic anions, while negatively charged compounds interact with organic cations, leading to the formation of molecules that are soluble in nonpolar organic solvents. As a result, the modified compounds can be effectively extracted from aqueous solutions [43].
To enhance the efficiency of the ion-pair formation process, specific reagents are used in IPE, which facilitate both the creation of ion pairs and the stabilization of the resulting complexes in the organic phase. Examples of such reagents include heptafluorobutyric acid, bis(2-ethylhexyl)phosphate, dioctylsulphosuccinate sodium salt, trifluoroacetic acid, and pentafluoropropionic acid. Their use not only enables the formation of ion pairs but also promotes the effective transfer of analytes into the organic solvent, thereby improving the extraction efficiency [44,45].
The QuEChERS method (quick, easy, cheap, effective, rugged, and safe) is based on liquid–liquid extraction techniques and is a modern approach to sample preparation, widely used in the analysis of pesticide residues in food [2,46]. Due to its advantages in line with the principles of “green chemistry”, this method quickly found application in the extraction of other contaminants, such as antibiotics, from various matrices. QuEChERS offers an eco-friendly alternative to traditional methods, providing fast and energy-efficient solutions without compromising analytical performance [47].
The QuEChERS method consists of an extraction and purification step. In the first step, the sample is mixed with acetonitrile and salts (most commonly anhydrous MgSO4 and NaCl). In the next step, the organic phase is purified using dispersive solid-phase extraction (d-SPE). Sorbents such as PSA and C18 are typically used to effectively remove fatty acids and other impurities, while anhydrous MgSO4 is used to eliminate residual water from the extract [48,49,50]. Key parameters of the method, such as solvents, extraction conditions, or sorbent types, can be easily adjusted and optimized depending on the sample being tested, making it effective in detecting antibiotics in various animal-derived matrices [51]. One modification involved the use of acetonitrile with formic acid for the extraction of 16 β-lactams from chicken muscle, with purification carried out using C18, PSA, and anhydrous MgSO4 [52]. In another study, a mixture of acetonitrile and water was employed for the extraction of cephalosporins from beef, followed by purification using PSA, C18, and anhydrous MgSO4 [53]. To analyze 23 β-lactams in eggs, raw milk, formula milk, and various meat and fish products, including baby food, acetonitrile with the addition of Na2SO4 and NaCl was used, while purification was performed using Na2SO4 and C18 sorbent [54]. These examples demonstrated that the QuEChERS method can be easily modified to suit different types of antibiotics and sample matrices, making it a versatile approach for various analytical applications.
Matrix solid-phase dispersion (MSPD) is an alternative to the QuEChERS method and was popular in residue analysis during the 1980s and 1990s. This technique enables simultaneous extraction of the sample and isolation of analytes in a single step. MSPD procedures involve the use of dispersing sorbents with chemically modified silica surfaces, such as C18 or C8 [55]. In the MSPD method, sorbents with particle sizes ranging from 40 to 100 μm are most commonly used. The sample is mixed with the sorbent in a ratio typically ranging from 1:1 to 1:4 (usually 0.5 g of sample and 2 g of dispersing sorbent) using a mortar and pestle made of glass or agate. After mixing, the sample–sorbent mixture is air-dried for 5–15 min and then placed between two frits in a syringe barrel and compressed using a plunger. To eliminate the air-drying step, untreated silica or sodium sulfate (Na2SO4) have been introduced as dispersing agents [37,43]. In the MSPD method, nonpolar solvents, such as hexane, are used to remove lipophilic matrix interferences, while polar solvents, like dichloromethane, alcohols, or hot water, are applied for the extraction of veterinary drugs, with caution to avoid analyte degradation.
Direct contact between the entire surface of the sample and the solvent allows for more effective washing and elution, and the use of solvents with different polarities enables sequential elution of compounds. An additional advantage of MSPD is the elimination of the need for protein precipitation and centrifugation steps [43]. Solid-phase extraction (SPE) is a key technique in analytical chemistry, particularly useful for analyzing trace amounts of substances like pharmaceuticals and pesticides in various matrices [43]. SPE allows for the enrichment of trace amounts, sample purification, and medium exchange. This method stands out for its low cost, short analysis time, high selectivity due to the wide range of available solid sorbents, and its reduced solvent consumption. It is also easy to automate [56,57]. However, SPE techniques have some limitations, such as unstable sorptive properties, variability between batches of sorbents with chemically modified surfaces, and a higher risk of contamination (originating from production materials) [57]. The choice of sorbent in the SPE method is crucial, as it affects key parameters like selectivity and affinity. The effectiveness of SPE largely depends on matching the sorbent to the properties of the analyte and the matrix. The decision should be based on the physicochemical characteristics of the analytes and the matrix, which define the interactions between the analyte and the sorbent [37]. This method offers a wide range of sorbents, including reversed-phase sorbents, normal-phase sorbents, ion-exchange sorbents, mixed-mode sorbents, functionalized polymer resins, immunosorbents, and molecularly imprinted polymers (MIPs) [58].
SPE is widely used in the analysis of antibiotic residues in various matrices due to its flexibility in adjusting parameters, such as sorbent selection, eluent composition, and purification conditions. Oasis HLB columns are frequently cited in the literature for their ability to retain both polar and nonpolar compounds, making them effective for analyzing a wide range of antibiotics [38,59,60,61,62,63]. Additionally, newer Oasis PRiME HLB columns have been introduced, which do not require conditioning and washing prior to elution [64,65,66]. Strata-X columns, chemically modified polymeric sorbents, are also commonly used for the analysis of antibiotics in complex matrices, offering high selectivity and chemical resistance [67,68].
SPE methods, due to their versatility and ability to selectively extract compounds, have become an essential tool in the analysis of antibiotic residues in biological materials.
Dispersive-SPE (d-SPE) is a simplified clean-up method in which a pre-extracted sample is mixed with a sorbent. The sorbent binds unwanted matrix components, allowing the target analytes to remain in the solution. After brief centrifugation, the resulting supernatant can be directly analyzed or concentrated if needed. This technique is characterized by its speed, simplicity, and low cost. Although it does not provide as thorough clean-up as traditional SPE, its efficiency, reproducibility, and ease of use make it a highly practical solution [43].
Molecularly imprinted polymers (MIPs) are synthetic materials that selectively bind to specific molecules by precisely replicating their shape and functional groups. They are increasingly used for preparing samples from complex environmental matrices, offering temperature and pH stability, reusability, and shorter preparation times. MIP columns are also utilized for the analysis of antibiotics in various matrices [69,70,71].
Recent studies, including a recent review focused on the application of magnetic molecularly imprinted polymers (MMIPs) for the extraction of veterinary drug residues from milk, have described the development and application of MMIPs [72]. MMIPs are an advancement of traditional MIPs by incorporating magnetic particles, enabling easy separation using a magnetic field and further improving the sample preparation process [72].
Microextraction by packed sorbent (MEPS) is a miniaturized form of solid-phase extraction (SPE), in which a small amount of sorbent, approximately 2 mg, is placed directly inside the syringe needle [73]. Compared to conventional SPE techniques, MEPS allows for the use of significantly smaller sample and solvent volumes and reduces the sample preparation time from several minutes to just 1–2 min [74]. The technique enables simultaneous extraction, pre-concentration, and clean-up, with a very low dead volume (<10 μL) that facilitates automated sample handling [73]. MEPS is applicable for sample volumes ranging from 10 to 1000 μL and can be directly coupled with analytical techniques, such as liquid chromatography (LC), gas chromatography (GC), LC-MS, or GC-MS [37,73]. It is available with different sorbent types, including SCX, SAX, C18, C8, and silica, and can be used in reversed-phase, normal-phase, ion-exchange, and mixed-mode applications [74].
5. Extraction of β-Lactams
An overview of the extraction methodologies for the determination of β-lactam antibiotics in different matrices is presented in Table 3. The studies are discussed in chronological order.

Table 3.
Extraction methodologies for the determination of β-lactams in different food samples.
Frédérique van Holthoon et al. [62] conducted a study in which eight kinds of penicillin (amoxicillin, ampicillin, penicillin G, penicillin V, oxacillin, cloxacillin, dicloxacillin, and nafcillin) were extracted from porcine muscle, kidney tissue, and milk. In this method, penicillins were converted to stable piperidine derivatives, followed by purification using solid-phase extraction with Oasis® HLB cartridges. Analysis was carried out by LC-MS/MS using a Symmetry C18 column. Accuracy for the extraction procedure ranged from 94% to 113% (muscle), 83% to 111% (kidney), and 87% to 103% (milk).
Zhang et al. [69] developed a method to extract three β-lactam antibiotics (penicillin V, amoxicillin, and oxacillin) from bovine milk using magnetic molecularly imprinted polymer (MMIP) extraction. Analysis was carried out by LC-MS/MS using a Symmetry C18 column. The obtained relative standard deviations of intra- and inter-day fluctuated between 3.2% and 8.3%, as well as between 3.6% and 9.8%. The β-lactam antibiotic recoveries obtained using this method ranged from 71.6% to 90.7%.
Three β-lactam antibiotics were also successfully extracted, but this time from chicken muscles (ampicillin, amoxicillin, and penicillin G), along with other groups of veterinary drugs, using liquid–liquid extraction (LLE) with a mixture of 2% trichloroacetic acid aqueous solution and acetonitrile, and analyzed by LC-MS/MS using a ZIC-HILIC column. For the developed method, recoveries for β-lactam antibiotics in the examined tissues ranged between 64% and 87%, with precision ≤ 15% RSD, and the LOQ values were 8.5 µg/kg for amoxicillin, 5 µg/kg for ampicillin, and 5 µg/kg for penicillin G [75].
Studies were conducted to develop a method for the analysis of β-lactam antibiotics, where seven penicillins (ampicillin, dicloxacillin, penicillin G, amoxicillin, nafcillin, oxacillin, and cloxacillin) and seven cephalosporins (cephalexin, cefoperazone, cefazolin, cephapirin, ceftiofur, cefquinome, and cephalonium) were extracted from cow milk using solid-phase extraction with Oasis® HLB cartridges and analyzed by LC-MS/MS using an Agilent Zorbax Eclipse XDB-C8 column and by UPLC-MS/MS using an Acquity UPLC BEH Shield RP18 column, with the presence of quinolones also being considered. The recoveries of β-lactam antibiotics were higher than 70% (except for amoxicillin), and for cephalosporins, the LOQ values for LC-MS/MS ranged from 0.3 to 125 µg/kg, while for penicillins, they ranged from 0.1 to 0.5 µg/kg. For UPLC-MS/MS, the LOQ values for cephalosporins ranged from 0.06 to 2.5 µg/kg, and for penicillins from 0.1 to 9 µg/kg [76].
Six β-lactam antibiotic residues (ceftiofur, penicillin G, penicillin V, oxacillin, cloxacillin, and dicloxacillin) were isolated from bovine milk using a rapid liquid–liquid extraction method with acetonitrile as the extraction solvent, developed by Jank et al. [77]. The compounds were analyzed by LC-MS/MS using a Synergy C18 column. The average accuracies obtained for the extracted compounds ranged from 104.1% to 109.9%, with LOQ values ranging from 1.0 ng/mL to 25 ng/mL.
Another research group, led by Pérez-Burgos [53], developed two methods for the extraction of cephalosporins from beef muscle (cephalexin, cephalonium, cefoperazone, cephapirin, cefquinome, cephazolin, and ceftiofur). The extraction and LC-MS/MS analysis were the same for both methods, with the difference being in the sample clean-up process. In the first approach, the QuEChERS method was applied using dispersive SPE kits containing PSA, C18 sorbents, and MgSO4. In the second approach, SPE was performed using Isolute ENV+ cartridges. Chromatographic separation was carried out on a Zorbax Eclipse XDB-C8 column with a Kromasil C8 precolumn. Comparing accuracies, the QuEChERS method provided slightly better results than the SPE method, with recovery only lower than 80% for cephalexin. The LOQ for the SPE method ranged from 0.1 to 10 µg/kg, while for the QuEChERS method, it ranged from 1.5 to 50 µg/kg, with the highest values obtained for cephalexin.
Muscle tissues from cattle, pigs, and chickens were utilized for studies on the extraction method of nine penicillin antibiotics, including penicillin G, penicillin V, amoxicillin, ampicillin, nafcillin, oxacillin, cloxacillin, dicloxacillin, and piperacillin (used as an internal standard). Extraction was carried out using LLE with water and acetonitrile, followed by sample purification using SPE with Isolute ENV+ cartridges. Chromatographic separation was performed on a Zorbax Eclipse XDB-C8 column with a Kromasil C8 precolumn. For the developed method, recoveries for all antibiotics in the examined animal tissues were obtained above 70%, except for amoxicillin at 50% recovery. The LOQ values ranged from 0.2 to 8 µg/kg, with the highest values observed for ampicillin and penicillin G in pig tissues [78].
Penicillin G, oxacillin, and cloxacillin were extracted from beef and milk using ion-pair extraction and a binary water–acetonitrile mixture. The first step was the removal of proteins and fats using acetone–acetonitrile solutions. Chromatographic separation was performed on an Xbridge™ C18 reversed-phase column, and detection was carried out by HPLC-UV at 215 nm. This method achieved detection limits (LOD) at the level of 1–2 ng/mL, good repeatability with RSD below 2%, and the average recoveries were higher than 85%. The LOQ values were 3 ng/mL for penicillin G, 3 ng/mL for oxacillin, and 7 ng/mL for cloxacillin [79,80].
Using an ultrasound-assisted matrix solid-phase dispersive extraction method, it was possible to successfully extract four penicillins, including amoxicillin, oxacillin, cloxacillin, and dicloxacillin, along with eight cephalosporins, such as ceftiofur, cefuroxime, cefotaxime, cefoperazone, cefaclor, cefadroxil, cephalexin, and cefazolin, from milk. The matrix solid-phase dispersive extraction (MSPD) procedure was conducted using the Oasis HLB sorbent. Chromatographic separation was performed by HPLC-PDA on an Inertsil ODS-3 column. Using this method, the recoveries for the analyzed antibiotics ranged from 85.0% to 115.7%, and the values of the relative standard deviation (RSD) were less than 12.7%. The LOQ values ranged from 19.2 to 46.5 µg/kg [81].
Maggi et al. [82] conducted research on catfish to develop an extraction method for eight penicillins (amoxicillin, ampicillin, cloxacillin, dicloxacillin, nafcillin, oxacillin, penicillin G, and penicillin V) using an online solid-phase extraction method with a C18 cartridge, followed by analysis with liquid chromatography coupled to ion trap tandem mass spectrometry. The symbiosis system of online SPE contained an automatic device that loaded, cleansed, and eluted the SPE cartridge and transferred analytes directly onto the column for analysis. Chromatographic separation was carried out on a Synergy Max column. The recoveries of β-lactam antibiotics at spike levels of 2.0, 10.0, and 50 µg/kg−1 varied between 72% and 92%. The samples showed precision values lower than 20%, except for amoxicillin.
In a targeted analysis of β-lactam antibiotic residues, twenty-two compounds were extracted from poultry muscle, including penicillins (amoxicillin, ampicillin, penicillin G, penicillin V, cloxacillin, dicloxacillin, nafcillin, and oxacillin), cephalosporins (ceftiofur, cefquinome, cefapirin, cefalexin, cefalonium, cefazolin, cefacetrile, and cefoperazone), and carbapenems (biapenem, doripenem, ertapenem, imipenem, meropenem, and faropenem). The sample purification was performed using solid-phase extraction (SPE) with a Phenomenex Strata-X cartridge. Analysis was carried out by a LC-MS/MS system. Separation was carried out using a Waters Acquity UPLC CSH C18 analytical column. The technique demonstrated acceptable quantitative results for all substances, achieving trueness ranging from 80% to 110% and within-laboratory reproducibility under 22% at the target concentration, with the exception of biapenem. For biapenem, the method was found to be appropriate only for qualitative analysis [68].
Nine β-lactam antibiotics (ampicillin, penicillin G, penicillin V, cephalexin, cefazolin, cefoperazone, cloxacillin, dicloxacillin, and oxacillin) were successfully extracted from ewe milk, as demonstrated by Cámara et al. [83]. The extraction was performed using SPE with Spe-ed C18 cartridges. Chromatographic analysis was carried out using an LC-UV-DAD system on a Supelcosil LC 18 DB column. The recovery of the studied β-lactams ranged from 79% to 96% with standard deviations between 0.5% and 4.9%. The LOQ values ranged from 3.4 to 8.6 μg/kg.
A research group led by Jank [40] successfully extracted fourteen β-lactam antibiotics (ampicillin, amoxicillin, penicillin G, penicillin V, oxacillin, cloxacillin, dicloxacillin, cefalonium, cefoperazone, cefapirin, ceftiofur, cefquinome, cefalexin, and nafcillin) from bovine milk using LLE. After extraction, the analytes were purified by adding C18 bulk sorbent directly to the sample. The analysis by LC-MS/MS was operated in positive mode. Chromatographic separation was obtained using a DuraShell RP column paired with a SecurityGuard Cartridge C18 guard column. Accuracy values ranged between 92% and 110%.
Dorival-García et al. [84] focused on extracting fourteen β-lactam antibiotics (cefoperazone, cephalexin, ceftiofur, cefazolin, cephapirin, cefquinome, cephalonium, amoxicillin, nafcillin, oxacillin, cloxacillin, ampicillin, dicloxacillin, and penicillin G) from raw cow milk. The extraction was performed using ultrasound-assisted extraction combined with dispersive-SPE, involving McIlvaine buffer (pH 6.0) and an acetonitrile:methanol mixture, followed by clean-up with PSA and anhydrous MgSO4. The analysis was performed by UHPLC-MS/MS with chromatographic separation achieved on an Acquity UPLC BEH C18 column. The recovery of the studied β-lactams ranged from 96.0% to 104.5%, with LOQ values ranging from 0.3 to 1.3 ng/g.
Using molecularly imprinted polymer (MIP) extraction, six β-lactam antibiotics (cephalexin, ceftiofur, cefazolin, cephapirin, cefquinome, and cephalonium) were extracted from bovine milk. The extraction was followed by analysis using a UHPLC-MS/MS system. Separation was achieved using an Acquity UPLC BEH™ C18 column. The results of the repeatability study showed that mean recoveries ranged from 62% to 100%, with RSDs below 6% for cephapirin, cephaloniu, cefquinome, and cefthiofur. Significantly lower values were obtained for cephalexin and cefazolin (recoveries: 15–28%), with RSD values lower than 6%. The LOQ for the analyzed compounds ranged from 0.4 to 12.5 µg/kg [70].
Another example of β-lactam extraction is a method that isolates sixteen β-lactam antibiotics, including penicillin G, cefalexin, ampicillin, penicillin V, penenethicillin, amoxicillin, methicillin, oxacillin, naficillin, cephapirin, cloxacillin, cefazolin, azlocillin, dicloxacillin, piperacillin, and ceftiofur, from pork muscle. The extraction was performed using matrix solid-phase dispersion with Oasis HLB sorbent, which was previously conditioned with acetonitrile, water, and sodium chloride solution. The analysis was carried out using a UPLC-MS/MS system, and chromatographic separation was performed on an Acquity UPLC HSS T3 C18 column (1.7 µm, 2.1 × 100 mm). The recoveries of β-lactam antibiotics were between 92% and 111%, and the RSDs were lower than 12%, with LOQ values ranging from 0.07 to 2.10 µg/kg [85].
Moretti et al. [86] successfully extracted 17 β-lactam antibiotics (desacetylcephapirin, amoxicillin, cephaphirin, cefquinome, cefacetrile, cefalonium, cefalexin, cefazolin, ampicillin, cefoperazone, ceftiofur, penicillin G, oxacillin, penicillin V, cloxacillin, dicloxacillin, and nafcillin) from meat using LC-MS/MS analysis, as part of a multiclass method detecting 62 antibiotics. The extraction involved the addition of EDTA, followed by LLE using an acetonitrile–water mixture (80:20, v/v) and pure acetonitrile. The analytes were separated using the Poroshell 120 EC-C18 column (100 × 3.0 mm; 2.7 μm) and Poroshell (2.1 × 5 mm) guard column. The average recoveries for β-lactam antibiotics in meat using this method ranged between 59% and 87%, with LOQ values of 2 µg/kg for all compounds, except for cefacetrile, which had an LOQ of 10 µg/kg.
Rocco et al. [41] used UHPLC-MS/MS detection to extract thirty β-lactam antibiotics, including cefadroxil, cefazolin, cephalexin, cefacetrile, cefalonium, cefoperazone, cefotaxime, cefquinome, cefuroxime, desacetyl cephapirin, desfuroylceftiofur cysteine disulfide, desfuroylceftiofur dimer, biapenem, doripenem, ertapenem, imipenem, meropenem, faropenem, amoxicillin, ampicillin, cloxacillin, dicloxacillin, mecillinam, methicillin, nafcillin, oxacillin, penicillin G, penicillin V, piperacillin, and ticarcillin, from bovine muscles. The extraction was performed by liquid–liquid extraction using water and acetonitrile, followed by a purification step with C18 sorbent. Separation was achieved with a stainless-steel CSH C18 column. The technique demonstrated trueness, which ranged between 69% and 143%. Precision was between 2.0% and 29.9% (within-laboratory reproducibility conditions).
In an effort to develop a method for detecting antibiotic residues in poultry, sixteen β-lactam antibiotics (ampicillin, amoxicillin, azlocillin, cefalexin, cefazolin, ceftiofur, cephapirin, cloxacillin, dicloxacillin, methicillin, nafcillin, oxacillin, penicillin G, penicillin V, penenethicillin, and piperacillin) were extracted from chicken muscle using UPLC-quadrupole-Orbitrap-MS. The QuEChERS method included extraction with acetonitrile containing 0.1% formic acid, using anhydrous magnesium sulfate and sodium acetate, followed by clean-up with end-capped C18, PSA, and anhydrous magnesium sulfate sorbents. Separation was accomplished using an HSS T3 C18 column. The recoveries ranged between 83% and 112% and the RSDs were < 15%, and LOQ values ranged from 0.03 to 0.59 µg/kg [52].
Turnipseed et al. [64] analyzed various fish species, including salmon, tilapia, and catfish, as well as shrimp and eel, to extract, among others, eight β-lactam antibiotics (amoxicillin, ampicillin, aspoxicillin, cloxacillin, dicloxacillin, oxacillin, penicillin G, and penicillin acid), using a multiclass method that also detects other veterinary drugs, using extraction with acetonitrile containing 0.2% p-toluenesulfonic acid monohydrate and 2% glacial acetic acid, followed by clean-up on an Oasis PRiME HLB cartridge. LC separation was carried out with the Supelco Ascentis Express C18 column, and the analysis was performed using LC-Q-Orbitrap HRMS. The average recoveries of the studied β-lactams were >90%, with standard deviations < 15%.
Seven β-lactam antibiotics (amoxicillin, ampicillin, cloxacillin, dicloxacillin, benzylpenicillin, cefquinome, and cefalexin) were extracted from heavy pigs’ urine and muscle using HPLC-MS/MS analysis. There were two methods of sample extraction, a different one for each matrix. For urine samples, SPE was applied after direct sample loading onto Oasis HLB cartridges, while for muscle samples, additional deproteinization and defatting steps were performed before SPE. Analytical separation was conducted using a Synergi Hydro-RP column and a Phonomenex C18 guard column. The mean recoveries for β-lactam antibiotics in the analyzed matrices ranged between 90% and 107% [61].
Bessaire et al. [54] extracted twenty-three β-lactam antibiotics (amoxicillin, ampicillin, aspoxicillin, cefacetril, cefadroxil, cefalexin, cefalonium, cefapirin, cefazolin, cefoperazone, cefquinome, ceftiofur, cefuroxime, cloxacillin, desacetylcefapirin, dicloxacillin, nafcillin, oxacillin, penicillin G, penicillin V, piperacillin, sulbactam, and tazobactam) from various foods of animal origin, including eggs, raw milk, processed dairy ingredients, infant formula, and meat- and fish-based products. The QuEChERS method involved extraction with acetonitrile in the presence of Na2SO4 and NaCl, followed by clean-up with Na2SO4 and C18 sorbents. Analysis was performed by LC-MS/MS using an Acquity BEH VanGuard precolumn attached to an Acquity BEH C18 column. Using this method, the average recoveries for the analyzed antibiotics ranged between 34% and 94%, with an average CV of 11%.
LC-MS/MS was used to detect five β-lactam antibiotics (ampicillin, cefazolin, oxacillin, penicillin G, and penicillin V), which were successfully extracted from fish, along with other groups of antibiotics. The extraction process for the antibiotics in the samples was carried out according to the previously developed method described by Gaugain-Juhel et al. Liquid–liquid extraction was performed using 5% trichloroacetic acid (TCA). A Millipore PVDF membrane with 0.45 μm pore size was used to filter the extract before LC-MS/MS analysis [39,42]. The separation was performed using an Agilent Technologies Zorbax Eclipse XDB C18. Even though the sensitivities of these analytes at a concentration of 0.5 MRL were satisfactory (greater than 95%), most of them exhibited high limit of detection (LOD) values, occasionally exceeding the MRL. Therefore, although the method is capable of monitoring these compounds, it cannot detect them at concentrations below the MRL [42].
A research group led by Karageorgou [87] extracted eight β-lactam antibiotics (amoxicillin, ampicillin, oxacillin, cloxacillin, ceftiofur, cefalonium, cefazolin, and cefapirin) from raw milk using the HPLC-DAD method. The extraction and purification were carried out using dispersive solid-phase extraction with preconditioned Plexa sorbent combined with QuEChERS salts containing magnesium sulfate, PSA, and C18EC. Separation was carried out using a Perfectsil ODS-2 analytical column. Counting of target analytes was conducted at the wavelength of optimum absorbance for each analyte: amoxicillin, ampicillin, cloxacillin, and oxacillin (240 nm), cefazolin, cefapirin, ceftiofur, and cefalonium (265 nm). The mean recovery rates ranged from 81.8% to 116.9%, with RSDs below 10.7% for all the examined agents.
A method for the extraction of eleven β-lactam antibiotics (amoxicillin, ampicillin, cefazolin, cephapirin, desacetyl cephapirin, cloxacillin, ceftiofur metabolite, dicloxacillin, nafcillin, oxacillin, and penicillin G), along with other classes of drugs, was developed for use with bovine tissues, including kidney, liver, and muscle. Liquid–liquid extraction was performed using a mixture of acetonitrile and water. The analysis was carried out by LC-MS/MS, and chromatographic separation was performed using a Waters Acquity HSS T3 analytical column and a 0.5 cm Waters Vanguard column. The average recoveries for β-lactam antibiotics ranged between 70% and 120% for most of the analytes, except for a few instances where the recoveries were higher or lower than the given range. The LOQ values ranged from 0.7 to 14 ng/g for kidney, from 1 to 22 ng/g for liver, and from 1 to 33 ng/g for muscle, with the highest values consistently observed for amoxicillin [88].
To extract seventeen β-lactam antibiotics, such as desacetylcephapyrin, amoxicillin, cephapyrin, cefquinome, cefacetrile, cefalonium, cefalexin, cefazolin, ampicillin, cefoperazone, ceftiofur, penicillin G, oxacillin, penicillin V, cloxacillin, dicloxacillin, and nafcillin, along with other classes of antibiotics, from muscle and milk, 1.50 g of each sample was weighed and placed into a Falcon tube. The sample preparation was first described by Moretti et al. [86,89,90]. The extraction involved LLE with acetonitrile/water and EDTA for muscle samples, and with acetonitrile and EDTA for milk samples, followed by a second extraction with acetonitrile for both matrices. LC-MS/MS detection was used. Separation was performed on an Agilent Poroshell 120 EC-C18 column with an Agilent Poroshell guard column. Using this method, the recoveries for the analyzed antibiotics ranged from 61% to 110%. The lowest recovery (61%) applied to ceftiofur extracted from milk, whereas the highest recovery (110%) applied to cloxacillin extracted from milk [89].
Studies have also been conducted on the extraction from chicken feathers. A research group led by Gajda [63] successfully extracted fifteen β-lactam antibiotics—amoxicillin, ampicillin, penicillin G, penicillin V, oxacillin, cloxacillin, nafcillin, dicloxacillin, cephapirin, cefoperazone, cephalexin, cefquinome, cefazolin, cefalonium, and ceftiofur, along with other classes of antibiotics. The extraction was performed using LLE with acetonitrile in the presence of oxalic acid and Na2EDTA, followed by SPE using Oasis HLB cartridges. The analysis was performed by UHPLC-MS/MS using an Agilent Zorbax SB-C18 and a Phenomenex octadecyl guard column for the separation. The gradient mobile phase consisted of acetonitrile and 0.025% HFBA. The recoveries for β-lactams in analyzed tissues ranged between 95% and 108%.
Di Rocco et al. [91] conducted a study to extract 32 β-lactam antibiotic residues from milk, including 12 penicillins (amoxicillin, ampicillin, cloxacillin, dicloxacillin, mecillinam, methicillin, nafcillin, oxacillin, penicillin G, penicillin V, piperacillin, and ticarcillin), 14 cephalosporins (cefacetrile, cefadroxil, cephalexin, cephapirin, cefalonium, cefazolin, cefoperazone, cefotaxime, cefquinome, ceftiofur, cefuroxime, desacetyl cephapirin, desfuroylceftiofur cysteine disulfide, and desfuroylceftiofur dimer), 5 carbapenems (biapenem, doripenem, ertapenem, imipenem, and meropenem), and faropenem. The extraction was performed by LLE (with acetonitrile) and purification by d-SPE (C18 sorbent). UHPLC-MS/MS detection was applied. The separation was carried out using an Agilent Zorbax Eclipse Plus Phenyl-Hexyl Rapid Resolution HD analytical column. Under conditions of within-laboratory repeatability, the accuracy ranged from 91% to 130%, while the precision varied from 1.4% to 38.6%.
Various animal-derived food products, including meat, kidneys, liver, bacon, milk, eggs, and honey, were used to extract residues of nineteen β-lactam antibiotics: amoxicillin, ampicillin, dicloxacillin, carbenicillin, cloxacillin, nafcillin, oxacillin, penicillin G, penicillin V, piperacillin, ticarcillin, cephalexin, cefalonium, cefoperazone, cefapirin, cefquinome, cefotaxime, ceftiofur, and cefuroxime. These residues were then analyzed using UHPLC-Q-TOF. Two extraction procedures based on LLE were applied, one using acetonitrile in the presence of NaCl and EDTA (first option), and the other using water with succinic acid and EDTA, followed by acetonitrile and ammonium sulfate (second option). The separation was performed with Acquity UPLC® BEN C18 columns. The second option of sample preparation was more suitable for milk, meat, liver, kidneys, eggs, and bacon, whereas the first option was preferable for honey (a larger number of analytes exhibited a matrix effect of less than 80%). Using this method, the recoveries for the analyzed antibiotics from different matrices ranged from 75% to 110%, and the values of RSD were ≤ 11% under the conditions of matrix calibration [92].
Cheng et al. [71] extracted ceftiofur from milk and muscle samples of chicken, pork, and beef using MISPE (molecularly imprinted solid-phase extraction), followed by HPLC-UV detection. For this purpose, after sample preparation, the analytes were loaded onto a cartridge packed with imprinted polymer, conditioned with methanol and water. The collected eluents were analyzed using HPLC-UV. Separation was performed using a reversed-phase C18 cartridge. UV detection was carried out at 292 nm. The average recoveries obtained using this method were greater than 91.9%, and the RSD was less than 8.5%, with an LOQ of 0.005 mg/L.
Sixty antibiotics, including sixteen β-lactam antibiotics (desacetylcephapirin, amoxicillin, cephapirin, cefquinome, cefalonium, cefalexin, cefazolin, ampicillin, cefoperazone, ceftiofur, penicillin G, oxacillin, penicillin V, cloxacillin, dicloxacillin, and nafcillin), were extracted from eggs. Two extraction steps based on LLE were applied: first using a mixture of acetonitrile and water with 0.05% formic acid in the presence of Na2EDTA, and second with pure acetonitrile. UHPLC-HRMS detection was applied. The separation was performed using a Poroshell 120 EC-C18 column and an Agilent Technologies Poroshell guard column. The recoveries for the antibiotics in the analyzed tissues ranged from 65% to 91% [93].
Hu et al. [65] extracted four β-lactam antibiotics (cloxacillin, dicloxacillin, penicillin G, and penicillin V), along with other classes of antibiotics, from cereals, meat, eggs, milk, vegetables, and fruits. Three extraction steps based on LLE were performed: the first with acetonitrile:water containing 0.1% formic acid, the second with acetonitrile:water containing 0.2% formic acid and a DisQue salt pack (MgSO4 and sodium acetate), and the third with acetonitrile:water containing 0.2% formic acid. Purification was carried out by SPE using Oasis PRiME HLB cartridges. UHPLC-MS/MS analysis was carried out. The separation was performed using the Waters BEH C8 column. The average recoveries at three spiking levels were as follows: 82 ± 26% for cereals, 77 ± 26% for meat, 70 ± 34% for eggs, 69 ± 31% for milk, 73 ± 29% for vegetables, and 62 ± 37% for fruits.
To detect fourteen β-lactam antibiotics, including amoxicillin, penicillin V, penicillin G, cloxacillin, oxacillin, dicloxacillin, ampicillin, nafcillin, cefazolin, cephalexin, ceftiofur, cefquinome, cefoperazone, and cefapirin, milk samples from cows, sheep, and goats were analyzed using an LC-Orbitrap-HRMS system, which, as a multiresidue method, also allowed for the detection of other antibiotics. The extraction was based on LLE with acetonitrile containing 2% formic acid and EDTA, followed by SPE clean-up on Oasis HLB PRiME cartridges. The separation was performed using a Poroshell 120 EC-C18 column with a Poroshell guard column. The method showed effectiveness at the target screening concentration, and the false positive rate was less than 5% [66].
Lakew et al. [94] extracted amoxicillin, ampicillin, and penicillin G (three β-lactam antibiotics, among other groups) from chicken tissues. The extraction was based on LLE using a mixture of acetonitrile and methanol. The analysis was performed using LC-UV detection. The separation was performed using Phenomenex Hypersil BDS-C18 in reversed-phase mode with isocratic elution. UV absorption was measured at a wavelength of 230 nm. The recoveries were acceptable, ranging from 96% to 102%. LOQs ranged from 0.297 to 0.574 μg/kg.
A research group led by Wu [95] used a modified QuEChERS extraction method to isolate a total of 52 β-lactam antibiotics, including cefetamet, cefotaxime, cefterampivoxil, faropenem, cefmenoxime, cefuroxime, cefazolin, cefathiamidine, cefodizime, ticarcillin, cefpiramide, cefamandole sodium, carbenicillin, cefoperazone, ceftiofur, penicillin g, methicillin, cefuroxime axetil, azlocillin, cefpodoxime proxetil, piperacillin, mezlocillin, cefetamet pivoxil, dicloxacillin sodium, flucloxacillin, cloxacillin, oxacillin sodium, penicillin v, cefadroxil, amoxicillin, ampicillin, cephradine, cephalexin, aspoxicillin, mecillinam, cefotiam, ceftizoxime, cefdinir, cefozopran, cilastatin, cefepime, ceftazidime, cefixime, ceftibuten, cefpirome, ceftriaxone, cefprozil hydrate, meropenem, aspoxicillin, nafcillin, biapenem, and cefapirin sodium, from samples of meat and poultry, aquatic products, milk, and eggs. The extraction was carried out by a modified QuEChERS method, involving extraction with a mixture of acetonitrile and water and clean-up with octadecyl silica (C18) sorbent. The filtered extract was injected into the UPLC-MS/MS system. Separation was carried out using an Agilent Zorbax SB-Aq column. The mean recoveries of β-lactam antibiotics at spike levels of 10, 20, and 50 µg/kg varied between 67.1% and 109.8%, with relative standard deviations ranging from 0.5% to 9.9%. LOQs of β-lactams ranged from 0.07 to 0.97 mg/kg.
The methods described in the paper, such as LC-MS/MS and extraction techniques, including LLE, SPE, and QuEChERS, have a great potential to become standard procedures in routine laboratory testing. With their high sensitivity, specificity, and ability to detect low concentrations of β-lactam antibiotics and other antibiotic groups in various matrices, they provide reliable results. This is important for meeting legal requirements concerning antibiotic residues in animal-derived food.
Routine laboratories can successfully implement these techniques, as equipment like HPLC and LC-MS/MS are widely available, and the extraction methods are relatively easy to apply. Additionally, they feature short analysis times and good repeatability of results, which makes them efficient tools for everyday laboratory work.
6. Conclusions
In conclusion, recent advancements in extraction and detection techniques for β-lactam antibiotics have significantly improved the sensitivity, precision, and reliability of analytical methods. Despite these achievements, the complexity of food matrices continues to pose significant challenges, often leading to unpredictable matrix effects that can interfere with accurate quantification. Therefore, further research is necessary to refine existing methods and develop more robust solutions to overcome these limitations.
Techniques such as LLE, SPE, IPE, QuEChERS, MIP, and MSPD are continuously being improved to optimize recovery rates and reduce matrix effects. LC-MS/MS, with its high sensitivity and selectivity, has become a cornerstone in the analysis of antibiotic residues, enabling increasingly lower detection limits and high precision and becoming essential for ensuring food safety as well as regulatory compliance.
As the field progresses, it is essential to further improve extraction techniques and enhance the performance and sensitivity of LC-MS/MS equipment to address the challenges posed by increasingly complex food matrices and emerging contaminants. Ongoing innovations underscore the urgent need for research focused on harmonizing and standardizing analytical methods across laboratories, as well as developing more selective and efficient extraction procedures. These advancements would enable the detection of progressively lower concentrations of substances, the identification of new compounds, and the development of multiresidue methods that facilitate the simultaneous analysis of multiple substance groups. Progress in these areas will directly impact the effectiveness of food safety monitoring and support regulatory decision-making, thereby contributing to enhanced public health protection.
Furthermore, collaboration between international research institutions is essential to facilitate the standardization of methods and ensure global applicability of food safety monitoring systems. Wider adoption of these improved extraction techniques and analytical methods will not only enhance food safety but also contribute to more equitable access to safe food products globally.
Author Contributions
Conceptualization J.P. and P.N., methodology J.P., writing—original draft J.P., writing—review and editing P.N. All authors have read and agreed to the published version of the manuscript.
Funding
The work of Joanna Pacyńska was supported by Grant No. DWD/3/1/2019, “Doktorat wdrożeniowy”, financed by the National Science Centre, Poland.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Moreno-Bondi, M.C.; Marazuela, M.D.; Herranz, S.; Rodriguez, E. An overview of sample preparation procedures for LC-MS multiclass antibiotic determination in environmental and food samples. Anal. Bioanal. Chem. 2009, 395, 921–946. [Google Scholar] [CrossRef]
- Zhang, C.; Deng, Y.; Zheng, J.; Zhang, Y.; Yang, L.; Liao, C.; Su, L.; Zhou, Y.; Gong, D.; Chen, L.; et al. The application of the QuEChERS methodology in the determination of antibiotics in food: A review. TrAC Trends Anal. Chem. 2019, 118, 517–537. [Google Scholar] [CrossRef]
- Rossi, R.; Saluti, G.; Moretti, S.; Diamanti, I.; Giusepponi, D.; Galarini, R. Multiclass methods for the analysis of antibiotic residues in milk by liquid chromatography coupled to mass spectrometry: A review. Food Addit. Contam. Part A 2018, 35, 241–257. [Google Scholar] [CrossRef] [PubMed]
- Ghimpețeanu, O.M.; Pogurschi, E.N.; Popa, D.C.; Dragomir, N.; Drăgotoiu, T.; Mihai, O.D.; Petcu, C.D. Antibiotic Use in Livestock and Residues in Food—A Public Health Threat: A Review. Foods 2022, 11, 1430. [Google Scholar] [CrossRef] [PubMed]
- Cañada-Cañada, F.; Muñoz de la Peña, A.; Espinosa-Mansilla, A. Analysis of antibiotics in fish samples. Anal. Bioanal. Chem. 2009, 395, 987–1008. [Google Scholar] [CrossRef]
- Peris-Vicente, J.; Peris-García, E.; Albiol-Chiva, J.; Durgbanshi, A.; Ochoa-Aranda, E.; Carda-Broch, S.; Bose, D.; Esteve-Romero, J. Liquid chromatography, a valuable tool in the determination of antibiotics in biological, food and environmental samples. Microchem. J. 2022, 177, 107309. [Google Scholar] [CrossRef]
- Pérez-Rodríguez, M.; Pellerano, R.G.; Pezza, L.; Pezza, H.R. An overview of the main foodstuff sample preparation technologies for tetracycline residue determination. Talanta 2018, 182, 1–21. [Google Scholar] [CrossRef]
- Lees, P.; Toutain, P.-L. Pharmacokinetics, Distribution, Bioavailability, and Relationship to Antibiotic Residues. In Chemical Analysis of Antibiotic Residues in Food; Wiley: Hoboken, NJ, USA, 2011; pp. 61–109. [Google Scholar] [CrossRef]
- Kennedy, D.G.; McCracken, R.J.; Cannavan, A.; Hewitt, S.A. Use of liquid chromatography-mass spectrometry in the analysis of residues of antibiotics in meat and milk. J. Chromatogr. A 1998, 812, 77–98. [Google Scholar] [CrossRef]
- Reeves, P.T. Antibiotics: Groups and Properties. In Chemical Analysis of Antibiotic Residues in Food; Wiley: Hoboken, NJ, USA, 2011; pp. 1–60. [Google Scholar]
- Etebu, E.; Arikekpar, I. Antibiotics: Classification and mechanisms of action with emphasis on molecular perspectives. Int. J. Appl. Microbiol. Biotechnol. Res. 2016, 4, 90–101. [Google Scholar]
- Samanidou, V.F.; Evaggelopoulou, E.N. Analytical strategies to determine antibiotic residues in fish. J. Sep. Sci. 2007, 30, 2549–2569. [Google Scholar] [CrossRef]
- Bargańska, Ż.; Namieśnik, J.; Ślebioda, M. Determination of antibiotic residues in honey. TrAC Trends Anal. Chem. 2011, 30, 1035–1041. [Google Scholar] [CrossRef]
- Martín, J.F.; Ullán, R.V.; García-Estrada, C. Regulation and compartmentalization of β-lactam biosynthesis. Microb. Biotechnol. 2010, 3, 285–299. [Google Scholar] [CrossRef] [PubMed]
- National Center for Biotechnology Information. PubChem Compound Summary for CID 5904, Penicillin G. 2025. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Penicillin-G (accessed on 6 April 2025).
- National Center for Biotechnology Information. PubChem Compound Summary for CID 6869, Penicillin V. 2025. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Penicillin-V (accessed on 6 April 2025).
- National Center for Biotechnology Information. PubChem Compound Summary for CID 33613, Amoxicillin. 2025. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Amoxicillin (accessed on 6 April 2025).
- National Center for Biotechnology Information. PubChem Compound Summary for CID 6249, Ampicillin. 2025. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Ampicillin (accessed on 6 April 2025).
- National Center for Biotechnology Information. PubChem Compound Summary for CID 6196, Oxacillin. 2025. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Oxacillin (accessed on 6 April 2025).
- National Center for Biotechnology Information. PubChem Compound Summary for CID 6098, Cloxacillin. 2025. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Cloxacillin (accessed on 7 April 2025).
- National Center for Biotechnology Information. PubChem Compound Summary for CID 8982, Nafcillin. 2025. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Nafcillin (accessed on 7 April 2025).
- National Center for Biotechnology Information. PubChem Compound Summary for CID 18381, Dicloxacillin. 2025. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Dicloxacillin (accessed on 7 April 2025).
- National Center for Biotechnology Information. PubChem Compound Summary for CID 43672, Piperacillin. 2025. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Piperacillin (accessed on 7 April 2025).
- National Center for Biotechnology Information. PubChem Compound Summary for CID 20824, Carbenicillin. 2025. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Carbenicillin (accessed on 7 April 2025).
- Samanidou, V.; Nisyriou, S. Multi-residue methods for confirmatory determination of antibiotics in milk. J. Sep. Sci. 2008, 31, 2068–2090. [Google Scholar] [CrossRef] [PubMed]
- Percival, K.M. Antibiotic Classification and Indication Review for the Infusion Nurse. J. Infus. Nurs. Off. Publ. Infus. Nurses Soc. 2017, 40, 55–63. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information. PubChem Compound Summary for CID 30699, Cephapirin. 2025. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Cephapirin (accessed on 7 April 2025).
- National Center for Biotechnology Information. PubChem Compound Summary for CID 6328657, Ceftiofur. 2025. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/6328657 (accessed on 7 April 2025).
- National Center for Biotechnology Information. PubChem Compound Summary for CID 5464355, Cefquinome. 2025. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Cefquinome (accessed on 7 April 2025).
- National Center for Biotechnology Information. PubChem Compound Summary for CID 21743, Cefalonium. 2025. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Cefalonium (accessed on 7 April 2025).
- National Center for Biotechnology Information. PubChem Compound Summary for CID 33255, Cefazolin. 2025. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Cefazolin (accessed on 7 April 2025).
- National Center for Biotechnology Information. PubChem Compound Summary for CID 27447, Cephalexin. 2025. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Cephalexin (accessed on 7 April 2025).
- National Center for Biotechnology Information. PubChem Compound Summary for CID 44187, Cefoperazone. 2025. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Cefoperazone (accessed on 7 April 2025).
- Hu, C.; Zhang, Y.; Zhou, Y.; Liu, Z.-F.; Meng, Q.; Feng, X.-S. A review of pretreatment and analysis of macrolides in food (Update Since 2010). J. Chromatogr. A 2020, 1634, 461662. [Google Scholar] [CrossRef]
- Bitas, D.; Kabir, A.; Locatelli, M.; Samanidou, V. Food Sample Preparation for the Determination of Sulfonamides by High-Performance Liquid Chromatography: State-of-the-Art. Separations 2018, 5, 31. [Google Scholar] [CrossRef]
- Nováková, L.; Vlcková, H. A review of current trends and advances in modern bio-analytical methods: Chromatography and sample preparation. Anal. Chim. Acta 2009, 656, 8–35. [Google Scholar] [CrossRef] [PubMed]
- Stolker, A.A.M.; Danaher, M. Sample Preparation: Extraction and Clean-Up. In Chemical Analysis of Antibiotic Residues in Food; Wiley: Hoboken, NJ, USA, 2011; pp. 125–152. [Google Scholar] [CrossRef]
- Kaufmann, A.; Butcher, P.; Maden, K.; Widmer, M. Quantitative multiresidue method for about 100 veterinary drugs in different meat matrices by sub 2-μm particulate high-performance liquid chromatography coupled to time of flight mass spectrometry. J. Chromatogr. A 2008, 1194, 66–79. [Google Scholar] [CrossRef]
- Gaugain-Juhel, M.; Delepine, B.; Gautier, S.; Fourmond, M.P.; Gaudin, V.; Hurtaud-Pessel, D.; Verdon, E.; Sanders, P. Validation of a liquid chromatography-tandem mass spectrometry screening method to monitor 58 antibiotics in milk: A qualitative approach. Food Addit. Contam. Part A Chem. Anal. Control. Expo. Risk Assess. 2009, 26, 1459–1471. [Google Scholar] [CrossRef]
- Jank, L.; Martins, M.T.; Arsand, J.B.; Hoff, R.B.; Barreto, F.; Pizzolato, T.M. High-throughput method for the determination of residues of β-lactam antibiotics in bovine milk by LC-MS/MS. Food Addit. Contam. Part A 2015, 32, 1992–2001. [Google Scholar] [CrossRef]
- Di Rocco, M.; Moloney, M.; O’Beirne, T.; Earley, S.; Berendsen, B.; Furey, A.; Danaher, M. Development and validation of a quantitative confirmatory method for 30 β-lactam antibiotics in bovine muscle using liquid chromatography coupled to tandem mass spectrometry. J. Chromatogr. A 2017, 1500, 121–135. [Google Scholar] [CrossRef]
- Guidi, L.R.; Santos, F.A.; Ribeiro, A.C.S.R.; Fernandes, C.; Silva, L.H.M.; Gloria, M.B.A. A simple, fast and sensitive screening LC-ESI-MS/MS method for antibiotics in fish. Talanta 2017, 163, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Kinsella, B.; O’Mahony, J.; Malone, E.; Moloney, M.; Cantwell, H.; Furey, A.; Danaher, M. Current trends in sample preparation for growth promoter and veterinary drug residue analysis. J. Chromatogr. A 2009, 1216, 7977–8015. [Google Scholar] [CrossRef]
- Fernandes, J.O.; Ferreira, M.A. Combined ion-pair extraction and gas chromatography–mass spectrometry for the simultaneous determination of diamines, polyamines and aromatic amines in Port wine and grape juice. J. Chromatogr. A 2000, 886, 183–195. [Google Scholar] [CrossRef] [PubMed]
- Carson, M.C. Ion-pair solid-phase extraction. J. Chromatogr. A 2000, 885, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Greenlees, K.J.; Friedlander, L.G.; Boxall, A. Antibiotic Residues in Food and Drinking Water, and Food Safety Regulations. In Chemical Analysis of Antibiotic Residues in Food; Wiley: Hoboken, NJ, USA, 2011; pp. 111–123. [Google Scholar] [CrossRef]
- Nannou, C.; Ofrydopoulou, A.; Heath, D.; Heath, E.; Lambropoulou, D. QuEChERS—A Green Alternative Approach for the Determination of Pharmaceuticals and Personal Care Products in Environmental and Food Samples. In Green Analytical Chemistry: Past, Present and Perspectives; Płotka-Wasylka, J., Namieśnik, J., Eds.; Springer: Singapore, 2019; pp. 395–430. [Google Scholar] [CrossRef]
- Lehotay, S.J. Quick, Easy, Cheap, Effective, Rugged, and Safe Approach for Determining Pesticide Residues. In Pesticide Protocols; Martínez Vidal, J.L., Frenich, A.G., Eds.; Humana Press: Totowa, NJ, USA, 2006; pp. 239–261. [Google Scholar] [CrossRef]
- Bruzzoniti, M.C.; Checchini, L.; De Carlo, R.M.; Orlandini, S.; Rivoira, L.; Del Bubba, M. QuEChERS sample preparation for the determination of pesticides and other organic residues in environmental matrices: A critical review. Anal. Bioanal. Chem. 2014, 406, 4089–4116. [Google Scholar] [CrossRef]
- Wilkowska, A.; Biziuk, M. Determination of pesticide residues in food matrices using the QuEChERS methodology. Food Chem. 2011, 125, 803–812. [Google Scholar] [CrossRef]
- Perestrelo, R.; Silva, P.; Porto-Figueira, P.; Pereira, J.A.M.; Silva, C.; Medina, S.; Câmara, J.S. QuEChERS—Fundamentals, relevant improvements, applications and future trends. Anal. Chim. Acta 2019, 1070, 1–28. [Google Scholar] [CrossRef]
- Chen, Q.; Pan, X.-D.; Huang, B.-F.; Han, J.-L. Quantification of 16 β-lactams in chicken muscle by QuEChERS extraction and UPLC-Q-Orbitrap-MS with parallel reaction monitoring. J. Pharm. Biomed. Anal. 2017, 145, 525–530. [Google Scholar] [CrossRef]
- Pérez-Burgos, R.; Grzelak, E.M.; Gokce, G.; Saurina, J.; Barbosa, J.; Barrón, D. Quechers methodologies as an alternative to solid phase extraction (SPE) for the determination and characterization of residues of cephalosporins in beef muscle using LC–MS/MS. J. Chromatogr. B 2012, 899, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Bessaire, T.; Mujahid, C.; Beck, A.; Tarres, A.; Savoy, M.C.; Woo, P.M.; Mottier, P.; Desmarchelier, A. Screening of 23 β-lactams in foodstuffs by LC-MS/MS using an alkaline QuEChERS-like extraction. Food Addit. Contam. Part A Chem. Anal. Control. Expo. Risk Assess. 2018, 35, 661–673. [Google Scholar] [CrossRef] [PubMed]
- Barker, S.A.; Long, A.R.; Short, C.R. Isolation of drug residues from tissues by solid phase dispersion. J. Chromatogr. A 1989, 475, 353–361. [Google Scholar] [CrossRef]
- Poole, C.F. New trends in solid-phase extraction. TrAC Trends Anal. Chem. 2003, 22, 362–373. [Google Scholar] [CrossRef]
- Poole, C.F.; Poole, S.K. Principles and Practice of Solid-Phase Extraction. Compr. Sampl. Sample Prep. 2012, 2, 273–297. [Google Scholar] [CrossRef]
- Buszewski, B.; Szultka, M. Past, Present, and Future of Solid Phase Extraction: A Review. Crit. Rev. Anal. Chem. 2012, 42, 198–213. [Google Scholar] [CrossRef]
- Becker, M.; Zittlau, E.; Petz, M. Residue analysis of 15 penicillins and cephalosporins in bovine muscle, kidney and milk by liquid chromatography–tandem mass spectrometry. Anal. Chim. Acta 2004, 520, 19–32. [Google Scholar] [CrossRef]
- Heller, D.N.; Nochetto, C.B.; Rummel, N.G.; Thomas, M.H. Development of Multiclass Methods for Drug Residues in Eggs: Hydrophilic Solid-Phase Extraction Cleanup and Liquid Chromatography/Tandem Mass Spectrometry Analysis of Tetracycline, Fluoroquinolone, Sulfonamide, and β-Lactam Residues. J. Agric. Food Chem. 2006, 54, 5267–5278. [Google Scholar] [CrossRef]
- Chiesa, L.M.; Nobile, M.; Panseri, S.; Arioli, F. Antibiotic use in heavy pigs: Comparison between urine and muscle samples from food chain animals analysed by HPLC-MS/MS. Food Chem. 2017, 235, 111–118. [Google Scholar] [CrossRef]
- van Holthoon, F.; Mulder, P.P.; van Bennekom, E.O.; Heskamp, H.; Zuidema, T.; van Rhijn, H.J. Quantitative analysis of penicillins in porcine tissues, milk and animal feed using derivatisation with piperidine and stable isotope dilution liquid chromatography tandem mass spectrometry. Anal. Bioanal. Chem. 2010, 396, 3027–3040. [Google Scholar] [CrossRef]
- Gajda, A.; Nowacka-Kozak, E.; Gbylik-Sikorska, M.; Posyniak, A. Multi-residues UHPLC–MS/MS analysis of 53 antibacterial compounds in poultry feathers as an analytical tool in food safety assurance. J. Chromatogr. B 2019, 1104, 182–189. [Google Scholar] [CrossRef]
- Turnipseed, S.B.; Storey, J.M.; Lohne, J.J.; Andersen, W.C.; Burger, R.; Johnson, A.S.; Madson, M.R. Wide-Scope Screening Method for Multiclass Veterinary Drug Residues in Fish, Shrimp, and Eel Using Liquid Chromatography–Quadrupole High-Resolution Mass Spectrometry. J. Agric. Food Chem. 2017, 65, 7252–7267. [Google Scholar] [CrossRef]
- Hu, M.; Ben, Y.; Wong, M.H.; Zheng, C. Trace Analysis of Multiclass Antibiotics in Food Products by Liquid Chromatography-Tandem Mass Spectrometry: Method Development. J. Agric. Food Chem. 2021, 69, 1656–1666. [Google Scholar] [CrossRef] [PubMed]
- Salis, S.; Rubattu, N.; Rubattu, F.; Cossu, M.; Sanna, A.; Chessa, G. Analytical Approaches in Official Food Safety Control: An LC-Orbitrap-HRMS Screening Method for the Multiresidue Determination of Antibiotics in Cow, Sheep, and Goat Milk. Molecules 2022, 27, 6162. [Google Scholar] [CrossRef]
- Stolker, A.A.; Rutgers, P.; Oosterink, E.; Lasaroms, J.J.; Peters, R.J.; van Rhijn, J.A.; Nielen, M.W. Comprehensive screening and quantification of veterinary drugs in milk using UPLC-ToF-MS. Anal. Bioanal. Chem. 2008, 391, 2309–2322. [Google Scholar] [CrossRef] [PubMed]
- Berendsen, B.J.A.; Gerritsen, H.W.; Wegh, R.S.; Lameris, S.; van Sebille, R.; Stolker, A.A.M.; Nielen, M.W.F. Comprehensive analysis of ß-lactam antibiotics including penicillins, cephalosporins, and carbapenems in poultry muscle using liquid chromatography coupled to tandem mass spectrometry. Anal. Bioanal. Chem. 2013, 405, 7859–7874. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, L.; Xu, Y.; Wang, H.; Zeng, Q.; Zhao, Q.; Ren, N.; Ding, L. Determination of β-lactam antibiotics in milk based on magnetic molecularly imprinted polymer extraction coupled with liquid chromatography-tandem mass spectrometry. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2010, 878, 3421–3426. [Google Scholar] [CrossRef]
- Baeza, A.N.; Urraca, J.L.; Chamorro, R.; Orellana, G.; Castellari, M.; Moreno-Bondi, M.C. Multiresidue analysis of cephalosporin antibiotics in bovine milk based on molecularly imprinted polymer extraction followed by liquid chromatography-tandem mass spectrometry. J. Chromatogr. A 2016, 1474, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Cheng, G.; Zhao, J.; Wang, X.; Yang, C.; Li, S.; Lu, T.; Li, X.; Wang, X.; Zhu, G. A highly sensitive and selective method for the determination of ceftiofur sodium in milk and animal-origin food based on molecularly imprinted solid-phase extraction coupled with HPLC-UV. Food Chem. 2021, 347, 129013. [Google Scholar] [CrossRef]
- Farooq, S.; Xu, L.; Ullah, S.; Li, J.; Nie, J.; Ping, J.; Ying, Y. Advancements and greenification potential of magnetic molecularly imprinted polymers for chromatographic analysis of veterinary drug residues in milk. Compr. Rev. Food Sci. Food Saf. 2024, 23, e13399. [Google Scholar] [CrossRef]
- Moein, M.M.; Abdel-Rehim, A.; Abdel-Rehim, M. Microextraction by packed sorbent (MEPS). TrAC Trends Anal. Chem. 2015, 67, 34–44. [Google Scholar] [CrossRef]
- Abdel-Rehim, M. Microextraction by packed sorbent (MEPS): A tutorial. Anal. Chim. Acta 2011, 701, 119–128. [Google Scholar] [CrossRef]
- Chiaochan, C.; Koesukwiwat, U.; Yudthavorasit, S.; Leepipatpiboon, N. Efficient hydrophilic interaction liquid chromatography-tandem mass spectrometry for the multiclass analysis of veterinary drugs in chicken muscle. Anal. Chim. Acta 2010, 682, 117–129. [Google Scholar] [CrossRef] [PubMed]
- Junza, A.; Amatya, R.; Barrón, D.; Barbosa, J. Comparative study of the LC–MS/MS and UPLC–MS/MS for the multi-residue analysis of quinolones, penicillins and cephalosporins in cow milk, and validation according to the regulation 2002/657/EC. J. Chromatogr. B 2011, 879, 2601–2610. [Google Scholar] [CrossRef]
- Jank, L.; Hoff, R.B.; Tarouco, P.C.; Barreto, F.; Pizzolato, T.M. β-lactam antibiotics residues analysis in bovine milk by LC-ESI-MS/MS: A simple and fast liquid–liquid extraction method. Food Addit. Contam. Part A 2012, 29, 497–507. [Google Scholar] [CrossRef] [PubMed]
- Macarov, C.A.; Tong, L.; Martínez-Huélamo, M.; Hermo, M.P.; Chirila, E.; Wang, Y.X.; Barrón, D.; Barbosa, J. Multi residue determination of the penicillins regulated by the European Union, in bovine, porcine and chicken muscle, by LC–MS/MS. Food Chem. 2012, 135, 2612–2621. [Google Scholar] [CrossRef]
- Kukusamude, C.; Santalad, A.; Boonchiangma, S.; Burakham, R.; Srijaranai, S.; Chailapakul, O. Mixed micelle-cloud point extraction for the analysis of penicillin residues in bovine milk by high performance liquid chromatography. Talanta 2010, 81, 486–492. [Google Scholar] [CrossRef] [PubMed]
- Kukusamude, C.; Burakham, R.; Chailapakul, O.; Srijaranai, S. High performance liquid chromatography for the simultaneous analysis of penicillin residues in beef and milk using ion-paired extraction and binary water–acetonitrile mixture. Talanta 2012, 92, 38–44. [Google Scholar] [CrossRef]
- Karageorgou, E.G.; Samanidou, V.F.; Papadoyannis, I.N. Ultrasound-assisted matrix solid phase dispersive extraction for the simultaneous analysis of β-lactams (four penicillins and eight cephalosporins) in milk by high performance liquid chromatography with photodiode array detection. J. Sep. Sci. 2012, 35, 2599–2607. [Google Scholar] [CrossRef]
- Maggi, L.; Hurtado de Mendoza, J.; Zalacain, A.; Bonetto, L.; Mocholí, F.A.; Carmona, M. On-line Solid-Phase Extraction Coupled to Liquid Chromatography–Ion Trap Tandem Mass Spectrometry for Determination of Penicillins in Catfish. Food Anal. Methods 2012, 5, 1047–1053. [Google Scholar] [CrossRef]
- Cámara, M.; Gallego-Picó, A.; Garcinuño, R.M.; Fernández-Hernando, P.; Durand-Alegría, J.S.; Sánchez, P.J. An HPLC-DAD method for the simultaneous determination of nine β-lactam antibiotics in ewe milk. Food Chem. 2013, 141, 829–834. [Google Scholar] [CrossRef]
- Dorival-García, N.; Junza, A.; Zafra-Gómez, A.; Barrón, D.; Navalón, A. Simultaneous determination of quinolone and β-lactam residues in raw cow milk samples using ultrasound-assisted extraction and dispersive-SPE prior to UHPLC−MS/MS analysis. Food Control 2016, 60, 382–393. [Google Scholar] [CrossRef]
- Huang, Z.; Pan, X.-D.; Huang, B.-f.; Xu, J.-J.; Wang, M.-L.; Ren, Y.-P. Determination of 15 β-lactam antibiotics in pork muscle by matrix solid-phase dispersion extraction (MSPD) and ultra-high pressure liquid chromatography tandem mass spectrometry. Food Control 2016, 66, 145–150. [Google Scholar] [CrossRef]
- Moretti, S.; Dusi, G.; Giusepponi, D.; Pellicciotti, S.; Rossi, R.; Saluti, G.; Cruciani, G.; Galarini, R. Screening and confirmatory method for multiclass determination of 62 antibiotics in meat. J. Chromatogr. A 2016, 1429, 175–188. [Google Scholar] [CrossRef]
- Karageorgou, E.; Christoforidou, S.; Ioannidou, M.; Psomas, E.; Samouris, G. Detection of β-Lactams and Chloramphenicol Residues in Raw Milk—Development and Application of an HPLC-DAD Method in Comparison with Microbial Inhibition Assays. Foods 2018, 7, 82. [Google Scholar] [CrossRef]
- Lehotay, S.J.; Lightfield, A.R. Simultaneous analysis of aminoglycosides with many other classes of drug residues in bovine tissues by ultrahigh-performance liquid chromatography-tandem mass spectrometry using an ion-pairing reagent added to final extracts. Anal. Bioanal. Chem. 2018, 410, 1095–1109. [Google Scholar] [CrossRef] [PubMed]
- Giusepponi, D.; Paoletti, F.; Barola, C.; Moretti, S.; Saluti, G.; Ianni, F.; Sardella, R.; Galarini, R. Transfer of a Multiclass Method for over 60 Antibiotics in Food from High Resolution to Low Resolution Mass Spectrometry. Molecules 2019, 24, 2935. [Google Scholar] [CrossRef]
- Moretti, S.; Cruciani, G.; Romanelli, S.; Rossi, R.; Saluti, G.; Galarini, R. Multiclass method for the determination of 62 antibiotics in milk. J. Mass Spectrom. JMS 2016, 51, 792–804. [Google Scholar] [CrossRef]
- Di Rocco, M.; Moloney, M.; Haren, D.; Gutierrez, M.; Earley, S.; Berendsen, B.; Furey, A.; Danaher, M. Improving the chromatographic selectivity of β-lactam residue analysis in milk using phenyl-column chemistry prior to detection by tandem mass spectrometry. Anal. Bioanal. Chem. 2020, 412, 4461–4475. [Google Scholar] [CrossRef] [PubMed]
- Amelin, V.G.; Bol’shakov, D.S.; Podkolzin, I.V. Rapid Screening and Determination of Residual Amounts of β-Lactam Antibiotics in Foods by Ultrahigh-Performance Liquid Chromatography–High-Resolution Quadrupole Time-of-Flight Mass Spectrometry. J. Anal. Chem. 2020, 75, 1177–1188. [Google Scholar] [CrossRef]
- Paoletti, F.; Sdogati, S.; Barola, C.; Giusepponi, D.; Moretti, S.; Galarini, R. Development and validation of a multiclass confirmatory method for the determination of over 60 antibiotics in eggs using liquid-chromatography high-resolution mass spectrometry. Food Control 2021, 127, 108109. [Google Scholar] [CrossRef]
- Lakew, A.; Assefa, T.; Woldeyohannes, M.; Megersa, N.; Chandravanshi, B.S. Development and validation of liquid chromatography method for simultaneous determination of multiclass seven antibiotic residues in chicken tissues. BMC Chem. 2022, 16, 5. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Guo, E.; Wang, M.; Wang, K.; Ma, L.; Lian, K. Determination of β-lactam antibiotics in animal derived foods by modified QuEChERS coupled with ultra performance liquid chromatography-tandem mass spectrometry. J. Food Compos. Anal. 2023, 122, 105437. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).