The Absence of a Very Long Chain Fatty Acid (VLCFA) in Lipid A Impairs Agrobacterium fabrum Plant Infection and Biofilm Formation and Increases Susceptibility to Environmental Stressors
Abstract
1. Introduction
2. Results
2.1. Genetic Characterisation of A. fabrum C58 acpXL–lpxXL Gene Cluster
2.1.1. The acpXL–lpxXL Gene Cluster Is Organised into Two Transcriptional Units
2.1.2. Genetic Characterisation of fabF2 and adhA2XL A. fabrum C58 Mutants
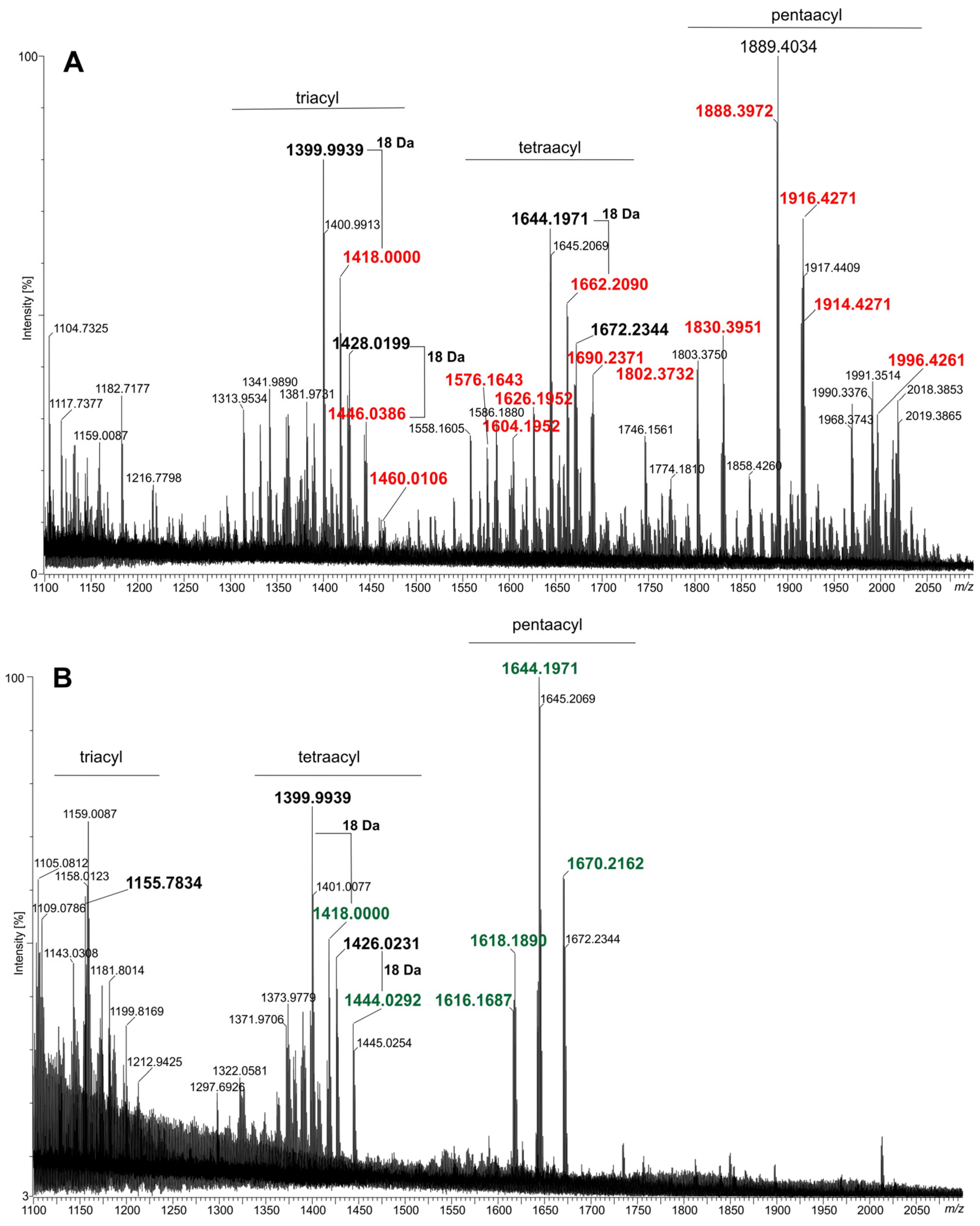
2.2. Lipids A of the fabF2XL and adhA2XL Mutants Are Deprived of VLCFAs
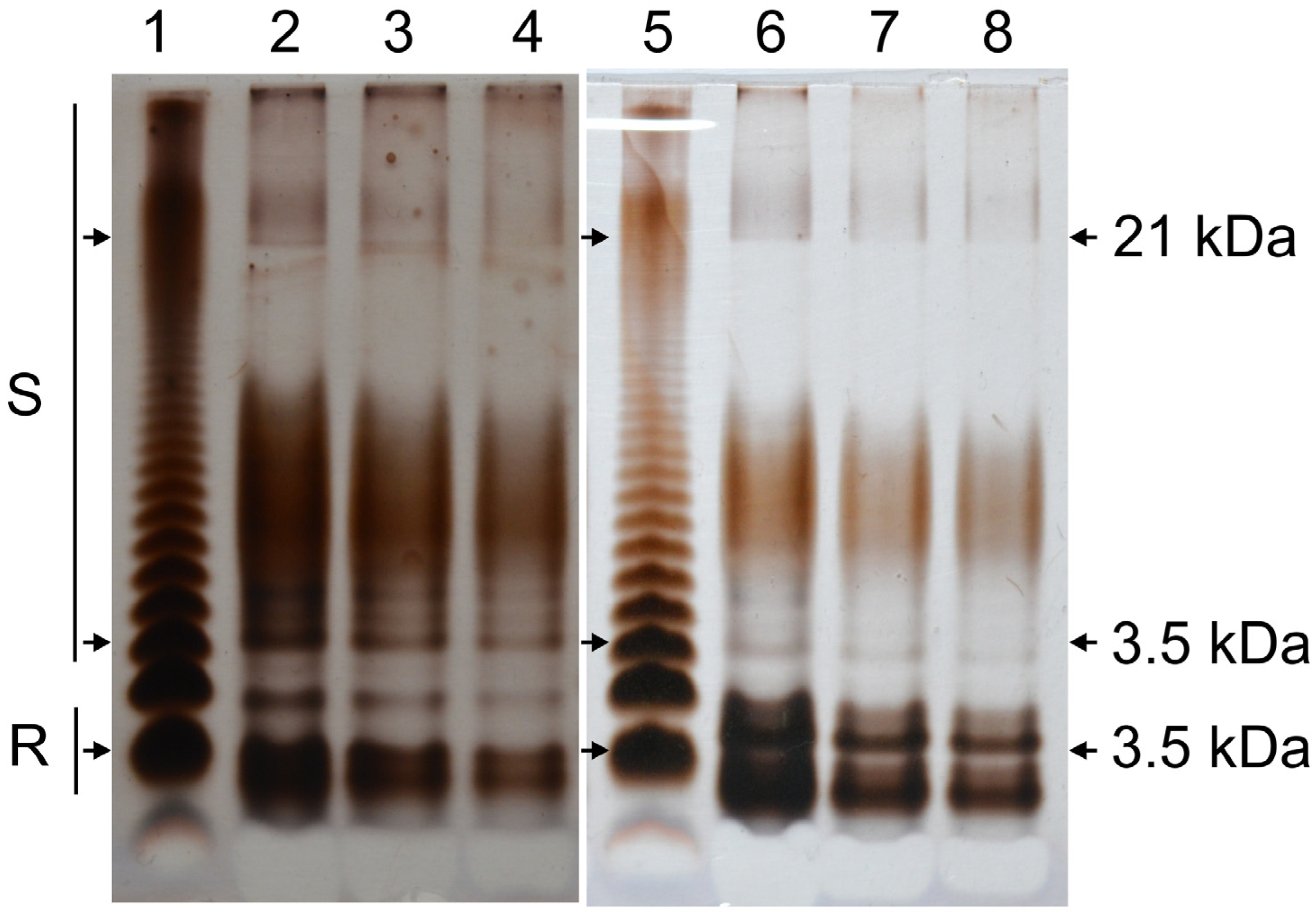
2.3. C58Δfab Mutant LPS Displays an Altered SDS-PAGE Profile
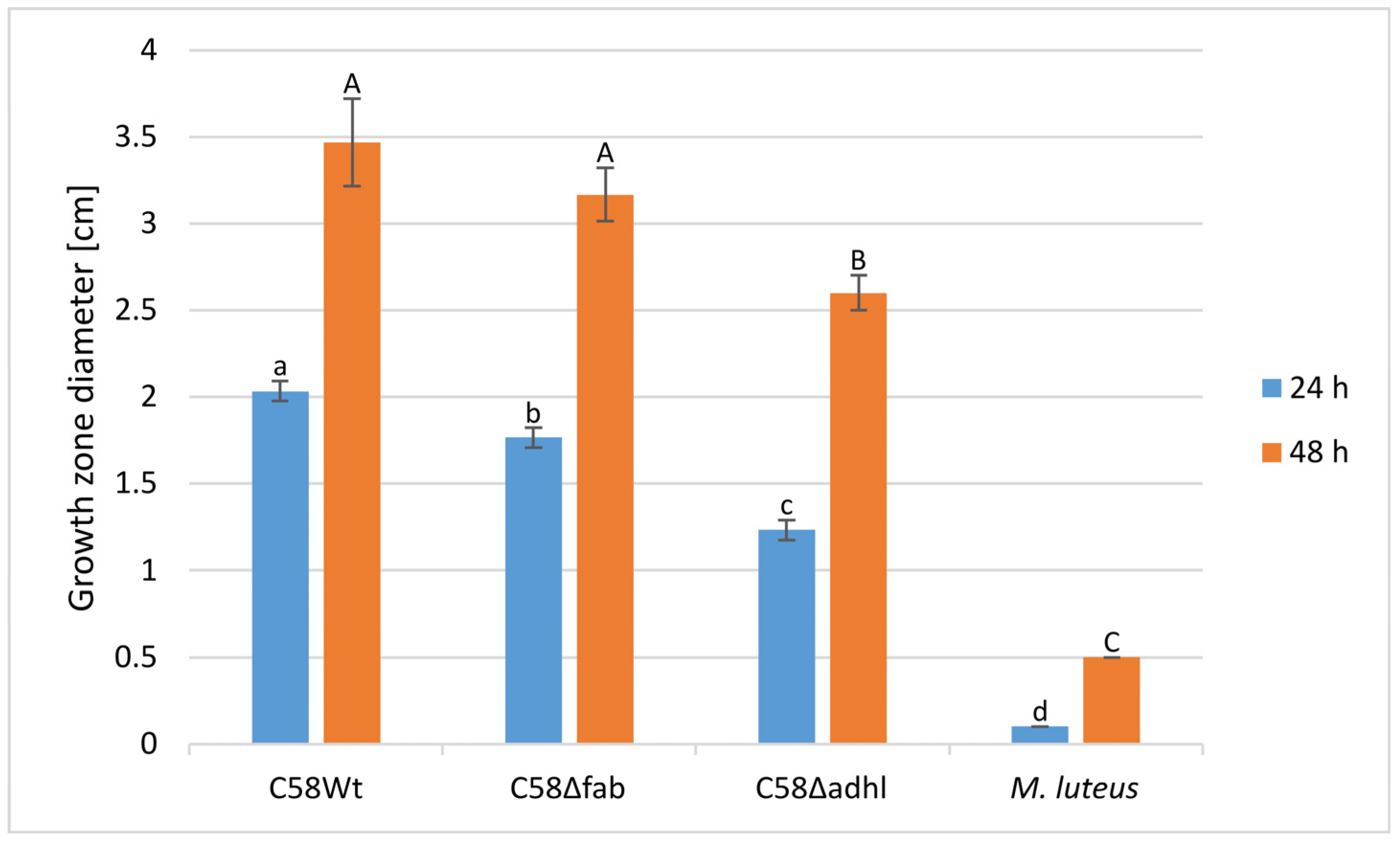
2.4. Sensitivity of A. fabrum Mutants to Stress Conditions
2.5. Swimming Motility of A. fabrum Strains
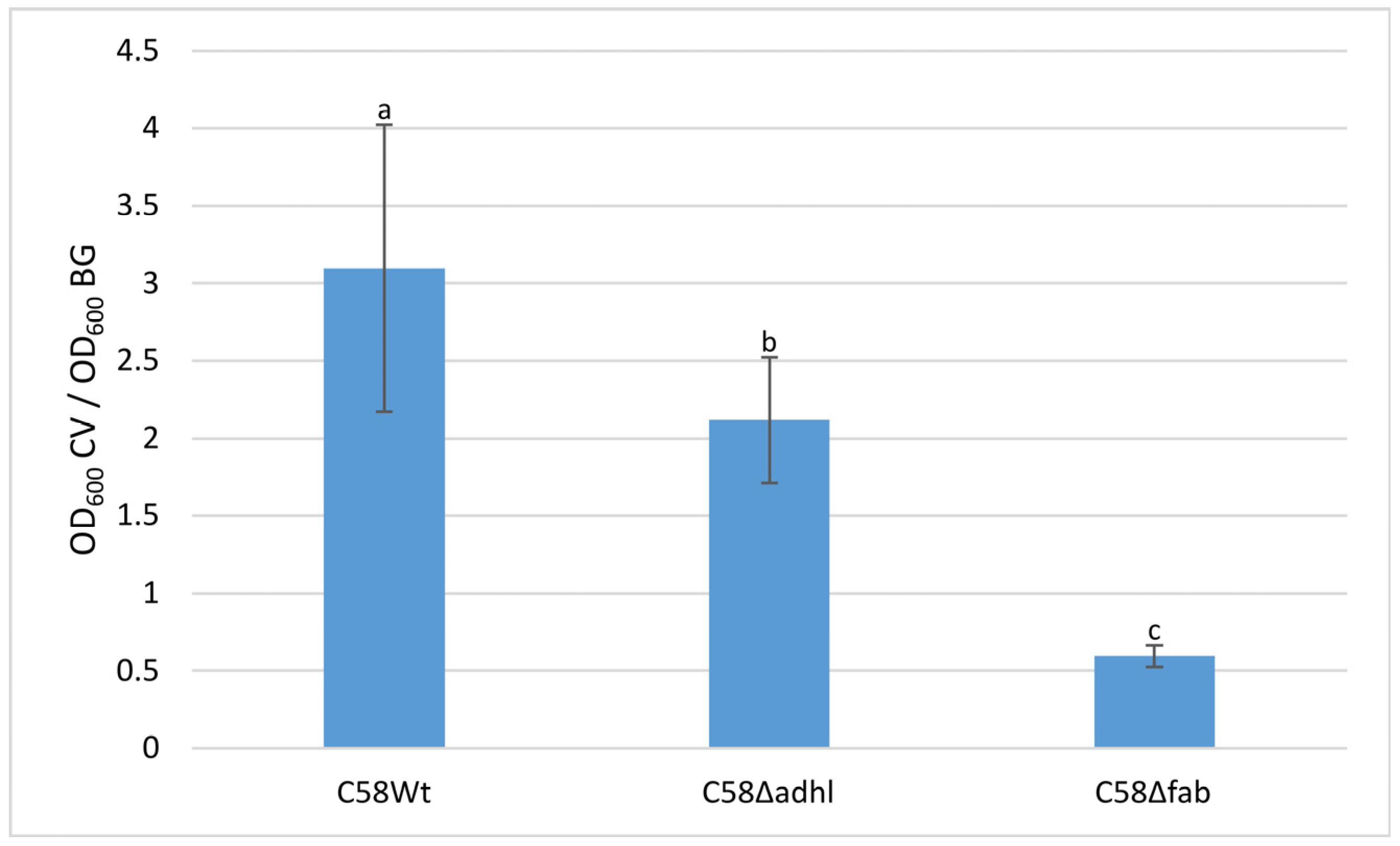
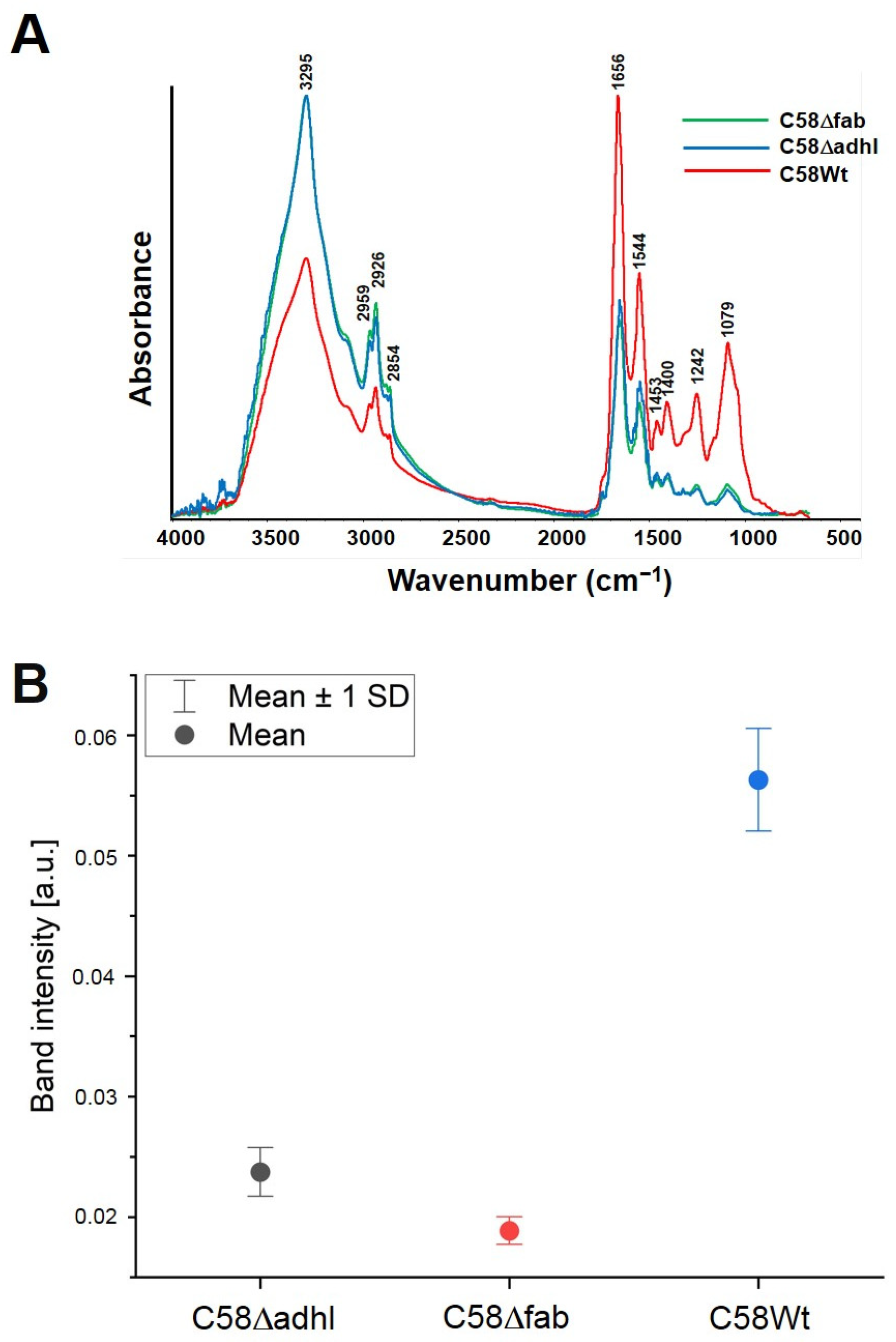
2.6. Quantitative Analysis of A. fabrum Biofilm Formation on an Abiotic Surface and FTIR Spectroscopy Analysis of Biofilm Formed
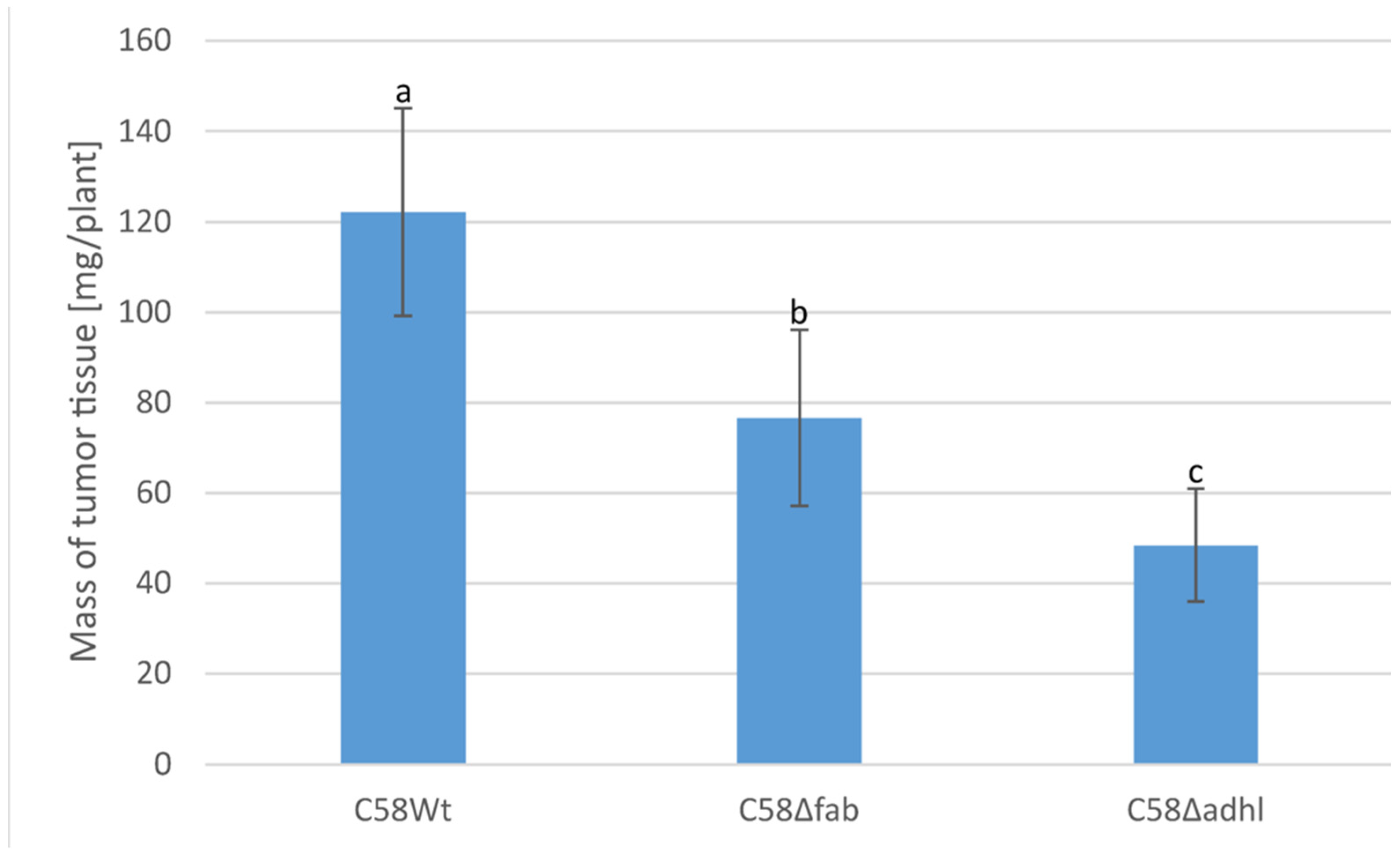
2.7. Infection of Tomato Seedlings by A. fabrum Strains
3. Discussion
4. Materials and Methods
4.1. Bacterial Strains and Culture Conditions
4.2. Bioinformatic Analyses
4.3. Total RNA Isolation and cDNA Synthesis
4.4. DNA Techniques
4.5. β-Galactosidase Activity Measurements of Transcriptional Fusions
4.6. Construction of the fabF2XL and adhlA2XL Insertional Mutants and Their Complemented Derivatives
4.7. Lipopolysaccharide Fatty Acids Analysis
4.8. Isolation of Lipids A
4.9. MALDI-TOF Mass Spectrometry of Lipid A Preparations
4.10. Phenotypic Analysis of Bacteria
4.11. Swimming Motility Assay
4.12. Quantitative Analysis of A. fabrum Biofilm Formation on an Abiotic Surface
4.13. FTIR Spectroscopy Analysis of A. fabrum Biofilm Formed on an Abiotic Surface
4.14. Plant Infection Test
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Young, J.M.; Kuykendall, L.D.; Martínez-Romero, E.; Kerr, A.; Sawada, H. A Revision of Rhizobium Frank 1889, with an Emended Description of the Genus, and the Inclusion of All Species of Agrobacterium Conn 1942 and Allorhizobium undicola de Lajudie et al. 1998 as New Combinations: Rhizobium radiobacter, R. rhizogenes, R. rubi, R. undicola and R. vitis. Int. J. Syst. Evol. Microbiol. 2001, 51, 89–103. [Google Scholar] [CrossRef] [PubMed]
- Mousavi, S.A.; Willems, A.; Nesme, X.; de Lajudie, P.; Lindström, K. Revised Phylogeny of Rhizobiaceae: Proposal of the Delineation of Pararhizobium Gen. Nov., and 13 New Species Combinations. Syst. Appl. Microbiol. 2015, 38, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Smith, E.F.; Townsend, C.O. A Plant-Tumor of Bacterial Origin. Science 1907, 25, 671–673. [Google Scholar] [CrossRef] [PubMed]
- Schrammeijer, B.; Beijersbergen, A.; Idler, K.B.; Melchers, L.S.; Thompson, D.V.; Hooykaas, P.J. Sequence Analysis of the Vir-Region from Agrobacterium tumefaciens Octopine Ti Plasmid pTi15955. J. Exp. Bot. 2000, 51, 1167–1169. [Google Scholar] [CrossRef]
- Bourras, S.; Rouxel, T.; Meyer, M. Agrobacterium tumefaciens Gene Transfer: How a Plant Pathogen Hacks the Nuclei of Plant and Nonplant Organisms. Phytopathology 2015, 105, 1288–1301. [Google Scholar] [CrossRef]
- Raetz, C.R.H.; Whitfield, C. Lipopolysaccharide Endotoxins. Annu. Rev. Biochem. 2002, 71, 635–700. [Google Scholar] [CrossRef]
- Nikaido, H. Molecular Basis of Bacterial Outer Membrane Permeability Revisited. Microbiol. Mol. Biol. Rev. 2003, 67, 593–656. [Google Scholar] [CrossRef]
- Choma, A.; Komaniecka, I.; Zebracki, K. Structure, Biosynthesis and Function of Unusual Lipids A from Nodule-Inducing and N2-Fixing Bacteria. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2017, 1862, 196–209. [Google Scholar] [CrossRef]
- Molinaro, A.; Holst, O.; Di Lorenzo, F.; Callaghan, M.; Nurisso, A.; D’Errico, G.; Zamyatina, A.; Peri, F.; Berisio, R.; Jerala, R.; et al. Chemistry of Lipid A: At the Heart of Innate Immunity. Chemistry 2015, 21, 500–519. [Google Scholar] [CrossRef]
- Silipo, A.; De Castro, C.; Lanzetta, R.; Molinaro, A.; Parrilli, M. Full Structural Characterization of the Lipid A Components from the Agrobacterium tumefaciens Strain C58 Lipopolysaccharide Fraction. Glycobiology 2004, 14, 805–815. [Google Scholar] [CrossRef]
- Bhat, U.R.; Forsberg, L.S.; Carlson, R.W. Structure of Lipid A Component of Rhizobium leguminosarum bv. phaseoli Lipopolysaccharide. Unique Nonphosphorylated Lipid A Containing 2-Amino-2-Deoxygluconate, Galacturonate, and Glucosamine. J. Biol. Chem. 1994, 269, 14402–14410. [Google Scholar] [CrossRef] [PubMed]
- Choma, A.; Komaniecka, I. Straight and Branched (ω-1)-Hydroxylated Very Long Chain Fatty Acids Are Components of Bradyrhizobium Lipid A. Acta Biochim. Pol. 2011, 58, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Komaniecka, I.; Choma, A.; Mazur, A.; Duda, K.A.; Lindner, B.; Schwudke, D.; Holst, O. Occurrence of an Unusual Hopanoid-Containing Lipid A among Lipopolysaccharides from Bradyrhizobium Species. J. Biol. Chem. 2014, 289, 35644–35655. [Google Scholar] [CrossRef]
- Silipo, A.; Vitiello, G.; Gully, D.; Sturiale, L.; Chaintreuil, C.; Fardoux, J.; Gargani, D.; Lee, H.-I.; Kulkarni, G.; Busset, N.; et al. Covalently Linked Hopanoid-Lipid A Improves Outer-Membrane Resistance of a Bradyrhizobium Symbiont of Legumes. Nat. Commun. 2014, 5, 5106. [Google Scholar] [CrossRef]
- Zamlynska, K.; Komaniecka, I.; Zebracki, K.; Mazur, A.; Sroka-Bartnicka, A.; Choma, A. Studies on Lipid A Isolated from Phyllobacterium trifolii PETP02T Lipopolysaccharide. Antonie Van Leeuwenhoek 2017, 110, 1413–1433. [Google Scholar] [CrossRef]
- Sharypova, L.A.; Niehaus, K.; Scheidle, H.; Holst, O.; Becker, A. Sinorhizobium meliloti acpXL Mutant Lacks the C28 Hydroxylated Fatty Acid Moiety of Lipid A and Does Not Express a Slow Migrating Form of Lipopolysaccharide. J. Biol. Chem. 2003, 278, 12946–12954. [Google Scholar] [CrossRef]
- Haag, A.F.; Wehmeier, S.; Muszyński, A.; Kerscher, B.; Fletcher, V.; Berry, S.H.; Hold, G.L.; Carlson, R.W.; Ferguson, G.P. Biochemical Characterization of Sinorhizobium meliloti Mutants Reveals Gene Products Involved in the Biosynthesis of the Unusual Lipid A Very Long-Chain Fatty Acid. J. Biol. Chem. 2011, 286, 17455–17466. [Google Scholar] [CrossRef]
- Marczak, M.; Żebracki, K.; Koper, P.; Horbowicz, A.; Wójcik, M.; Mazur, A. A New Face of the Old Gene: Deletion of the PssA, Encoding Monotopic Inner Membrane Phosphoglycosyl Transferase in Rhizobium leguminosarum, Leads to Diverse Phenotypes That Could Be Attributable to Downstream Effects of the Lack of Exopolysaccharide. Int. J. Mol. Sci. 2023, 24, 1035. [Google Scholar] [CrossRef]
- Żebracki, K.; Horbowicz, A.; Marczak, M.; Turska-Szewczuk, A.; Koper, P.; Wójcik, K.; Romańczuk, M.; Wójcik, M.; Mazur, A. Exopolysaccharide Biosynthesis in Rhizobium leguminosarum bv. trifolii Requires a Complementary Function of Two Homologous Glycosyltransferases PssG and PssI. Int. J. Mol. Sci. 2023, 24, 4248. [Google Scholar] [CrossRef]
- Gudlavalleti, S.K.; Forsberg, L.S. Structural Characterization of the Lipid A Component of Sinorhizobium Sp. NGR234 Rough and Smooth Form Lipopolysaccharide. Demonstration That the Distal Amide-Linked Acyloxyacyl Residue Containing the Long Chain Fatty Acid Is Conserved in Rhizobium and Sinorhizobium sp. J. Biol. Chem. 2003, 278, 3957–3968. [Google Scholar] [CrossRef]
- Ferguson, G.P.; Datta, A.; Carlson, R.W.; Walker, G.C. Importance of Unusually Modified Lipid A in Sinorhizobium Stress Resistance and Legume Symbiosis. Mol. Microbiol. 2005, 56, 68–80. [Google Scholar] [CrossRef] [PubMed]
- Vanderlinde, E.M.; Muszyński, A.; Harrison, J.J.; Koval, S.F.; Foreman, D.L.; Ceri, H.; Kannenberg, E.L.; Carlson, R.W.; Yost, C.K. Rhizobium Leguminosarum Biovar Viciae 3841, Deficient in 27-Hydroxyoctacosanoate-Modified Lipopolysaccharide, Is Impaired in Desiccation Tolerance, Biofilm Formation and Motility. Microbiology 2009, 155, 3055–3069. [Google Scholar] [CrossRef]
- Bourassa, D.V.; Kannenberg, E.L.; Sherrier, D.J.; Buhr, R.J.; Carlson, R.W. The Lipopolysaccharide Lipid A Long-Chain Fatty Acid Is Important for Rhizobium leguminosarum Growth and Stress Adaptation in Free-Living and Nodule Environments. Mol. Plant Microbe Interact. 2017, 30, 161–175. [Google Scholar] [CrossRef]
- Nikaido, H.; Vaara, M. Molecular Basis of Bacterial Outer Membrane Permeability. Microbiol. Rev. 1985, 49, 1–32. [Google Scholar] [CrossRef] [PubMed]
- Gieroba, B.; Krysa, M.; Wojtowicz, K.; Wiater, A.; Pleszczyńska, M.; Tomczyk, M.; Sroka-Bartnicka, A. The FT-IR and Raman Spectroscopies as Tools for Biofilm Characterization Created by Cariogenic Streptococci. Int. J. Mol. Sci. 2020, 21, 3811. [Google Scholar] [CrossRef] [PubMed]
- Kamnev, A.A.; Tugarova, A.V. Specificities of the Fourier Transform Infrared Spectroscopic Methodology and Interpretation of Spectroscopic Data in Microbiological Analyses. J. Anal. Chem. 2023, 78, 1320–1332. [Google Scholar] [CrossRef]
- Kassem, A.; Abbas, L.; Coutinho, O.; Opara, S.; Najaf, H.; Kasperek, D.; Pokhrel, K.; Li, X.; Tiquia-Arashiro, S. Applications of Fourier Transform-Infrared Spectroscopy in Microbial Cell Biology and Environmental Microbiology: Advances, Challenges, and Future Perspectives. Front. Microbiol. 2023, 14, 1304081. [Google Scholar] [CrossRef]
- Naumann, D.; Helm, D.; Labischinski, H. Microbiological Characterizations by FT-IR Spectroscopy. Nature 1991, 351, 81–82. [Google Scholar] [CrossRef]
- Derenne, A.; Vandersleyen, O.; Goormaghtigh, E. Lipid Quantification Method Using FTIR Spectroscopy Applied on Cancer Cell Extracts. Biochim. Biophys. Acta 2014, 1841, 1200–1209. [Google Scholar] [CrossRef]
- Carlson, R.W.; Forsberg, L.S.; Kannenberg, E.L. Lipopolysaccharides in Rhizobium-Legume Symbioses. In Subcellular Biochemistry; Springer: Berlin/Heidelberg, Germany, 2010; Volume 53, pp. 339–386. [Google Scholar] [CrossRef]
- Haag, A.F.; Wehmeier, S.; Beck, S.; Marlow, V.L.; Fletcher, V.; James, E.K.; Ferguson, G.P. The Sinorhizobium meliloti LpxXL and AcpXL Proteins Play Important Roles in Bacteroid Development within Alfalfa. J. Bacteriol. 2009, 191, 4681–4686. [Google Scholar] [CrossRef]
- Haag, A.F.; Arnold, M.F.F.; Myka, K.K.; Kerscher, B.; Dall’Angelo, S.; Zanda, M.; Mergaert, P.; Ferguson, G.P. Molecular Insights into Bacteroid Development during Rhizobium-Legume Symbiosis. FEMS Microbiol. Rev. 2013, 37, 364–383. [Google Scholar] [CrossRef] [PubMed]
- Vedam, V.; Kannenberg, E.L.; Haynes, J.G.; Sherrier, D.J.; Datta, A.; Carlson, R.W. A Rhizobium leguminosarum AcpXL Mutant Produces Lipopolysaccharide Lacking 27-Hydroxyoctacosanoic Acid. J. Bacteriol. 2003, 185, 1841–1850. [Google Scholar] [CrossRef] [PubMed]
- Vedam, V.; Kannenberg, E.; Datta, A.; Brown, D.; Haynes-Gann, J.G.; Sherrier, D.J.; Carlson, R.W. The Pea Nodule Environment Restores the Ability of a Rhizobium Leguminosarum Lipopolysaccharide acpXL Mutant to Add 27-Hydroxyoctacosanoic Acid to Its Lipid A. J. Bacteriol. 2006, 188, 2126–2133. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, G.P.; Jansen, A.; Marlow, V.L.; Walker, G.C. BacA-Mediated Bleomycin Sensitivity in Sinorhizobium meliloti Is Independent of the Unusual Lipid A Modification. J. Bacteriol. 2006, 188, 3143–3148. [Google Scholar] [CrossRef]
- Bertani, G. Studies on Lysogenesis. I. The Mode of Phage Liberation by Lysogenic Escherichia coli. J. Bacteriol. 1951, 62, 293–300. [Google Scholar] [CrossRef]
- Vincent, J.M. A Manual for the Practical Study of Root-Nodule Bacteria; Illustrated ed.; IBP Handbook No. 15; Blackwell Scientific Publications: Oxford, UK; Edinburgh, UK, 1970; ISBN 978-0-632-06410-6. [Google Scholar]
- Kowalczuk, E.; Lorkiewicz, Z. Transfer of RP4 and R68.45 Factors to Rhizobium. Acta Microbiol. Pol. 1979, 28, 221–229. [Google Scholar]
- Żebracki, K.; Koper, P.; Wójcik, M.; Marczak, M.; Mazur, A. Transcriptomic Response of Rhizobium leguminosarum to Acidic Stress and Nutrient Limitation Is Versatile and Substantially Influenced by Extrachromosomal Gene Pool. Int. J. Mol. Sci. 2024, 25, 11734. [Google Scholar] [CrossRef]
- Taboada, B.; Estrada, K.; Ciria, R.; Merino, E. Operon-Mapper: A Web Server for Precise Operon Identification in Bacterial and Archaeal Genomes. Bioinformatics 2018, 34, 4118–4120. [Google Scholar] [CrossRef]
- Reese, M.G. Application of a Time-Delay Neural Network to Promoter Annotation in the Drosophila melanogaster Genome. Comput. Chem. 2001, 26, 51–56. [Google Scholar] [CrossRef]
- Solovyev, V.; Salamov, A. Automatic Annotation of Microbial Genomes and Metagenomic Sequences. In Metagenomics and Its Applications in Agriculture, Biomedicine and Environmental Studies; Li, R.W., Ed.; Genetics—Research and Issues; Nova Biomedical: Hauppauge, NY, USA, 2011; pp. 61–78. ISBN 978-1-61668-682-6. [Google Scholar]
- Sambrook, J.; Russell, D.W. Molecular Cloning: A Laboratory Manual, 3rd ed.; Lab Manual Edition; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 2001; ISBN 978-0-87969-577-4. [Google Scholar]
- Cooley, M.B.; D’Souza, M.R.; Kado, C.I. The virC and virD Operons of the Agrobacterium Ti Plasmid Are Regulated by the Ros Chromosomal Gene: Analysis of the Cloned Ros Gene. J. Bacteriol. 1991, 173, 2608–2616. [Google Scholar] [CrossRef]
- Żebracki, K.; Koper, P.; Marczak, M.; Skorupska, A.; Mazur, A. Plasmid-Encoded RepA Proteins Specifically Autorepress Individual repABC Operons in the Multipartite Rhizobium leguminosarum bv. trifolii Genome. PLoS ONE 2015, 10, e0131907. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.H. Assay of β-Galactosidase. In Experiments in Molecular Genetics; Miller, J.H., Ed.; Cold Spring Harbor Laboratory Press: New York, NY, USA, 1972; pp. 352–355. ISBN 0-87969-106-9. [Google Scholar]
- Cervantes-Rivera, R.; Pedraza-López, F.; Pérez-Segura, G.; Cevallos, M.A. The Replication Origin of a repABC Plasmid. BMC Microbiol. 2011, 11, 158. [Google Scholar] [CrossRef] [PubMed]
- Kovach, M.E.; Elzer, P.H.; Hill, D.S.; Robertson, G.T.; Farris, M.A.; Roop, R.M.; Peterson, K.M. Four New Derivatives of the Broad-Host-Range Cloning Vector pBBR1MCS, Carrying Different Antibiotic-Resistance Cassettes. Gene 1995, 166, 175–176. [Google Scholar] [CrossRef] [PubMed]
- Que, N.L.; Lin, S.; Cotter, R.J.; Raetz, C.R. Purification and Mass Spectrometry of Six Lipid A Species from the Bacterial Endosymbiont Rhizobium etli. Demonstration of a Conserved Distal Unit and a Variable Proximal Portion. J. Biol. Chem. 2000, 275, 28006–28016. [Google Scholar] [CrossRef]
- Bligh, E.G.; Dyer, W.J. A Rapid Method of Total Lipid Extraction and Purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef]
- Silipo, A.; Molinaro, A.; Sturiale, L.; Dow, J.M.; Erbs, G.; Lanzetta, R.; Newman, M.-A.; Parrilli, M. The Elicitation of Plant Innate Immunity by Lipooligosaccharide of Xanthomonas campestris. J. Biol. Chem. 2005, 280, 33660–33668. [Google Scholar] [CrossRef]
- Beringer, J.E. R Factor Transfer in Rhizobium leguminosarum. J. Gen. Microbiol. 1974, 84, 188–198. [Google Scholar] [CrossRef]
- Morton, E.R.; Fuqua, C. Phenotypic Analyses of Agrobacterium. Curr. Protoc. Microbiol. 2012, 25, 3D.3.1–3D.3.14. [Google Scholar] [CrossRef]
- Christensen, G.D.; Simpson, W.A.; Younger, J.J.; Baddour, L.M.; Barrett, F.F.; Melton, D.M.; Beachey, E.H. Adherence of Coagulase-Negative Staphylococci to Plastic Tissue Culture Plates: A Quantitative Model for the Adherence of Staphylococci to Medical Devices. J. Clin. Microbiol. 1985, 22, 996–1006. [Google Scholar] [CrossRef]
- Ricciardelli, A.; Casillo, A.; Vergara, A.; Balasco, N.; Corsaro, M.M.; Tutino, M.L.; Parrilli, E. Environmental Conditions Shape the Biofilm of the Antarctic Bacterium Pseudoalteromonas haloplanktis TAC125. Microbiol. Res. 2019, 218, 66–75. [Google Scholar] [CrossRef]
- Baker, M.J.; Trevisan, J.; Bassan, P.; Bhargava, R.; Butler, H.J.; Dorling, K.M.; Fielden, P.R.; Fogarty, S.W.; Fullwood, N.J.; Heys, K.A.; et al. Using Fourier Transform IR Spectroscopy to Analyze Biological Materials. Nat. Protoc. 2014, 9, 1771–1791. [Google Scholar] [CrossRef]
- Escobar, M.A.; Civerolo, E.L.; Summerfelt, K.R.; Dandekar, A.M. RNAi-Mediated Oncogene Silencing Confers Resistance to Crown Gall Tumorigenesis. Proc. Natl. Acad. Sci. USA 2001, 98, 13437–13442. [Google Scholar] [CrossRef]



| Strain | C58Wt | C58Δfab | C58Δfab-C | C58Δadhl | C58Δadhl-C | |
|---|---|---|---|---|---|---|
| Fatty Acid | ||||||
| 16:0 | 5.8 | 10.5 | 5.9 | 6.7 | 4.3 | |
| 14:0-(3OH) | 23.9 | 33.0 | 8.8 | 44.4 | 21.4 | |
| 18:2 | 1.4 | 0.1 | 10.0 | 0.1 | 6.7 | |
| 18:1 1 | 8.7 | 8.9 | 45.1 | 4.4 | 24.4 | |
| 18:1 2 | 15.3 | 12.9 | 11.2 | 15.2 | 11.3 | |
| 18:0 | 6.3 | 6.7 | 7.1 | 0.7 | 4.0 | |
| 16:0-(3OH) | 20.4 | 16.7 | 7.1 | 21.8 | 16.1 | |
| 18:1-(3OH) | 2.6 | 8.8 | Tr. | 6.5 | 4.0 | |
| 18:0-(3OH) | 2.7 | 2.4 | Tr. | 0.1 | 2.9 | |
| 26:0-(25-OH) | - | - | - | - | 2.3 | |
| 28:0-(27OH) | 11.0 | - | 4.8 | - | 2.7 | |
| 30:0-(29OH) | 2.0 | - | - | - | - | |
| Strain | NaCl 2% | DOC 100 µg/mL | EDTA-Na2 1 mM | SDS 0.5 mM | pH 5.7 | pH 6.3 | pH 8.0 | PolyB 6 µg/mL | Elevated Temperature from 28 °C to 37 °C |
|---|---|---|---|---|---|---|---|---|---|
| C58Wt | 162 A ± 3 | 97 A ± 2 | 117 Aa ± 4 | 101 Aa ± 3 | 70 a ± 2 | 70 a ± 2 | 80 Aa ± 9 | 89 Aa ± 1 | 87 ± 5 |
| C58Δfab | 132 B± 10 | 70 B ± 4 | 91 B ± 2 | 18 B ± 0.5 | 69 ± 2 | 71 ± 1 | 66 B ± 2 | 60 B ± 5 | 82 ± 10 |
| C58Δfab-C | 96 C ± 8 | 110 C ± 5 | 96 B ± 4 | 37 C ± 3 | 71 ± 2 | 75 ± 2 | 81 A ± 2 | 70 B ± 8 | 91 ± 4 |
| C58Δadhl | 147 ± 14 | 124 ± 10 | 80 b ± 4 | 24 b ± 2 | 51 b ± 3 | 50 b ± 4 | 48 b ± 5 | 34 b ± 2 | 98 ± 8 |
| C58Δadhl-C | 138 ± 14 | 117 ± 15 | 85 b ± 5 | 85 a ± 9 | 58 b ± 3 | 56 b ± 1 | 61 c ± 0.5 | 46 c ± 5 | 81 ± 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Komaniecka, I.; Żebracki, K.; Mazur, A.; Suśniak, K.; Sroka-Bartnicka, A.; Swatek, A.; Choma, A. The Absence of a Very Long Chain Fatty Acid (VLCFA) in Lipid A Impairs Agrobacterium fabrum Plant Infection and Biofilm Formation and Increases Susceptibility to Environmental Stressors. Molecules 2025, 30, 1080. https://doi.org/10.3390/molecules30051080
Komaniecka I, Żebracki K, Mazur A, Suśniak K, Sroka-Bartnicka A, Swatek A, Choma A. The Absence of a Very Long Chain Fatty Acid (VLCFA) in Lipid A Impairs Agrobacterium fabrum Plant Infection and Biofilm Formation and Increases Susceptibility to Environmental Stressors. Molecules. 2025; 30(5):1080. https://doi.org/10.3390/molecules30051080
Chicago/Turabian StyleKomaniecka, Iwona, Kamil Żebracki, Andrzej Mazur, Katarzyna Suśniak, Anna Sroka-Bartnicka, Anita Swatek, and Adam Choma. 2025. "The Absence of a Very Long Chain Fatty Acid (VLCFA) in Lipid A Impairs Agrobacterium fabrum Plant Infection and Biofilm Formation and Increases Susceptibility to Environmental Stressors" Molecules 30, no. 5: 1080. https://doi.org/10.3390/molecules30051080
APA StyleKomaniecka, I., Żebracki, K., Mazur, A., Suśniak, K., Sroka-Bartnicka, A., Swatek, A., & Choma, A. (2025). The Absence of a Very Long Chain Fatty Acid (VLCFA) in Lipid A Impairs Agrobacterium fabrum Plant Infection and Biofilm Formation and Increases Susceptibility to Environmental Stressors. Molecules, 30(5), 1080. https://doi.org/10.3390/molecules30051080







