Abstract
Simple substitutions on the donor or acceptor units in radicals is an effective method to improve luminescent properties. However, the luminescence efficiency of radicals has not yet reached satisfactory levels through simple molecular structure modification. In this study, two [4-(N-Carbazolyl)-2,6-dichlorophenyl] bis(2,4,6-trichlorophenyl)methyl (Cz-TTM) radical derivatives (Mes2Cz-TTM, Mes2Cz-Mes2TTM) were synthesized and characterized by modifying the carbazole (donor) and tris-2,4,6-trichlorophenylmethyl radical (acceptor) units with 2,4,6-trimethylphenyl groups. The different substitutions showed varying influences on photoluminescence quantum efficiency (PLQE) compared to the Cz-TTM parent radical. The donor-only substitution suppressed the PLQE (39%) in Mes2Cz-TTM. In contrast, Mes2Cz-Mes2TTM exhibited a significantly higher PLQE of 92.6%, compared to the 68% PLQE of the Cz-TTM parent radical in toluene. Additionally, thermostability and photostability were improved with both donor and acceptor substitutions. The photophysical properties, molecular orbitals, and electrochemical behaviors were also systematically explored. This strategy provides a feasible approach to achieve high luminescence efficiency in radicals through simple substitutions on donor and acceptor units.
1. Introduction
Due to the distinctive doublet emission mechanism, organic radicals possess inherent advantages as luminescent materials. With the exception of a few newly developed luminescent radicals, the chlorinated triphenylmethyl (trityl) radicals and their derivatives have been extensively studied [1,2,3,4,5,6,7]. These include tris(2,4,6-trichlorophenyl)methyl (TTM) radicals [8,9,10,11,12,13,14,15,16,17,18,19,20,21], perchloro triphenylmethyl (PTM) radicals [22,23,24], pyridyl-containing triphenylmethyl (PyBTM) radicals [25,26,27,28,29,30,31], (N-carbazolyl) bis(2,4,6-trichlorophenyl)methyl (CzBTM) radicals [32,33,34], and so on. Traditional PTM or TTM derivatives typically feature donor–acceptor structures, with an electron-rich donor (carbazole or triphenylamine, etc.) and an electron-deficient acceptor (PTM or TTM radical core). Some studies had documented significant enhancements in the luminescent properties of these radicals [35,36,37,38,39,40,41,42,43]. However, radicals with high photoluminescence quantum efficiency (PLQE) are still relatively rare compared to other types of organic luminescent materials. Luis Juliá et al. first incorporated carbazole (electron donor) into a TTM radical discovering an efficient red light-emitting radical [17]. However, the terminal modifications of the carbazole donor unit with phenyl derivatives or halogen atoms had a minimal impact on the luminescent efficiency of the radicals in latter works [42,44,45]. In contrast, direct bonding to the radical acceptor core was proved to be more effective [35,36,39,40,41]. However, due to the low luminescence efficiency of the parent radicals (PyBTM, CzBTM), the improvement in PLQE did not meet expectations. Recently, Li et al. reported nearly 100% PLQE radical emitters by fine-tuning the effective donor-acceptor distance in [4-(N-Carbazolyl)-2,6-dichlorophenyl] bis(2,4,6-trichlorophenyl)methyl (Cz-TTM) radical analogues [46]. This suggests that the design strategy of modifying the donor-acceptor radical system to achieve high PLQE is promising. In this study, Cz-TTM with 68% PLQE in toluene was used as the parent radical. Two new Cz-TTM derivatives (Mes2Cz-TTM, Mes2Cz-Mes2TTM) were synthesized by modifying the carbazole donor and TTM radical acceptor unit with 2,4,6-trimethylphenyl (Mes) (Figure 1). In Mes2Cz-TTM, the substitution of Mes on the carbazole donor significantly lowered the luminescence efficiency (39%) compared to the minimal impact with benzene substitution (electron-donating) [44] and the negative impact with the halogen atom (electron-withdrawing) [42,45]. Surprisingly, Mes2Cz-Mes2TTM, with further substitution on the TTM radical acceptor, achieved an excellent PLQE (92.6%). Additionally, both radicals exhibited enhanced photostability and thermostability due to the introduction of donor and acceptor substitutions. This simple molecular design strategy is proved to be highly effective in achieving luminescent radicals with excellent luminescence efficiency.
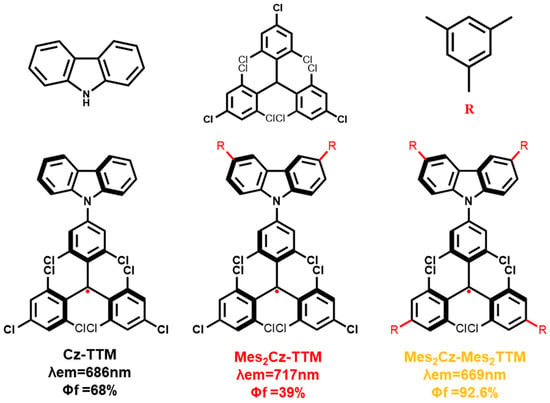
Figure 1.
Molecular structures of Cz-TTM, Mes2Cz-TTM, and Mes2Cz-Mes2TTM.
2. Results and Discussion
2.1. Synthesis and Structure Characterization
Mes2Cz-TTM and Mes2Cz-Mes2TTM were synthesized using commercially available reagents. The detailed synthetic routes were described in the Supporting Information (Figure S1). The molecular structures and compositions of these target radicals were characterized by MALDI-TOF (Figure S2) and Fourier transform infrared spectra (FT-IR) (Figure S3). The absorption peaks ranging from 1600 cm−1 to 1400 cm−1 mainly originated from benzene rings and the bonds that connect the central carbon atom of the radical to the adjacent benzene rings. The presence of the unpaired electron in the radicals (g values around 2.002–2.003) was confirmed by electron paramagnetic resonance (EPR) (Figure S4).
2.2. Photophysical Properties
The ultraviolet-visible (UV-Vis) absorption spectra and photoluminescence (PL) spectra of Mes2Cz-TTM and Mes2Cz-Mes2TTM were measured in toluene solvent (1 × 10−5 M) (Figure 2a,b). The Cz-TTM parent radical was also measured as a reference. Similar to Cz-TTM, the absorption band around 376 nm was attributed to the characteristic absorption of carbon-centered radicals. A broad and weaker absorption band at longer wavelengths (608 nm) was primarily due to the intramolecular charge transfer (CT) state. As Mes was introduced, increasing the electron-donating ability of the carbazole unit, the enhanced charge transfer effect resulted in a red shift of the absorption band (626 nm) from the carbazole donor to the TTM radical acceptor in Mes2Cz-TTM. When Mes was attached to the dichlorophenyl group of the TTM radical core, the long-wavelength absorption band shifted back to the initial wavelength (610 nm), which was associated with the weakened donor-acceptor character due to the decreased electron-accepting ability of the TTM radical core in Mes2Cz-Mes2TTM. On the other hand, the PL spectra of the target radicals followed a similar trend to the UV-Vis absorption spectra. Both radicals exhibited red fluorescence emission (Figure 2c). The emission band of Mes2Cz-TTM exhibited a bathochromic shift (717 nm) due to the effect of donor substitution. With the substitution of the TTM radical acceptor, a blue shift in emission (669 nm) was observed, resulting from the weaker electron-accepting TTM radical core. Moreover, we concluded that the effect of acceptor substitution on the shift in emission was more pronounced. The absorption bands showed no solvent dependence behavior, while the emission bands shifted to longer wavelengths with increasing solvent polarity, indicating the CT excited-state character (Figures S5 and S6).
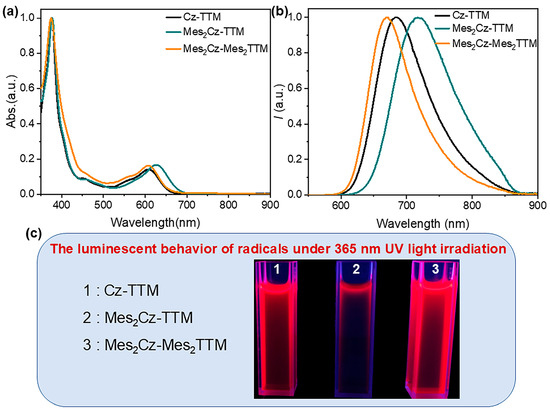
Figure 2.
(a) The normalized UV-Vis absorption spectra of Cz-TTM, Mes2Cz-TTM, and Mes2Cz-Mes2TTM in toluene solution (1 × 10−5 M) at room temperature; (b) the normalized PL spectra of Cz-TTM, Mes2Cz-TTM, and Mes2Cz-Mes2TTM in toluene solution (1 × 10−5 M) at room temperature; and (c) the luminescent behavior of radicals under 365 nm UV light irradiation in toluene solvent.
The absolute PLQE values in toluene were measured using an integrating sphere. The different substitutions on the donor and acceptor units resulted in opposite trends. The Mes substitution significantly suppressed the PLQE in Mes2Cz-TTM (39%). The suppression on luminescence efficiency was more obvious than the previous Cz-TTM derivatives, especially the terminal modification of carbazole with phenyl derivatives. The simple substitution on carbazole donor may not be an effective method. However, when the additional Mes group was directly attached to the TTM radical core, the PLQE of Mes2Cz-Mes2TTM (92.6%) dramatically increased compared to the PLQE of Cz-TTM (68%). To explain these results, the transient photoluminescence decay spectra were measured in toluene. Mes2Cz-TTM radical exhibited shorter lifetime and Mes2Cz-Mes2TTM radical exhibited a longer lifetime compared to Cz-TTM (Figure 3). The radiative rate constant (kr) and non-radiative rate constant (knr) for Mes2Cz-TTM and Mes2Cz-Mes2TTM were also calculated according to Equations (1) and (2) (Table 1). The kr of Cz-TTM, Mes2Cz-TTM, and Mes2Cz-Mes2TTM were on the same order, which was due to the similar transition oscillator strength (Table S4). Therefore, the knr played the main role in determining luminescence efficiency. Interestingly, distorted molecular space structure did not lead to lower knr in Mes2Cz-TTM. In contrast, the possible flipping motions may cause larger thermal deactivation paths. Mes2Cz-Mes2TTM showed lower knr than Cz-TTM. On the one hand, the direct attachment of Mes to TTM radical acceptor impeded the vibronic coupling of the luminescence core. On the other hand, the larger energy gap suppressing the internal conversion to the ground state also resulted in lower knr.
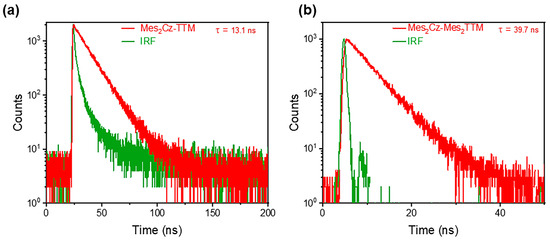
Figure 3.
Transient fluorescence decay of Mes2Cz-TTM (a) and Mes2Cz-Mes2TTM (b) in toluene solution (1 × 10−5 M).

Table 1.
Photophysical parameters of Cz-TTM, Mes2Cz-TTM, and Mes2Cz-Mes2TTM in toluene.
2.3. Stability
The thermostabilities of the radicals were compared using thermogravimetric analysis (TGA) under ambient and nitrogen atmospheres, respectively (Figure S7). The differences in decomposition temperatures (Td, corresponding to 5% weight loss) were attributed to the different substitution sites. Mes2Cz-TTM with a modified carbazole unit exhibited poorer stability than Cz-TTM. The cause for the significant decrease in stability was unclear. In contrast, the higher decomposition temperature of Mes2Cz-Mes2TTM was due to the substituents replacing the reactive chlorine sites in the TTM radical core, which are key to its reactivity.
Radicals were also monitored under continuous irradiation with a 375 nm xenon lamp in toluene to measure the half-life (t1/2) values (Figure 4). Compared to Cz-TTM (2.9 × 105 s), a longer t1/2 for Mes2Cz-TTM (3.9 × 105 s) and Mes2Cz-Mes2TTM (8.7 × 105 s) indicated stronger photostabilities. As the number of substitution groups increased, photostability also showed an increasing trend (Cz-TTM < Mes2Cz-TTM < Mes2Cz-Mes2TTM). The enhanced photostability was attributed to the more stable excited state resulting from larger conjugation and steric hindrance. Also, the introduction of 2,4,6-trimethylphenyl can effectively reduce the reactions at active sites, such as the 3,6 positions of the carbazole and the ortho positions of the radical benzene ring.
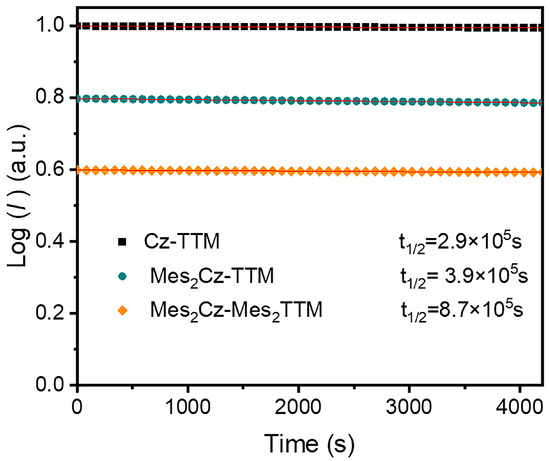
Figure 4.
Photostability of Cz-TTM, Mes2Cz-TTM, and Mes2Cz-Mes2TTM.
2.4. Electrochemistry
Cyclic voltammetry (CV) was performed to assess the redox potentials of Mes2Cz-TTM and Mes2Cz-Mes2TTM. Both compounds exhibited reversible redox behavior in dichloromethane (Figure 5). In Mes2Cz-TTM, the standard reduction potential and oxidation potential (−0.99 V, 0.53 V) were lower than Cz-TTM (−0.98 V, 0.55 V). The minimal decrease in potential was attributed to the weak electron-donating effect of the Mes fragments due to the torsion angles between the carbazole and Mes. The reduction/oxidation peaks also shifted to obviously lower potentials (−1.40 V/0.26 V) in Mes2Cz-Mes2TTM. The result was attributed to the direct connection between Mes and the TTM radical core, which decreases its electron-deficient character. The energy levels of the α-SOMO and β-SUMO were calculated based on the redox potentials. Based on results, the energy levels (α-SOMO, β-SUMO) of Mes2Cz-TTM (−5.33 V, −3.81 V) were higher than Cz-TTM (−5.35 V, −3.82 V). Mes2Cz-Mes2TTM (−5.06 V, −3.40 V) was significantly higher than Cz-TTM and Mes2Cz-TTM. The data suggested that the donor and acceptor substitutions increased the frontier energy levels of the radicals. The 20 cycles of CV scans demonstrated that both radicals had excellent electrochemical stability (Figure S8).
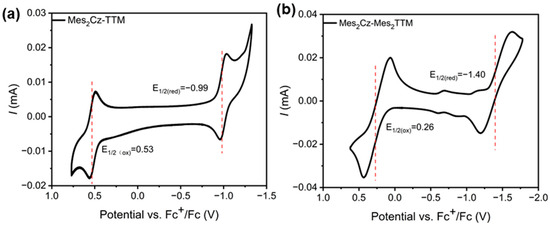
Figure 5.
Cyclic voltammetry (CV) curves of Mes2Cz-TTM (a) and Mes2Cz-Mes2TTM (b) in dichloromethane. (The red lines indicate the positions of the half-wave potential.)
2.5. Theoretical Calculations
To investigate the effects of donor and acceptor substitutions on the frontier molecular orbitals (MOs) and electron density distribution of Cz-TTM type radicals, density functional theory (DFT) calculations (B3LYP/6-31G(d,p)) were performed. The optimized structures of Cz-TTM, Mes2Cz-TTM, and Mes2Cz-Mes2TTM revealed negligible change (Tables S2 and S3). The donor and acceptor substitutions did not significantly affect the structure of the Cz-TTM core. Although the dihedral angles between Mes and the aromatic nuclei of carbazole or the TTM radical core remained distorted, the donor and acceptor substitutions also resulted in a modest shift in the absorption and emission bands.
Mes2Cz-TTM and Mes2Cz-Mes2TTM exhibited similar spin-density distributions (Figure S9). The unpaired electron, primarily located on the TTM radical core and partially extending to the carbazole unit, was not obviously affected by the donor and acceptor substitutions. The MOs of Cz-TTM, Mes2Cz-TTM, and Mes2Cz-Mes2TTM showed that the positions of the singly occupied molecular orbital (α-SOMO) and singly unoccupied molecular orbital (β-SUMO) remained largely unchanged. The α-SOMO was mainly located on the TTM radical core and the carbazole unit, while the β-SUMO was distributed over the TTM radical core, with slight extension to the carbazole unit (Figure 6). The energy levels of the α-SOMO and β-SUMO in Mes2Cz-TTM (−5.42 eV, −3.36 eV) were slightly higher than Cz-TTM (−5.45 eV, −3.36 eV) with the substitution on the carbazole unit. Mes2Cz-Mes2TTM exhibited significantly higher α-SOMO (−5.25 eV) and β-SUMO (−3.06 eV) energy levels due to the additional substitution on the TTM radical acceptor. The results were consistent with the electrochemistry. Time-dependent DFT (TD-DFT) calculations (B3LYP/6-31G(d,p)) were also performed to further explore the excited states. The results showed that the doublet excited states (D1) of Cz-TTM, Mes2Cz-TTM, and Mes2Cz-Mes2TTM originated from the 171β→172β, 235β→236β, and 283β→284β transitions, respectively (Table S4). The increasing trend in energy levels (Cz-TTM < Mes2Cz-TTM < Mes2Cz-Mes2TTM) and transition energies of D1 (Mes2Cz-TTM < Cz-TTM < Mes2Cz-Mes2TTM) confirmed the shift trend in the emission bands.
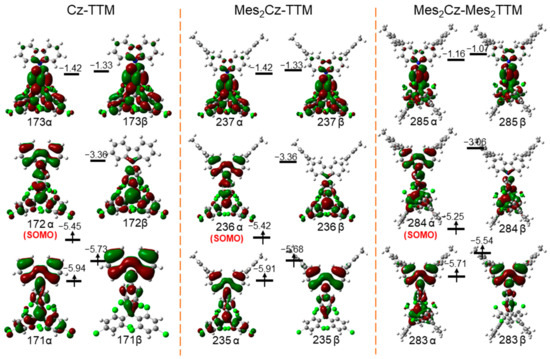
Figure 6.
Frontier molecular orbitals calculated by DFT calculations of Cz-TTM, Mes2Cz-TTM, and Mes2Cz-Mes2TTM. (Red and dark green distributions on molecule represent electron cloud distributions; green, blue, gray, and white balls represent Cl, N, C, and H atoms, respectively.)
3. Materials and Methods
Mes2Cz-TTM and Mes2Cz-Mes2TTM radicals were prepared according to the detailed synthesis routes in Supporting Information. All raw materials and chemical reagents used in this work were procured from ERNEGI and Xilong Science Co., Ltd. (Shanghai, China) without further purification. The mass spectra measurement was performed on MALDI-TOF (ion source 1: 20 kV, ion source 2: 17.65 kV, lens: 8 kV, reflector: 20.98 kV, reflector 2: 11.09 kV, pulsed ion extraction: 170 ns, DCTB as the matrix in the MALDI-TOF testing). Infrared (IR) spectra were collected on a BRUKER TENSOR 27 spectrophotometer (Bruker Corporation, Bruck, Germany) using KBr pellets. The EPR testing was finished with Bruker A320 spectrometer (Bruker Corporation, Bruck, Germany) (radicals in solid state and 0.1 M dichloromethane solution, sweep width: 100 G, frequency: 9.852902 GHz, power: 19.83 mW, resolution in X: 1024, number of X-scans: 1, temperature: room temperature). The UV-Vis spectra were obtained with a Shimadzu UV-1900i UV-Vis spectrometer (Shimadzu (Suzhou) Instruments Co., Ltd., Suzhou, China) (slit width: 2 um). The photoluminescence spectra were acquired with the Shimadzu RF-6000 spectrometer (Shimadzu Corporation, Kyoto, Japan) (excitation bandwidth: 3.0 nm; emission bandwidth: 3.0 nm; scan speed: 600 nm/min). The PL decays were measured on the Edinburgh FLS1000 spectrometer (Edinburgh Company, Edinburgh, UK), and the absolute PLQEs were measured on the identical apparatus via the integrating sphere method (slit width: 50 um). The DFT and TD-DFT calculations were run on Gaussian16 C.02 commercial software [47]. The TGA tests in air and nitrogen atmospheres were performed with TA INSTRUMENTS Q600 instrument (PERKINELMER, Waltham, MA, USA) (heating rate: 10 °C/min). The electrochemistry data were obtained with CH Instruments CHI660E electrochemical analyzer (scan rate: 300 mVs−1; supporting electrolyte: 0.1 M (Bu4N)PF6; working, counter, and reference electrodes: glassy graphite, platinum, and Ag/AgCl; ferrocene was added as an internal standard in the measurement). The photostability was tested under continuous xenon lamp irradiation, using the Shimadzu RF-6000 spectrometer (Shimadzu Corporation, Kyoto, Japan) (excitation bandwidth: 3.0 nm, emission bandwidth: 3.0 nm, integration time: 10 ms).
3.1. Synthesis of Compound Mes2Cz-TTM
Mes2Cz-TTM: MALDI-TOF (m/z): [M]+ calcd. for C49H34Cl8N˙, 920.42; found, 920.75. IR(KRI): 2960(s), 2925(m), 2867(m), 1610(m), 1575(m), 1504(m), 1458(m), 1371(m), 1319(w), 1265(m), 1243(w), 1189(w), 1083(w), 1035(w), 877(m), 804(m).
3.2. Synthesis of Compound Mes2Cz-Mes2TTM
Mes2Cz-Mes2TTM: MALDI-TOF (m/z): [M]+ calcd. for C67H56Cl6N˙, 1087.89; found, 1088.06. IR(KRI): 2952(m), 2920(m), 2856(w), 1612(m), 1577(m), 1506(s), 1473(m), 1375(m), 1284(w), 1263(w), 1220(w), 1188(w), 1033(w), 875(m), 852(m), 808(w), 742(w).
4. Conclusions
In summary, we successfully designed and synthesized two Cz-TTM radical derivatives (Mes2Cz-TTM and Mes2Cz-Mes2TTM) by incorporating Mes groups into the carbazole donor and TTM radical acceptor units of Cz-TTM. The two radicals exhibited distinct luminescence efficiencies due to the different substitution positions. The sole donor substitution on the carbazole unit led to a reduced PLQE in Mes2Cz-TTM (39%). However, Mes2Cz-Mes2TTM achieved a significantly higher PLQE of 92.6%, which is notably greater than that of Cz-TTM (68%). The introduction of substitutions on the donor and acceptor sites also improved the thermostability and photostability. Theoretical calculations were carried out to explain the differences in the luminescent properties. This work demonstrates that simple modifications on the donor and acceptor units in radicals with donor–acceptor structure are effective in enhancing the PLQE of luminescent radicals.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/molecules30061191/s1, Figure S1: The synthetic routes of Mes2Cz-TTM and Mes2Cz-Mes2TTM; Figure S2: Mass spectrometry of Mes2Cz-TTM and Mes2Cz-Mes2TTM; Figure S3: FT-IR spectra of Mes2Cz-TTM and Mes2Cz-Mes2TTM; Figure S4: EPR spectra of Mes2Cz-TTM and Mes2Cz-Mes2TTM in dichloromethane solution and powder at room temperature. Figure S5: UV-Vis absorption spectra of Mes2Cz-TTM in solutions of different polarities (10−5 M); Figure S6: UV-Vis absorption spectra of Mes2Cz-Mes2TTM in solutions of different polarities (10−5 M); Figure S7: TGA curve of Mes2Cz-TTM and Mes2Cz-Mes2TTM; Figure S8: Cyclic voltammetry (CV) curves of Mes2Cz-TTM and Mes2Cz-Mes2TTM for multiple (20-turn) cycles.; Figure S9: Spin densities of Cz-TTM, Mes2Cz-TTM and Mes2Cz-Mes2TTM by DFT calculations.; Table S1: Redox potentials and corresponding orbital energy levels calculated theoretically and measured experimentally of Cz-TTM, Mes2Cz-TTM, and Mes2Cz-Mes2TTM; Table S2: The values of characteristic torsion angles in radical molecules in theoretical calculations; Table S3: The bond lengths of radical molecules in theoretical calculations; Table S4: The parameters corresponding to the D1 transition in the TD-DFT calculation results of radicals.
Author Contributions
Conceptualization, L.Z. and X.A.; data curation, S.G., J.G., and L.Z.; formal analysis, S.G., J.G., and L.Z.; investigation, S.G., J.G., L.Z., and X.A.; methodology, S.G., J.G., and L.Z.; project administration, L.Z. and X.A.; supervision, L.Z. and X.A.; validation, L.Z. and X.A.; visualization, S.G., J.G., L.Z., and X.A.; writing—original draft, S.G., J.G., and L.Z.; writing—review and editing, S.G., J.G., L.Z., and X.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (nos. 22105054 and 22265009), Hainan Provincial Natural Science Foundation of China (no. 222QN221), Collaborative Innovation Center Foundation of Hainan University (no. XTCX2022XXC02), South China Sea New Star Innovation Talent Platform Project (NHXXRCXM202307), and the Hainan University Start-Up Fund.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material; further inquiries can be directed to the corresponding author.
Acknowledgments
S.G., J.G., L.Z., and X.A. are grateful for the financial support, as detailed above. The authors are thankful for the support from the Analytical and Testing Center of Hainan University.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Zheng, W.; Li, X.; Baryshnikov, G.V.; Shan, X.; Siddique, F.; Qian, C.; Zhao, S.; Wu, H. Bright Free-Radical Emission in Ionic Liquids. Angew. Chem. Int. Ed. 2023, 62, e202305925. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Gong, J.; Alam, P.; Ma, C.; Wang, Y.; Guo, J.; Zeng, Z.; He, Z.; Sung, H.H.Y.; Williams, I.D.; et al. A Simple Approach to Achieve Organic Radicals with Unusual Solid-State Emission and Persistent Stability. CCS Chem. 2022, 4, 1912–1920. [Google Scholar] [CrossRef]
- Wang, Z.; Zou, X.; Xie, Y.; Zhang, H.; Hu, L.; Chan, C.C.S.; Zhang, R.; Guo, J.; Kwok, R.T.K.; Lam, J.W.Y.; et al. A nonconjugated radical polymer with stable red luminescence in the solid state. Mater. Horiz. 2022, 9, 2564–2571. [Google Scholar] [CrossRef] [PubMed]
- She, P.; Qin, Y.; Zhou, Y.; Zheng, X.; Li, F.; Liu, S.; Ma, Y.; Zhao, Q.; Wong, W.Y. Photoactivated Circularly Polarized Luminescent Organic Radicals in Doped Amorphous Polymer. Angew. Chem. Int. Ed. 2024, 63, e202403660. [Google Scholar] [CrossRef]
- Mu, Y.; Liu, Y.; Tian, H.; Ou, D.; Gong, L.; Zhao, J.; Zhang, Y.; Huo, Y.; Yang, Z.; Chi, Z. Sensitive and Repeatable Photoinduced Luminescent Radicals from A Simple Organic Crystal. Angew. Chem. Int. Ed. 2021, 60, 6367–6371. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, Y.L.; Chen, C.; Ren, Y.Y.; Han, Y.F. A platform for blue-luminescent carbon-centered radicals. Nat. Commun. 2022, 13, 5367. [Google Scholar] [CrossRef]
- Abdurahman, A.; Hele, T.J.H.; Gu, Q.; Zhang, J.; Peng, Q.; Zhang, M.; Friend, R.H.; Li, F.; Evans, E.W. Understanding the luminescent nature of organic radicals for efficient doublet emitters and pure-red light-emitting diodes. Nat. Mater. 2020, 19, 1224–1229. [Google Scholar] [CrossRef]
- Zhu, Z.; Kuang, Z.; Shen, L.; Wang, S.; Ai, X.; Abdurahman, A.; Peng, Q. Dual Channel Emissions of Kasha and Anti-Kasha from a Single Radical Molecule. Angew. Chem. Int. Ed. 2024, 63, e202410552. [Google Scholar]
- Zhao, Y.; Abdurahman, A.; Zhang, Y.; Zheng, P.; Zhang, M.; Li, F. Highly Efficient Multifunctional Luminescent Radicals. CCS Chem. 2022, 4, 722–731. [Google Scholar] [CrossRef]
- Yuan, J.W.; Peng, Q.C.; Fu, J.C.; Yang, Q.; Gao, Z.Y.; Wang, Z.Y.; Li, K.; Zang, S.Q.; Tang, B.Z. Highly Efficient Stable Luminescent Radical-Based X-ray Scintillator. J. Am. Chem. Soc. 2023, 145, 27095–27102. [Google Scholar] [CrossRef]
- Yan, C.; An, D.; Chen, W.; Zhang, N.; Qiao, Y.; Fang, J.; Lu, X.; Zhou, G.; Liu, Y. Stable Diarylamine-Substituted Tris(2,4,6-trichlorophenyl)methyl Radicals: One-Step Synthesis, Near-Infrared Emission, and Redox Chemistry. CCS Chem. 2022, 4, 3190–3203. [Google Scholar] [CrossRef]
- Rui, X.; Ota, W.; Sato, T.; Furukori, M.; Nakayama, Y.; Hosokai, T.; Hisamura, E.; Nakamura, K.; Matsuda, K.; Nakao, K.; et al. Carbazole-Dendronized Luminescent Radicals. Angew. Chem. Int. Ed. 2023, 62, e202302550. [Google Scholar]
- Shi, J.; Xu, W.; Yu, H.; Wang, X.; Jin, F.; Zhang, Q.; Zhang, H.; Peng, Q.; Abdurahman, A.; Wang, M. A Highly Luminescent Metallo-Supramolecular Radical Cage. J. Am. Chem. Soc. 2023, 145, 24081–24088. [Google Scholar] [CrossRef]
- Peng, Q.; Obolda, A.; Zhang, M.; Li, F. Organic Light-Emitting Diodes Using a Neutral pi Radical as Emitter: The Emission from a Doublet. Angew. Chem. Int. Ed. 2015, 54, 7091–7095. [Google Scholar] [CrossRef] [PubMed]
- Mayorga Burrezo, P.; Jimenez, V.G.; Blasi, D.; Ratera, I.; Campana, A.G.; Veciana, J. Organic Free Radicals as Circularly Polarized Luminescence Emitters. Angew. Chem. Int. Ed. 2019, 58, 16282–16288. [Google Scholar] [CrossRef]
- Li, F.; Gillett, A.J.; Gu, Q.; Ding, J.; Chen, Z.; Hele, T.J.H.; Myers, W.K.; Friend, R.H.; Evans, E.W. Singlet and triplet to doublet energy transfer: Improving organic light-emitting diodes with radicals. Nat. Commun. 2022, 13, 2744. [Google Scholar] [CrossRef]
- Gamero, V.; Velasco, D.; Latorre, S.; López-Calahorra, F.; Brillas, E.; Juliá, L. [4-(N-Carbazolyl)-2,6-dichlorophenyl]bis(2,4,6-trichlorophenyl)methyl radical an efficient red light-emitting paramagnetic molecule. Tetrahedron Lett. 2006, 47, 2305–2309. [Google Scholar] [CrossRef]
- Cho, H.H.; Gorgon, S.; Hung, H.C.; Huang, J.Y.; Wu, Y.R.; Li, F.; Greenham, N.C.; Evans, E.W.; Friend, R.H. Efficient and Bright Organic Radical Light-Emitting Diodes with Low Efficiency Roll-Off. Adv. Mater. 2023, 35, e2303666. [Google Scholar] [CrossRef]
- Chang, X.; Arnold, M.E.; Blinder, R.; Zolg, J.; Wischnat, J.; van Slageren, J.; Jelezko, F.; Kuehne, A.J.C.; von Delius, M. A Stable Chichibabin Diradicaloid with Near-Infrared Emission. Angew. Chem. Int. Ed. 2024, 63, e202404853. [Google Scholar] [CrossRef]
- Abdurahman, A.; Wang, J.; Zhao, Y.; Li, P.; Shen, L.; Peng, Q. A Highly Stable Organic Luminescent Diradical. Angew. Chem. Int. Ed. 2023, 62, e202300772. [Google Scholar] [CrossRef]
- Velasco, D.; Castellanos, S.; López, M.; López-Calahorra, F.; Brillas, E.; Juliá, L. Red organic light-emitting radical adducts of carbazole and tris (2, 4, 6-trichlorotriphenyl) methyl radical that exhibit high thermal stability and electrochemical amphotericity. J. Org. Chem. 2007, 72, 7523–7532. [Google Scholar] [CrossRef]
- Ballester, M.; Castaner, J.; Riera, J.; Ibanez, A.; Pujadas, J. Inert carbon free radicals. 2. Monofunctionalized tetradecachlorotriphenylmethyl radicals and related compounds. J. Org. Chem. 1982, 47, 259–264. [Google Scholar] [CrossRef]
- Heckmann, A.; Dümmler, S.; Pauli, J.; Margraf, M.; Köhler, J.; Stich, D.; Lambert, C.; Fischer, I.; Resch-Genger, U. Highly fluorescent open-shell NIR dyes: The time-dependence of back electron transfer in triarylamine-perchlorotriphenylmethyl radicals. J. Phys. Chem. C 2009, 113, 20958–20966. [Google Scholar] [CrossRef]
- Guo, H.; Peng, Q.; Chen, X.K.; Gu, Q.; Dong, S.; Evans, E.W.; Gillett, A.J.; Ai, X.; Zhang, M.; Credgington, D.; et al. High stability and luminescence efficiency in donor-acceptor neutral radicals not following the Aufbau principle. Nat. Mater. 2019, 18, 977–984. [Google Scholar] [CrossRef]
- Matsuoka, R.; Kimura, S.; Kusamoto, T. Solid-State Room-Temperature Near-Infrared Photoluminescence of a Stable Organic Radical. ChemPhotoChem 2021, 5, 669–673. [Google Scholar] [CrossRef]
- Kimura, S.; Tanushi, A.; Kusamoto, T.; Kochi, S.; Sato, T.; Nishihara, H. A luminescent organic radical with two pyridyl groups: High photostability and dual stimuli-responsive properties, with theoretical analyses of photophysical processes. Chem. Sci. 2018, 9, 1996–2007. [Google Scholar] [CrossRef] [PubMed]
- Kimura, S.; Kusamoto, T.; Kimura, S.; Kato, K.; Teki, Y.; Nishihara, H. Magnetoluminescence in a Photostable, Brightly Luminescent Organic Radical in a Rigid Environment. Angew. Chem. Int. Ed. 2018, 57, 12711–12715. [Google Scholar] [CrossRef]
- Kato, K.; Kimura, S.; Kusamoto, T.; Nishihara, H.; Teki, Y. Luminescent Radical-Excimer: Excited-State Dynamics of Luminescent Radicals in Doped Host Crystals. Angew. Chem. Int. Ed. 2019, 58, 2606–2611. [Google Scholar] [CrossRef]
- Hattori, Y.; Kusamoto, T.; Nishihara, H. Highly photostable luminescent open-shell (3,5-dihalo-4-pyridyl)bis(2,4,6-trichlorophenyl)methyl radicals: Significant effects of halogen atoms on their photophysical and photochemical properties. RSC Adv. 2015, 5, 64802–64805. [Google Scholar] [CrossRef]
- Hattori, Y.; Kusamoto, T.; Nishihara, H. Enhanced luminescent properties of an open-shell (3,5-dichloro-4-pyridyl)bis(2,4,6-trichlorophenyl)methyl radical by coordination to gold. Angew. Chem. Int. Ed. 2015, 54, 3731–3734. [Google Scholar] [CrossRef]
- Hattori, Y.; Kusamoto, T.; Nishihara, H. Luminescence, Stability, and Proton Response of an Open-Shell (3,5-Dichloro-4-pyridyl)bis(2,4,6-trichlorophenyl)methyl Radical. Angew. Chem. 2014, 126, 12039–12042. [Google Scholar] [CrossRef]
- Wu, C.; Lu, C.; Yu, S.; Zhang, M.; Zhang, H.; Zhang, M.; Li, F. Highly Efficient Near-Infrared Luminescent Radicals with Emission Peaks over 750 nm. Angew. Chem. Int. Ed. 2024, 63, e202412483. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Zhang, L.; Cui, Z.; Ai, X. Improving the Luminescence and Stability of Carbon-Centered Radicals by Kinetic Isotope Effect. Molecules 2023, 28, 4805. [Google Scholar] [CrossRef]
- Ai, X.; Chen, Y.; Feng, Y.; Li, F. A Stable Room-Temperature Luminescent Biphenylmethyl Radical. Angew. Chem. Int. Ed. 2018, 57, 2869–2873. [Google Scholar] [CrossRef] [PubMed]
- Murto, P.; Li, B.; Fu, Y.; Walker, L.E.; Brown, L.; Bond, A.D.; Zeng, W.; Chowdhury, R.; Cho, H.H.; Yu, C.P.; et al. Steric Control of Luminescence in Phenyl-Substituted Trityl Radicals. J. Am. Chem. Soc. 2024, 146, 13133–13141. [Google Scholar] [CrossRef]
- Murto, P.; Chowdhury, R.; Gorgon, S.; Guo, E.; Zeng, W.; Li, B.; Sun, Y.; Francis, H.; Friend, R.H.; Bronstein, H. Mesitylated trityl radicals, a platform for doublet emission: Symmetry breaking, charge-transfer states and conjugated polymers. Nat. Commun. 2023, 14, 4147. [Google Scholar] [CrossRef]
- Lu, C.; Cho, E.; Cui, Z.; Gao, Y.; Cao, W.; Bredas, J.L.; Coropceanu, V.; Li, F. Towards Efficient and Stable Donor-Acceptor Luminescent Radicals. Adv. Mater. 2023, 35, e2208190. [Google Scholar] [CrossRef]
- Liu, C.H.; Hamzehpoor, E.; Sakai-Otsuka, Y.; Jadhav, T.; Perepichka, D.F. A Pure-Red Doublet Emission with 90% Quantum Yield: Stable, Colorless, Iodinated Triphenylmethane Solid. Angew. Chem. Int. Ed. 2020, 59, 23030–23034. [Google Scholar] [CrossRef]
- Hattori, Y.; Kitajima, R.; Ota, W.; Matsuoka, R.; Kusamoto, T.; Sato, T.; Uchida, K. The simplest structure of a stable radical showing high fluorescence efficiency in solution: Benzene donors with triarylmethyl radicals. Chem. Sci. 2022, 13, 13418–13425. [Google Scholar] [CrossRef]
- Hattori, Y.; Kitajima, R.; Baba, A.; Yamamoto, K.; Matsuoka, R.; Kusamoto, T.; Uchida, K. Effects of hydrocarbon substituents on highly fluorescent bis(4-phenylphenyl)pyridylmethyl radical derivatives. Mater. Adv. 2023, 4, 5149–5159. [Google Scholar] [CrossRef]
- Gou, Q.; Guan, J.; Zhang, L.; Ai, X. Phenyl Derivatives Modulate the Luminescent Properties and Stability of CzBTM-Type Radicals. Molecules 2024, 29, 2900. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Arnold, M.; Kittel, Y.; Blinder, R.; Jelezko, F.; Kuehne, A.J.C. 2,7-Substituted N-Carbazole Donors on Tris(2,4,6-trichlorophenyl)methyl Radicals with High Quantum Yield. Adv. Opt. Mater. 2022, 10, 2102101. [Google Scholar] [CrossRef]
- Ai, X.; Evans, E.W.; Dong, S.; Gillett, A.J.; Guo, H.; Chen, Y.; Hele, T.J.H.; Friend, R.H.; Li, F. Efficient radical-based light-emitting diodes with doublet emission. Nature 2018, 563, 536–540. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, K.; Xiaotian, R.; Nakamura, K.; Furukori, M.; Hosokai, T.; Anraku, K.; Nakao, K.; Albrecht, K. Photostability of luminescent tris(2,4,6-trichlorophenyl)methyl radical enhanced by terminal modification of carbazole donor. Chem. Commun. 2022, 58, 13443–13446. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, K.; Matsuda, K.; Xiaotian, R.; Furukori, M.; Miyata, S.; Hosokai, T.; Anraku, K.; Nakao, K.; Albrecht, K. Effects of halogen atom substitution on luminescent radicals: A case study on tris(2,4,6-trichlorophenyl)methyl radical-carbazole dyads. Faraday Discuss. 2024, 250, 192–201. [Google Scholar] [CrossRef]
- Lu, C.; Cho, E.; Wan, K.; Wu, C.; Gao, Y.; Coropceanu, V.; Brédas, J.L.; Li, F. Achieving Nearly 100% Photoluminescence Quantum Efficiency in Organic Radical Emitters by Fine-Tuning the Effective Donor-Acceptor Distance. Adv. Funct. Mater. 2024, 34, 2314811. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16 Rev. C.02; Gaussian, Inc.: Wallingford, CT, USA, 2019. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).