Loading of Oregano Oil in Natural Nanogel and Preliminary Studies on Its Antiviral Activity on Betacoronavirus 1
Abstract
1. Introduction
2. Results and Discussion
2.1. Encapsulation of Oregano Oil in the Nanogel and Characterization of the System
2.2. Studies of the Antiviral Activity of the Loaded Nanoparticles
3. Materials and Methods
3.1. Materials
3.2. Preparation of Oregano Oil-Loaded Nanogel
3.3. Characterization of the Loaded Nanogel
3.4. In Vitro Release Study
3.5. Cultivation of MDBK Cells and Propagation of Betacoronavirus 1
3.6. In Vitro Cytotoxicity of Encapsulated Oregano Oil on MDBK Cells
3.7. Determination of Antiviral Activity
3.8. Mathematical Model for Calculation of Median Inhibitory and Maximal Non-Toxic Concentration
3.9. Determination of the Bovine Coronavirus Titer with Droplet Digital (ddPCR) After Treatment with OrO-NG or NG
3.10. Statistical Evaluation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Abouelezz, K.; Abou-Hadied, M.; Yuan, J.; Elokil, A.A.; Wang, G.; Wang, S.; Wang, J.; Bian, G. Nutritional Impacts of Dietary Oregano and Enviva Essential Oils on the Performance, Gut Microbiota and Blood Biochemicals of Growing Ducks. Animal 2019, 13, 2216–2222. [Google Scholar] [CrossRef]
- Betancourt, L.; Hume, M.; Rodríguez, F.; Nisbet, D.; Sohail, M.U.; Afanador-Tellez, G. Effects of Colombian Oregano Essential Oil (Lippia origanoides Kunth) and Eimeria Species on Broiler Production and Cecal Microbiota. Poult. Sci. 2019, 98, 4777–4786. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Galicia, I.A.; Arras-Acosta, J.A.; Huerta-Jimenez, M.; Rentería-Monterrubio, A.L.; Loya-Olguin, J.L.; Carrillo-Lopez, L.M.; Tirado-Gallegos, J.M.; Alarcon-Rojo, A.D. Natural Oregano Essential Oil May Replace Antibiotics in Lamb Diets: Effects on Meat Quality. Antibiotics 2020, 9, 248. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.; Wu, J.; Zhang, L.; Liu, L.; Casper, D.P.; Jiao, T.; Liu, T.; Wang, J.; Lang, X.; Song, S.; et al. Effects of Oregano Essential Oil on the Ruminal pH and Microbial Population of Sheep. PLoS ONE 2019, 14, e0217054. [Google Scholar] [CrossRef]
- Boskovic, M.; Djordjevic, J.; Glisic, M.; Ciric, J.; Janjic, J.; Zdravkovic, N.; Krnjaic, D.; Baltic, M.Z. The Effect of Oregano (Origanum vulgare) Essential Oil on Four Salmonella Serovars and Shelf Life of Refrigerated Pork Meat Packaged under Vacuum and Modified Atmosphere. J. Food Process. Preserv. 2020, 44, e14311. [Google Scholar] [CrossRef]
- Gonçalves Cattelan, M.; Bonatto Machado De Castilhos, M.; Juliana Pinsetta Sales, P.; Leite Hoffmann, F. Antibacterial Activity of Oregano Essential Oil against Foodborne Pathogens. Nutr. Food Sci. 2013, 43, 169–174. [Google Scholar] [CrossRef]
- Ignatova-Ivanova, T.; Yotova, I.; Ivanov, R. In Vitro Study of Biological Activity Essential Oil of Origanum vulgare L. Subsp. Vulgare L. J. Chem. Pharm. Res. 2016, 8, 958–962. [Google Scholar]
- Pesavento, G.; Calonico, C.; Bilia, A.R.; Barnabei, M.; Calesini, F.; Addona, R.; Mencarelli, L.; Carmagnini, L.; Di Martino, M.C.; Lo Nostro, A. Antibacterial Activity of Oregano, Rosmarinus and Thymus Essential Oils against Staphylococcus aureus and Listeria monocytogenes in Beef Meatballs. Food Control 2015, 54, 188–199. [Google Scholar] [CrossRef]
- Veenstra, J.P.; Johnson, J.J. Oregano (Origanium vulgare) Extract for Food Preservation and Improving Gastrointestinal Health. Int. J. Nutr. 2019, 3, 43–52. [Google Scholar] [CrossRef]
- Pontes-Quero, G.M.; Esteban-Rubio, S.; Pérez Cano, J.; Aguilar, M.R.; Vázquez-Lasa, B. Oregano Essential Oil Micro- and Nanoencapsulation with Bioactive Properties for Biotechnological and Biomedical Applications. Front. Bioeng. Biotechnol. 2021, 9, 703684. [Google Scholar] [CrossRef]
- Mediouni, S.; Jablonski, J.A.; Tsuda, S.; Barsamian, A.; Kessing, C.; Richard, A.; Biswas, A.; Toledo, F.; Andrade, V.M.; Even, Y.; et al. Oregano Oil and Its Principal Component, Carvacrol, Inhibit HIV-1 Fusion into Target Cells. J. Virol. 2020, 94. [Google Scholar] [CrossRef]
- Deng, H.; Deng, Y.; Song, T.; Pang, L.; Zhu, S.; Ren, Z.; Guo, H.; Xu, Z.; Zhu, L.; Geng, Y.; et al. Evaluation of the Activity and Mechanisms of Oregano Essential Oil against PRV in Vivo and in Vitro. Microb. Pathog. 2024, 194, 106791. [Google Scholar] [CrossRef]
- Gilling, D.H.; Kitajima, M.; Torrey, J.R.; Bright, K.R. Antiviral Efficacy and Mechanisms of Action of Oregano Essential Oil and Its Primary Component Carvacrol against Murine Norovirus. J. Appl. Microbiol. 2014, 116, 1149–1163. [Google Scholar] [CrossRef] [PubMed]
- Koch, C.; Reichling, J.; Schneele, J.; Schnitzler, P. Inhibitory Effect of Essential Oils against Herpes Simplex Virus Type 2. Phytomedicine 2008, 15, 71–78. [Google Scholar] [CrossRef]
- Ma, L.; Yao, L. Antiviral Effects of Plant-Derived Essential Oils and Their Components: An Updated Review. Molecules 2020, 25, 2627. [Google Scholar] [CrossRef] [PubMed]
- Pilau, M.R.; Alves, S.H.; Weiblen, R.; Arenhart, S.; Cueto, A.P.; Lovato, L.T. Antiviral Activity of the Lippia Graveolens (Mexican oregano) Essential Oil and Its Main Compound Carvacrol against Human and Animal Viruses. Braz. J. Microbiol. Publ. Braz. Soc. Microbiol. 2011, 42, 1616–1624. [Google Scholar] [CrossRef]
- Husain, I.; Ahmad, R.; Siddiqui, S.; Chandra, A.; Misra, A.; Srivastava, A.; Ahamad, T.; Khan, M.F.; Siddiqi, Z.; Trivedi, A.; et al. Structural Interactions of Phytoconstituent(s) from Cinnamon, Bay Leaf, Oregano, and Parsley with SARS-CoV-2 Nucleocapsid Protein: A Comparative Assessment for Development of Potential Antiviral Nutraceuticals. J. Food Biochem. 2022, 46, e14262. [Google Scholar] [CrossRef]
- Katsoulos, P.D.; Karatzia, M.A.; Dovas, C.I.; Filioussis, G.; Papadopoulos, E.; Kiossis, E.; Arsenopoulos, K.; Papadopoulos, T.; Boscos, C.; Karatzias, H. Evaluation of the In-Field Efficacy of Oregano Essential Oil Administration on the Control of Neonatal Diarrhea Syndrome in Calves. Res. Vet. Sci. 2017, 115, 478–483. [Google Scholar] [CrossRef]
- Preuss, H.G.; Aruoma, O.I. Suggestions for Combatting COVID-19 by Natural Means in the Absence of Standard Medical Regimens. J. Am. Coll. Nutr. 2020, 40, 95. [Google Scholar] [CrossRef]
- Debnath, S.; Debnath, B.; Debnath, P. Carvacrol: A PLpro Inhibitor of SARS-CoV-2 Is a Natural Weapon for COVID-19. Chem. Proc. 2022, 12, 11. [Google Scholar] [CrossRef]
- Yoncheva, K.; Benbassat, N.; Zaharieva, M.M.; Dimitrova, L.; Kroumov, A.; Spassova, I.; Kovacheva, D.; Najdenski, H.M. Improvement of the Antimicrobial Activity of Oregano Oil by Encapsulation in Chitosan—Alginate Nanoparticles. Molecules 2021, 26, 7017. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Liu, P.; Ye, K.; He, Y.; Chen, S.; Yuan, A.; Chen, F.; Yang, W. Preparation, Characterization, In Vitro Release, and Antibacterial Activity of Oregano Essential Oil Chitosan Nanoparticles. Foods 2022, 11, 3756. [Google Scholar] [CrossRef] [PubMed]
- Vehapi, M.; Yilmaz, A.; Özçimen, D. Fabrication of Oregano-Olive Oil Loaded PVA/Chitosan Nanoparticles via Electrospraying Method. J. Nat. Fibers 2021, 18, 1359–1373. [Google Scholar] [CrossRef]
- Souza, J.R.; Bonfim, K.S.; Lorevice, M.V.; Correa, D.S.; Mattoso, L.H.C.; de Moura, M.R. Antibacterial Properties of Oregano Essential Oil Encapsulated in Poly(ε-Caprolactone) Nanoparticles. Adv. Sci. Eng. Med. 2020, 12, 864–869. [Google Scholar] [CrossRef]
- Yousefi, M.; Mohammadi, V.G.; Shadnoush, M.; Khorshidian, N.; Mortazavian, A.M. Zingiber officinale Essential Oil-Loaded Chitosan-Tripolyphosphate Nanoparticles: Fabrication, Characterization and in-Vitro Antioxidant and Antibacterial Activities. Food Sci. Technol. Int. 2022, 28, 592–602. [Google Scholar] [CrossRef]
- de Oliveira, E.F.; Paula, H.C.B.; de Paula, R.C.M. Alginate/Cashew Gum Nanoparticles for Essential Oil Encapsulation. Colloids Surf. B Biointerfaces 2014, 113, 146–151. [Google Scholar] [CrossRef]
- Mori-Mestanza, D.; Valqui-Rojas, I.; Caetano, A.C.; Culqui-Arce, C.; Cruz-Lacerna, R.; Cayo-Colca, I.S.; Castro-Alayo, E.M.; Balcázar-Zumaeta, C.R. Physicochemical Properties of Nanoencapsulated Essential Oils: Optimizing D-Limonene Preservation. Polymers 2025, 17, 348. [Google Scholar] [CrossRef]
- Suhail, M.; Rosenholm, J.M.; Minhas, M.U.; Badshah, S.F.; Naeem, A.; Khan, K.U.; Fahad, M. Nanogels As Drug-Delivery Systems: A Comprehensive Overview. Ther. Deliv. 2019, 10, 697–717. [Google Scholar] [CrossRef]
- Yin, Y.; Hu, B.; Yuan, X.; Cai, L.; Gao, H.; Yang, Q. Nanogel: A Versatile Nano-Delivery System for Biomedical Applications. Pharmaceutics 2020, 12, 290. [Google Scholar] [CrossRef]
- Souza, E.J.D.D.; Pacheco, C.D.O.; Costa, I.H.D.L.; Dias, A.R.G.; Zavareze, E.D.R. Applications of Nanotechnology in Essential Oil Protection to Extend the Shelf Life of Fruits and Vegetables: A Review. Food Control 2025, 170, 111044. [Google Scholar] [CrossRef]
- Hosseini, S.F.; Zandi, M.; Rezaei, M.; Farahmandghavi, F. Two-Step Method for Encapsulation of Oregano Essential Oil in Chitosan Nanoparticles: Preparation, Characterization and in Vitro Release Study. Carbohydr. Polym. 2013, 95, 50–56. [Google Scholar] [CrossRef]
- Granata, G.; Stracquadanio, S.; Leonardi, M.; Napoli, E.; Malandrino, G.; Cafiso, V.; Stefani, S.; Geraci, C. Oregano and Thyme Essential Oils Encapsulated in Chitosan Nanoparticles as Effective Antimicrobial Agents against Foodborne Pathogens. Molecules 2021, 26, 4055. [Google Scholar] [CrossRef] [PubMed]
- Filippov, S.K.; Khusnutdinov, R.; Murmiliuk, A.; Inam, W.; Zakharova, L.Y.; Zhang, H.; Khutoryanskiy, V.V. Dynamic Light Scattering and Transmission Electron Microscopy in Drug Delivery: A Roadmap for Correct Characterization of Nanoparticles and Interpretation of Results. Mater. Horiz. 2023, 10, 5354–5370. [Google Scholar] [CrossRef]
- Naumenko, K.; Zahorodnia, S.; Pop, C.V.; Rizun, N. Antiviral Activity of Silver Nanoparticles against the Influenza A Virus. J. Virus Erad. 2023, 9, 100330. [Google Scholar] [CrossRef] [PubMed]
- Reichling, J.; Koch, C.; Stahl-Biskup, E.; Sojka, C.; Schnitzler, P. Virucidal Activity of a Beta-Triketone-Rich Essential Oil of Leptospermum scoparium (Manuka Oil) against HSV-1 and HSV-2 in Cell Culture. Planta Med. 2005, 71, 1123–1127. [Google Scholar] [CrossRef] [PubMed]
- Astani, A.; Reichling, J.; Schnitzler, P. Screening for Antiviral Activities of Isolated Compounds from Essential Oils. Evid.-Based Complement. Altern. Med. ECAM 2011, 2011, 253643. [Google Scholar] [CrossRef]
- Wu, S.; Patel, K.B.; Booth, L.J.; Metcalf, J.P.; Lin, H.-K.; Wu, W. Protective Essential Oil Attenuates Influenza Virus Infection: An in Vitro Study in MDCK Cells. BMC Complement. Altern. Med. 2010, 10, 69. [Google Scholar] [CrossRef]
- Garozzo, A.; Timpanaro, R.; Stivala, A.; Bisignano, G.; Castro, A. Activity of Melaleuca alternifolia (Tea Tree) Oil on Influenza Virus A/PR/8: Study on the Mechanism of Action. Antivir. Res. 2011, 89, 83–88. [Google Scholar] [CrossRef]
- Lai, W.-L.; Chuang, H.-S.; Lee, M.-H.; Wei, C.-L.; Lin, C.-F.; Tsai, Y.-C. Inhibition of Herpes Simplex Virus Type 1 by Thymol-Related Monoterpenoids. Planta Med. 2012, 78, 1636–1638. [Google Scholar] [CrossRef]
- Ultee, A.; Bennik, M.H.J.; Moezelaar, R. The Phenolic Hydroxyl Group of Carvacrol Is Essential for Action against the Food-Borne Pathogen Bacillus Cereus. Appl. Environ. Microbiol. 2002, 68, 1561–1568. [Google Scholar] [CrossRef]
- Cox, S.D.; Markham, J.L. Susceptibility and Intrinsic Tolerance of Pseudomonas Aeruginosa to Selected Plant Volatile Compounds. J. Appl. Microbiol. 2007, 103, 930–936. [Google Scholar] [CrossRef] [PubMed]
- García-García, R.; López-Malo, A.; Palou, E. Bactericidal Action of Binary and Ternary Mixtures of Carvacrol, Thymol, and Eugenol against Listeria Innocua. J. Food Sci. 2011, 76, M95–M100. [Google Scholar] [CrossRef] [PubMed]
- ISO 10993-5:2009; Biological Evaluation of Medical Devices—Part 5: Tests for In Vitro Cytotoxicity, in ICS 11.100.20. International Organization for Standardization: Geneva, Switzerland, 2017. Available online: https://www.iso.org/standard/36406.html (accessed on 19 January 2024).
- Zaharieva, M.M.; Foka, P.; Karamichali, E.; Kroumov, A.D.; Philipov, S.; Ilieva, Y.; Kim, T.C.; Podlesniy, P.; Manasiev, Y.; Kussovski, V.; et al. Photodynamic Inactivation of Bovine Coronavirus with the Photosensitizer Toluidine Blue O. Viruses 2023, 16, 48. [Google Scholar] [CrossRef] [PubMed]
- Radeva, L.; Zaharieva, M.M.; Spassova, I.; Kovacheva, D.; Pencheva-El Tibi, I.; Najdenski, H.; Yoncheva, K. Biopolymeric Nanogel as a Drug Delivery System for Doxorubicin—Improved Drug Stability and Enhanced Antineoplastic Activity in Skin Cancer Cells. Pharmaceuticals 2024, 17, 186. [Google Scholar] [CrossRef]
- Partheniadis, I.; Karakasidou, P.; Vergkizi, S.; Nikolakakis, I. Spectroscopic Examination and Release of Microencapsulated Oregano Essential Oil. ADMET DMPK 2017, 5, 224–233. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid Colorimetric Assay for Cellular Growth and Survival: Application to Proliferation and Cytotoxicity Assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Indrayanto, G.; Putra, G.S.; Suhud, F. Validation of in-Vitro Bioassay Methods: Application in Herbal Drug Research. In Profiles of Drug Substances, Excipients and Related Methodology; Al-Majed, A.A., Ed.; Academic Press: Cambridge, MA, USA, 2021; Volume 46, pp. 273–307. [Google Scholar]
- Chou, T.-C.; Martin, N. A Computer Program for Quantitation of Synergism and Antagonism in Drug Combinations, and the Determination of IC50 and ED50 Values; ComboSyn Inc: Paramus, NJ, USA, 2005; p. 68. [Google Scholar]
- Zaharieva, M.; Trochopoulos, A.; Dimitrova, L.; Berger, M.; Najdenski, H.; Konstantinov, S.; Kroumov, A. New Insights in Routine Procedure for Mathematical Evaluation of in Vitro Cytotoxicity Data from Cancer Cell Lines. Int. J. Bioautomation 2018, 22, 87–106. [Google Scholar] [CrossRef]
- Horwood, P.F.; Mahony, T.J. Multiplex Real-Time RT-PCR Detection of Three Viruses Associated with the Bovine Respiratory Disease Complex. J. Virol. Methods 2011, 171, 360–363. [Google Scholar] [CrossRef]
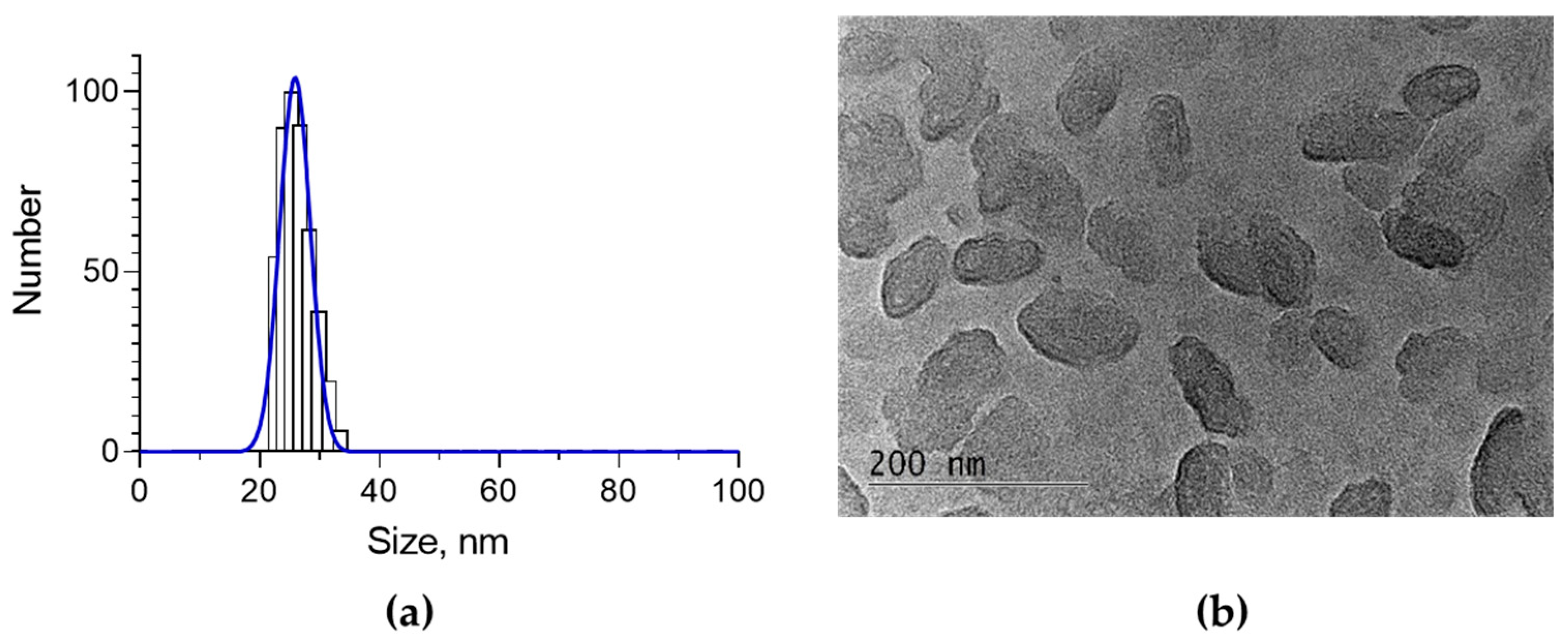

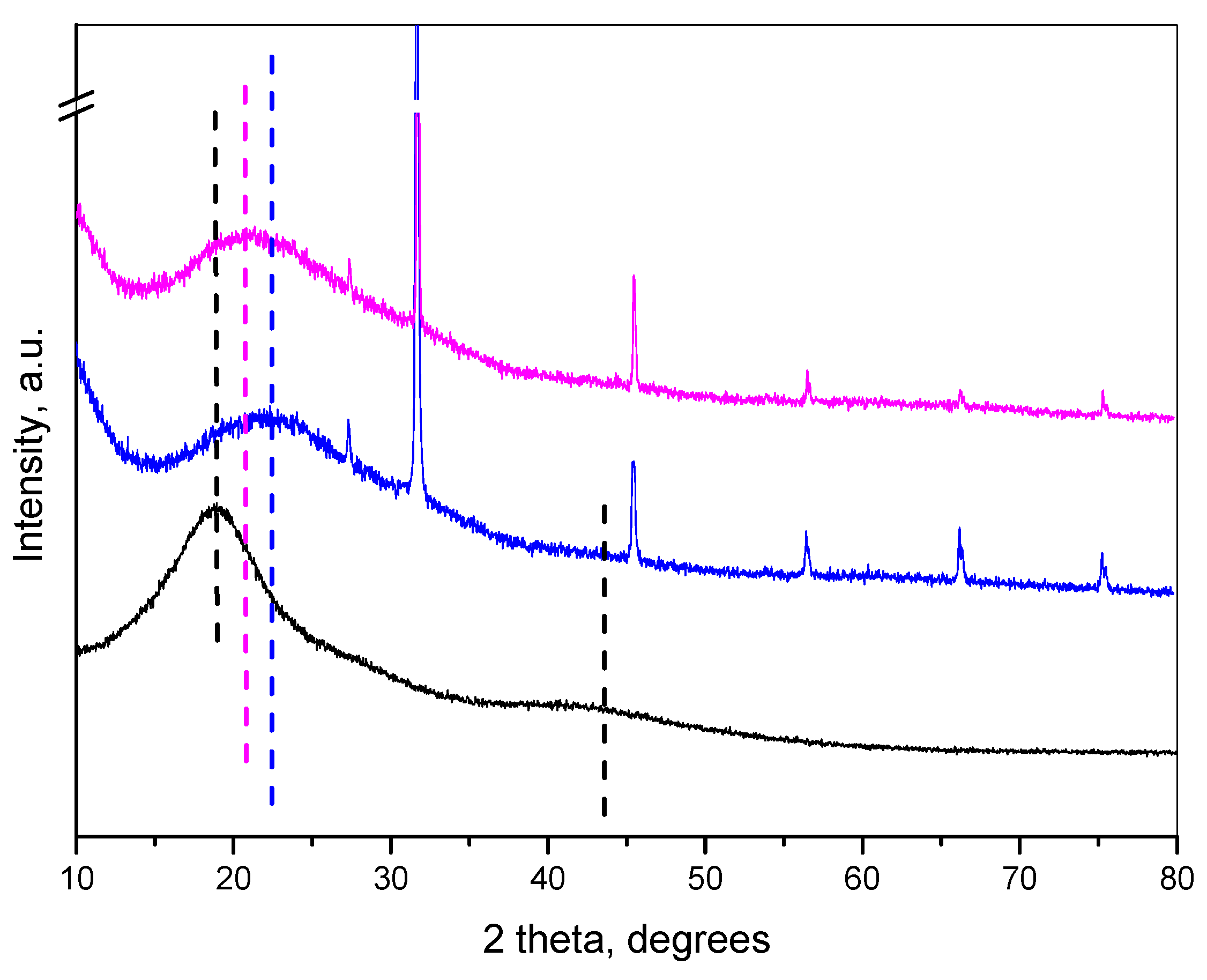
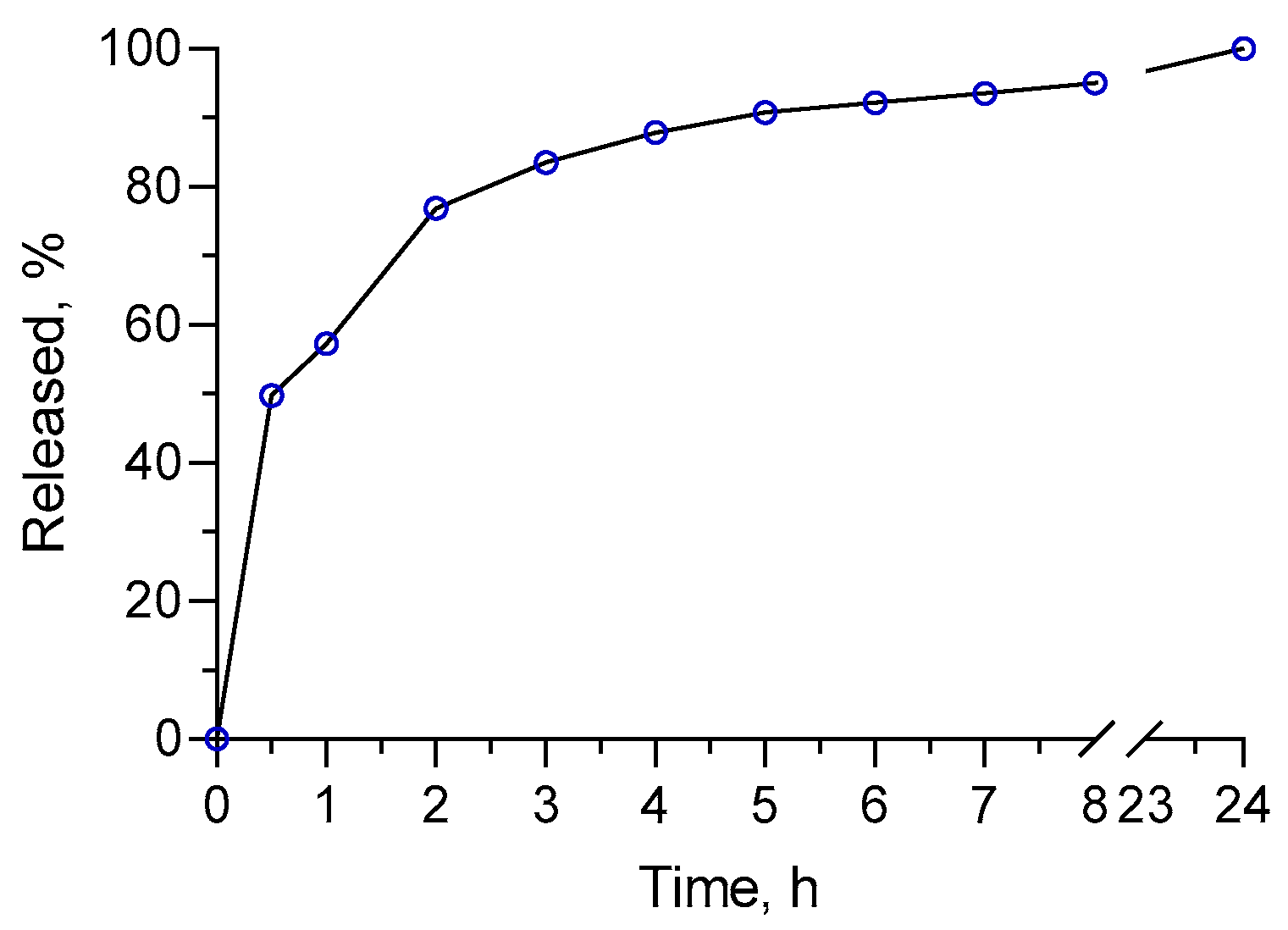


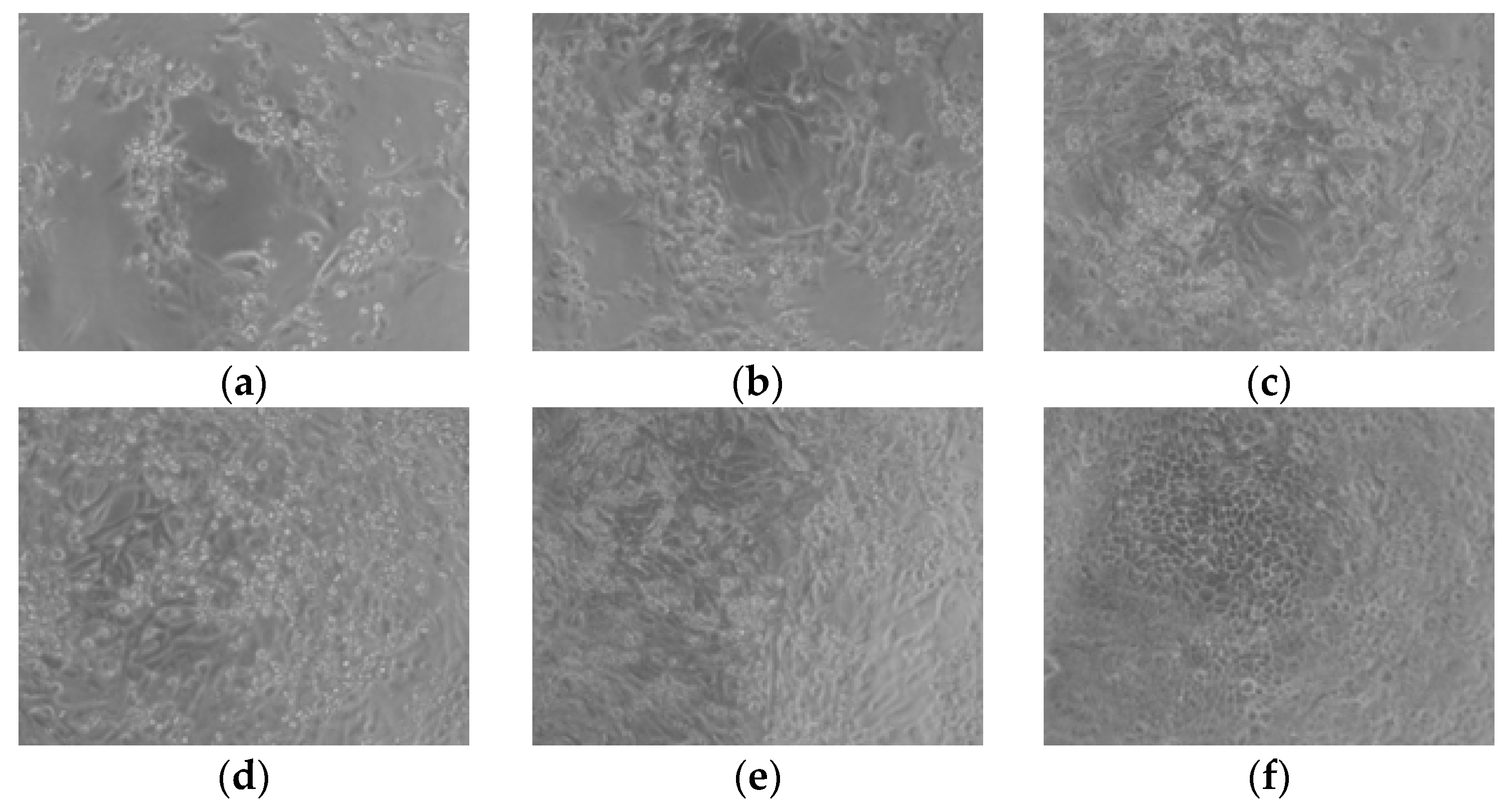

| Sample | Size, nm | PDI | Zeta Potential, mV | EE, % |
|---|---|---|---|---|
| Empty NG | 29.2 ± 5 | 0.241 | 35.5 | - |
| OrO-NG | 25.9 ± 2.6 | 0.242 | 20.6 | 39.2 |
| Parameters | Cytotoxicity | Antiviral Activity |
|---|---|---|
| IC50, 90 h | 38.55 µg/mL | - |
| 100% vital cells | 20 µg/mL | - |
| EC50 (treatment) | - | 17.75 µg/mL |
| R | 0.990 | 0.995 |
| m | 0.48 | 0.68 |
| Selectivity index | 2.17 | |
| Well | Sample | Copies per ddPCR Reaction | Copies per Sample (100 µL) |
|---|---|---|---|
| C01 | Untreated control | 0 | 0.00 |
| D01 | Untreated control | 0 | 0.00 |
| E01 | BCoV dilution 10−1 | 14,180 | 7.85 × 105 |
| F01 | BCoV dilution 10−2 | 1060 | 5.86 × 104 |
| G01 | BCoV dilution 10−3 | 104 | 5.75 × 103 |
| H01 | BCoV dilution 10−4 | 6.2 | 3.43 × 102 |
| A03 | OrO-NG 25 µg/mL dilution 10−1 | 420 | 2.32 × 103 |
| B03 | OrO-NG 25 µg/mL dilution 10−2 | 28 | 1.55 × 102 |
| C03 | OrO-NG 25 µg/mL dilution 10−3 | 0 | 0.00 |
| D03 | OrO-NG 25 µg/mL dilution 10−4 | 0 | 0.00 |
| A05 | NG 25 µg/mL dilution 10−1 | 12,700 | 7.03 × 105 |
| B05 | NG 25 µg/mL dilution 10−2 | 1232 | 6.82 × 104 |
| C05 | NG 25 µg/mL dilution 10−3 | 132 | 7.30 × 103 |
| D05 | NG 25 µg/mL dilution 10−4 | 6 | 3.32 × 102 |
| A06 | PCR water | 0 | 0.00 |
| B06 | PCR water | 0 | 0.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Radeva, L.; Zaharieva, M.M.; Naydenska, S.; Foka, P.; Karamichali, E.; Koufogeorgou, E.I.; Georgopoulou, U.; Philipov, S.; Kroumov, A.; Najdenski, H.; et al. Loading of Oregano Oil in Natural Nanogel and Preliminary Studies on Its Antiviral Activity on Betacoronavirus 1. Molecules 2025, 30, 1939. https://doi.org/10.3390/molecules30091939
Radeva L, Zaharieva MM, Naydenska S, Foka P, Karamichali E, Koufogeorgou EI, Georgopoulou U, Philipov S, Kroumov A, Najdenski H, et al. Loading of Oregano Oil in Natural Nanogel and Preliminary Studies on Its Antiviral Activity on Betacoronavirus 1. Molecules. 2025; 30(9):1939. https://doi.org/10.3390/molecules30091939
Chicago/Turabian StyleRadeva, Lyubomira, Maya M. Zaharieva, Sevda Naydenska, Pelagia Foka, Erini Karamichali, Efthymia Ioanna Koufogeorgou, Urania Georgopoulou, Stanislav Philipov, Alexander Kroumov, Hristo Najdenski, and et al. 2025. "Loading of Oregano Oil in Natural Nanogel and Preliminary Studies on Its Antiviral Activity on Betacoronavirus 1" Molecules 30, no. 9: 1939. https://doi.org/10.3390/molecules30091939
APA StyleRadeva, L., Zaharieva, M. M., Naydenska, S., Foka, P., Karamichali, E., Koufogeorgou, E. I., Georgopoulou, U., Philipov, S., Kroumov, A., Najdenski, H., Spassova, I., Kovacheva, D., & Yoncheva, K. (2025). Loading of Oregano Oil in Natural Nanogel and Preliminary Studies on Its Antiviral Activity on Betacoronavirus 1. Molecules, 30(9), 1939. https://doi.org/10.3390/molecules30091939












