New Insights into the Metabolic Profile and Cytotoxic Activity of Kigelia africana Stem Bark
Abstract
1. Introduction
2. Results and Discussion
2.1. UHPLC-HRMS Profiling of Secondary Metabolites in K. africana Stem Bark Extract
2.1.1. Phenolic Acids and Flavonoids
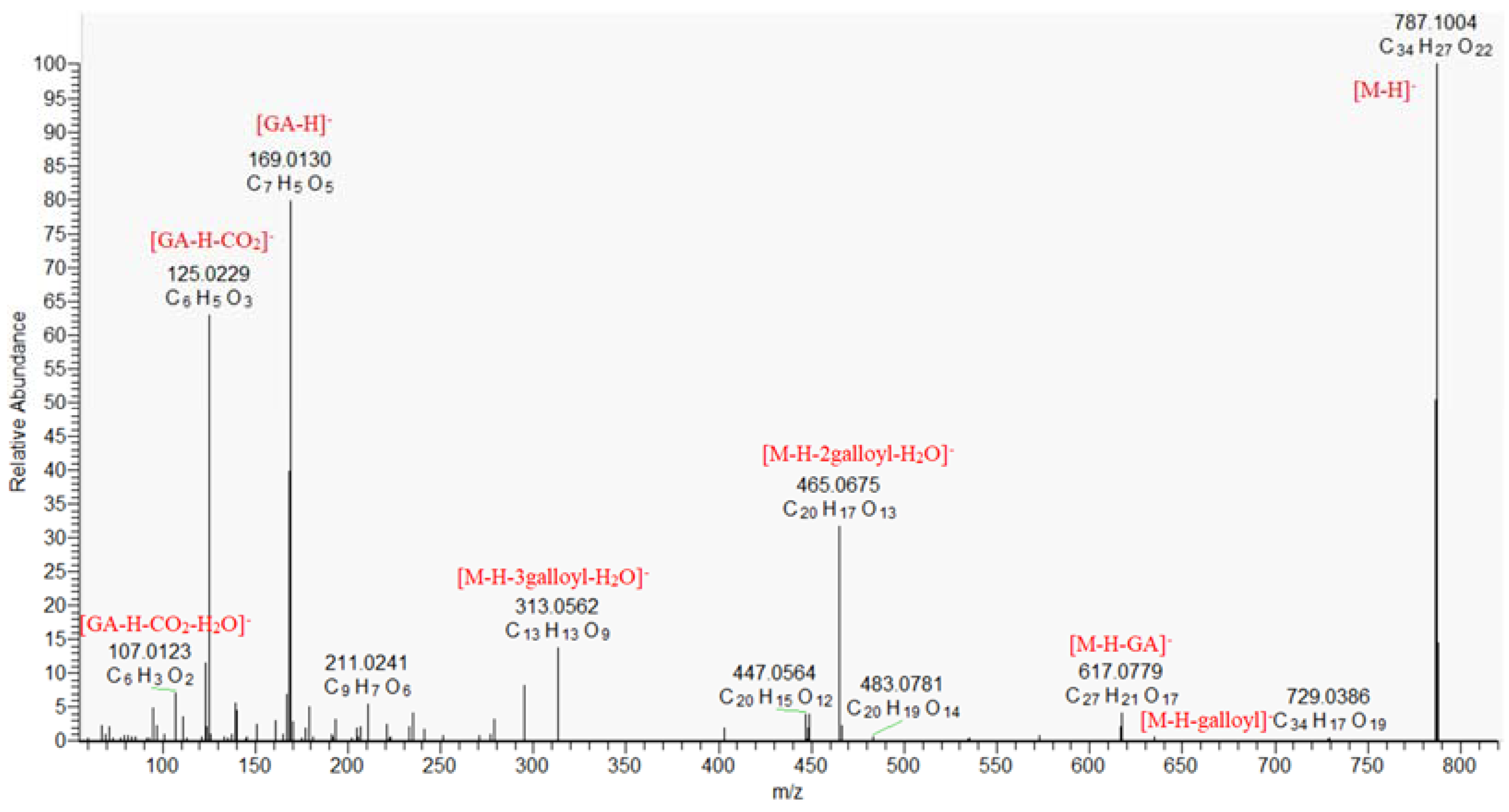
2.1.2. Gallotannins
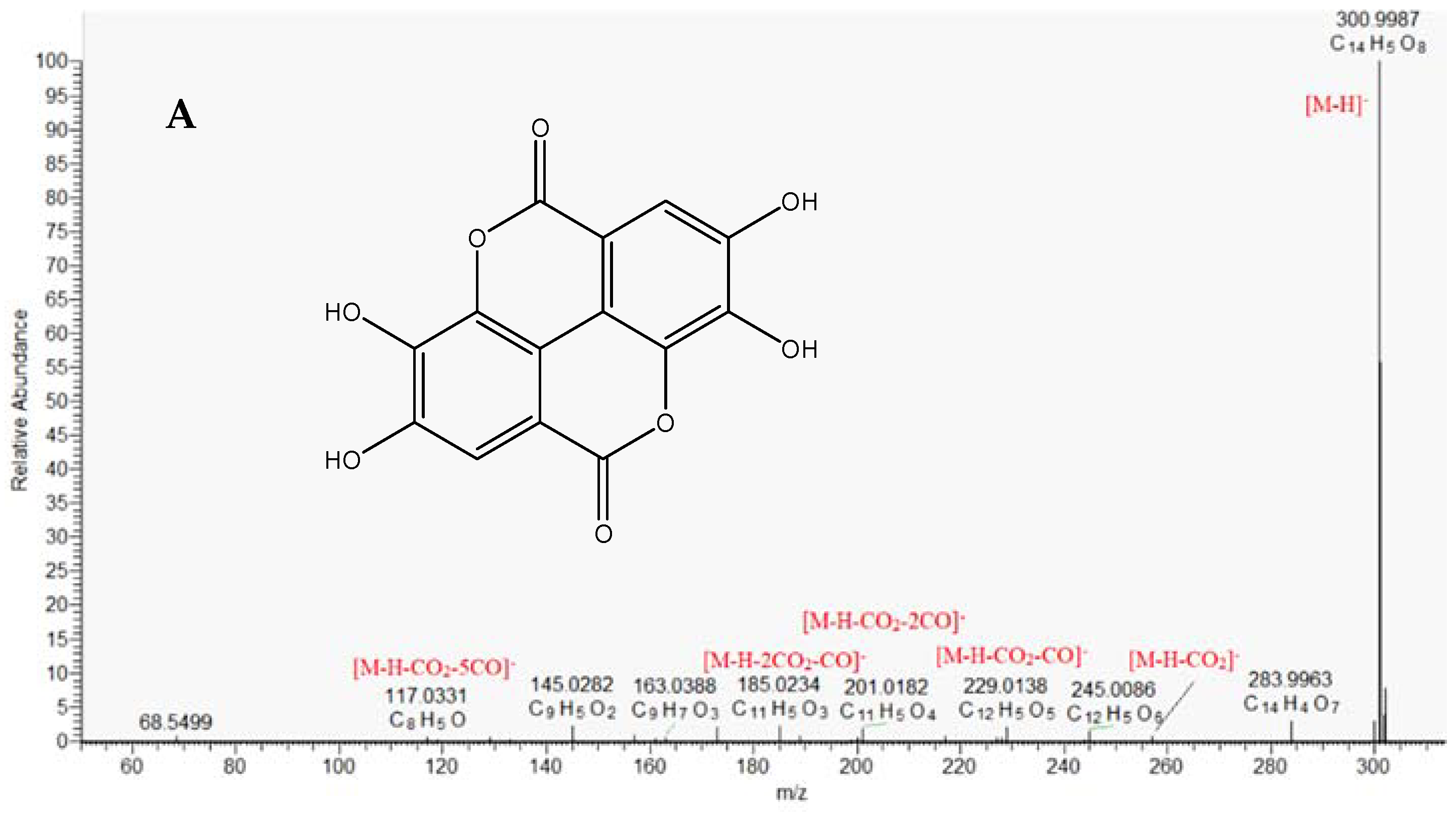
2.1.3. Ellagitannins
2.1.4. Phenylethanoid Glycosides and Iridoids
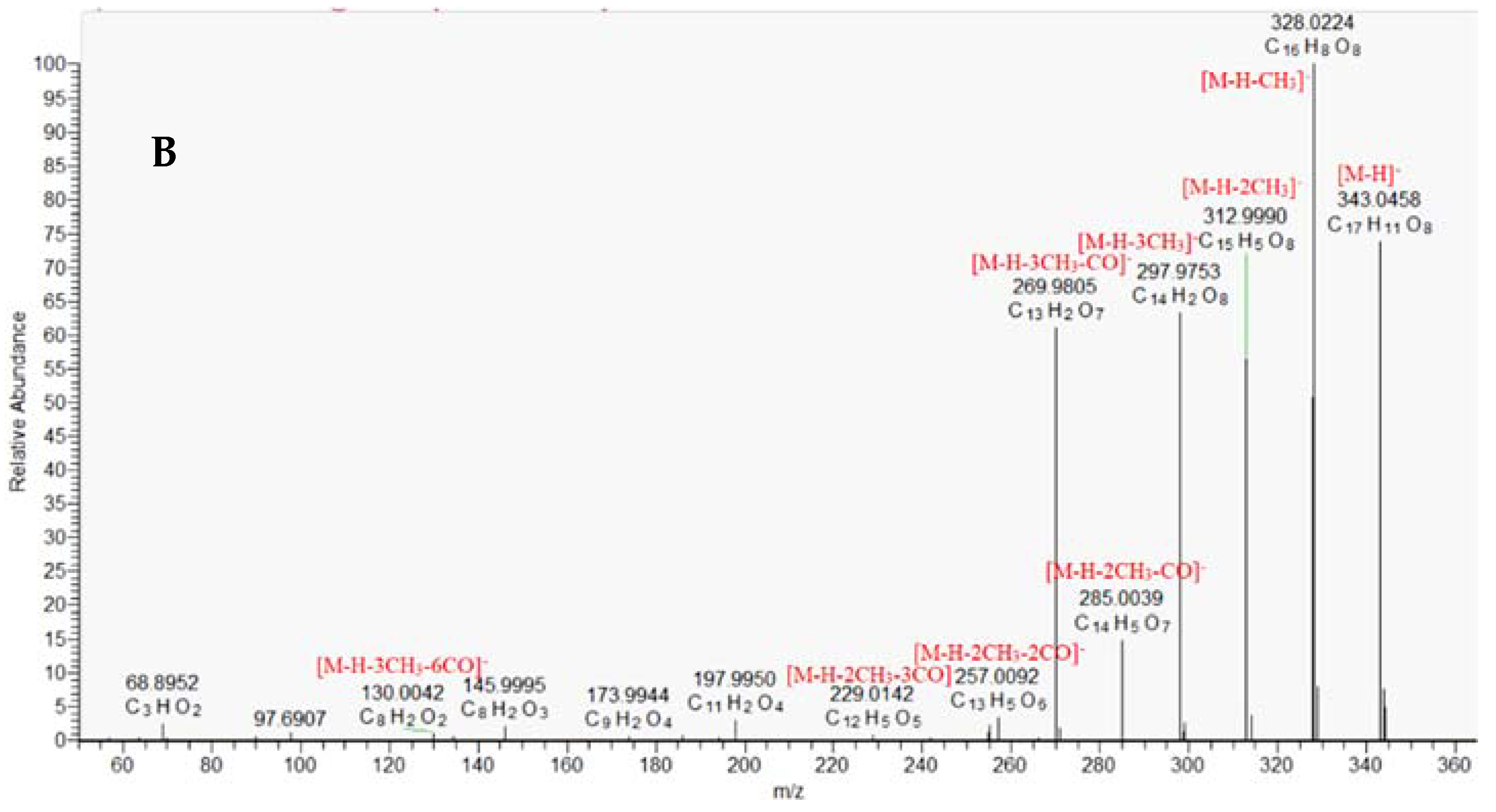
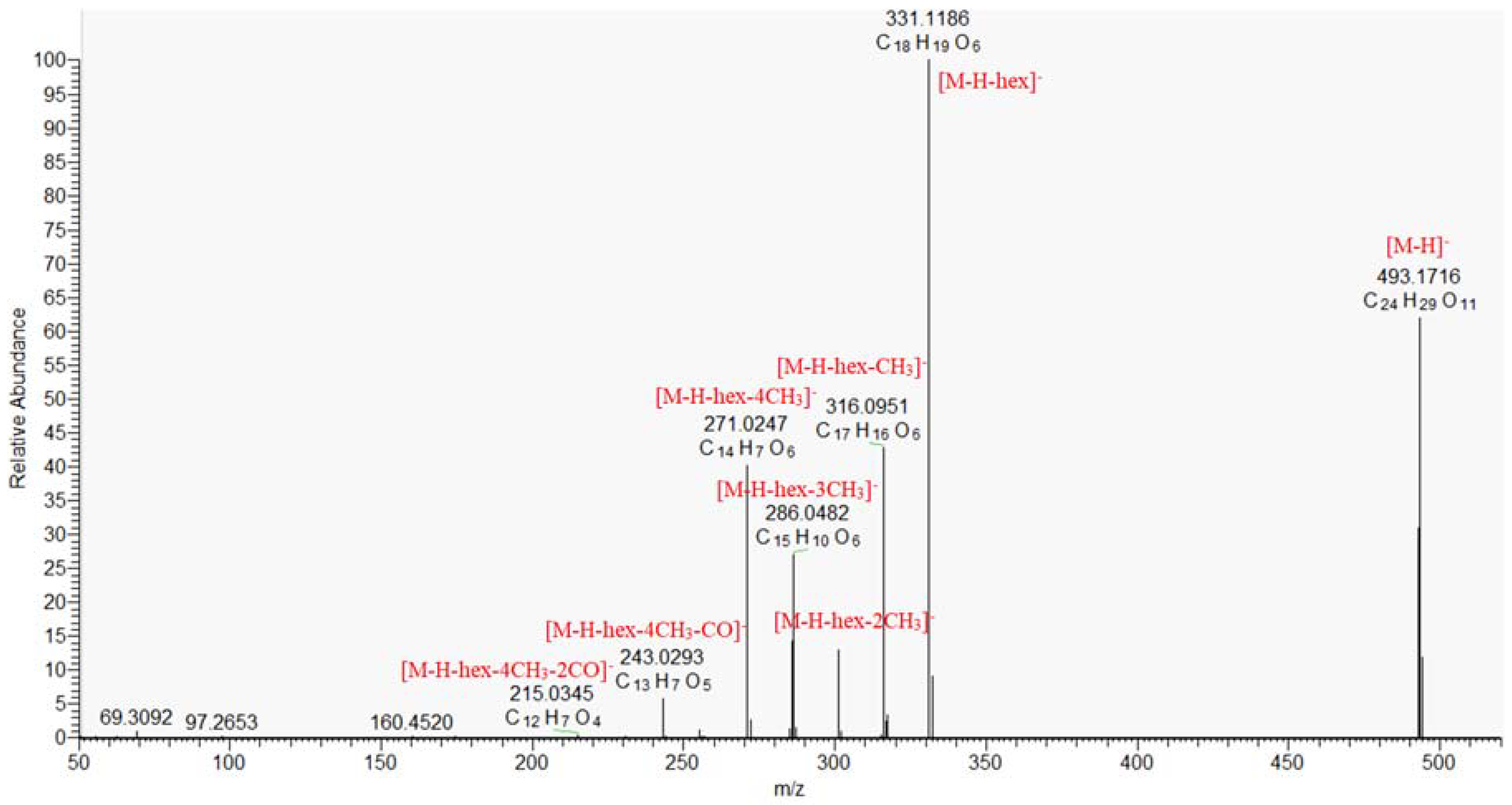
2.1.5. Naphthoquinones and Anthracene Derivatives
2.2. In Vitro Cytotoxicity of K. africana Stem Bark Extract
3. Materials and Methods
3.1. Plant Material
3.2. Sample Extraction
3.3. Chemicals
3.4. UHPLC-HRMS
3.5. MTT Cell Viability Assay
3.6. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bello, I.; Shehu, M.W.; Musa, M.; Zaini Asmawi, M.; Mahmud, R. Kigelia africana (Lam.) Benth. (Sausage Tree): Phytochemistry and Pharmacological Review of a Quintessential African Traditional Medicinal Plant. J. Ethnopharmacol. 2016, 189, 253–276. [Google Scholar] [CrossRef] [PubMed]
- Vicidomini, C.; Palumbo, R.; Moccia, M.; Roviello, G.N. Oxidative Processes and Xenobiotic Metabolism in Plants: Mechanisms of Defense and Potential Therapeutic Implications. JoX 2024, 14, 1541–1569. [Google Scholar] [CrossRef] [PubMed]
- Falode, J.A.; Akinmoladun, A.C.; Olaleye, M.T. Ameliorative Property of Kigelia Africana Crude and Flavonoid Leaf Extracts on Aluminum-Induced Hepatotoxicity in Albino Rats. Comp. Clin. Pathol. 2019, 28, 1495–1506. [Google Scholar] [CrossRef]
- Akunyili, D.N.; Houghton, P.J.; Raman, A. Antimicrobial Activities of the Stembark of Kigelia Pinnata. J. Ethnopharmacol. 1991, 35, 173–177. [Google Scholar] [CrossRef]
- Arkhipov, A.; Sirdaarta, J.; Matthews, B.; Cock, I.E. Metabolomic Profiling of Kigelia Africana Extracts with Anti-Cancer Activity by High Resolution Tandem Mass Spectroscopy. Pharmacogn. Commun. 2014, 4, 10–32. [Google Scholar]
- Akunyili, D.N.; Houghton, P.J. Meroterpenoids and Naphthaquinones from Kigelia Pinnata. Phytochemistry 1993, 32, 1015–1018. [Google Scholar] [CrossRef]
- Khan, M.F.; Dixit, P.; Jaiswal, N.; Tamrakar, A.K.; Srivastava, A.K.; Maurya, R. Chemical Constituents of Kigelia Pinnata Twigs and Their GLUT4 Translocation Modulatory Effect in Skeletal Muscle Cells. Fitoterapia 2012, 83, 125–129. [Google Scholar] [CrossRef]
- Gouda, Y.G.; Abdel-baky, A.M.; Darwish, F.M.; Mohamed, K.M.; Kasai, R.; Yamasaki, K. Iridoids from Kigelia Pinnata DC. Fruits. Phytochemistry 2003, 63, 887–892. [Google Scholar] [CrossRef]
- Houghton, P.J.; Jâger, A.K. The Sausage Tree (Kigelia pinnata): Ethnobotany and Recent Scientific Work. South Afr. J. Bot. 2002, 68, 14–20. [Google Scholar] [CrossRef]
- Jabeen, B.; Riaz, N. Isolation and Characterization of Limonoids from Kigelia Africana. Z. Für Naturforschung B 2013, 68, 1041–1048. [Google Scholar] [CrossRef]
- Govindachari, T.R.; Patankar, S.J.; Viswanathan, N. Isolation and Structure of Two New Dihydroisocoumarins from Kigelia pinnata. Phytochemistry 1971, 10, 1603–1606. [Google Scholar] [CrossRef]
- Inoue, K.; Inouye, H.; Chen, C.-C. A Naphthoquinone and a Lignan from the Wood of Kigelia pinnata. Phytochemistry 1981, 20, 2271–2276. [Google Scholar] [CrossRef]
- Joshi, K.C.; Singh, P.; Taneja, S.; Cox, P.J.; Allan Howie, R.; Thomson, R.H. New Terpenoid Aldehydes from Kigelia pinnata: Crystal Structure of Pinnatal. Tetrahedron 1982, 38, 2703–2708. [Google Scholar] [CrossRef]
- Gouda, Y.G.; Abdel-baky, A.M.; Mohamed, K.M.; Darwish, F.M.; Kasai, R.; Yamasaki, K. Phenylpropanoid and Phenylethanoid Derivatives from Kigelia pinnata DC. Fruits. Nat. Prod. Res. 2006, 20, 935–939. [Google Scholar] [CrossRef]
- Higgins, C.; Bell, T.; Delbederi, Z.; Feutren-Burton, S.; McClean, B.; O’Dowd, C.; Watters, W.; Armstrong, P.; Waugh, D.; Van Den Berg, H. Growth Inhibitory Activity of Extracted Material and Isolated Compounds from the Fruits of Kigelia pinnata. Planta Med. 2010, 76, 1840–1846. [Google Scholar] [CrossRef] [PubMed]
- Falode, J.A.; Akinmoladun, A.C.; Olaleye, M.T.; Akindahunsi, A.A. Sausage Tree (Kigelia africana) Flavonoid Extract Is Neuroprotective in AlCl 3 -Induced Experimental Alzheimer’s Disease. Pathophysiology 2017, 24, 251–259. [Google Scholar] [CrossRef]
- Arena, K.; Rigano, F.; Mangraviti, D.; Cacciola, F.; Occhiuto, F.; Dugo, L.; Dugo, P.; Mondello, L. Exploration of Rapid Evaporative-Ionization Mass Spectrometry as a Shotgun Approach for the Comprehensive Characterization of Kigelia africana (Lam) Benth. Fruit. Molecules 2020, 25, 962. [Google Scholar] [CrossRef]
- Fagbohun, O.F.; Olawoye, B.; Ademakinwa, A.N.; Oriyomi, O.V.; Fagbohun, O.S.; Fadare, O.A.; Msagati, T.A.M. UHPLC/GC-TOF-MS Metabolomics, MTT Assay, and Molecular Docking Studies Reveal Physostigmine as a New Anticancer Agent from the Ethyl Acetate and Butanol Fractions of Kigelia africana (Lam.) Benth. Fruit Extracts. Biomed. Chromatogr. 2021, 35, e4979. [Google Scholar] [CrossRef] [PubMed]
- Swati, D.; Vishwatej, P.; Vinay, S.; Bhupesh, P. LC-MS Analysis of Kigelia Pinnata (JACQ) DC. Root Bark- A Multi-Potent Medicinal Plant. Ayushdhara 2023, 10, 60–67. [Google Scholar] [CrossRef]
- Nabatanzi, A.; Nkadimeng, S.M.; Lall, N.; Kabasa, J.D.; McGaw, L.J. Ethnobotany, Phytochemistry and Pharmacological Activity of Kigelia africana (Lam.) Benth. (Bignoniaceae). Plants 2020, 9, 753. [Google Scholar] [CrossRef]
- Assanti, G.; Kaur, R.; Nizard, S.; Pollack, E.; Rafferty, B.; Priano, C.; Fernández Romero, J.A.; Koroch, A.R. Biology, Chemistry, and Pharmacological Activity of Kigelia Africana (Bignoniaceae) and Garcinia Kola (Clusiaceae)—A Review. J. Med. Act. Plants 2022, 11, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Weiss, C.R.; Moideen, S.V.K.; Croft, S.L.; Houghton, P.J. Activity of Extracts and Isolated Naphthoquinones from Kigelia pinnata against Plasmodium falciparum. J. Nat. Prod. 2000, 63, 1306–1309. [Google Scholar] [CrossRef]
- Momekova, D.; Momekov, G.; Pencheva, I.; Konstantinov, S. Antineoplastic Activity of a Methanolic Extract from Kigelia Pinnata DC Stem Bark. J. Cancer Ther. Res. 2012, 1, 17. [Google Scholar] [CrossRef]
- Sumner, L.W.; Amberg, A.; Barrett, D.; Beale, M.H.; Beger, R.; Daykin, C.A.; Fan, T.W.-M.; Fiehn, O.; Goodacre, R.; Griffin, J.L.; et al. Proposed Minimum Reporting Standards for Chemical Analysis: Chemical Analysis Working Group (CAWG) Metabolomics Standards Initiative (MSI). Metabolomics 2007, 3, 211–221. [Google Scholar] [CrossRef]
- Gevrenova, R.; Zheleva-Dimitrova, D.; Balabanova, V.; Voynikov, Y.; Sinan, K.I.; Mahomoodally, M.F.; Zengin, G. Integrated Phytochemistry, Bio-Functional Potential and Multivariate Analysis of Tanacetum macrophyllum (Waldst. & Kit.) Sch.Bip. and Telekia speciosa (Schreb.) Baumg. (Asteraceae). Ind. Crops Prod. 2020, 155, 112817. [Google Scholar] [CrossRef]
- Sinan, K.I.; Zengin, G.; Zheleva-Dimitrova, D.; Etienne, O.K.; Fawzi Mahomoodally, M.; Bouyahya, A.; Lobine, D.; Chiavaroli, A.; Ferrante, C.; Menghini, L.; et al. Qualitative Phytochemical Fingerprint and Network Pharmacology Investigation of Achyranthes Aspera Linn. Extracts. Molecules 2020, 25, 1973. [Google Scholar] [CrossRef]
- Hooi Poay, T.; Sui Kiong, L.; Cheng Hock, C. Characterisation of Galloylated Cyanogenic Glucosides and Hydrolysable Tannins from Leaves of Phyllagathis rotundifolia by LC-ESI-MS/MS. Phytochem. Anal. 2011, 22, 516–525. [Google Scholar] [CrossRef] [PubMed]
- Mena, P.; Calani, L.; Dall’Asta, C.; Galaverna, G.; García-Viguera, C.; Bruni, R.; Crozier, A.; Del Rio, D. Rapid and Comprehensive Evaluation of (Poly)Phenolic Compounds in Pomegranate (Punica granatum L.) Juice by UHPLC-MSn. Molecules 2012, 17, 14821–14840. [Google Scholar] [CrossRef]
- Singh, A.; Bajpai, V.; Kumar, S.; Sharma, K.R.; Kumar, B. Profiling of Gallic and Ellagic Acid Derivatives in Different Plant Parts of Terminalia arjuna by HPLC-ESI-QTOF-MS/MS. Nat. Prod. Commun. 2016, 11, 239–244. [Google Scholar] [CrossRef]
- Ss, S.; Sm, D.; Ma, A. Antibacterial and Toxicity Evaluation of Stem Bark Extract of Kigelia africana (Lam.) Benth. Glob. Acad. J. Agri. Biosci. 2022, 4, 86–91. [Google Scholar] [CrossRef]
- Pires, F.B.; Dolwitsch, C.B.; Dal Prá, V.; Faccin, H.; Monego, D.L.; Carvalho, L.M.D.; Viana, C.; Lameira, O.; Lima, F.O.; Bressan, L.; et al. Qualitative and Quantitative Analysis of the Phenolic Content of Connarus Var. Angustifolius, Cecropia obtusa, Cecropia palmata and Mansoa alliacea Based on HPLC-DAD and UHPLC-ESI-MS/MS. Rev. Bras. De Farmacogn. 2017, 27, 426–433. [Google Scholar] [CrossRef]
- Sarr, A.; Dieng, S.I.M.; Diatta-Badji, K.; Mbaye, A.I.; Diatta, W.; Ka, A.; Fall, A.D.; Bassène, E. Phytochemical Screening and Determination of Polyphenols in the Hydro-Ethanolic Extract of Trunk Bark and Its Fractions of Stereospermum kunthianium Cham (Bignoniaceae). APRJ 2021, 7, 1–9. [Google Scholar] [CrossRef]
- Costa, R.; Albergamo, A.; Pellizzeri, V.; Dugo, G. Phytochemical Screening by LC-MS and LC-PDA of Ethanolic Extracts from the Fruits of Kigelia africana (Lam.) Benth. Nat. Prod. Res. 2017, 31, 1397–1402. [Google Scholar] [CrossRef] [PubMed]
- Nakano, K.; Maruyama, K.; Murakami, K.; Takaishi, Y.; Tomimatsu, T. Iridoids from Tabebuia Avellanedae. Phytochemistry 1993, 32, 371–373. [Google Scholar] [CrossRef]
- Zhan, C.; Xiong, A.; Shen, D.; Yang, L.; Wang, Z. Characterization of the Principal Constituents of Danning Tablets, a Chinese Formula Consisting of Seven Herbs, by an UPLC-DAD-MS/MS Approach. Molecules 2016, 21, 631. [Google Scholar] [CrossRef] [PubMed]
- Ye, M.; Han, J.; Chen, H.; Zheng, J.; Guo, D. Analysis of Phenolic Compounds in Rhubarbs Using Liquid Chromatography Coupled with Electrospray Ionization Mass Spectrometry. J. Am. Soc. Mass Spectrom. 2007, 18, 82–91. [Google Scholar] [CrossRef]
- Moideen, S.V.K.; Houghton, P.J.; Rock, P.; Croft, S.L.; Aboagye-Nyame, F. Activity of Extracts and Naphthoquinones from Kigelia Pinnata Against Trypanosoma Brucei Brucei and Trypanosoma Brucei Rhodesiense. Planta Med. 1999, 65, 536–540. [Google Scholar] [CrossRef]
- Onegi, B.; Kraft, C.; Köhler, I.; Freund, M.; Jenett-Siems, K.; Siems, K.; Beyer, G.; Melzig, M.F.; Bienzle, U.; Eich, E. Antiplasmodial Activity of Naphthoquinones and One Anthraquinone from Stereospermum kunthianum. Phytochemistry 2002, 60, 39–44. [Google Scholar] [CrossRef]
- Lazare Sidjui, S.; Zeuko´o Menkem, E.; Toghueo, R.; Noté, O.; Mahiou-Leddet, V.; Herbette, G.; Fekam Boyom, F.; Ollivier, E.; Folefoc, G.N. Secondary Metabolites from Jacaranda mimosifolia and Kigelia africana (Bignoniaceae) and Their Anticandidal Activity. Rec. Nat. Prod. 2014, 8, 307–311. [Google Scholar]
- Shkondrov, A.; Krasteva, I.; Ionkova, I.; Popova, P.; Zarev, Y.; Mihaylova, R.; Konstantinov, S. Production of Saponins from in Vitro Cultures of Astragalus glycyphyllos and Their Antineoplastic Activity. Biotechnol. Biotechnol. Equip. 2019, 33, 1413–1418. [Google Scholar] [CrossRef]
- Ilango, S.; Sahoo, D.K.; Paital, B.; Kathirvel, K.; Gabriel, J.I.; Subramaniam, K.; Jayachandran, P.; Dash, R.K.; Hati, A.K.; Behera, T.R.; et al. A Review on Annona Muricata and Its Anticancer Activity. Cancers 2022, 14, 4539. [Google Scholar] [CrossRef] [PubMed]
- Cosquillo-Rafael, M.F.; Placencia-Medina, M.D.; Miranda-Tomasevich, T.Y.; Moreno-Hinojosa, M.; Retuerto-Figueroa, M.G. Efecto Citotóxico y Genotóxico in Vitro Del Extracto Crudo y Etanólico Del Rizoma de Curcuma longa L. Rev. Peru. Med. Exp. Salud. Publica. 2020, 37, 454–461. [Google Scholar] [CrossRef]
- Nisar, A.; Mamat, A.S.; Hatim Mohamed Dzahir, M.I.; Aslam, M.S.; Ahmad, M.S. Antioxidant and Total Phenolic Content of Catharanthus Roseus Using Deep Eutectic Solvent. Recent Adv. Biol. Med. 2017, 03, 7. [Google Scholar] [CrossRef]
- Jackson, S.J.; Houghton, P.J.; Retsas, S.; Photiou, A. In Vitro Cytotoxicity of Norviburtinal and Isopinnatal from Kigelia pinnata Against Cancer Cell Lines. Planta Med. 2000, 66, 758–761. [Google Scholar] [CrossRef] [PubMed]
- Okon, E.; Gaweł-Bęben, K.; Jarzab, A.; Koch, W.; Kukula-Koch, W.; Wawruszak, A. Therapeutic Potential of 1,8-Dihydroanthraquinone Derivatives for Breast Cancer. IJMS 2023, 24, 15789. [Google Scholar] [CrossRef]
- Rahman, M.M.; Islam, M.R.; Akash, S.; Shohag, S.; Ahmed, L.; Supti, F.A.; Rauf, A.; Aljohani, A.S.M.; Al Abdulmonem, W.; Khalil, A.A.; et al. Naphthoquinones and Derivatives as Potential Anticancer Agents: An Updated Review. Chem. Biol. Interact. 2022, 368, 110198. [Google Scholar] [CrossRef]
- Wellington, K.W. Understanding Cancer and the Anticancer Activities of Naphthoquinones—A Review. RSC Adv. 2015, 5, 20309–20338. [Google Scholar] [CrossRef]
- Malik, M.S.; Alsantali, R.I.; Jassas, R.S.; Alsimaree, A.A.; Syed, R.; Alsharif, M.A.; Kalpana, K.; Morad, M.; Althagafi, I.I.; Ahmed, S.A. Journey of Anthraquinones as Anticancer Agents—A Systematic Review of Recent Literature. RSC Adv. 2021, 11, 35806–35827. [Google Scholar] [CrossRef]
- Zhang, X.P.; Ritke, M.K.; Yalowich, J.C.; Slovak, M.L.; Ho, J.P.; Collins, K.I.; Annable, T.; Arceci, R.J.; Durr, F.E.; Greenberger, L.M. P-Glycoprotein Mediates Profound Resistance to Bisantrene. Oncol. Res. 1994, 6, 291–301. [Google Scholar]
- Wardana, A.P.; Abdjan, M.I.; Aminah, N.S.; Fahmi, M.Z.; Siswanto, I.; Kristanti, A.N.; Saputra, M.A.; Takaya, Y. 3,4,3′-Tri- O -Methylellagic Acid as an Anticancer Agent: In Vitro and in Silico Studies. RSC Adv. 2022, 12, 29884–29891. [Google Scholar] [CrossRef]
- Chung, Y.-C.; Lu, L.-C.; Tsai, M.-H.; Chen, Y.-J.; Chen, Y.-Y.; Yao, S.-P.; Hsu, C.-P. The Inhibitory Effect of Ellagic Acid on Cell Growth of Ovarian Carcinoma Cells. Evid. Based Complement. Altern. Med. 2013, 2013, 306705. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Z.; Zhou, B.; Jin, L.; Yu, H.; Liu, L.; Liu, Y.; Qin, C.; Xie, S.; Zhu, F. In Vitro Antioxidant and Antiproliferative Effects of Ellagic Acid and Its Colonic Metabolite, Urolithins, on Human Bladder Cancer T24 Cells. Food Chem. Toxicol. 2013, 59, 428–437. [Google Scholar] [CrossRef]
- Vanella, L.; Di Giacomo, C.; Acquaviva, R.; Barbagallo, I.; Li Volti, G.; Cardile, V.; Abraham, N.; Sorrenti, V. Effects of Ellagic Acid on Angiogenic Factors in Prostate Cancer Cells. Cancers 2013, 5, 726–738. [Google Scholar] [CrossRef]
- Umesalma, S.; Nagendraprabhu, P.; Sudhandiran, G. Antiproliferative and Apoptotic-Inducing Potential of Ellagic Acid against 1,2-Dimethyl Hydrazine-Induced Colon Tumorigenesis in Wistar Rats. Mol. Cell Biochem. 2014, 388, 157–172. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Chen, H.-S.; Wang, L.-F.; Bai, M.-H.; Wang, Y.-C.; Jiang, X.-F.; Liu, M. Ellagic Acid Exerts Anti-Proliferation Effects via Modulation of Tgf-Β/Smad3 Signaling in MCF-7 Breast Cancer Cells. Asian Pac. J. Cancer Prev. 2014, 15, 273–276. [Google Scholar] [CrossRef]
- Mandal, S.; Stoner, G.D. Inhibition of N-Nitrosobenzylmethylamine-Induced Esophageal Tumorigenesis in Rats by Ellagic Acid. Carcinogenesis 1990, 11, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Teel, R.W.; Babcock, M.S.; Dixit, R.; Stoner, G.D. Ellagic Acid Toxicity and Interaction with Benzo[a]Pyrene and Benzo[a]Pyrene 7,8-Dihydrodiol in Human Bronchial Epithelial Cells. Cell Biol. Toxicol. 1986, 2, 53–62. [Google Scholar] [CrossRef]
- Ramírez De Molina, A.; Vargas, T.; Molina, S.; Sánchez, J.; Martínez-Romero, J.; González-Vallinas, M.; Martín-Hernández, R.; Sánchez-Martínez, R.; Gómez De Cedrón, M.; Dávalos, A.; et al. The Ellagic Acid Derivative 4,4′-Di- O -Methylellagic Acid Efficiently Inhibits Colon Cancer Cell Growth through a Mechanism Involving WNT16. J. Pharmacol. Exp. Ther. 2015, 353, 433–444. [Google Scholar] [CrossRef]


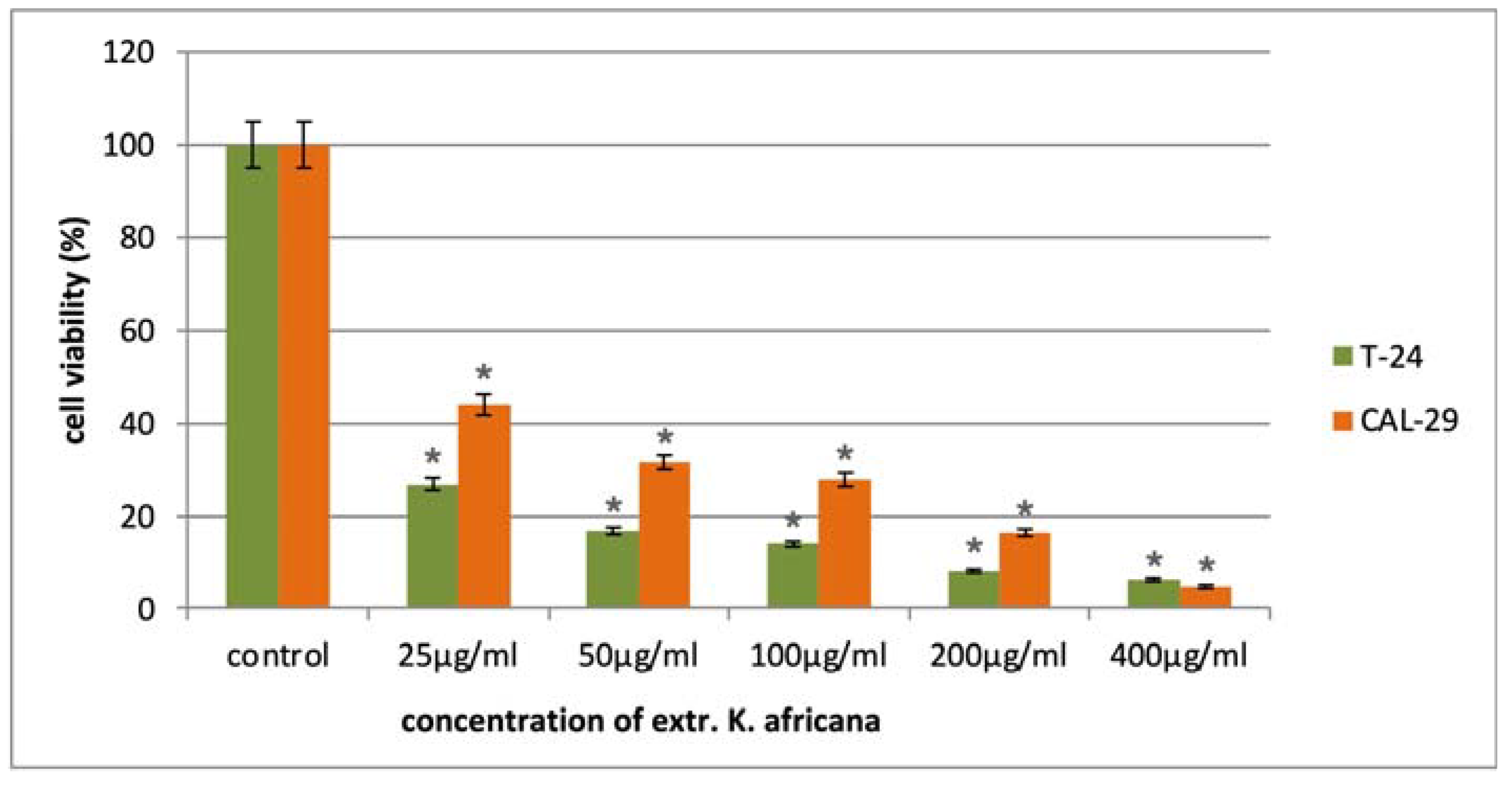
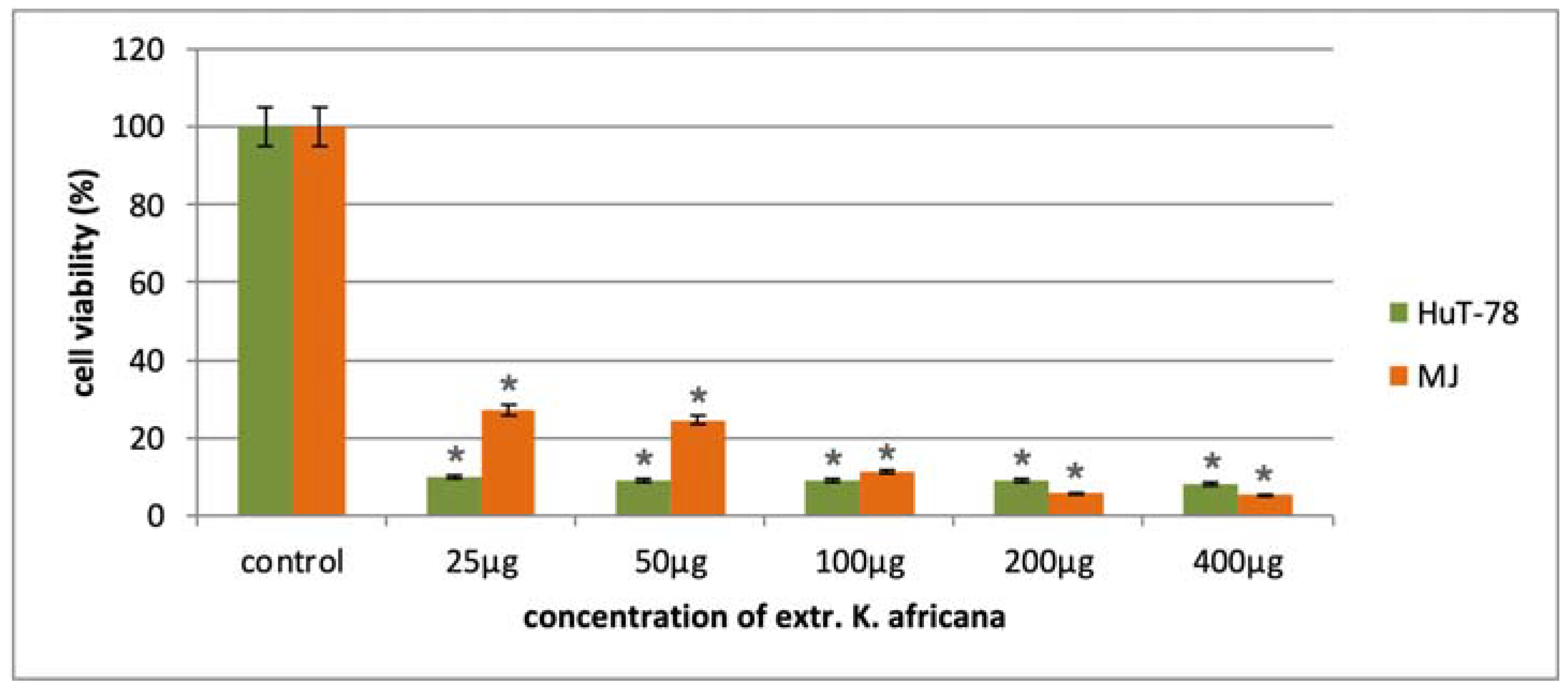
| No | Identified/Tentatively Annotated Compound | Molecular Formula | Exact Mass [M − H] | Fragmentation Pattern in (-) ESI-MS/MS | tR (min) | Δ ppm | Level of Confidence |
|---|---|---|---|---|---|---|---|
| Phenolic acids, flavonoids and derivatives | |||||||
| 1. | gentisic acid a | C7H6O4 | 153.0193 | 153.0184 (14.5), 109.0279 (100), 81.0328 (1.6) | 3.26 | −8.574 | 1 |
| 2. | dihydroxybenzoic acid O-pentoside | C12H14O8 | 285.0616 | 285.0615 (92.9), 153.0180 (42.6), 152.0101 (65.7), 123.0070 (0.7), 108.0200 (100), 85.0280 (0.6) | 4.18 | −0.458 | 2 |
| 3. | p-hydroxybenzoic acid a | C7H6O3 | 137.0244 | 137.0232 (100), 119.0121 (0.9), 108.0201 (6.1), 93.0330 (22.6), 81.0329 (2.5) | 4.66 | −8.738 | 1 |
| 4. | caffeic acid a | C9H8O4 | 179.0350 | 179.0343 (19.9), 135.0437 (100), 161.1659 (1.1), 107.0486 (1.3) | 6.73 | −3.865 | 1 |
| 5. | brevifolin | C12H8O6 | 247.0248 | 247.0242 (100), 219.0292 (2.9), 201.0181 (0.5), 191.0339 (9.7), 173.0230 (3.1), 163.0389 (2.1), 145.0280 (3.7), 135.0435 (1.1), 119.0488 (1.4), 107.0485 (0.2) | 9.02 | −2.393 | 2 |
| 6. | luteolin a | C15H10O6 | 285.0405 | 285.0404 (100), 151.0023 (6.3), 133.0282 (21.4), 107.0122 (5.7) | 16.62 | −0.320 | 1 |
| Gallotannins | |||||||
| 7. | galloyl hexose isomer I | C13H16O10 | 331.0671 | 331.0670 (100), 271.0670 (1.0), 241.0361 (1.2), 311.0242 (14.3), 169.0129 (40.3), 151.0024 (13.9), 125.0229 (13.8), 107.0123 (4.5) | 1.27 | −0.332 | 2 |
| 8. | galloyl hexose isomer II | C13H16O10 | 331.0671 | 331.0670 (16.3), 271.0458 (100), 241.0343 (1.4), 211.0240 (30.2), 169.0130 (24.9), 125.0229 (19.3) | 1.38 | −0.151 | 2 |
| 9. | gallic acid a | C7H6O5 | 169.0142 | 169.0134 (35.79), 125.0229 (100), 123.0071 (0.94), 107.0122 (1.1), 97.0279 (4.11) | 1.68 | −5.186 | 1 |
| 10. | methyl gallate | C8H8O5 | 183.0299 | 183.0292 (100), 168.0052 (13.1), 140.0101 (10.9), 127.0020 (2.5), 111.0072 (6.6) | 5.35 | −3.751 | 2 |
| 11. | digalloyl hexose | C20H20O14 | 483.0780 | 483.0786 (100), 331.0675 (3.0), 313.0569 (16.7), 271.0462 (52.6), 211.0220 (14.4), 169.0133 (48.6), 125.0229 (36.4) | 5.81 | 1.266 | 2 |
| 12. | trigalloyl hexose | C27H24O18 | 635.0879 | 635.0895 (90.0), 483.0808 (0.6), 465.0673 (100), 447.0559 (0.5), 313.0565 (47.6), 295.0457 (5.2), 241.0346 (2.2), 211.0240 (8.6), 193.0130 (4.4), 169.0129 (73.5), 125.0229 (60.9), 107.0122 (9.4) | 8.22 | 0.776 | 2 |
| 13. | tetragalloyl hexose | C34H28O22 | 787.0999 | 787.1002 (100), 635.0847 (0.7), 617.0803 (6.0), 465.0673 (29.8), 447.0556 (2.7), 403.0667 (0.9), 313.0559 (12.5), 300.9977 (0.5), 271.0448 (0.8), 295.0461 (8.5), 251.0532 (0.5), 211.0234 (5.9), 193.0129 (2.1), 169.0130 (75.6), 125.0229 (65.6) | 10.92 | 0.349 | 2 |
| Ellagitannins | |||||||
| 14. | HHDP-hexose isomer I | C20H18O14 | 481.0624 | 481.0623 (100), 300.9989 (66.3), 275.0197 (36.9), 257.0085 (6.1), 229.0136 (10.6), 201.0185 (6.1), 173.0233 (2.8), 145.0289 (2.5), 123.0074 (1.6) | 0.83 | −0.246 | 2 |
| 15. | HHDP-hexose isomer II | C20H18O14 | 481.0624 | 481.0624 (100), 421.0404 (0.4), 300.9988 (76.5), 275.0196 (40.3), 257.0087 (6.4), 229.0137 (11.7), 201.0184 (5.0), 173.0233 (3.2), 145.0278 (1.9), 123.0069 (1.8) | 1.15 | 0.128 | 2 |
| 16. | pedunculagin isomer I | C34H24O22 | 783.0686 | 783.0694 (68.4), 481.0621 (0.9), 300.9988 (100), 275.0196 (40.5), 249.0402 (5.4), 229.0136 (14.6), 145.0285 (3.5), 123.0068 (0.7) | 2.94 | 0.964 | 2 |
| 17. | castalin/vescalin | C27H20O18 | 631.05768 | 631.0579 (100), 450.9943 (88.2), 432.9821 (4.0), 425.0160 (6.3), 407.0040 (1.1), 379.0106 (2.3), 351.0152 (3.2), 323.0195 (2.8), 295.0253 (2.3), 279.0285 (0.6), 267.0292 (2.0) | 3.78 | 0.370 | 2 |
| 18. | punicalin α/β | C34H22O22 | 781.0530 | 781.0535 (100), 448.9783 (48.3), 420.9829 (5.4), 392.9886 (52.1), 298.9839 (0.8), 600.9926 (0.7), 364.9933 (7.3), 336.9989 (19.7), 309.040 (3.4), 300.9981 (0.6), 265.0138 (3.1), 237.0179 (2.9), 123.0071 (0.6) | 3.99 | 0.685 | 2 |
| 19. | punicalin α/β | C34H22O22 | 781.0530 | 781.0538 (100), 600.9902 (0.8), 448.9786 (53.9), 420.9838 (4.5), 392.9891 (56.9), 364.9934 (5.1), 336.9993 (19.2), 321.0037 (2.5), 281.0090 (3.0), 237.0186 (2.6), 209.0237 (1.3), 166.9978 (0.8), 123.0069 (1.2), 299.9911 (0.9) | 4.16 | 1.069 | 2 |
| 20. | 2-O-galloylpunicalin | C41H26O26 | 933.0640 | 933.0644 (91.2), 889.3828 (0.3), 781.0530 (44.4), 721.0327 (2.3), 600.9899 (13.5), 450.9941 (100), 425.0152 (10.4), 300.9986 (32.3), 249.0177 (0.5), 229.0132 (3.2), 145.0280 (6.6) | 4.18 | 0.468 | 2 |
| 21. | punicalgin α/β/γ | C24H14O15 | 541.0260 | 541.0261 (100), 450.9945 (3.4), 300.9987 (40.9), 275.0195 (17.0), 229.0139 (7.2), 173.0227 (2.6), 145.0282 (2.3) | 4.19 | 0.124 | 2 |
| 22. | valoneic acid dilactone | C21H10O13 | 469.0048 | 469.0045 (48.8), 425.0150 (100), 407.0042 (15.1), 379.0101 (5.6), 351.0135 (2.6), 335.0205 (2.4), 307.0253 (1.5), 300.9968 (3.7), 299.9908 (15.9), 251.0348 (1.7), 223.0401 (0.6), 207.0450 (0.9), 172.0160 (0.6), 145.0281 (0.5) | 4.25 | −0.860 | 3 |
| 23. | pedunculagin isomer I | C34H24O22 | 783.0686 | 783.0696 (69.6), 481.0630 (0.8), 300.9989 (100), 275.0197 (51.7), 249.0404 (7.8), 229.0138 (11.8), 145.0285 (2.9), 123.0075 (0.9) | 4.56 | 1.270 | 2 |
| 24. | galloyl-HHDP-hexose | C27H22O18 | 633.0733 | 633.0738 (100), 300.9988 (81.0), 275.0197 (55.6), 257.0090 (8.2), 229.0138 (13.7), 201.0184 (6.8), 169.0132 (4.8), 125.0228 (3.9) | 4.66 | 0.716 | 2 |
| 25. | punicalgin α/β/γ | C24H14O15 | 541.0260 | 541.0259 (100), 300.9987 (41.99), 275.0197 (21.3), 229.0133 (5.4), 173.0231 (2.8), 125.0229 (2.1) | 5.64 | −0.098 | 2 |
| 26. | galloyl-HHDP-hexose isomer | C27H22O18 | 633.07333 | 633.0737 (100), 300.9988 (75.0), 275.0196 (48.4), 257.0080 (7.7), 229.0136 (12.5), 201.0183 (5.7), 169.0127 (4.5), 125.0229 (4.6), 107.0124 (1.6) | 6.13 | 0.621 | 2 |
| 27. | tellimagrandin I | C34H26O22 | 785.0843 | 785.0850 (95.8), 483.0754 (0.8), 313.0577 (1.6), 300.9987 (100), 275.0496 (35.5), 249.0401 (30.6), 229.0134 (10.6), 169.0129(17.4), 125.0230 (16.6), 107.0120 (2.6) | 7.37 | 0.923 | 2 |
| 28. | punicalgin α/β/γ | C24H14O15 | 541.0260 | 541.0259 (100), 450.9940 (43.3), 425.0150 (27.3), 300.9987 (32.9), 229.0137 (10.5), 169.0131 (6.9), 125.0231 (15.3), 173.0233 (6.8) | 9.08 | −0.098 | 2 |
| 29. | methylvaloneic acid dilactone | C22H12O13 | 483.0205 | 483.0204 (100), 450.9944 (57.5), 432.09844 (5.2), 407.0046 (4.1), 379.0081 (2.8), 299.9908 (2.4) | 9.19 | −0.318 | 2 |
| 30. | ellagic acid-O-hexoside | C20H16O13 | 463.0518 | 463.0526 (100), 300.9991 (83.3), 299.9922 (32.1), 107.0343 (2.7) | 9.20 | 1.590 | 2 |
| 31. | 3,4,8,9,10-pentahydroxy dibenzo[b,d]pyran-6-on | C13H8O7 | 275.0197 | 275.0196 (100), 257.0088 (9.6), 247.0242 (1.0), 229.0134 (10.3), 219.0290 (1.4), 203.0339 (3.5), 191.0338 (2.2), 185.0233 (1.9), 173.0233 (2.9), 145.0278 (1.8), 129.0331 (0.3), 101.0382 (0.3) | 9.22 | −0.385 | 2 |
| 32. | gallagic acid dilactone (terminalin) | C28H10O16 | 600.9896 | 600.9901 (100), 300.9991 (10.9), 298.9830 (22.6), 270.9883 (14.7), 257.0099 (0.4), 242.9931 (6.5), 229.0154 (1.7), 201.0182 (0.7), 185.0231 (0.6) | 10.16 | 0.737 | 2 |
| 33. | ellagic acid-O-hexoside isomer | C20H16O13 | 463.052 | 463.0525 (100), 373.0215 (16.2), 343.0091 (9.9), 315.0135 (12.9), 300.9991 (1.6), 299.9909 (13.2), 285.0038 (2.1) | 10.99 | 1.461 | 2 |
| 34. | ellagic acid-O-pentoside | C19H14O12 | 433.0412 | 433.0414 (100), 300.9987 (77.2), 299.9911 (42.4), 283.9970 (1.2), 243.9996 (1.9), 185.0230 (1.8) | 10.95 | 0.372 | 2 |
| 35. | methylellagic acid-O-hexoside | C21H18O13 | 477.0674 | 477.0674 (100), 315.0157 (76.9), 314.0067 (7.3), 299.9903 (68.0), 270.9878 (8.7) | 11.36 | −0.050 | 2 |
| 36. | ellagic acid a | C14H6O8 | 300.9990 | 300.9991 (100), 245.0664 (1.5), 257.0074 (1.1), 229.0131 (4.1), 201.0184 (2.9), 185.0234 (2.3), 145.0281 (2.8), 117.0333 (0.4) | 11.57 | 0.858 | 1 |
| 37. | methylellagic acid-O-pentoside | C20H16O12 | 447.0569 | 447.0572 (100), 315.0145 (80.1), 314.0072 (4.2), 299.9912 (64.5), 298.9819 (12.8), 270.9869 (19.5) | 13.40 | 0.718 | 2 |
| 38. | methylellagic acid | C15H8O8 | 315.0146 | 315.0150 (84.6), 299.9912 (100), 270.9866 (2.3), 242.9920 (1.9), 200.0096 (1.7), 300.9955 (11.5) | 14.45 | 2.443 | 2 |
| 39. | dimethylellagic acid-O-pentoside | C21H18O12 | 461.0725 | 461.0735 (100), 329.0271 (7.9), 328.0230 (54.1), 314.0027 (2.8), 312.9988 (33.9), 297.9762 (30.4), 285.0045 (8.2), 269.9809 (21.9) | 14.73 | 2.041 | 2 |
| 40. | methylellagic acid isomer | C15H8O8 | 315.0146 | 315.0149 (100), 299.9912 (89.1), 242.9920 (1.7), 200.0092 (2.1), 300.9946 (6.3) | 14.78 | 1.872 | 2 |
| 41. | dimethylellagic acid | C16H10O8 | 329.0303 | 329.0305 (100), 314.0070 (76.0), 298.9834 (28.8), 285.0030 (6.9), 270.9883 (49.4), 242.9905 (5.4), 214.9963 (1.4), 315.0101 (7.7), 312.9991 (17.8) | 18.18 | 1.609 | 2 |
| 42. | dimethylellagic acid isomer I | C16H10O8 | 329.0303 | 329.0303 (94.6), 314.0071 (100), 298.9835 (30.6), 270.9986 (54.5), 242.9902 (8.4), 315.0095 (10.4), 312.9980 (3.8) | 18.58 | 1.883 | 2 |
| 43. | dimethylellagic acid isomer II | C16H10O8 | 329.0303 | 329.0304 (100), 314.0070 (43.7), 315.0094 (3.1), 312.9990 (18.8), 298.9836 (19.9), 285.0052 (8.6), 270.9888 (27.6), 242.9902 (1.7) | 19.04 | 1.974 | 2 |
| 44. | trimethylellagic acid | C17H12O8 | 343.0459 | 343.0461 (98.2), 328.0227 (100), 312.9994 (48.5), 297.9756 (38.1), 285.0042 (9.9), 269.9805 (29.7), 197.9932 (2.4), 145.9990 (0.7) | 22.90 | 0.232 | 2 |
| Phenylethanoid glycosides and iridoids | |||||||
| 45. | echinacoside | C35H46O20 | 785.2510 | 785.2520 (66.4), 623.2211 (8.9), 461.1661 (1.5), 179.0337 (1.9), 161.0231 (100), 133.0280 (43.7), 123.0435 (3.2), 135.0436 (12.3) | 9.71 | 1.329 | 2 |
| 46. | verminoside | C24H28O13 | 523.1457 | 523.1464 (100), 361.0936 (0.9), 343.0828 (0.9), 247.0587 (4.2), 180.0364 (0.9), 179.0337 (18.4), 163.0387 (12.9), 161.0231 (75.3), 135.0437 (24.1), 133.0281 (28.9) | 11.70 | 2.171 | 2 |
| 47. | verbascoside/acteoside | C29H36O15 | 623.1981 | 623.1995 (72.2), 461.1653 (3.9), 315.1112 (1.7), 179.0338 (2.0), 161.0232 (100), 153.0542 (1.1), 135.0438 (6.7), 133.0280 (35.7) | 11.99 | 2.209 | 2 |
| 48. | verbascoside/acteoside | C29H36O15 | 623.1981 | 623.1994 (87.8), 461.1672 (11.1), 315.1119 (2.1), 179.0335 (2.7), 161.0233 (100), 153.0548 (0.3), 135.0436 (16.1), 133.0231 (29.9) | 13.02 | 2.016 | 2 |
| 49. | methoxybenzoylajugol | C23H30O11 | 481.1715 | 481.1724 (50.2), 319.1180 (100), 304.0956 (17.1), 152.0101 (1.1), 138.0309 (34.3), 137.0230 (35.0), 109.0280 (12.4) | 16.35 | 1.715 | 3 |
| Naphthoquinones and anthracene derivatives | |||||||
| 50. | pentahydroxy-1,4-naptoquinone | C10H6O7 | 237.0041 | 237.0035 (22.3), 193.0132 (100), 149.0229 (91.5), 121.0278 (66.2), 107.0127 (0.6), 93.0329 (2.8), 77.0381 (0.6) | 4.36 | −2.598 | 3 |
| 51. | dimethoxy-hydroxy-dihydroanthraquinone | C16H14O5 | 285.0768 | 285.0767 (42.9), 270.0532 (84.5), 255.0295 (100), 227.0343 (57.9), 199.0392 (4.8) | 17.66 | −0.445 | 3 |
| 52. | dihydroxy-methoxyantraquinone | C15H10O5 | 269.0455 | 269.0457 (100), 254.0199 (6.4), 253.0142 (31.2), 237.0163 (9.9), 226.0241 (9.0), 185.0225 (4.4), 161.0228 (8.7) | 18.01 | 0.756 | 3 |
| 53. | hydroxy-tetramethoxy-dihydroanthraquinone-O-hexoside | C24H30O11 | 493.1715 | 493.1724 (56.0), 331.1190 (100), 316.0954 (50.7), 301.0721 (13.6), 286.0484 (28.8), 271.0250 (48.0), 257.0435 (0.8), 227.0307 (0.5), 243.0296 (4.9) | 18.21 | 1.795 | 3 |
| 54. | dimethoxy-hydroxy-dihydroanthraquinone | C16H14O5 | 285.0768 | 285.0768 (24.1), 270.0533 (100), 255.0296 (2.4), 227.0341 (36.9), 199.0365 (2.9), 178.9915 (3.3) | 21.26 | −0.340 | 3 |
| 55. | pinnatal/isopinnatal/sterekunthal A | C20H18O5 | 337.1082 | 337.1081 (100), 309.1129 (3.0), 307.0972 (2.6), 294.0889 (0.3), 279.0661 (1.2), 251.0345 (6.6), 237.0551 (29.6), 223.0395 (5.3), 209.0604 (1.8), 195.0432 (0.2), 160.0149 (0.4) | 22.52 | −0.169 | 2 |
| 56. | hydroxy-trimethoxy- dihydroanthraquinone | C17H16O5 | 299.0925 | 299.0926 (92.5), 284.0692 (100), 269.0455 (99.8), 254.0221 (40.8), 241.0477 (5.4), 230.0210 (15.0), 226.0266 (67.2), 198.0302 (6.4), 92.9940 (1.7) | 22.99 | 0.311 | 3 |
| 57. | pinnatal/isopinnatal/sterekunthal A | C20H18O5 | 337.1082 | 337.1081 (100), 321.0780 (0.3), 307.0999 (0.3), 295.2039 (0.3), 267.0670 (1.1), 279.0670 (0.2), 251.0348 (0.4), 237.0551 (36.9), 223.0394 (5.9), 209.0600 (1.2), 198.0022 (0.2), 163.0394 (0.2) | 23.14 | −0.169 | 2 |
| 58. | tetramethoxy-hydroxy-dihydroanthraquinone | C18H20O6 | 331.1187 | 331.1190 (100), 316.0953 (95.6), 301.0717 (64.1), 286.0482 (22.6), 271.0248 (76.3), 243.0295 (25.0), 227.0329 (1.6), 215.0324 (3.9), 199.0365 (1.1) | 23.21 | 0.841 | 3 |
| 59. | kigelinol/isokigelinol | C19H16O4 | 307.0976 | 307.0973 (100), 289.0868 (23.9), 274.0633 (9.8), 261.0920 (0.3), 246.0672 (0.2), 237.1321 (0.2) | 23.42 | −0.854 | 2 |
| 60. | pinnatal/isopinnatal/sterekunthal A | C20H18O5 | 337.1082 | 337.1082 (100), 309.1133 (7.4), 307.0974 (5.9), 294.0902 (1.0), 279.0660 (1.2), 251.0349 (11.0), 237.0552 (18.4), 223.0395 (7.9), 209.0600 (2.5), 195.0446 (0.2), 173.0235 (0.4), 160.0150 (0.4) | 23.51 | −0.169 | 2 |
| 61. | dimethoxy-dihydroanthraquinone | C16H14O4 | 269.0819 | 269.0819 (56.8), 254.0583 (100), 239.0320 (8.5), 228.9896 (15.3), 211.0394 (65.9), 182.9854 (2.3), 154.9913 (6.9) | 23.84 | 0.029 | 3 |
| 62. | kigelinol/isokigelinol | C19H16O4 | 307.0976 | 307.0974 (100), 289.0869 (25.0), 274.0634 (9.9), 261.0910 (0.3), 246.0679 (0.4) | 24.00 | −0.463 | 2 |
| 63. | tetramethoxy-hydroxy-dihydroanthraquinone isomer | C18H20O6 | 331.1187 | 331.1190 (100), 316.0953 (95.6), 301.0717 (64.1), 286.0482 (22.6), 271.0248 (76.3), 243.0295 (25.0), 227.0329 (1.6), 215.0324 (3.9), 199.0365 (1.1) | 24.12 | 0.841 | 3 |
| Cell Line | MCF-7 | MDA-MB-231 | T-24 | CAL-29 | HUT-78 | MJ | HEK-293 |
|---|---|---|---|---|---|---|---|
| K. africana | 30.3 ± 5.3 | 29.5 ± 4.8 | 10.2 ± 1.5 | 24.8 ± 3.9 | 4.6 ± 1.6 | 11.5 ± 2.5 | 237.5 ± 13.6 |
| SI * | 7.8 | 8.0 | 23.3 | 9.6 | 51.6 | 20.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheleva-Dimitrova, D.; Mihaylova, R.; Nikolova, M.; Singh, N.; Konstantinov, S. New Insights into the Metabolic Profile and Cytotoxic Activity of Kigelia africana Stem Bark. Molecules 2025, 30, 1388. https://doi.org/10.3390/molecules30061388
Zheleva-Dimitrova D, Mihaylova R, Nikolova M, Singh N, Konstantinov S. New Insights into the Metabolic Profile and Cytotoxic Activity of Kigelia africana Stem Bark. Molecules. 2025; 30(6):1388. https://doi.org/10.3390/molecules30061388
Chicago/Turabian StyleZheleva-Dimitrova, Dimitrina, Rositsa Mihaylova, Maria Nikolova, Nisha Singh, and Spiro Konstantinov. 2025. "New Insights into the Metabolic Profile and Cytotoxic Activity of Kigelia africana Stem Bark" Molecules 30, no. 6: 1388. https://doi.org/10.3390/molecules30061388
APA StyleZheleva-Dimitrova, D., Mihaylova, R., Nikolova, M., Singh, N., & Konstantinov, S. (2025). New Insights into the Metabolic Profile and Cytotoxic Activity of Kigelia africana Stem Bark. Molecules, 30(6), 1388. https://doi.org/10.3390/molecules30061388








