Abstract
To discover novel fungicides with good inhibitory effects on plant fungal diseases, twenty-five 3-indolyl-3-hydroxy oxindole derivatives (3a–3y) were synthesized. These newly derivatives were characterized by NMR and HRMS. Their antifungal activities against five plant pathogenic fungi were assessed in vitro. Most of the compounds exhibited moderate to excellent antifungal activities against the five pathogenic fungi. Notably, compounds 3t, 3u, 3v, and 3w displayed remarkable and broad-spectrum antifungal activities comparable to or superior to those of the fungicides carvacrol (CA) and phenazine-1-carboxylic acid (PCA). Among them, compound 3u displayed the most excellent antifungal activity against Rhizoctonia solani Kühn (R. solani), with an EC50 of 3.44 mg/L, which was superior to CA (7.38 mg/L) and PCA (11.62 mg/L). Preliminary structure–activity relationship (SAR) results indicated that the introduction of I, Cl, or Br substituents at position 5 of the 3-hydroxy-2-oxindole and indole rings is crucial for compounds to exhibit good antifungal activity. The in vivo antifungal activity assay showed that compound 3u has good curative effects against R. solani. The current results suggest that these compounds are capable of serving as promising lead compounds.
1. Introduction
Plant pathogenic fungi pose a severe threat to global agriculture and food security, causing devastating yield losses and substantial economic damage through diseases such as rice sheath blight (caused by Rhizoctonia solani), rice blast (Pyricularia oryzae/Magnaporthe oryzae), gray mold (Botrytis cinerea), southern corn leaf blight (Bipolaris maydis), and anthracnose (Colletotrichum gloeosporioides) [1,2]. These pathogens employ diverse infection strategies: R. solani, a soil-borne fungus, persists in soil for extended periods and infects multiple plant tissues, leading to widespread destruction of cereals, legumes, and forage crops [3,4]. P. oryzae targets all aerial parts of rice plants throughout their growth cycle [5], while B. maydis severely impairs photosynthetic efficiency by damaging functional leaves, with yield losses exceeding 58% in susceptible corn varieties [6,7]. Notably, C. gloeosporioides and B. cinerea not only reduce postharvest quality but also accelerate the evolution of fungicide resistance through their rapid adaptive mechanisms [8,9,10]. Although chemical fungicides remain the primary control strategy, their prolonged application has exacerbated critical issues such as pesticide resistance, residue accumulation, and ecological degradation [11,12,13]. Consequently, there is an urgent demand for eco-friendly alternatives with novel modes of action to achieve sustainable crop protection and mitigate reliance on conventional agrochemicals [14].
Natural products are instrumental in the development of new pesticides, offering numerous models and templates for their design [15]. The 3-hydroxy-2-oxindole structural unit has been identified in various bioactive natural products and molecules (Figure 1), including convolutamydine A, donaxaridine, dioxibrassinine, arundaphine, maremycin, and cladoquinazoline [16,17,18,19,20]. These compounds exhibit anti-tumor, anti-cancer, antioxidant, anti-inflammatory, and proteasome inhibition activities [16,17,20]. Although research on the antifungal activity of 3-hydroxy-2-oxoindole compounds is relatively scarce, there are still some literature reports on the inhibitory effects of these compounds on fungi. For example, the maremycin analogue isolated from the endophytic actinobacterium associated with infected soybeans and obtained from the Streptomyces capitiformicae (KX777629) strain DDPA2-14 exhibits high activity against Sclerotinia sclerotiorum, with an EC50 value of 3.70 mg/L [21].
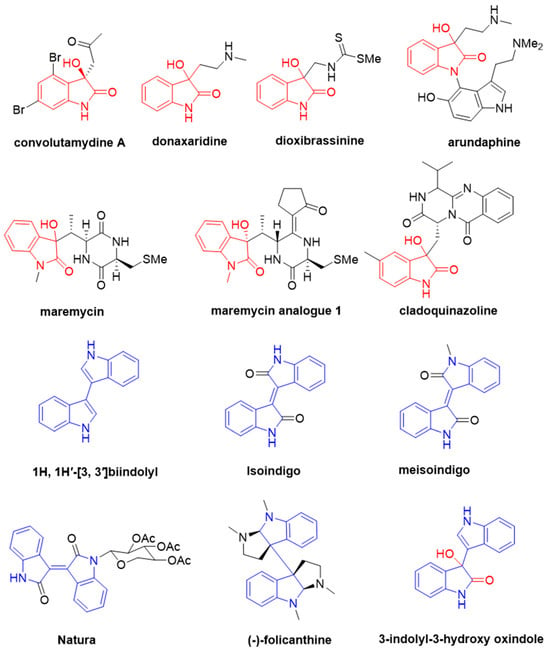
Figure 1.
Representative natural 3-hydroxy-2-oxindole and bisindole alkaloids.
Bisindole alkaloids, composed of two monomeric indole alkaloid units and widely distributed in nature primarily as metabolites produced by terrestrial and marine organisms (Figure 1) [22,23], exhibit a wide array of biological and pharmacological activities, including antibacterial [24,25] and antifungal properties [26]. Importantly, bisindole alkaloids tend to exhibit higher biological activity compared to their individual monomers [27]. The 3,3′-bisindole motif is a crucial structural component in bisindole alkaloids, a class of natural products known for their complex molecular structures and promising biological activities [28,29]. This class of natural products encompasses 1H, 1H′-[3, 3′]biindolyl, isoindigo, and hexahydropyrroloindole, among others (Figure 1). 1H, 1H′-[3, 3′]biindolyl, a natural product from the terrestrial fungus Gliocladium catenulatum, has specific antibacterial activity against the honey bee enthomopathogenic bacterium P. larvae. As a compound structurally isomeric with the well-known indigo, isoindigo not only enjoys widespread applications in the pharmaceutical field but also serves as a critical component in various functional materials [30,31]. Its derivatives, such as meisoindigo, have been applied in the treatment of chronic myeloid leukemia in China [32]. Natura is an efficient inhibitor of cell cycle-dependent kinases (CDKs) [33]. The hexahydropyrroloindole-type compound (-)-folicanthine was isolated from the active methanol extract of the seeds of Chimonanthus praecox Link and exhibits significant inhibitory activity against five plant pathogenic fungi: Exserohilum turcicum, Bipolaris maydis, Alternaria solani, Sclerotinia sclerotiorum, and Fusarium oxysporum [34].
3-Indolyl-3-hydroxy oxindoles, which consist of a bisindole and a 3-hydroxy-2-oxindole unit, are significant substrates for the investigation of biological activities [35] and serve as valuable synthetic intermediates for drug candidates and alkaloids [36]. These compounds have demonstrated notable anti-proliferative effects against various cancer cell lines, including leukemia (U937, THP-1), lung (A549), and breast cancer (MCF7) cells [37]. Polymethylene-linked 3-indolyl-3-hydroxy-2-oxindole dimers exhibit selectivity as butyrylcholinesterase (BChE) inhibitors [38]. Research into other biological activities is currently less reported. Recently, we reported that compounds bearing a bisindole structure, specifically bis(indolyl)-hydrazide-hydrazone derivatives, demonstrated potent antifungal activity against various plant fungal diseases [39]. In this study, we fused the 3-hydroxy oxindole and bisindole structures to synthesize 25 3-indolyl-3-hydroxy oxindoles and assessed their antifungal properties against five pathogenic fungi: Rhizoctonia solani Kühn (R. solani), Pyricularia oryzae Cav. (P. oryzae), Colletotrichum gloeosporioides Penz. (C. gloeosporioides), Botrytis cinerea Pers.:Fr. (B. cinerea), and Bipolaris maydis (Nishik.) Shoemaker (B. maydis). Among the synthesized compounds, 3u demonstrated the most potent antifungal activity against R. solani, with an efficacy surpassing both carvacrol (CA) and the commercial fungicide phenazine-1-carboxylic acid (PCA). To further explore its practical application potential, the in vivo antifungal performance of compound 3u was assessed using a broad bean leaf bioassay, where PCA served as the positive control. Additionally, we analyzed the structure–activity relationship of these compounds.
2. Results
2.1. Chemistry
As shown in Scheme 1, the general synthetic route for the preparation of 3-indolyl-3-hydroxy oxindole derivatives (3a–3y) is consistent with the method reported by Prathima et al. [38]. This method uses water as the solvent and diethanolamine as the catalyst to synthesize R-configured 3-indolyl-3-hydroxy oxindole derivatives from isatin (1) and indole (2). A total of twenty-five synthesized derivatives (3a–3y) were prepared, with yields ranging from 28% to 90%.
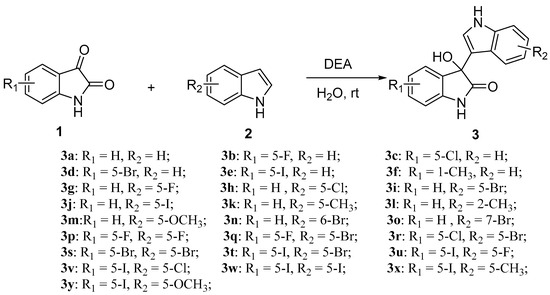
Scheme 1.
Synthesis route for 3-indolyl-3-hydroxy oxindoles.
2.2. In Vitro Antifungal Activity
The inhibitory effects of 3-indolyl-3-hydroxy oxindole derivatives on five plant pathogenic fungi are presented in Table 1. The antifungal activities of the target compounds were assessed in vitro against the mycelium growth of the phytopathogens R. solani, P. oryzae, C. gloeosporioides, B. cinerea, and B. maydis at concentrations of 50 mg/L, using the commercialized fungicides carvacrol (CA) and phenazine-1-carboxylic acid (shenqinmycin, PCA) as positive controls. The results showed that most of the synthesized compounds exhibited moderate antifungal activity against the five tested fungi. According to the data presented in Table 1, compound 3u showed favorable antifungal activities against R. solani, with inhibition rates of 100% at 50 mg/L, which was superior to that of CA (91.56%) and PCA (81.07%). The inhibitory activity of compounds 3t (82.48%) and 3w (87.37%) is comparable to that of the positive control. For P. oryzae, compounds 3t, 3u, 3v, and 3w demonstrated good antifungal activities (inhibition rate > 75%), which were better than that of carvacrol (74.94%) and PCA (53.64%) at 50 mg/L. The compounds 3t and 3w exhibited moderate inhibitory activity against C. gloeosporioides at a concentration of 50 mg/L, with inhibition rates of 61.62% and 66.67%, respectively. This performance is comparable to that of CA (62.65%) but lower than that of PCA (77.96%). Three compounds exhibited inhibition rates against B. cinerea that exceeded 80% at a concentration of 50 mg/L. Among them, compound 3u achieved an inhibition rate of 91.05%, which was superior to that of CA (84.38%) and PCA (81.86%). Compounds 3h and 3i showed inhibition rates of 80.82% and 80.42%, respectively, which were comparable to those of CA and PCA. Regarding B. maydis, the four compounds 3t, 3u, 3v, and 3w exhibited significant antifungal activity, with inhibition rates of 86.56%, 81.13%, 92.22%, and 89.85%, respectively, at a concentration of 50 mg/L, which is superior to CA’s 64.36% and comparable to PCA’s 98.01%. In general, compounds 3t, 3u, 3v, and 3w exhibited noteworthy broad-spectrum antifungal activities against the five fungi.

Table 1.
Preliminary in vitro antifungal activity of compounds against five fungi at 50 mg/L.
To more thoroughly study the inhibitory performance of the target compounds on plant pathogenic fungi, the EC50 values of the compounds with favorable inhibition rates at a concentration of 50 mg/L were tested. As shown in Table 2, the title compounds showed good antifungal activities against R. solani, P. oryzae, B. cinerea, and B. maydis. For R. solani, compound 3u demonstrated the highest inhibitory activity, with an EC50 of 3.44 mg/L, which was superior to CA (7.38 mg/L) and PCA (11.62 mg/L). Compounds 3v and 3w exhibited EC50 values of 14.72 and 15.69 mg/L for P. oryzae, respectively, which were superior to CA and PCA (25.30 and 64.53 mg/L, respectively). Compounds 3h and 3u showed EC50 values of 12.05 and 11.89 mg/L for B. cinerea, respectively, which were superior to CA and PCA (21.36 and 14.75 mg/L, respectively). Compounds 3t, 3u, 3v, and 3w demonstrated EC50 values of 13.62, 12.76, 13.47, and 10.55 mg/L, respectively, for B. maydis, which were all superior to CA (18.58 mg/L) and higher than PCA (3.09 mg/L). It is noteworthy that compounds 3t, 3u, 3v, and 3w exhibited broad-spectrum antifungal activities that were comparable to or superior to those of the fungicides CA and PCA, thereby qualifying them as priority candidates for further study.

Table 2.
EC50 values (mg/L) of selected compounds against R. solani, P. oryzae, B. cinerea, and B. maydis in vitro.
2.3. In Vivo Antifungal Activity
As summarized in Table 2, compound 3u exhibited the most potent antifungal activity against R. solani, with an EC50 value of 3.44 mg/L, demonstrating superior efficacy to both carvacrol (CA; EC50 = 7.38 mg/L) and phenazine-1-carboxylic acid (PCA; EC50 = 11.62 mg/L).
To further investigate its practical potential, the in vivo antifungal performance of compound 3u was evaluated using a broad bean leaf bioassay, with the commercial fungicide PCA as the positive control. As illustrated in Figure 2 and Table 3, compound 3u displayed promising curative effects, achieving control efficacies of 58.41% and 81.93% at concentrations of 100 and 200 mg/L, respectively. Although these values were slightly lower than those of PCA (71.62% and 83.84% at the same concentrations), the high activity at 200 mg/L suggests comparable therapeutic potential under elevated dosage conditions.
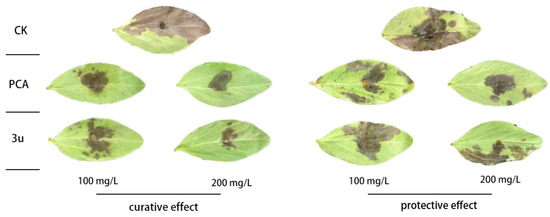
Figure 2.
In vivo protective effects of compound 3u and PCA (phenazine-1-carboxylic acid) against R. solani. CK, control.

Table 3.
Curative and protective activities of 3u against R. solani in vivo a,b.
In contrast, the protective efficacy of compound 3u was relatively limited, with control rates of 16.30% and 28.46% at 100 and 200 mg/L, respectively. This performance was significantly inferior to PCA, which showed protective efficacies of 53.43% and 61.18% at corresponding concentrations. The marked disparity between curative and protective effects implies that compound 3u may function primarily through direct antifungal action rather than systemic induction of plant defense mechanisms.
These findings highlight compound 3u as a promising candidate for therapeutic intervention against R. solani, while further structural optimization may be required to enhance its preventive capabilities.
3. Discussion
The target compounds were synthesized following the method reported by Prathima et al. [38], wherein diethanolamine was identified as an efficient catalyst for the aqueous-phase synthesis of 3-indolyl-3-hydroxy oxindole derivatives. While our synthetic yields (ranging from 28% to 90%) were moderately lower than those described in the literature (80–98%) [30], this discrepancy likely arose from losses during purification steps, particularly recrystallization and column chromatography. Given that our primary objective focused on evaluating the biological activity of these compounds, further optimization of synthetic yields was not pursued. Notably, although the method reported by Prathima et al. [38] demonstrated the catalytic superiority of diethanolamine over other amine catalysts in water, the underlying mechanism remains unexplored. We hypothesize that the emulsifying properties of diethanolamine may enhance the solubility of isatin and indole derivatives in aqueous media, thereby facilitating the electrophilic addition. Furthermore, the stereochemical configuration of the asymmetric carbon adjacent to the hydroxyl group was unambiguously assigned as R based on X-ray crystallographic data from analogous compounds reported by Prathima et al. [38].
By analyzing the results in Table 1, we found that the compounds studied in this work exhibited higher sensitivity to R. solani, P. oryzae, B. cinerea, and B. maydis compared to C. gloeosporioides. Based on the data listed in Table 1 and Table 2, we conducted an analysis of the in vitro structure–activity relationship of the target compounds. When the ring of the isatin fragment is substituted with Cl, Br, or I, the inhibitory activity of the compound is higher. In comparison, when the ring of the indole fragment is substituted with Cl and Br, the compound exhibits stronger antifungal activity; compounds with F, I, and other substituents typically have lower inhibitory activity (as shown in Figure 3). It was found that compounds with substitution at position 5 are the most active; for example, compound 3i (5-Br) showed a much higher inhibition rate against B. cinerea at 50 mg/L concentration than compounds 3n (6-Br) and 3o (7-Br). When both the isatin and indole rings have substituents, compounds composed of an I substituent at position 5 of the isatin and Cl, Br, and I substituents at position 5 of the indole exhibit excellent broad-spectrum antifungal activity. Compounds with other substituents generally have lower antifungal activity. These research results indicate that iodine substitution plays a crucial role in the antifungal activity of the compounds.
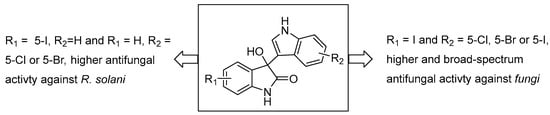
Figure 3.
In vitro structure–activity relationship of 3-indolyl-3-hydroxy oxindoles.
The structure–activity relationships (SARs) of bis-indole compounds have been extensively studied in multiple reports [40,41,42,43,44,45,46,47], and while some discrepancies exist among certain findings [40], the overall trend indicates that halogen-substituted bis-indole compounds generally exhibit enhanced antibacterial activity [43,44,45,46,47]. Our study demonstrates that indole-ring halogen-substituted compounds possess superior antifungal activity, consistent with the previous literature [43,44,45,46,47]. For example, Guo et al. observed that brominated nortopsentin analogues exhibited higher antifungal efficacy against Alternaria solani [45], while Rehberg et al. reported that 5-chlorinated bis-indole derivatives showed the strongest antibacterial activity against methicillin-resistant Staphylococcus aureus [46]. Similarly, Yan et al. found that F-, Cl-, and Br-substituted compounds displayed stronger antibacterial effects against two Gram-positive strains, Staphylococcus aureus and Bacillus subtilis. However, the influence of specific substitution positions on biological activity remains unclear; Huang et al. noted that 4-substituted bis-indole compounds exhibited stronger antifungal activity [39], whereas Guo et al. focused on substitutions at the 5th and 6th positions [45], and Rehberg et al. investigated substitution at the 5th position [46]. In contrast, Yan et al. observed potent antibacterial activity for halogenated compounds at positions 4, 5, 6, and 7 [47], while our results indicated that 5-substituted compounds exhibit better antifungal activity. Additionally, dihalogenated compounds often display enhanced biological activity [47], aligning with our findings. Notably, most previously reported bis-indole derivatives lacked iodine (I)-substituted analogues, whereas our study revealed that I-substituted compounds exhibit superior biological activity. These findings emphasize that both the specific position of substitution and the nature of the substituent significantly impact compound bioactivity, providing a promising avenue for the development of more effective antifungal agents.
4. Materials and Methods
4.1. General Information
All indole compounds were purchased from Shanghai Energy Chemical Technology Co., Ltd. (Shanghai, China). and Shanghai Adamas Beta Chemical Reagent Co., Ltd. (Shanghai, China). Shenqinmycin (phenazine-1-carboxylic acid, 98%) was purchased from Meryer (Shanghai) Biochemical Technology Co., Ltd. (Shanghai, China). Other reagents and solvents were of reagent grade or purified according to standard methods before use. Analytical thin-layer chromatography (TLC) was performed with silica gel plates using silica gel 60 GF254 (Qingdao Haiyang Chemical Co., Ltd., Qingdao, China). The 1H NMR (400 MHz) and 13C NMR (101 MHz) were recorded on an Bruker AVANCE NEO 400MHZ FT-NMR spectrometer (Bruker Corporation, Billerica, MA, USA) with DMSO-d6 as the solvent and TMS as the internal standard. 1H NMR and 13C NMR chemical shifts are reported in ppm (δ), with the solvent (DMSO-d6) peaks employed as the internal standard (3.33 ppm for 1H and 39.52 ppm for 13C). Data are reported as follows: chemical shift, multiplicity (s = singlet, brs = broad singlet, d = doublet, t = triplet, q = quartet, m = multiplet, dd = doublet of doublets, dt = doublet of triplets, and td = triplet of doublets), coupling constants (Hz), and integration. High-resolution mass spectra (HRMS) were determined with a Thermo Fisher Scientific Q Exactive Focus (Thermo Fisher Scientific Inc., Waltham, MA, USA).
4.2. Synthetic Procedures
The synthesis of 3-indolyl-3-hydroxy oxindole derivatives 3 was according to the method described by Prathima et al. [38]. At room temperature, indole (2 mmol) and diethanolamine (20 mol%) were slowly added to a solution of isatin (2 mmol) in water (8 mL). After the reaction was complete as monitored by TLC, the reaction mixture was extracted with ethyl acetate and washed with a brine solution. The organic layer was dried over anhydrous Na2SO4 and concentrated under reduced pressure to obtain a crude product, which was subsequently purified through recrystallization from ethanol or isolated via column chromatography to obtain the compound. The chemical structure of the title compounds was characterized and confirmed by 1H NMR, 13C NMR, and HRMS. The characterization data of compounds 3a–3y are listed as follows:
3-hydroxy-3-(1H-indol-3-yl)indolin-2-one (3a). Isolated yield: 68%; orange-yellow solid; 1H NMR (400 MHz, DMSO-d6) δ 10.94 (s, 1H), 10.30 (s, 1H), 7.35–7.27 (m, 2H), 7.22 (m, 2H), 7.04 (d, J = 2.6 Hz, 1H), 7.00 (t, J = 8.1 Hz, 1H), 6.93 (t, J = 7.5, 1H), 6.88 (d, J = 7.6 Hz, 1H), 6.84 (t, J = 8.1 Hz, 1H), 6.31 (s, 1H). 13C NMR (101 MHz, DMSO-d6) δ 178.43, 141.65, 136.78, 133.42, 129.02, 124.90, 124.74, 123.49, 121.66, 121.03, 120.30, 118.44, 115.42, 111.46, 109.59, 74.89.
5-fluoro-3-hydroxy-3-(1H-indol-3-yl)indolin-2-one (3b). Isolated yield: 51%; white solid; 1H NMR (400 MHz, DMSO-d6) δ 10.98 (d, J = 2.6 Hz, 1H), 10.33 (s, 1H), 7.38–7.26 (m, 2H), 7.12–6.94 (m, 4H), 6.91–6.78 (m, 2H), 6.45 (s, 1H). 13C NMR (101 MHz, DMSO-d6) δ 178.37, 159.23, 156.87, 137.79 (d), 136.81, 135.18 (d), 124.78, 123.62, 121.16, 120.19, 118.61, 115.41-114.83 (t), 112.26 (d), 111.58, 110.50 (d), 75.21 (d).
5-chloro-3-hydroxy-3-(1H-indol-3-yl)indolin-2-one (3c). Isolated yield: 65%; pale-yellow solid; 1H NMR (400 MHz, DMSO-d6) δ 11.00 (d, J = 2.7 Hz, 1H), 10.46 (s, 1H), 7.37–7.29 (m, 2H), 7.27 (dd, J = 8.3, 2.2 Hz, 1H), 7.17 (d, J = 2.2 Hz, 1H), 7.07 (d, J = 2.5 Hz, 1H), 7.05–6.96 (m, 1H), 6.94–6.81 (m, 2H), 6.49 (s, 1H). 13C NMR (101 MHz, DMSO-d6) δ 178.05, 140.53, 136.81, 135.47, 128.90, 125.67, 124.70, 124.63, 123.61, 121.20, 120.07, 118.67, 114.68, 111.63, 111.23, 74.99.
5-bromo-3-hydroxy-3-(1H-indol-3-yl)indolin-2-one (3d). Isolated yield: 89%; pale-yellow solid; 1H NMR (400 MHz, DMSO-d6) δ 11.00 (s, 1H), 10.47 (s, 1H), 7.40 (dd, J = 8.2, 2.1 Hz, 1H), 7.36–7.21 (m, 3H), 7.07 (d, J = 2.6 Hz, 1H), 7.01 (t, J = 7.7 Hz, 1H), 6.86 (t, J = 8.7 Hz, 2H), 6.49 (s, 1H). 13C NMR (101 MHz, DMSO-d6) δ 177.92, 140.94, 136.81, 135.87, 131.75, 127.35, 124.68, 123.60, 121.21, 120.02, 118.69, 114.68, 113.36, 111.78, 111.65, 74.95.
3-hydroxy-3-(1H-indol-3-yl)-5-iodoindolin-2-one (3e). Isolated yield: 93%; pale-yellow solid; 1H NMR (400 MHz, DMSO-d6) δ 11.00 (d, J = 2.7 Hz, 1H), 10.46 (s, 1H), 7.56 (dd, J = 8.1, 1.9 Hz, 1H), 7.42 (d, J = 1.9 Hz, 1H), 7.31 (t, J = 8.3 Hz, 2H), 7.07 (d, J = 2.5 Hz, 1H), 7.01 (t, J = 7.6 Hz, 1H), 6.86 (t, J = 7.5 Hz, 1H), 6.74 (d, J = 8.1 Hz, 1H), 6.46 (s, 1H). 13C NMR (101 MHz, DMSO-d6) δ 177.71, 141.41, 137.55, 136.79, 136.13, 132.87, 124.65, 123.56, 121.19, 119.95, 118.67, 114.75, 112.29, 111.65, 84.47, 74.78.
3-hydroxy-3-(1H-indol-3-yl)-1-methylindolin-2-one (3f). Isolated yield: 90%; pale-yellow solid; 1H NMR (400 MHz, DMSO-d6) 10.95 (d, J = 2.6 Hz, 1H), 7.27 (m, 4H), 7.05–6.92 (m, 4H), 6.80 (td, J = 8.2, 1.0 Hz, 1H), 6.39 (s, 1H), 3.10 (s, 3H). 13C NMR (101 MHz, DMSO-d6) δ 176.66, 143.11, 136.82, 132.76, 129.21, 124.89, 124.34, 123.65, 122.43, 121.14, 120.32, 118.59, 115.16, 111.55, 108.57, 74.68, 26.01.
3-(5-fluoro-1H-indol-3-yl)-3-hydroxyindolin-2-one (3g). Isolated yield: 54%; pale-yellow solid; 1H NMR (400 MHz, DMSO-d6) δ 11.05 (d, J = 2.7 Hz, 1H), 10.28 (s, 1H), 7.29 (dd, J = 8.8, 4.6 Hz, 1H), 7.26–7.18 (m, 2H), 7.09 (dd, J = 10.5, 2.6 Hz, 1H), 7.00 (d, J = 2.6 Hz, 1H), 6.98–6.91 (m, 1H), 6.87-6.82 (m, 2H), 6.35 (s, 1H). 13C NMR (101 MHz, DMSO-d6) δ 178.31, 157.59, 155.29, 141.66, 133.52, 132.97, 129.21, 125.57, 125.26 (d), 124.81, 121.80, 115.62 (d), 112.53, 112.44, 109.70-109.21 (t), 105.27 (d), 74.70.
3-(5-chloro-1H-indol-3-yl)-3-hydroxyindolin-2-one (3h). Isolated yield: 66%; pale-yellow solid; 1H NMR (400 MHz, DMSO-d6) 11.15 (d, J = 2.5 Hz, 1H), 10.31 (s, 1H), 7.49 (d, J = 2.1 Hz, 1H), 7.32 (d, J = 8.6 Hz, 1H), 7.24 (td, J = 7.4, 1.3 Hz, 2H), 7.01 (dd, J = 8.6, 2.1 Hz, 1H), 6.99–6.92 (m, 2H), 6.90–6.84 (m, 1H), 6.39 (s, 1H). 13C NMR (101 MHz, DMSO-d6) δ 178.25, 141.65, 135.33, 132.90, 129.26, 126.21, 125.29, 124.80, 123.12, 121.84, 121.11, 120.06, 115.33, 113.09, 109.72, 74.67.
3-(5-bromo-1H-indol-3-yl)-3-hydroxyindolin-2-one (3i). Isolated yield: 49%; yellow solid; 1H NMR (400 MHz, DMSO-d6) δ 11.15 (d, J = 2.7 Hz, 1H), 10.30 (s, 1H), 7.66 (d, J = 1.9 Hz, 1H), 7.30–7.18 (m, 3H), 7.11 (dd, J = 8.6, 2.0 Hz, 1H), 6.99–6.91 (m, 2H), 6.86 (d, J = 7.4 Hz, 1H), 6.38 (s, 1H). 13C NMR (101 MHz, DMSO-d6) δ 178.24, 141.66, 135.56, 132.89, 129.28, 126.95, 125.11, 124.81, 123.64, 123.17, 121.85, 115.27, 113.58, 111.20, 109.72, 74.68. HRMS (ESI): m/z [M+Na]+ calcd for [C16H11BrN2O2Na]+: 364.9896, found: 364.9882.
3-(5-iodo-1H-indol-3-yl)-3-hydroxyindolin-2-one (3j). Isolated yield: 28%; yellow solid; 1H NMR (400 MHz, DMSO-d6) δ 11.13 (s, 1H), 10.31 (s, 1H), 7.89 (d, J = 1.7 Hz, 1H), 7.30–7.20 (m, 3H), 7.18 (d, J = 8.5 Hz, 1H), 6.97 (td, J = 7.5, 1.1 Hz, 1H), 6.91–6.83 (m, 2H), 6.38 (s, 1H). 13C NMR (101 MHz, DMSO-d6) δ 178.21, 141.64, 135.89, 132.92, 129.45, 129.24, 129.03, 127.86, 124.78, 124.58, 121.82, 114.95, 114.05, 109.68, 82.54, 74.70.
3-hydroxy-3-(5-methyl-1H-indol-3-yl)indolin-2-one (3k). Isolated yield: 93%; yellow solid; 1H NMR (400 MHz, DMSO-d6) δ 10.80 (d, J = 2.7 Hz, 1H), 10.27 (s, 1H), 7.29–7.10 (m, 4H), 6.97–6.73 (m, 4H), 6.26 (s, 1H), 2.23 (s, 3H). 13C NMR (101 MHz, DMSO-d6) δ 178.49, 141.63, 135.19, 133.50, 128.98, 126.64, 125.24, 124.73, 123.50, 122.66, 121.65, 120.14, 114.92, 111.18, 109.56, 74.94, 21.45.
3-hydroxy-3-(2-methyl-1H-indol-3-yl)indolin-2-one (3l). Isolated yield: 69%; orange-yellow solid; 1H NMR (400 MHz, DMSO-d6) δ 10.84 (s, 1H), 10.31 (s, 1H), 7.32–7.06 (m, 3H), 6.94–6.86 (m, 4H), 6.70 (t, J = 7.4 Hz, 1H), 6.24 (s, 1H), 2.38 (s, 3H). 13C NMR (101 MHz, DMSO-d6) δ 178.66, 141.57, 134.84, 134.13, 133.42, 128.98, 126.61, 124.92, 121.68, 119.78, 119.18, 118.16, 110.23, 109.60, 109.41, 75.86, 13.30.
3-hydroxy-3-(5-methoxy-1H-indol-3-yl)indolin-2-one (3m). Isolated yield: 61%; grayish-white solid; 1H NMR (400 MHz, DMSO-d6) δ 10.78 (d, J = 2.7 Hz, 1H), 10.27 (s, 1H), 7.31–7.10 (m, 3H), 6.96 (d, J = 2.6 Hz, 1H), 6.93 (td, J = 7.5, 1.0 Hz, 1H), 6.89–6.83 (m, 1H), 6.79 (d, J = 2.5 Hz, 1H), 6.65 (dd, J = 8.8, 2.5 Hz, 1H), 6.28 (s, 1H), 3.57 (s, 3H). 13C NMR (101 MHz, DMSO-d6) δ 178.43, 152.72, 141.69, 133.35, 131.98, 129.04, 125.35, 124.82, 124.21, 121.70, 115.00, 112.02, 110.86, 109.54, 102.64, 74.93, 55.15.
3-(6-bromo-1H-indol-3-yl)-3-hydroxyindolin-2-one (3n). Isolated yield: 31%; pale-yellow solid; 1H NMR (400 MHz, DMSO-d6) δ 11.07 (d, J = 2.7 Hz, 1H), 10.30 (s, 1H), 7.49 (d, J = 1.9 Hz, 1H), 7.36 (d, J = 8.6 Hz, 1H), 7.25–7.15 (m, 2H), 7.03–6.96 (m, 2H), 6.92 (td, J = 7.5, 1.1 Hz, 1H), 6.85 (dd, J = 8.1, 1.1 Hz, 1H), 6.37 (s, 1H). 13C NMR (101 MHz, DMSO-d6) δ 178.24, 141.64, 137.72, 133.06, 129.20, 124.76, 124.57, 124.11, 122.40, 121.79, 121.42, 115.81, 114.08, 113.94, 109.72, 74.70.
3-(7-bromo-1H-indol-3-yl)-3-hydroxyindolin-2-one (3o). Isolated yield: 68%; pale-yellow solid; 1H NMR (400 MHz, DMSO-d6) δ 11.19 (d, J = 2.7 Hz, 1H), 10.34 (s, 1H), 7.35 (d, J = 8.0 Hz, 1H), 7.27–7.16 (m, 3H), 7.03 (d, J = 2.6 Hz, 1H), 6.93 (td, J = 7.5, 1.1 Hz, 1H), 6.87 (dd, J = 7.7, 1.0 Hz, 1H), 6.81 (t, J = 7.8 Hz, 1H), 6.43 (s, 1H). 13C NMR (101 MHz, DMSO-d6) δ178.15, 141.69, 135.03, 132.98, 129.28, 126.65, 124.78, 124.65, 123.74, 121.83, 120.06, 119.98, 116.97, 109.76, 104.14, 74.75.
5-fluoro-3-hydroxy-3-(5-fluoro-1H-indol-3-yl)indolin-2-one (3p). Isolated yield: 90%; pale-yellow solid; 1H NMR (400 MHz, DMSO-d6) δ 11.09 (d, J = 2.7 Hz, 1H), 10.32 (s, 1H), 7.30 (dd, J = 8.9, 4.6 Hz, 1H), 7.15 (dd, J = 10.5, 2.6 Hz, 1H), 7.11–6.99 (m, 3H), 6.86 (m, 2H), 6.49 (s, 1H). 13C NMR (101 MHz, DMSO-d6) δ 178.26, 159.28, 157.65, 156.92, 155.36, 137.80 (d), 134.69 (d), 133.55, 125.66, 125.17 (d), 115.60-115.01 (q), 112.64-112.26 (q), 110.61 (d), 109.48 (q), 105.27 (d), 75.01 (d).
5-fluoro-3-hydroxy-3-(5-bromo-1H-indol-3-yl)indolin-2-one (3q). Isolated yield: 65%; pale-yellow solid; 1H NMR (400 MHz, DMSO-d6) δ 11.21 (d, J = 2.6 Hz, 1H), 10.34 (s, 1H), 7.72 (d, J = 2.0 Hz, 1H), 7.29 (d, J = 8.6 Hz, 1H), 7.14 (dd, J = 8.6, 2.0 Hz, 1H), 7.12–7.02 (m, 2H), 6.97 (d, J = 2.4 Hz, 1H), 6.86 (dd, J = 7.9, 4.4 Hz, 1H), 6.53 (s, 1H). 13C NMR (101 MHz, DMSO-d6) δ 178.17, 156.91, 137.80 (d), 135.59, 134.67 (d), 126.86, 125.22, 123.75, 123.16, 115.55 (d), 114.65, 113.64, 112.40 (d), 111.29, 110.62 (d), 74.97.
3-(5-bromo-1H-indol-3-yl)-5-chloro-3-hydroxyindolin-2-one (3r). Isolated yield: 63%; pale-yellow solid; 1H NMR (400 MHz, DMSO-d6) δ 11.22 (d, J = 2.7 Hz, 1H), 10.47 (s, 1H), 7.72 (d, J = 2.0 Hz, 1H), 7.30 (dd, J = 8.4, 2.6 Hz, 2H), 7.22 (d, J = 2.3 Hz, 1H), 7.14 (dd, J = 8.6, 2.0 Hz, 1H), 6.97 (d, J = 2.6 Hz, 1H), 6.89 (d, J = 8.2 Hz, 1H), 6.56 (s, 1H). 13C NMR (101 MHz, DMSO-d6) δ 177.85, 140.54, 135.60, 134.88, 129.14, 126.80, 125.80, 125.20, 124.74, 123.78, 123.07, 114.49, 113.69, 111.32 (2C), 74.76.
3-(5-bromo-1H-indol-3-yl)-5-bromo-3-hydroxyindolin-2-one (3s). Isolated yield: 81%; white solid; 1H NMR (400 MHz, DMSO-d6) δ 11.21 (d, J = 2.7 Hz, 1H), 10.48 (s, 1H), 7.71 (d, J = 2.1 Hz, 1H), 7.43 (dd, J = 8.3, 2.1 Hz, 1H), 7.37–7.21 (m, 2H), 7.14 (dd, J = 8.6, 2.0 Hz, 1H), 6.97 (d, J = 2.5 Hz, 1H), 6.84 (d, J = 8.2 Hz, 1H), 6.56 (s, 1H). 13C NMR (101 MHz, DMSO-d6) δ 177.71, 140.95, 135.59, 135.27, 131.99, 127.45, 126.78, 125.19, 123.79, 123.03, 114.50, 113.71, 113.48, 111.86, 111.33, 74.72.
3-hydroxy-5-iodo-3-(5-bromo-1H-indol-3-yl)indolin-2-one (3t). Isolated yield: 29%; pale-yellow solid; 1H NMR (400 MHz, DMSO-d6) δ 11.22 (d, J = 2.6 Hz, 1H), 10.47 (s, 1H), 7.69 (d, J = 2.1 Hz, 1H), 7.60 (dd, J = 8.1, 1.9 Hz, 1H), 7.48 (d, J = 1.8 Hz, 1H), 7.31 (d, J = 8.6 Hz, 1H), 7.16 (dd, J = 8.6, 2.0 Hz, 1H), 6.98 (d, J = 2.6 Hz, 1H), 6.75 (d, J = 8.1 Hz, 1H), 6.54 (s, 1H). 13C NMR (101 MHz, DMSO-d6) δ177.50, 141.40, 137.77, 135.57, 135.53, 132.95, 126.75, 125.16, 123.76, 122.95, 114.57, 113.70, 112.35, 111.30, 84.59, 74.54.
3-hydroxy-5-iodo-3-(5-fluoro-1H-indol-3-yl)indolin-2-one (3u). Isolated yield: 61%; pale-yellow solid; 1H NMR (400 MHz, DMSO-d6) δ 11.10 (d, J = 2.8 Hz, 1H), 10.44 (s, 1H), 7.57 (dd, J = 8.1, 1.8 Hz, 1H), 7.46 (d, J = 1.8 Hz, 1H), 7.31 (dd, J = 8.8, 4.7 Hz, 1H), 7.11 (dd, J = 10.4, 2.6 Hz, 1H), 7.03 (d, J = 2.6 Hz, 1H), 6.87 (td, J = 9.2, 2.6 Hz, 1H), 6.73 (d, J = 8.1 Hz, 1H), 6.49 (s, 1H). 13C NMR (101 MHz, DMSO-d6) δ 177.59, 157.65, 155.36, 141.40, 137.73, 135.65, 133.54, 132.95, 125.60-124.99 (t), 114.96 (d), 112.67 (d), 112.36, 109.51 (d), 105.06 (d), 84.57, 74.59. HRMS (ESI): m/z [M+Na]+ calcd for [C16H10FIN2O2Na]+: 430.9663, found: 430.9665.
3-hydroxy-5-iodo-3-(5-chloro-1H-indol-3-yl)indolin-2-one (3v). Isolated yield: 57%; white solid; 1H NMR (400 MHz, DMSO-d6) δ 11.20 (s, 1H), 10.46 (s, 1H), 7.59 (dd, J = 8.1, 1.8 Hz, 1H), 7.51 (d, J = 2.1 Hz, 1H), 7.47 (d, J = 1.8 Hz, 1H), 7.34 (d, J = 8.6 Hz, 1H), 7.03 (dd, J = 8.6, 2.1 Hz, 1H), 7.00 (d, J = 2.3 Hz, 1H), 6.74 (d, J = 8.1 Hz, 1H), 6.53 (s, 1H). 13C NMR (101 MHz, DMSO-d6) δ 177.53, 141.40, 137.77, 135.56, 135.35, 132.96, 126.01, 125.34, 123.26, 121.26, 119.85, 114.65, 113.24, 112.36, 84.60, 74.55. HRMS (ESI): m/z [M+Na]+ calcd for [C16H10ClIN2O2Na]+: 446.9368, found: 446.9383.
3-hydroxy-5-iodo-3-(5-iodo-1H-indol-3-yl)indolin-2-one (3w). Isolated yield: 36%; white solid; 1H NMR (400 MHz, DMSO-d6) δ 11.18 (s, 1H), 10.46 (s, 1H), 7.91 (s, 1H), 7.60 (dd, J = 8.2, 1.8 Hz, 1H), 7.51 (s, 1H), 7.30 (d, J = 8.6 Hz, 1H), 7.20 (d, J = 8.6 Hz, 1H), 6.93 (s, 1H), 6.75 (d, J = 8.1 Hz, 1H), 6.52 (s, 1H). 13C NMR (101 MHz, DMSO-d6) δ 177.48, 141.38, 137.73, 135.90, 135.56, 132.94, 129.24, 129.15, 127.65, 124.63, 114.25, 114.16, 112.30, 84.53, 82.66, 74.56. HRMS (ESI): m/z [M-H]− calcd for [C16H9I2N2O2]−: 514.8759, found: 514.9234.
3-hydroxy-5-iodo-3-(5-methyl-1H-indol-3-yl)indolin-2-one (3x). Isolated yield: 40%; white solid; 1H NMR (400 MHz, DMSO-d6) 10.85 (d, J = 2.6 Hz, 1H), 10.43 (s, 1H), 7.53 (dd, J = 8.1, 1.8 Hz, 1H), 7.40 (d, J = 1.9 Hz, 1H), 7.18 (d, J = 8.3 Hz, 1H), 7.16–7.11 (m, 1H), 6.95 (d, J = 2.6 Hz, 1H), 6.82 (dd, J = 8.3, 1.7 Hz, 1H), 6.71 (d, J = 8.1 Hz, 1H), 6.41 (s, 1H), 2.23 (s, 3H). 13C NMR (101 MHz, DMSO-d6) δ 177.79, 141.40, 137.51, 136.20, 135.21, 132.89, 126.90, 125.00, 123.59, 122.84, 119.80, 114.26, 112.26, 111.36, 84.48, 74.83, 21.49.
3-hydroxy-5-iodo-3-(5-methoxy-indol-3-yl)indolin-2-one (3y). Isolated yield: 56%; white solid; 1H NMR (400 MHz, DMSO-d6) 10.87 (d, J = 2.7 Hz, 1H), 10.45 (s, 1H), 7.58 (dd, J = 8.1, 1.8 Hz, 1H), 7.48 (d, J = 1.8 Hz, 1H), 7.23 (d, J = 8.8 Hz, 1H), 7.02 (d, J = 2.6 Hz, 1H), 6.81 (d, J = 2.5 Hz, 1H), 6.76 (d, J = 8.1 Hz, 1H), 6.70 (dd, J = 8.8, 2.5 Hz, 1H), 6.45 (s, 1H), 3.62 (s, 3H). 13C NMR (101 MHz, DMSO-d6) 177.66, 152.81, 141.41, 137.50, 135.99, 132.95, 131.96, 125.10, 124.25, 114.29, 112.18(2C), 110.96, 102.32, 84.42, 74.77, 55.17.
4.3. Biological Assay
Five plant pathogenic fungi, including Rhizoctonia solani Kühn, Pyricularia oryzae Cav., Colletotrichum gloeosporioides Penz., Botrytis cinerea Pers.:Fr., and Bipolaris maydis (Nishik.) Shoemaker, were selected for testing the efficacy of newly synthesized compounds. These fungi are detrimental to crops such as grains, fruits, and vegetables, exhibiting a variety of harmful effects. The control agents for comparison were the agricultural fungicides carvacrol (CA) and phenazine-1-carboxylic acid (shenqinmycin, PCA). The in vitro antifungal activity of these compounds was assessed using the mycelial growth rate method. The in vivo antifungal activity of compound 3u against R. solani was evaluated through a modified broad bean leaf bioassay, following the procedures outlined in the Chinese National Agricultural Industry Standard [48]. Detailed test procedures are available in the Supplementary Materials.
5. Conclusions
A series of twenty-five novel 3-indolyl-3-hydroxy oxindole derivatives (3a–3y) were successfully synthesized and evaluated for their antifungal activity against plant pathogenic fungi, with most compounds exhibiting moderate to excellent inhibitory effects; notably, 3t, 3u, 3v, and 3w emerged as potent broad-spectrum candidates, matching or surpassing the efficacies of commercial fungicides carvacrol (CA) and phenazine-1-carboxylic acid (PCA). Compound 3u demonstrated exceptional activity against Rhizoctonia solani (EC50 = 3.44 mg/L), outperforming both CA (7.38 mg/L) and PCA (11.62 mg/L). The practical potential of 3u was further validated through an in vivo broad bean leaf bioassay, where it achieved control efficacies of 58.41% and 81.93% at concentrations of 100 and 200 mg/L, respectively, slightly lower than PCA (71.62% and 83.84%) but confirming its significant therapeutic potential under elevated dosage conditions. Structure–activity relationship (SAR) analysis revealed that halogen substituents (I, Cl, and Br) at position 5 of the 3-hydroxy-2-oxindole and indole rings play a critical role in enhancing antifungal potency. These findings highlight the potential of 3-indolyl-3-hydroxy oxindole derivatives, particularly compound 3u, as promising lead compounds for next-generation agricultural fungicides, warranting further studies on mechanistic investigations, field trials, and structural optimization for practical applications in plant disease management.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/molecules30051079/s1. Figure S1: In vitro antifungal activity evaluation; Figure S2: In vivo antifungal activity evaluation; Table S1. Preliminary in vitro antifungal activity and statistical analysis of compounds against five fungi at 50 mg/L; Figure S3: Copies of 1H, 13C NMR, and HRMS spectra of compounds.
Author Contributions
F.S. and M.Y. designed the study; F.S. and K.D. conducted the synthetic experiments; K.D., J.T., R.Y., Q.L. and Y.Y. performed the in vitro antifungal experiments; F.S. and Z.B. analyzed the data; Z.B. and L.F. supervised the antifungal experiments; F.S. and J.T. wrote the initial draft of the manuscript; F.S. and M.Y. supervised the whole project and contributed to the final version. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Yunnan Provincial Fund Project under Grant number 202401CF070069 and 202101AT070262 as well as the Yunnan Provincial Agricultural Basic Research Joint Special Project under Grant number 202301BD070001-162.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article and Supplementary Materials.
Acknowledgments
The authors would like to thank Genhua Yang for providing the Rhizoctonia solani, Pengfei He for providing the Bipolaris maydis, and Yi Wang for providing the Pyricularia oryzae.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Doehlemann, G.; Ökmen, B.; Zhu, W.J.; Sharon, A. Plant Pathogenic Fungi. Microbiol. Spectr. 2017, 5, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Dean, R.; Kan, J.V.; Pretorius, Z.A.; Hammond-Kosack, K.E.; Pietro, A.D.; Spanu, P.D.; Rudd, J.J.; Dickman, M.; Kahmann, R.; Ellis, J.; et al. The Top 10 fungal pathogens in molecular plant pathology. Mol. Plant Pathol. 2012, 13, 414–430. [Google Scholar] [CrossRef]
- Akber, M.A.; Mubeen, M.; Sohail, M.A.; Khan, S.W.; Solanki, M.K.; Khalid, R.; Abbas, A.; Divvela, P.K.; Zhou, L. Global distribution, traditional and modern detection, diagnostic, and management approaches of Rhizoctonia solani associated with legume crops. Front. Microbiol. 2023, 13, 1091288. [Google Scholar] [CrossRef]
- Senapati, M.; Tiwari, A.; Sharma, N.; Chandra, P.; Bashyal, B.M.; Ellur, R.K.; Bhowmick, P.K.; Bollinedi, H.; Vinod, K.K.; Singh, A.K.; et al. Rhizoctonia solani Kühn Pathophysiology: Status and Prospects of Sheath Blight Disease Management in Rice. Front. Plant Sci. 2022, 13, 881116. [Google Scholar] [CrossRef]
- Asibi, A.E.; Chai, Q.; Coulter, J.A. Rice Blast: A Disease with Implications for Global Food Security. Agronomy 2019, 9, 451. [Google Scholar] [CrossRef]
- Nsibo, D.L.; Barnes, I.; Berger, D.K. Recent advances in the population biology and management of maize foliar fungal pathogens Exserohilum turcicum, Cercospora zeina and Bipolaris maydis in Africa. Front. Plant Sci. 2024, 15, 1404483. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.L.; Gan, L.; Lan, C.Z.; Liu, X.F.; Liu, W.D.; Yang, X.J. Population structure and mixed reproductive strategies in Bipolaris maydis from single and multiple corn cultivars in Fujian Province, China. Front. Plant Sci. 2023, 14, 1232414. [Google Scholar] [CrossRef] [PubMed]
- Peralta-Ruiz, Y.; Rossi, C.; Grande-Tovar, C.D.; Chaves-López, C. Green Management of Postharvest Anthracnose Caused by Colletotrichum gloeosporioides. J. Fungi 2023, 9, 623. [Google Scholar] [CrossRef]
- Shao, W.Y.; Zhao, Y.F.; Ma, Z.H. Advances in Understanding Fungicide Resistance in Botrytis cinerea in China. Phytopathology 2021, 111, 455–463. [Google Scholar] [CrossRef] [PubMed]
- Bi, K.; Liang, Y.; Mengiste, T.; Sharon, A. Killing softly: A roadmap of Botrytis cinerea pathogenicity. Trends Plant Sci. 2023, 28, 211–222. [Google Scholar] [CrossRef] [PubMed]
- Bauske, M.J.; Mallik, I.; Yellareddygari SK, R.; Gudmestad, N.C. Spatial and temporal distribution of mutations conferring QoI and SDHI Resistance in Alternaria solani Across the United States. Plant Dis. 2018, 102, 349–358. [Google Scholar] [CrossRef]
- Ishii, H. Fungicide resistance in plant pathogens: Principles and a guide to practical management. Neth. J. Plant Pathol. 2015, 87, 233–255. [Google Scholar]
- Yin, Y.N.; Miao, J.Q.; Shao, W.Y.; Liu, X.L.; Zhao, Y.F.; Ma, Z.H. Fungicide Resistance: Progress in Understanding Mechanism, Monitoring, and Management. Phytopathology 2023, 113, 707–718. [Google Scholar] [CrossRef]
- Guo, S.X.; He, F.; Song, B.A.; Wu, J. Future direction of agrochemical development for plant disease in China. Food Energy Secur. 2021, 10, e293. [Google Scholar] [CrossRef]
- Sparks, T.C.; Hahn, D.R.; Garizi, N.V. Natural products, their derivatives, mimics and synthetic equivalents: Role in agrochemical discovery. Pest Manag. Sci. 2017, 73, 700–715. [Google Scholar] [CrossRef]
- Xie, k.; Li, A.; Kong, B.R.; Chen, Z.C.; Du, W.; Chen, Y.C. Recent Advances in Asymmetric Addition Reactions to Isatins. Synthesis 2025, 57, 937–952. [Google Scholar] [CrossRef]
- Karpe, S.A.; Mondal, D. Synthesis of 3-Hydroxy-2-oxindole and 2, 5-Diketopiperazine Cores as Privileged Scaffolds of Indole Alkaloids. ChemistrySelect 2022, 7, e202202516. [Google Scholar] [CrossRef]
- Kamano, Y.; Zhang, H.P.; Ichihara, Y.; Kizu, H.; Komiyama, K.; Pettit, G.R. Convolutamydine A, a novel bioactive hydroxyoxindole alkaloid from marine bryozoan Amathia convolute. Tetrahedron Lett. 1995, 36, 2783–2784. [Google Scholar] [CrossRef]
- Kawasaki, T.; Nagaoka, M.; Satoh, T.; Okamoto, A.; Ukon, R.; Ogawa, A. Synthesis of 3-hydroxyindolin-2-one alkaloids, (±)-donaxaridine and (±)-convolutamydines A and E, through enolization–Claisen rearrangement of 2-allyloxyindolin-3-ones. Tetrahedron 2004, 60, 3493–3503. [Google Scholar] [CrossRef]
- Peddibhotla, S. 3-Substituted-3-hydroxy-2-oxindole, an Emerging New Scaffold for Drug Discovery with Potential Anti-Cancer and other Biological Activities. Curr. Bioact. Compd. 2009, 5, 20–38. [Google Scholar] [CrossRef]
- Liu, C.X.; Zhuang, X.X.; Yu, Z.Y.; Wang, Z.Y.; Wang, Y.J.; Guo, X.W.; Xiang, W.S.; Huang, S.X. Community Structures and Antifungal Activity of Root-Associated Endophytic Actinobacteria of Healthy and Diseased Soybean. Microorganisms 2019, 7, 243. [Google Scholar] [CrossRef]
- Rahman, M.T.; Tiruveedhula, V.V.N.P.B.; Cook, J.M. Synthesis of Bisindole Alkaloids from the Apocynaceae Which Contain a Macroline or Sarpagine Unit: A Review. Molecules 2016, 21, 1525. [Google Scholar] [CrossRef] [PubMed]
- Bao, B.Q.; Sun, Q.S.; Yao, X.S.; Hong, J.K.; Lee, C.O.; Cho, H.Y.; Jung, J.H. Bisindole Alkaloids of the Topsentin and Hamacanthin Classes from a Marine Sponge Spongosorites sp. J. Nat. Prod. 2007, 70, 2–8. [Google Scholar] [CrossRef] [PubMed]
- Imran, S.; Taha, M.; Ismail, N.H.; Khan, K.M.; Naz, F.; Hussain, M.; Tauseef, S. Synthesis of novel bisindolylmethane Schiff bases and their antibacterial activity. Molecules 2014, 19, 11722–11740. [Google Scholar] [CrossRef] [PubMed]
- Strigácová, J.; Hudecová, D.; Mikulášová, M.; Varečka, L.; Lásiková, A.; Végh, D. Novel oxindole derivatives and their biological activity. Folia Microbiol. 2001, 46, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Campana, R.; Sisti, M.; Sabatini, L.; Lucarini, S. Marine bisindole alkaloid 2, 2-bis(6-bromo-3-indolyl)ethylamine to control and prevent fungal growth on building material: A potential antifungal agent. Appl. Microbiol. Biotechnol. 2019, 103, 5607–5616. [Google Scholar] [CrossRef]
- Pandey, K.; Rahman, M.; Cook, J. Bisindole Alkaloids from the Alstonia Species: Recent Isolation, Bioactivity, Biosynthesis, and Synthesis. Molecules 2021, 26, 3459. [Google Scholar] [CrossRef] [PubMed]
- Tasdan, Y.; Mei, G.J.; Lu, Y.X. Enantioselective synthesis of mixed 3, 3′-bisindoles via a phosphine-catalyzed umpolung c-addition of 3′-indolyl-3-oxindoles to allenoates. Sci. Bull. 2020, 65, 557–563. [Google Scholar] [CrossRef]
- Ruiz-Sanchis, P.; Savina, S.A.; Albericio, F.; Álvarez, M. Structure, Bioactivity and Synthesis of Natural Products with Hexahydropyrrolo [2,3-b]indole. Chem. Eur. J. 2011, 17, 1388–1408. [Google Scholar] [CrossRef]
- Bogdanov, A.V.; Musin, L.I.; Mironov, V.F. Advances in the synthesis and application of isoindigo derivatives. ARKIVOC Online J. Org. Chem. 2015, 6, 362–392. [Google Scholar] [CrossRef]
- Liu, M.L.; Qiu, S.Z.; Ye, Y.; Yin, G.D. Mild and efficient synthesis of isoindigo derivatives catalyzed by Lewis acid. Tetrahedron Lett. 2016, 57, 5856–5858. [Google Scholar] [CrossRef]
- Xiao, Z.J.; Wang, Y.; Lu, L.; Li, Z.J.; Peng, Z.; Han, Z.C.; Hao, Y.S. Anti-angiogenesis effects of meisoindigo on chronic myelogenous leukemia in vitro. Leuk. Res. 2006, 30, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Sassatelli, M.; Saab, E.; Anizon, F.; Prudhomme, M.; Moreau, P. Synthesis of glycosyl-isoindigo derivatives. Tetrahedron Lett. 2004, 45, 4827–4830. [Google Scholar] [CrossRef][Green Version]
- Zhang, J.W.; Gao, J.M.; Xu, T.; Zhang, X.C.; Ma, Y.T.; Jarussophon, S.; Konishi, Y. Antifungal activity of alkaloids from the seeds of Chimonanthus praecox. Chem. Biodivers. 2009, 6, 838–845. [Google Scholar] [CrossRef]
- Tayade, Y.A.; Patil, D.R.; Wagh, Y.B.; Jangle, A.D.; Dalal, D.S. An efficient synthesis of 3-indolyl-3-hydroxy oxindoles and 3, 3-di(indolyl)indolin-2-ones catalyzed by sulfonated β-CD as a supramolecular catalyst in water. Tetrahedron Lett. 2015, 56, 666–673. [Google Scholar] [CrossRef] [PubMed]
- Dalal, K.S.; Wagh, Y.B.; Tayade, Y.A.; Dalal, D.S.; Chaudhari, B.L. Hen Egg White Lysozyme Catalyzed Efficient Synthesis of 3-Indolyl-3-hydroxy Oxindole in Aqueous Ethanol. Catal. Lett. 2018, 148, 3335–3341. [Google Scholar] [CrossRef]
- Kristin, M.R.; Eckroat, T.J. Selective butyrylcholinesterase inhibition by isatin dimers and 3-indolyl-3-hydroxy-2-oxindole dimers. Bioorg. Med. Chem. Lett. 2022, 77, 129037. [Google Scholar]
- Prathima, P.S.; Rajesh, P.; Rao, J.V.; Kailash, U.S.; Sridhar, B.; Rao, M.M. “On water” expedient synthesis of 3-indolyl-3-hydroxy oxindole derivatives and their anticancer activity in vitro. Eur. J. Med. Chem. 2014, 84, 155e159. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.W.; Tang, J.R.; Dang, K.R.; Tang, J.Y.; Li, E.X.; Fan, L.M.; Ye, M.; Wu, G.X.; Su, F.W. Design and synthesis of bis(indolyl)-hydrazide-hydrazone derivatives and their antifungal activities against plant pathogen fungi. Nat. Prod. Res. 2024, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Gao, Y.Y.; Zhang, M.J.; Ding, X.; Wang, Z.W.; Ma, D.J.; Wang, Q.M. Streptindole and Its Derivatives as Novel Antiviral and Anti-Phytopathogenic Fungus Agents. J. Agric. Food Chem. 2020, 68, 7839–7849. [Google Scholar] [CrossRef]
- Tantak, M.P.; Gupta, V.; Nikhil, K.; Arun, V.; Singh, R.P.; Jha, P.N.; Shah, K.; Kumar, D. Sequential one-pot synthesis of bis(indolyl)glyoxylamides: Evaluation of antibacterial and anticancer activities. Bioorganic Med. Chem. Lett. 2016, 26, 3167–3171. [Google Scholar] [CrossRef] [PubMed]
- Gehrmann, R.; Hertlein, T.; Hopke, E.; Ohlsen, K.; Lalk, M.; Hilgeroth, A. Novel Small-Molecule Hybrid-Antibacterial Agents against S. aureus and MRSA Strains. Molecules 2022, 27, 61. [Google Scholar] [CrossRef]
- Kim, A.; Kim, M.J.; Noh, T.H.; Hong, J.K.; Liu, Y.H.; Wei, X.Y.; Jung, J.H. Synthesis and antibacterial evaluation of hamacanthin B analogues. Bioorganic Med. Chem. Lett. 2016, 26, 5013–5017. [Google Scholar] [CrossRef]
- Campana, R.; Mangiaterra, G.; Tiboni, M.; Frangipani, E.; Biavasco, F.; Lucarini, S.; Citterio, B. A Fluorinated Analogue of Marine Bisindole Alkaloid 2,2-Bis(6-bromo-1H-indol-3-yl)ethanamine as Potential Anti-Biofilm Agent and Antibiotic Adjuvant Against Staphylococcus aureus. Pharmaceuticals 2020, 13, 210. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.C.; Hao, Y.N.; Ji, X.F.; Wang, Z.W.; Liu, Y.X.; Ma, D.J.; Li, Y.Q.; Pang, H.L.; Ni, J.P.; Wang, Q.M. Optimization, Structure−Activity Relationship, and Mode of Action of Nortopsentin Analogues Containing Thiazole and Oxazole Moieties. J. Agric. Food Chem. 2019, 67, 10018–10031. [Google Scholar] [CrossRef]
- Rehberg, N.; Sommer, G.A.; Drießen, D.; Kruppa, M.; Adeniyi, E.T.; Chen, S.; Wang, L.; Wolf, K.; Tasch, B.O.A.; Ioerger, T.R.; et al. Nature-Inspired (di)Azine-Bridged Bisindole Alkaloids with Potent Antibacterial In Vitro and In Vivo Efficacy against Methicillin-Resistant Staphylococcus aureus. J. Med. Chem. 2020, 63, 12623–12641. [Google Scholar] [CrossRef]
- Yan, X.; Tang, Y.D.; He, F.; Yu, S.J.; Liu, X.G.; Bao, J.; Zhang, H. Synthesis and assessment of bisindoles as a new class of antibacterial Agents. Monatshefte Chem. 2020, 151, 971–979. [Google Scholar] [CrossRef]
- NY/T 1156.5-2006; Chinese National Agricultural Industry Standard, Pesticides Guidelines for Laboratory Bioactivity Tests, Part 5: Detached Leaf Test for Fungicide Inhibition of Rhizoctonia solani on Faba Bean. Chinese Academy of Agricultural Sciences: Beijing, China.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).