Abstract
The use of elicitors during germination is a strategy to enhance the nutritional quality and biofunctional properties of various legumes, such as chickpeas, which are important sources of proteins and bioactive compounds. The objective of this study was to evaluate the effect of the use of chitosan (CH) and sucrose (SU) during sprouting on protein content, in vitro protein digestibility (IVPD), total phenolic content (TPC), and antioxidant activity (AOX). For this purpose, soaking time, elicitor concentration (CH or SU), and sprouting time were optimized to obtain maximum values for the response variables. The results showed that the optimal conditions for achieving increases in nutritional and biofunctional properties were 1 h of soaking, 0.35% w/v, and 5 days of sprouting for CH, and 2.55 h of soaking, 1% w/v, and 5 days of sprouting for SU. Under these conditions, protein content increased by 7–12%, IVPD by 78–86%, TPC by 379–327%, and AOX by 115% for CH and SU, respectively. Additionally, morphological changes were observed in the cellular structure of chickpea cotyledons, but no changes were detected in the crystalline structure of starch. These results contribute to the understanding of the effect of CH and SU in modifying the nutritional and biofunctional properties of chickpeas.
1. Introduction
Chickpea (Cicer arietinum L.) is the third-most cultivated legume in the world after soybean and bean. It belongs to the Leguminosae family. This legume contains significant amounts of protein (18–25%), carbohydrates (40–50%), lipids (4–6%), dietary fiber (25–27%), and minerals (3–4%) such as phosphorus, zinc, magnesium, calcium, and iron. It also provides essential vitamins, including riboflavin, niacin, thiamin, and folate [1,2,3,4,5]. Furthermore, it contains phenolic compounds such as catechin, chlorogenic acid, coumaric acid, ferulic acid, rutin, kaempferol 3-glucoside, cyanidin 3-glucoside, and quercetin; however, the content of phenolic compounds in chickpeas (1.44 mg GAE/g) is lower compared to in other legumes such as lentils (7.34 mg GAE/g) [6,7]. Additionally, chickpea contains bioactive compounds with antioxidant activity that may help prevent chronic diseases such as cardiovascular diseases, obesity, and cancer [8,9].
For consumption, legumes can be processed using various methods, including extractive, thermal, and biological treatments [10]. Germination is an effective biological method that enhances the nutritional value of seeds by improving protein digestibility and increasing bioactive compounds, such as polyphenols, which act as antioxidants in foods [11,12].
Germination is a complex process involving physiological and biochemical changes, including water absorption, changes in subcellular structure, root and sprout growth, the formation of the enzyme system, and the degradation of or changes in storage substances [12]. The germination conditions of legumes influence a series of morphological, physiological, and biochemical characteristics of plants and the content of phytochemicals, such as phenolic compounds. These compounds play an important role in seed dispersal and plant defense responses [13].
On the other hand, elicitors are substances that are applied in small quantities to activate transcriptional factors in seeds, regulating the expression of genes related to the production of secondary metabolites [14]. Elicitors promote the synthesis of antioxidant compounds due to the activation of the enzyme phenylalanine ammonia-lyase, which is involved in the biosynthesis of phenolic compounds [13].
Among the most used elicitors are molecules such as salicylic acid, chitosan, glutamic acid, hydrogen peroxide, and sucrose, along with treatments such as UV-B radiation, hydric stress, and ultrasound [15,16,17]. However, it is crucial to identify the type of elicitor, its concentration, and the duration of contact to ensure that it does not negatively impact seed germination.
This study evaluated the effect of elicitor concentration, specifically chitosan (CH) and sucrose (SU), and soaking and sprouting times on protein content, in vitro protein digestibility (IVPD), total phenolic content (TPC), and antioxidant activity (AOX) in chickpea. CH concentrations ranged from 0.1% to 0.5% (w/v), while SU concentrations ranged from 1% to 3% (w/v), soaking time ranged from 1 to 3 h, and sprouting time ranged from 1 to 5 days.
2. Results and Discussion
2.1. Protein Content
The protein content of chickpeas sprouted using CH as an elicitor ranged from 18.97 to 23.36 g/100 g (Table 1), while that of chickpeas treated with SU ranged from 18.08 to 23.52 g/100 g (Table 2). According to the ANOVA (Tables S1 and S5), sprouting time (C) was the factor that presented the most significant effect (p ≤ 0.05) on protein content for both elicitors. This effect can be observed in Pareto charts (Figures S1 and S2). On the other hand, the response surface plots (Figure 1a and Figure 2a) show that the protein content increases with increasing germination time. Similar protein content values have been reported in sprouted chickpeas without the use of elicitors (20.95–24.2 g/100 g) [2].

Table 1.
Box–Behnken matrix or analysis of parameters: protein content, in vitro protein digestibility (IVPD), total phenolic content (TPC), and antioxidant activity (AOX) of chitosan-elicited chickpeas.

Table 2.
Box–Behnken matrix or analysis of parameters: protein content, in vitro protein digestibility (IVPD), total phenolic content (TPC), and antioxidant activity (AOX) of sucrose-elicited chickpeas.
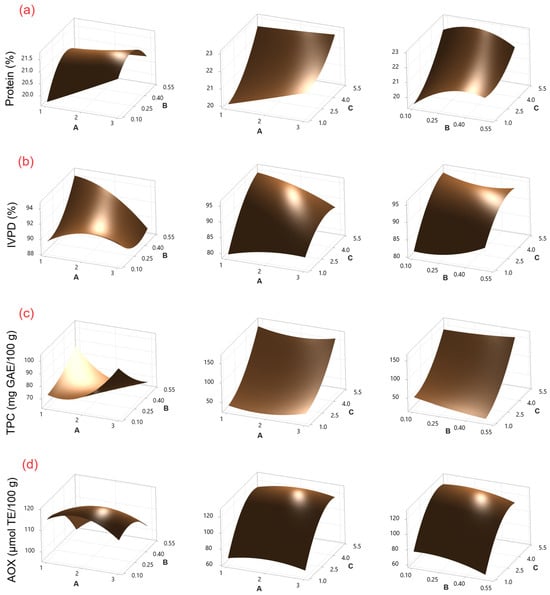
Figure 1.
Surface response plots for (a) protein content, (b) IVPD, (c) TPC, and (d) AOX in chickpea sprouts treated with CH as an elicitor. Experimental factors: A = soaking time (h); B = CH concentration (% w/v); and C = sprouting time (days).
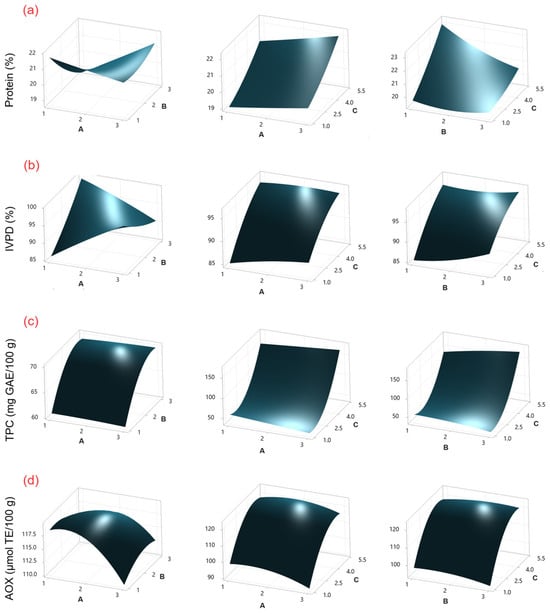
Figure 2.
Surface response plots for (a) protein content, (b) IVPD, (c) TPC, and (d) AOX in chickpea sprouts treated with SU as an elicitor. Experimental factors: A = soaking time (h); B = SU concentration (% w/v); and C = sprouting time (days).
The application of CH and SU as elicitors to chickpeas has not been documented; however, Peñas et al. [18] reported that using chitosan as an elicitor during lentil sprouting had no significant effects compared to sprouting with water after eight days (~27 g/100 g). Similarly, it has been reported that protein content remained similar with and without the use of sucrose as an elicitor in sprouted buckwheat over ten days [19]. Based on these results and statistical analysis, it was observed that at the studied concentrations, CH and SU did not have a significant effect on protein content during seed sprouting. On the other hand, it has been reported that with an increase in sprouting time, protein content increased in chickpeas (~20–23 g/100 g), which may be attributed to protein synthesis from low-molecular-weight peptides [12,20,21].
2.2. In Vitro Protein Digestibility (IVPD)
The use of CH and SU as elicitors during germination has been studied in chickpeas, lentils, and mung beans [13,22,23]. However, changes in protein digestibility during the process were not investigated in the studies. In this study, IVPD values for CH ranged from 78.63% to 96.82% (Table 1), and those for SU ranged from 82.36% to 99.51% (Table 2). According to the ANOVA (Tables S2 and S6), for CH, the factors sprouting time (C), sprouting time–sprouting time (CC), and soaking time–sprouting time (AC) showed a significant effect (p ≤ 0.05), while for SU, the factors sprouting time (C) and soaking time–sucrose concentration (AB) were significant. This effect can be observed in Pareto charts (Figures S1 and S2). On the other hand, response surface plots (Figure 1b and Figure 2b) show that IVPD increases with increasing germination time. The results are similar to those reported by Khattak et al. [24] (~80% IVPD on day 5), and by Khalil et al. [21] (95% IVPD on day 4) for chickpea sprouting.
It has been reported that germination increases protein digestibility [12]. This may be related to protein hydrolysis during germination, which facilitates the release of amino acids from the protein structure, making them more digestible and absorbable in the body. Additionally, a reduction in antinutritional factors, such as protease inhibitors and phytic acid, has been observed, leading to high IVPD values, which are considered indicators of high-quality proteins [4,25,26].
2.3. Total Phenolic Content (TPC)
The TPC concentration for CH ranged from 39.18 to 175.10 mg GAE/100 g (Table 1), while for SU, it ranged from 45.45 to 180.69 mg GAE/100 g (Table 2). According to the ANOVA (Tables S3 and S7), for CH and SU, the factors of sprouting time (C) and the interaction of sprouting time–sprouting time (CC) showed a significant effect (p ≤ 0.05). Additionally, for SU, sucrose concentration–sprouting time (BC) was also significant. This effect can be observed in Pareto charts (Figures S1 and S2). On the other hand, TPC increases with increasing germination time as shown by the response surface plots (Figure 1c and Figure 2c). The resulting values are lower than those reported by Mesfin et al. [27] (272.5 mg GAE/100 g) after three days of chickpea sprouting without the use of elicitors.
Peñas et al. [18] reported that 50 ppm of CH significantly increased TPC in sprouted lentils over eight days (from ~3 to 3.9 mg GAE/g with CH). Similarly, Yu et al. [13] reported that SU concentrations of 2% and 3% increased TPC by up to 28% in sprouted mung beans. Additionally, it has been reported that 3% SU increased the total polyphenol content by 54% in buckwheat [28].
The increase in TPC may be attributed to the activation of endogenous hydrolases, such as phenylalanine ammonia-lyase, a key enzyme in phenol synthesis [29]. Additionally, during germination, the degradation of cell wall components occurs, releasing bound phenolic compounds [30].
With respect to elicitors, CH and SU can influence the germination of legumes by modulating key metabolic pathways, including the phenylpropanoid pathway and the pentose phosphate pathway. The phenylpropanoid pathway is essential for the production of secondary metabolites such as phenolic compounds, which play an important role in the stress response, and in the activation of plant defense mechanisms during germination [14,18]. The elicitors, used alone or in combination, improve germination efficiency and stress resilience in legumes. Further transcriptomic and metabolomic studies could precisely map these interactions.
2.4. Antioxidant Activity (AOX)
AOX values for CH ranged from 64.92 to 120.65 μmol TE/100 g, while for SU, they ranged from 91.08 to 120.1365 μmol TE/100 g. According to the ANOVA (Tables S4 and S8), for CH, the factors sprouting time (C), sprouting time–sprouting time (CC), and chitosan concentration (B) were significant (p ≤ 0.05), while for SU, the factors sprouting time (C), sprouting time–sprouting time (CC), soaking time (A), and soaking time–soaking time (AA) were significant. This effect can be observed in Pareto charts (Figures S1 and S2). On the other hand, the response surface plots (Figure 1d and Figure 2d) show that AOX increases with increasing germination time. The results are similar to those reported by Linares-Castañeda et al. [1] (121.18 μmol TE/100 g) in sprouted chickpeas treated with hydrogen peroxide as an elicitor after five days of sprouting.
Additionally, Mendoza-Sánchez et al. [31] reported that 7 µM CH increased antioxidant capacity in germinated beans by up to 30%. Likewise, Yu et al. [13] demonstrated that SU (1%, 2%, and 3%) increased antioxidant properties by 38% in sprouted mung beans.
Antioxidant activity can be attributed to the increase in phenolic compounds during sprouting and elicitation because elicitors stimulate the pentose phosphate and phenylpropanoid pathways. These compounds can neutralize excess free radicals and maintain intracellular balance in the body, thereby exerting protective effects against oxidative stress [28,32].
2.5. Optimization and Validation of the Sprouting and Elicitation Process
The four response variables were maximized for each elicitor (Figures S3 and S4), obtaining the optimal conditions for sprouting and elicitation (Table 3). The optimal sprouting conditions using CH as an elicitor were 1 h of soaking in 0.35% w/v and 5 days of sprouting (D = 0.92). Meanwhile, for SU as an elicitor, the optimal conditions were 2.55 h of soaking in 1% w/v and 5 days of sprouting (D = 0.89). Subsequently, chickpeas were sprouted under these optimized conditions, and the corresponding flours were obtained to validate the predicted values. The results showed that the experimentally obtained values were similar to those predicted by the model (Table 4). The use of CH increased IVPD by 78%, TPC by 379%, and AOX by 115% compared to raw seeds. Additionally, it increased IVPD by 14%, TPC by 39%, and AOX by 1% compared to CCH. On the other hand, the use of SU increased protein content by 12%, IVPD by 86%, TPC by 327%, and AOX by 115% compared to raw seeds. Additionally, it increased protein content by 9%, IVPD by 8%, TPC by 9%, and AOX by 1% compared to CSU.

Table 3.
Optimization of experimental designs.

Table 4.
Experimental validation of sprouted and elicited samples with chitosan and sucrose.
2.5.1. Proximate Chemical Analysis
During germination and elicitation, macronutrients such as proteins, lipids, and carbohydrates serve as essential energy sources for seed growth, resulting in changes in chemical composition [33,34]. According to the results in Table 5, lipid content ranged from 6.69 to 8.27 g/100 g. Similar values (6–8 g/100 g) have been reported by Khalil et al. [21] in sprouted chickpeas. Additionally, lipid content significantly increased (p ≤ 0.05) by 24% with CH and 15% with SU compared to raw seeds. With the addition of the elicitor, an increase of 12% with CH and 2% with SU was observed compared to the control treatments (CCH and CSU), respectively. In seeds, storage lipids are metabolized to provide the necessary energy for germination, which can lead to a decrease in lipid content. However, an increase can be attributed to the synthesis of structural lipids (such as phospholipids) for the formation of new membranes [11,35].

Table 5.
Proximate chemical analysis of raw, sprouted and elicited samples with CH and SU (g/100 g DM).
For crude fiber (Table 5), values ranged from 1.16 to 2.11 g/100 g. Khalil et al. [21] reported higher crude fiber values (4–6 g/100 g) in sprouted chickpeas. As observed, fiber content decreased by 40% when CH was used as an elicitor and by 6% with SU compared to raw seeds. Meanwhile, in the control treatments, fiber content increased by 45% with CH and 3% with SU. A 55% reduction in fiber content has been reported during sprouting in pigeon pea, indicating that the effects of sprouting and elicitor use depend on the type of legume studied [36,37]. Additionally, the reduction in crude fiber may be attributed to enzyme activity (such as cellulases, hemicellulases, and pectinases), which degrades the cell wall [38,39].
Ash content ranged from 2.74 to 3.16 g/100 g. Similar ash values (2–3 g/100 g) have been reported in sprouted chickpeas [21]. In this study, an increase of up to 10% in ash content was observed when SU and CSU were used, compared to raw seeds and the other two treatments. SU and CSU involved a longer soaking time (2.55 h) compared to CH and CCH (1 h). This increase may be attributed to increased phytase activity, releasing minerals bound to protein-based compounds [11].
NFE content ranged from 64.32 to 67.93 g/100 g. Khalil et al. [21] reported similar values (~60 g/100 g) in sprouted chickpeas. As observed in Table 5, the NFE content decreased by 3% in the presence of CH and by 10% when SU was used compared to raw seeds. Additionally, NFE content decreased by 3% with SU compared to CSU. These changes may be due to carbohydrate hydrolysis, as chickpeas are primarily composed of starch, which is hydrolyzed by enzymes (α-amylase, β-amylase, and maltase) into oligosaccharides, disaccharides, and monosaccharides for various biochemical activities during germination [28,37].
2.5.2. Confocal Laser Scanning Microscopy (CLSM)
In addition to the nutritional and bioactive compound changes occurring during seed germination, structural changes also occur in cotyledon cells [11]. Through CLSM, cell walls, intercellular spaces, lipid bodies, carbohydrates, and structural proteins were identified in the analyzed samples. Generally, differences were observed in cotyledon cells between raw samples and samples treated with CH, CCH, SU, and CSU (Figure 3). It has been reported that germination induces changes in cell wall structure and the cytoplasmic matrix [40].
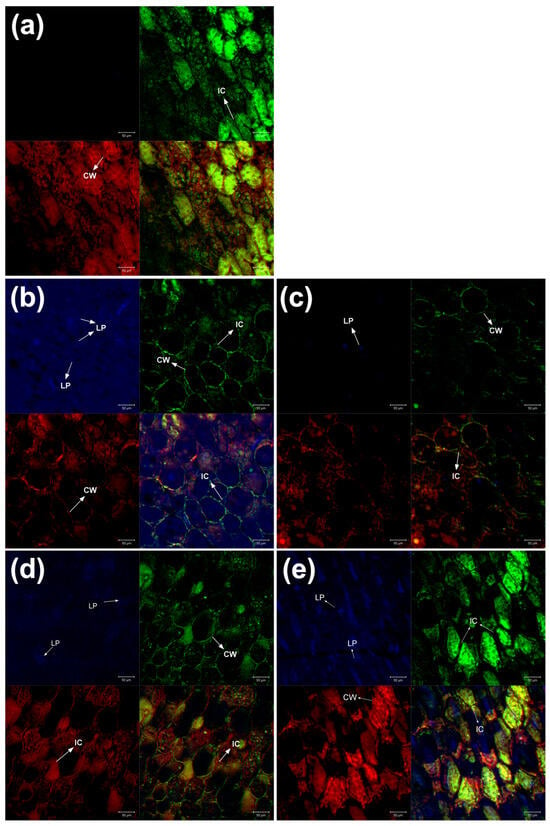
Figure 3.
CLSM images (20X) of chickpea cotyledons. Fluorescent markers: Rhodamine (red), FITC (green), and Auramine O (blue). (a) Raw, (b) CH, (c) CCH, (d) SU, and (e) CSU. Abbreviations: CW: cell wall; IC: intercellular space; LP: lipid body.
The plant cell wall consists of several polysaccharides (cellulose, hemicelluloses, and pectins), as well as structural proteins and phenolic compounds [41]. The cotyledon cell sizes in the samples were approximately 50 μm, and similar cell sizes have been reported in bean cotyledons [42].
In the raw sample (Figure 3a), slightly compact and ellipsoid-shaped cell structures were observed. Chickpeas have been reported to exhibit this type of ellipsoidal cell [43]. In contrast, in the CH sample (Figure 3b), irregularly shaped lipid bodies were observed, as well as semi-spherical cells and an increase in intercellular spaces. In the CCH sample (Figure 3c), very few lipid bodies and semi-spherical cells were identified. In the SU sample (Figure 3d), hexagon-shaped cell structures were observed, and no lipid bodies were detected in the analyzed region. Regarding CSU (Figure 3e), the cells showed an ellipsoidal shape with an increase in intercellular spaces.
Structural changes in cotyledon cells may be due to the enzymatic degradation of the cell wall induced by germination [44]. It has been reported that germination increases intercellular spaces [11] and results in loosely packed cells [45]. Additionally, the low presence of lipids may be due to the naturally low lipid content in legume cotyledons [46].
Changes in cellular structures can impact the bioavailability of certain nutrients. An increase in intercellular spaces and alterations to the cell wall can enhance the release and absorption of these nutrients. Additionally, the concentration of some bioactive compounds, like phenolic compounds, may increase as they are released from the matrices in which they are typically found [47].
2.5.3. X-Ray Diffraction (XRD)
Figure 4 shows the XRD pattern of the raw sample and the sprouted and elicited samples. The intensity of sample peaks decreased after germination, which has also been reported by Kaur and Prasad [48] in sprouted chickpeas. The analysis showed a type C pattern (a combination of types A and B), which is characteristic of legume starches [48]. Additionally, no changes were observed in peak positions, which were around 15°, 17°, 18°, 20°, 23°, 31°, and 33°. Similar patterns have been reported in chickpeas [49] and sprouted peas [50].
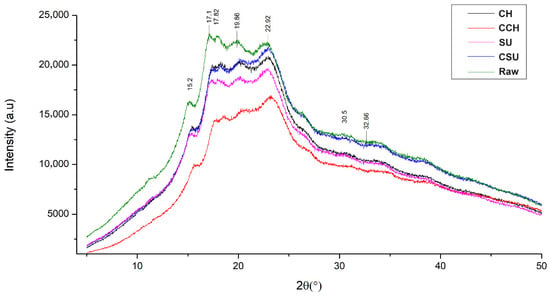
Figure 4.
XRD of raw and sprouted and elicited samples. Abbreviations: CH = chitosan; CCH = control chitosan; SU = sucrose; CSU = control sucrose.
XRD patterns serve as an analytical technique to evaluate the crystalline and amorphous nature of compounds present in flour samples [50]. Sharp peaks represent crystalline regions, while diffuse peaks represent the amorphous region [4].
Furthermore, relative crystallinity in the raw sample was 64.78% and decreased to 50.48% in CH. However, in CCH, SU, and CSU, relative crystallinity increased to 65.56%, 68.23%, and 66.24%, respectively. These different degrees of crystallinity indicate differences in the chemical structure and composition of starches. During germination, starch granule hydrolysis occurs in a non-uniform manner, with amorphous regions being hydrolyzed first, followed by crystalline regions. As a result, crystallinity index values depend on the specific region being hydrolyzed, which may lead to either an increase or decrease in starch granule crystallinity [51].
For example, a decrease in the crystallinity index has been reported in sprouted pea [50] and chickpea flours, attributed to the reduction in crystalline regions due to starch granule hydrolysis by amylases produced during germination [4]. The breakdown of protein structures also reduces the α-helix structure, leading to an increase in a disordered structure [45]. However, Kaur and Prasad [48] reported an increase in crystallinity in mung beans during 12 h of sprouting.
3. Materials and Methods
3.1. Biological Material and Reagents
Chickpea seeds were purchased from the local market, originating from the state of Sinaloa, Mexico. The seeds were selected by removing foreign materials and damaged seeds. Likewise, 100 g of chickpea was used for each experimental treatment.
Chitosan with a medium molecular weight (190,000–310,000 Da) and a degree of deacetylation between 75 and 85%, sucrose, Folin–Ciocalteu reagent, 2,2-diphenyl-1-picrylhydrazyl (DPPH), 6-hydroxy-2,3,7,8-tetramethylchroman-2-carboxylic acid (TROLOX), gallic acid, Bradford reagent, pepsin (E.C. 3.4.23.1, PP-77163, 800–2500 units/mg protein, from pig stomach), and pancreatin (P-1750, 4X USP, from pig pancreas) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Methanol was obtained from Reasol (Mexico City, Mexico). All other chemicals were of analytical grade and sourced from JT Baker (Phillipsburg, NJ, USA).
3.2. Experimental Design
To optimize the sprouting and elicitation process, Box–Behnken designs with four central points were used. The evaluated experimental factors were soaking time (A), elicitor concentration (B), and sprouting time (C), as described in Table 1 and Table 2. The response variables were protein content, IVPD, TPC, and AOX.
Sprouting and Elicitation of Chickpea
The selected seeds were disinfected with 0.5% v/v sodium hypochlorite (NaClO), and then rinsed with purified water to remove residual NaClO. The elicitation treatment was applied to the seeds during imbibition, following the specified soaking time (1–3 h) and concentration of each elicitor (0.1–0.5% w/v for CH and 1–3% w/v for SU), with agitation every 15 min. Germination was carried out at 28 °C and 70% relative humidity until the sprouting time (1–5 days) was completed for each treatment. Finally, the samples were dried at 50 °C for 24 h and then milled and sieved through a 60-mesh sieve (250 μm) to obtain the flour.
In Vitro Protein Digestibility (IVPD)
The methodology established by Linares-Castañeda et al. [1] was used. The IVPD on a dry matter basis was estimated as follows:
Extraction and Determination of Total Phenolic Content (TPC)
Extracts were prepared in aqueous methanol (80% v/v) containing HCl (pH 2.8). The extraction of total phenolics was carried out for 16 h in darkness (4 °C), followed by centrifugation at 4000 g for 20 min, and the supernatant was collected. Total phenolic content was estimated using the Folin–Ciocalteau method, using gallic acid as the standard, and absorbance was measured at 765 nm [52]. Results are expressed as mg gallic acid equivalent/100 g dry matter (mg GAE/100 g DM).
Antioxidant Activity (AOX)
The analysis was performed according to the method described by Brand-Williams et al. [53] adapted to a multiwell plate. The AOX of the extract was evaluated using the stable radical DPPH in methanolic solution and Trolox for the standard curve. After 30 min of incubation in the dark, absorbance was measured at 517 nm. Results are expressed in μmol Trolox equivalents per 100 g of dry matter (μmol TE/100 g DM).
3.3. Optimization and Validation
Based on the results from the Box–Behnken designs, the sprouting process was optimized for elicitation with chitosan and sucrose; all four study variables were maximized. The predicted optimal conditions were experimentally validated for CH and SU. Additionally, a germination control was established for each elicitor using purified water under the same soaking and germination conditions, that is, 1 h of soaking in purified water and 5 days of germination for the chitosan control (CCH) and 2.55 h of soaking in purified water and 5 days of germination for the sucrose control (CSU), while the raw seed was also analyzed (Table 3).
3.3.1. Proximate Composition
The moisture, crude protein, lipids, crude fiber, and ash content of chickpea flour samples were determined using the standard Association of Official Analytical Chemists (AOAC) method [54]. The nitrogen-free extract (NFE) was measured using the difference method. All the results are reported as g/100 g of dry matter (g/100 g DM).
3.3.2. Confocal Laser Scanning Microscopy (CLSM)
The microstructure of chickpea cotyledons was investigated using CLSM according to the methodology described by Linares-Castañeda et al. [1].
3.3.3. X-Ray Diffraction (XRD)
The samples were analyzed using a Rigaku MiniFlex diffractometer (Rigaku Holdings Corporation, Tokyo, Japan) to obtain X-ray diffraction patterns with Cu Kα radiation (λ = 1.54056 Å). The results of the XRD analysis were the 2θ diffraction angles, intensity, and d-spacing. The relative percentage of crystallinity of the samples was determined from the area between the crystalline and amorphous regions in the XRD patterns using OriginPro 8.1 software (OriginLab, Northampton, MA, USA).
3.4. Statistical Analysis
Analysis of variance (ANOVA) was conducted (p ≤ 0.05), and Tukey’s test was used to determine significant differences at p ≤ 0.05. Minitab software version 21.2 (Minitab Inc., State College, PA, USA) was used.
4. Conclusions
The findings of this study demonstrate that CH and SU are effective elicitors in enhancing the nutritional and functional properties of chickpea sprouts.
The results demonstrated that both elicitors, under optimal conditions, significantly increased IVPD, TPC, and AOX compared to raw seeds. The protein content was found to be significantly increased only when SU was used as an elicitor. The optimal conditions for CH were 1 h of soaking, a concentration of 0.35% w/v, and 5 days of sprouting, while for SU, the optimal conditions were 2.55 h of soaking, a concentration of 1% w/v, and 5 days of sprouting. These conditions allowed for the maximum values of protein (22.10–23.00%), IVPD (92.30–96.58%), TPC (163.15–145.44 mg GAE/100 g), and AOX (120.32–120.62 μmol TE/100 g). Additionally, the use of elicitors can modify the cellular morphology of chickpea cotyledons and the relative crystallinity of the starch.
CH and SU treatments provide a cost-effective and scalable method for producing nutrient-rich chickpea sprouts for functional foods, dietary supplements, or plant protein ingredients. The increase in phenolic compounds and improved protein digestibility may help manage chronic diseases by enhancing antioxidant activity and metabolic health.
This work bridges agricultural innovation and nutritional science, providing actionable strategies to improve legume-based foods in the face of global health challenges. Using elicitors like CH and SU aligns with sustainable food production goals, leveraging natural compounds to maximize crop value without genetic modification.
This study advances the understanding of legume biofortification by optimizing germination conditions with chitosan and sucrose. It delivers a practical, eco-friendly solution to enhance the dietary quality of chickpeas—a crop critical for food security and nutrition.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/molecules30081775/s1, Figures S1 and S2: Pareto charts for chitosan and sucrose as elicitors during chickpea sprouting. (a): Protein content; (b): in vitro protein digestibility (IVPD); (c): total phenolic content (TPC); and (d): antioxidant activity (AOX). A: Soaking time; B: chitosan concentration; and C: sprouting time. Figures S3 and S4: Optimal conditions for chitosan and sucrose as elicitors in chickpea germination. Tables S1–S8: Analysis of variance (ANOVA) for chitosan and sucrose across all studied variables. Tables S9 and S10: Statistical parameters used for the analysis of means by the Tukey test.
Author Contributions
Conceptualization, A.L.-C., L.J.C.-R. and C.J.-M.; methodology, A.L.-C., A.E.C.-O. and L.J.C.-R.; validation, L.J.C.-R., R.M.-E. and X.M.S.-C.; formal analysis, C.J.-M., X.M.S.-C., L.J.C.-R. and R.M.-E.; investigation, A.E.C.-O. and X.M.S.-C.; data curation, X.M.S.-C., L.J.C.-R. and C.J.-M.; writing—original draft preparation, A.L.-C. and L.J.C.-R.; writing—review and editing, L.J.C.-R., C.J.-M., X.M.S.-C., A.E.C.-O. and R.M.-E. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Secretaría de Investigación y Posgrado [SIP 20240612 and 20240631], of the Instituto Politécnico Nacional, México.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data are available in the Supplementary Materials.
Acknowledgments
The authors thank Jonathan Vera Pérez and Dulce Maribel Martínez Cortés for their generous advice on RXD analysis. Alejandra Linares Castañeda thanks the Instituto Politécnico Nacional and the Secretaría de Ciencia Humanidades Tecnología e innovación (SECIHTI) for the grant provided [964186].
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Linares-Castañeda, A.; Jiménez-Martínez, C.; Cedillo-Olivos, A.E.; Cruz-Narváez, Y.; Corzo-Ríos, L.J. Effects of Hydrogen Peroxide (H2O2) Elicitation on Protein Content and Digestibility, Phenolic Compounds, and Antioxidant Activity in Sprouted Chickpeas (Cicer arietinum L.). Appl. Food Res. 2025, 5, 100785. [Google Scholar] [CrossRef]
- Linares-Castañeda, A.; Jiménez-Martínez, C.; Sánchez-Chino, X.M.; Pérez-Pérez, V.; Cid-Gallegos, M.S.; Corzo-Ríos, L.J. Modifying of Non-Nutritional Compounds in Legumes: Processing Strategies and New Technologies. Food Chem. 2025, 463, 141603. [Google Scholar] [CrossRef]
- Sarita; Mehrotra, S.; Dimkpa, C.O.; Goyal, V. Survival Mechanisms of Chickpea (Cicer arietinum) under Saline Conditions. Plant Physiol. Biochem. 2023, 205, 108168. [Google Scholar] [CrossRef] [PubMed]
- Sofi, S.A.; Rafiq, S.; Singh, J.; Mir, S.A.; Sharma, S.; Bakshi, P.; McClements, D.J.; Mousavi Khaneghah, A.; Dar, B.N. Impact of Germination on Structural, Physicochemical, Techno-Functional, and Digestion Properties of Desi Chickpea (Cicer arietinum L.) Flour. Food Chem. 2023, 405, 135011. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, J.; Zhu, C.; Singh, R.P.; Chen, W. Chickpea: Its Origin, Distribution, Nutrition, Benefits, Breeding, and Symbiotic Relationship with Mesorhizobium Species. Plants 2024, 13, 429. [Google Scholar] [CrossRef] [PubMed]
- Caprioli, G.; Nzekoue, F.K.; Giusti, F.; Vittori, S.; Sagratini, G. Optimization of an Extraction Method for the Simultaneous Quantification of Sixteen Polyphenols in Thirty-One Pulse Samples by Using HPLC-MS/MS Dynamic-MRM Triple Quadrupole. Food Chem. 2018, 266, 490–497. [Google Scholar] [CrossRef]
- Sharma, K.R.; Giri, G. Quantification of Phenolic and Flavonoid Content, Antioxidant Activity, and Proximate Composition of Some Legume Seeds Grown in Nepal. Int. J. Food Sci. 2022, 2022, 4629290. [Google Scholar] [CrossRef]
- Rizvi, N.B.; Aleem, S.; Khan, M.R.; Ashraf, S.; Busquets, R. Quantitative Estimation of Protein in Sprouts of Vigna radiate (Mung Beans), Lens culinaris (Lentils), and Cicer arietinum (Chickpeas) by Kjeldahl and Lowry Methods. Molecules 2022, 27, 814. [Google Scholar] [CrossRef]
- Singh, J.P.; Singh, B.; Kaur, A. Bioactive Compounds of Legume Seeds. In Bioactive Compounds in Underutilized Vegetables and Legumes; Murthy, H.N., Paek, K.Y., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 1–21. [Google Scholar]
- Amoah, I.; Ascione, A.; Muthanna, F.M.S.; Feraco, A.; Camajani, E.; Gorini, S.; Armani, A.; Caprio, M.; Lombardo, M. Sustainable Strategies for Increasing Legume Consumption: Culinary and Educational Approaches. Foods 2023, 12, 2265. [Google Scholar] [CrossRef]
- Atudorei, D.; Stroe, S.-G.; Codină, G.G. Impact of Germination on the Microstructural and Physicochemical Properties of Different Legume Types. Plants 2021, 10, 592. [Google Scholar] [CrossRef]
- Liu, S.; Wang, W.; Lu, H.; Shu, Q.; Zhang, Y.; Chen, Q. New Perspectives on Physiological, Biochemical and Bioactive Components during Germination of Edible Seeds: A Review. Trends Food Sci. Technol. 2022, 123, 187–197. [Google Scholar] [CrossRef]
- Yu, J.; Lee, H.; Heo, H.; Jeong, H.S.; Sung, J.; Lee, J. Sucrose-Induced Abiotic Stress Improves the Phytochemical Profiles and Bioactivities of Mung Bean Sprouts. Food Chem. 2023, 400, 134069. [Google Scholar] [CrossRef] [PubMed]
- Aloo, S.O.; Ofosu, F.K.; Oh, D.-H. Elicitation: A New Perspective into Plant Chemo-Diversity and Functional Property. Crit. Rev. Food Sci. Nutr. 2021, 63, 4522–4540. [Google Scholar] [CrossRef] [PubMed]
- Ampofo, J.O.; Ngadi, M. Ultrasonic Assisted Phenolic Elicitation and Antioxidant Potential of Common Bean (Phaseolus vulgaris) Sprouts. Ultrason. Sonochem. 2020, 64, 104974. [Google Scholar] [CrossRef]
- Doss, A.; Esther, A.; Rajalakshmi, R. Influence of UV-B Treatment on the Accumulation of Free Phenols and Tannins in the Legumes of Abrus precatorius L. and Vigna mungo (L.) Hepper. Phytomed. Plus 2022, 2, 100189. [Google Scholar] [CrossRef]
- Liu, H.K.; Kang, Y.F.; Zhao, X.Y.; Liu, Y.P.; Zhang, X.W.; Zhang, S.J. Effects of Elicitation on Bioactive Compounds and Biological Activities of Sprouts. J. Funct. Foods 2019, 53, 136–145. [Google Scholar] [CrossRef]
- Peñas, E.; Limón, R.I.; Martínez-Villaluenga, C.; Restani, P.; Pihlanto, A.; Frias, J. Impact of Elicitation on Antioxidant and Potential Antihypertensive Properties of Lentil Sprouts. Plant Foods Hum. Nutr. 2015, 70, 401–407. [Google Scholar] [CrossRef]
- Jeong, H.; Sung, J.; Yang, J.; Kim, Y.; Jeong, H.S.; Lee, J. Effect of Sucrose on the Functional Composition and Antioxidant Capacity of Buckwheat (Fagopyrum esculentum M.) Sprouts. J. Funct. Foods 2018, 43, 70–76. [Google Scholar] [CrossRef]
- Chinma, C.E.; Adedeji, O.E.; Etim, I.I.; Aniaka, G.I.; Mathew, E.O.; Ekeh, U.B.; Anumba, N.L. Physicochemical, Nutritional, and Sensory Properties of Chips Produced from Germinated African Yam Bean (Sphenostylis stenocarpa). LWT 2021, 136, 110330. [Google Scholar] [CrossRef]
- Khalil, A.W.; Zeb, A.; Mahmood, F.; Tariq, S.; Khattak, A.B.; Shah, H. Comparison of Sprout Quality Characteristics of Desi and Kabuli Type Chickpea Cultivars (Cicer arietinum L.). LWT-Food Sci. Technol. 2007, 40, 937–945. [Google Scholar] [CrossRef]
- Lyu, C.; Zhang, X.; Huang, L.; Yuan, X.; Xue, C.; Chen, X. Widely Targeted Metabolomics Analysis Characterizes the Phenolic Compounds Profiles in Mung Bean Sprouts under Sucrose Treatment. Food Chem. 2022, 395, 133601. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Ramírez, I.F.; Escobedo-Alvarez, D.E.; Mendoza-Sánchez, M.; Rocha-Guzmán, N.E.; Reynoso-Camacho, R.; Acosta-Gallegos, J.A.; Ramos-Gómez, M. Phytochemical Profile and Composition of Chickpea (Cicer arietinum L.): Varietal Differences and Effect of Germination under Elicited Conditions. Plants 2023, 12, 3093. [Google Scholar] [CrossRef] [PubMed]
- Khattak, A.B.; Zeb, A.; Bibi, N. Impact of Germination Time and Type of Illumination on Carotenoidcontent, Protein Solubility and in Vitro Protein Digestibility of Chickpea (Cicer arietinum L.) Sprouts. Food Chem. 2008, 109, 797–801. [Google Scholar] [CrossRef] [PubMed]
- Di, Y.; Li, X.; Chang, X.; Gu, R.; Duan, X.; Liu, F.; Liu, X.; Wang, Y. Impact of Germination on Structural, Functional Properties and in Vitro Protein Digestibility of Sesame (Sesamum indicum L.) Protein. LWT 2022, 154, 112651. [Google Scholar] [CrossRef]
- Sharma, N.; Sahu, J.K.; Joshi, S.; Khubber, S.; Bansal, V.; Bhardwaj, A.; Bangar, S.P.; Bal, L.M. Modulation of Lentil Antinutritional Properties Using Non-Thermal Mediated Processing Techniques—A Review. J. Food Compos. Anal. 2022, 109, 104498. [Google Scholar] [CrossRef]
- Mesfin, N.; Belay, A.; Amare, E. Effect of Germination, Roasting, and Variety on Physicochemical, Techno-Functional, and Antioxidant Properties of Chickpea (Cicer arietinum L.) Protein Isolate Powder. Heliyon 2021, 7, e08081. [Google Scholar] [CrossRef]
- Sim, U.; Sung, J.; Lee, H.; Heo, H.; Jeong, H.S.; Lee, J. Effect of Calcium Chloride and Sucrose on the Composition of Bioactive Compounds and Antioxidant Activities in Buckwheat Sprouts. Food Chem. 2020, 312, 126075. [Google Scholar] [CrossRef]
- Mao, H.; Yuan, S.; Li, Q.; Zhao, X.; Zhang, X.; Liu, H.; Yu, M.; Wang, M. Influence of Germination on the Bioactivity, Structural, Functional and Volatile Characteristics of Different Chickpea Flours. Food Chem. X 2024, 21, 101195. [Google Scholar] [CrossRef]
- He, L.; Yang, Y.; Ren, L.; Bian, X.; Liu, X.; Chen, F.; Tan, B.; Fu, Y.; Zhang, X.; Zhang, N. Effects of Germination Time on the Structural, Physicochemical and Functional Properties of Brown Rice. Int. J. Food Sci. Technol. 2022, 57, 1902–1910. [Google Scholar] [CrossRef]
- Mendoza-Sánchez, M.; Guevara-González, R.G.; Castaño-Tostado, E.; Mercado-Silva, E.M.; Acosta-Gallegos, J.A.; Rocha-Guzmán, N.E.; Reynoso-Camacho, R. Effect of Chemical Stress on Germination of Cv Dalia Bean (Phaseolus vularis L.) as an Alternative to Increase Antioxidant and Nutraceutical Compounds in Sprouts. Food Chem. 2016, 212, 128–137. [Google Scholar] [CrossRef]
- Randhir, R.; Shetty, K. Elicitation of the Proline-Linked Pentose Phosphate Pathway Metabolites and Antioxidant Enzyme Response by Ascorbic Acid in Dark Germinated Fava Bean Sprouts. J. Food Biochem. 2007, 31, 485–508. [Google Scholar] [CrossRef]
- Lucas-Aguirre, J.C.; Quintero-Castaño, V.D.; Beltrán-Bueno, M.; Rodríguez-García, M.E. Study of the Changes on the Physicochemical Properties of Isolated Lentil Starch during Germination. Int. J. Biol. Macromol. 2024, 267, 131468. [Google Scholar] [CrossRef]
- Gawlik-Dziki, U.; Dziki, D.; Nowak, R.; Świeca, M.; Olech, M.; Pietrzak, W. Influence of Sprouting and Elicitation on Phenolic Acids Profile and Antioxidant Activity of Wheat Seedlings. J. Cereal Sci. 2016, 70, 221–228. [Google Scholar] [CrossRef]
- Francis, H.; Debs, E.; Koubaa, M.; Alrayess, Z.; Maroun, R.G.; Louka, N. Sprouts Use as Functional Foods. Optimization of Germination of Wheat (Triticum aestivum L.), Alfalfa (Medicago sativa L.), and Radish (Raphanus sativus L.) Seeds Based on Their Nutritional Content Evolution. Foods 2022, 11, 1460. [Google Scholar] [CrossRef]
- Anaemene, D.; Fadupin, G. Anti-Nutrient Reduction and Nutrient Retention Capacity of Fermentation, Germination and Combined Germination-Fermentation in Legume Processing. Appl. Food Res. 2022, 2, 100059. [Google Scholar] [CrossRef]
- Kavitha, S.; Parimalavalli, R. Effect of Processing Methods on Proximate Composition of Cereal and Legume Flours. J. Hum. Nutr. Food Sci. 2014, 2, 1051. [Google Scholar]
- Sharma, S.; Sahni, P. Germination Behaviour, Techno-Functional Characteristics, Antinutrients, Antioxidant Activity and Mineral Profile of Lucerne as Influenced by Germination Regimes. J. Food Meas. Charact. 2021, 15, 1796–1809. [Google Scholar] [CrossRef]
- de Souza, T.S.P.; Kawaguti, H.Y. Cellulases, Hemicellulases, and Pectinases: Applications in the Food and Beverage Industry. Food Bioproc. Tech. 2021, 14, 1446–1477. [Google Scholar] [CrossRef]
- Zahir, M.; Fogliano, V.; Capuano, E. Soybean Germination Limits the Role of Cell Wall Integrity in Controlling Protein Physicochemical Changes during Cooking and Improves Protein Digestibility. Food Res. Int. 2021, 143, 110254. [Google Scholar] [CrossRef]
- Hu, X.; Zhang, G.; Hamaker, B.R.; Miao, M. The Contribution of Intact Structure and Food Processing to Functionality of Plant Cell Wall-Derived Dietary Fiber. Food Hydrocoll. 2022, 127, 107511. [Google Scholar] [CrossRef]
- Berg, T.; Singh, J.; Hardacre, A.; Boland, M.J. The Role of Cotyledon Cell Structure during in Vitro Digestion of Starch in Navy Beans. Carbohydr. Polym. 2012, 87, 1678–1688. [Google Scholar] [CrossRef]
- Li, C.; Hu, Y.; Zhang, B. Plant Cellular Architecture and Chemical Composition as Important Regulator of Starch Functionality in Whole Foods. Food Hydrocoll. 2021, 117, 106744. [Google Scholar] [CrossRef]
- Ge, X.; Saleh, A.S.M.; Jing, L.; Zhao, K.; Su, C.; Zhang, B.; Zhang, Q.; Li, W. Germination and Drying Induced Changes in the Composition and Content of Phenolic Compounds in Naked Barley. J. Food Compos. Anal. 2021, 95, 103594. [Google Scholar] [CrossRef]
- Mitharwal, S.; Chauhan, K. Impact of Germination on the Proximate Composition, Functional Properties, and Structural Characteristics of Black Soybean (Glycine max L. Merr). J. Food Process Preserv. 2022, 46, e17202. [Google Scholar] [CrossRef]
- Wang, J.; Liu, C.; Zheng, X.; Hong, J.; Sun, B.; Liu, M. The Structural Integrity of Endosperm/Cotyledon Cells and Cell Modification Affect Starch Digestion Properties. Food Funct. 2023, 14, 6784–6801. [Google Scholar] [CrossRef] [PubMed]
- Di Stefano, E.; Tsopmo, A.; Oliviero, T.; Fogliano, V.; Udenigwe, C.C. Bioprocessing of Common Pulses Changed Seed Microstructures, and Improved Dipeptidyl Peptidase-IV and α-Glucosidase Inhibitory Activities. Sci. Rep. 2019, 9, 15308. [Google Scholar] [CrossRef]
- Kaur, R.; Prasad, K. Elucidation of Chickpea Hydration, Effect of Soaking Temperature, and Extent of Germination on Characteristics of Malted Flour. J. Food Sci. 2022, 87, 2197–2210. [Google Scholar] [CrossRef]
- Kaur, R.; Prasad, K. Elucidation of Temperature Dependent Hydration Behaviour of Chickpea Seeds: Prerequisite for Germination. Biocatal. Agric. Biotechnol. 2023, 50, 102669. [Google Scholar] [CrossRef]
- Lakshmipathy, K.; Buvaneswaran, M.; Rawson, A.; Chidanand, D.V. Effect of Dehulling and Germination on the Functional Properties of Grass Pea (Lathyrus sativus) Flour. Food Chem. 2024, 449, 139265. [Google Scholar] [CrossRef]
- Liu, Y.; Su, C.; Saleh, A.S.M.; Wu, H.; Zhao, K.; Zhang, G.; Jiang, H.; Yan, W.; Li, W. Effect of Germination Duration on Structural and Physicochemical Properties of Mung Bean Starch. Int. J. Biol. Macromol. 2020, 154, 706–713. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of Total Phenols and Other Oxidation Substrates and Antioxidants by Means of Folin-Ciocalteu Reagent. In Methods in Enzymology, Oxidants and Antioxidants Part A; Academic Press: Cambridge, MA, USA, 1999; Volume 299, pp. 152–178. [Google Scholar]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- AOAC. AOAC Official Methods of Analysis, 16th ed.; Asociation of Official Analytical Chemist International: Gaitherstourg, MD, USA, 2002. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).