Abstract
Resveratrol, a naturally occurring phenolic stilbene molecule, has been intensively researched for its anti-inflammatory, anticancer, antioxidant, antibacterial, and neuroprotective properties. However, due to its limited absorption and probable hepatotoxicity, it is difficult to employ directly as a medication, limiting its therapeutic applicability. Over the last five years, numerous structural changes in resveratrol have been widely studied, resulting in considerable improvements in pharmacological activity and drug availability. This work reviews the biological activities and structure–activity relationships (SARs) of resveratrol derivatives, with the goal of providing useful insights for the discovery of new resveratrol derivatives.
1. Introduction
Resveratrol (3,5,4′-trihydroxy-trans-stilbene, RSV, Figure 1) is a naturally occurring non-flavonoid polyphenolic compound first isolated from Eustoma odorata roots in 1940. Researchers have identified RSV in various plants, such as grapes, mulberries, and peanuts [1,2,3]. The structure of the compound is composed of two hydroxybenzene rings, each with phenolic characteristics, which are bonded together by means of an ethylene connection. In the RSV molecule, the vinyl bridge is responsible for the existence of two stereoisomeric configurations, namely, the cis- and trans- forms. Among these configurations, the trans- form is not only more abundant in plant systems but also demonstrates a higher degree of biological activity [4,5]. RSV acts as a self-protective factor in plants, helping them withstand adversities such as UV light, mechanical damage, and fungal infections. RSV has various biological activities in humans, including anti-inflammatory, anticancer, antioxidant, neuroprotective, and antibacterial properties, among others [6,7,8,9,10,11,12]. However, RSV’s low bioavailability in vivo, poor aqueous solubility, photosensitivity, chemical instability, rapid metabolism, potential hepatotoxicity, potential anticoagulant effect, etc., have combined to limit its development for clinical use and commercialization [13,14,15,16,17].
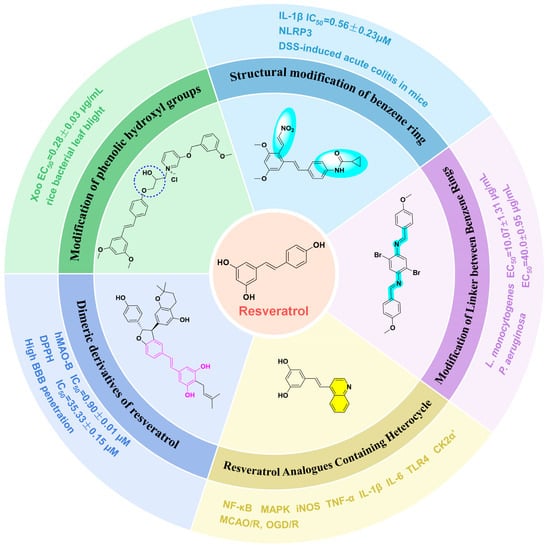
Figure 1.
Resveratrol and its structural modification types.
Medicinal chemists have conducted extensive research to address these issues, including modifications of phenolic hydroxyl groups and benzene rings, alterations to the linkers between benzene rings, the introduction of heterocyclic compounds, and the development of dimeric derivatives of RSV [18,19,20,21,22]. The purpose behind implementing these chemical modifications is to optimize the bioavailability, stability, and solubility of RSV, as well as to refine its targeting capabilities, with the ultimate goal of enhancing its potential use within the realm of pharmaceuticals. In addition, scientists have conducted in vitro cellular, pharmacological, and pharmacokinetic analyses and other experiments on these derivatives to study their mechanisms of action [23,24,25].
The present study provides an overview of advancements in RSV derivatives’ roles in combating inflammation, cancer, oxidative stress, infections, and neurodegeneration over the past half-decade, with a focus on those compounds that exhibit superior benefits. Its goal is to offer a scientific foundation for the creation of new RSV analogs with improved biological activities.
2. Modification of Phenolic Hydroxyl Groups
The phenolic hydroxyl group of RSV is highly susceptible to oxidation (Figure 2), and the modification of one or multiple phenolic hydroxyl groups on RSV with protective groups such as methoxy, ester, amino, benzene sulfonyl, glycoside, etc., can improve its stability, solubility, bioactivity, bioavailability, and targeting [26].
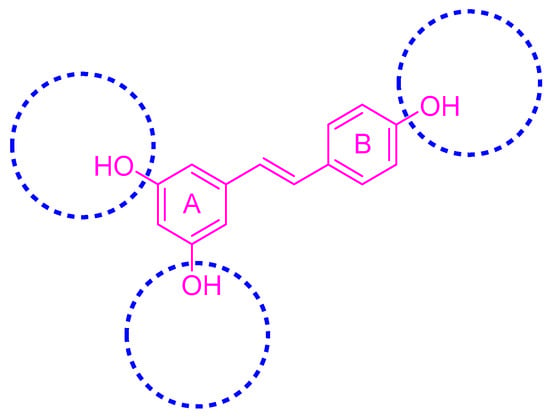
Figure 2.
Modification sites of RSV’s phenolic hydroxyl groups.
Pterostilbene (PTS, Table 1) is a natural dimethyl derivative of RSV. PTS also possesses anti-inflammatory, antioxidant, antitumor, and neuroprotective activities. In many cases, pterostilbene exhibits significantly higher biological activity than RSV. PTS is distinguished by its low molecular weight and high lipid solubility, which enable it to easily cross the blood–brain barrier (BBB) and be swiftly absorbed and dispersed throughout the body. PTS has appropriate metabolic stability and bioavailability and has no significant toxic effects [27,28,29].
1,2,3-Triazoles are widely used in approved antimicrobial drugs for their increased interaction with targets and increased water solubility. Therefore, Tang et al. (2019) introduced the 1,2,3-triazole structure into the pterostilbene parent nucleus to develop novel agents targeting methicillin-resistant Staphylococcus aureus (MRSA) infections. Among them, Compound 1 (Table 1) had the highest anti-MRSA action, with minimum inhibitory concentration (MIC) values of 1.2–2.4 μg/mL and minimum bactericidal concentration (MBC) values of 19.5–39 μg/mL. The ability of Compound 1 to inhibit DNA polymerase was examined, and it was found that Compound 1 could completely inhibit DNA polymerase activity at a concentration of 0.4 μg/mL. This suggests that Compound 1 may inhibit MRSA by acting on DNA polymerase [30].
According to earlier research, 1,3,4-oxadiazole thioether sulfone analogs have good antibacterial action [31]. Peng et al. (2023) introduced this structure on the RSV backbone, and synthesized derivatives mostly showed good antibacterial activity. Compound 2 (Table 1) exhibited median effective concentrations (EC50s) of 4.76 ± 0.09 μg/mL against Xanthomonas oryzae pv. oryzae (Xoo) and 8.85 ± 1.22 μg/mL against Xanthomonas oryzae pv. oryzicola (Xoc). Compound 2 also has the ability to interfere with the normal growth patterns of bacteria by diminishing the creation of biofilms and the production of extracellular polysaccharides (EPSs), and by causing an increase in the permeability of the cell membrane [32].
Among the isopropanol RSV derivatives synthesized by Qi et al. (2022), Compound 3 (Table 1) showed excellent antimicrobial effect, with EC50s of 0.88 ± 0.026 μg/mL for Xoo and 5.71 ± 0.11 μg/mL for Xanthomonas axonopodis pv. citri (Xac) [33]. Subsequently, among the novel β-hydroxy pyridinium salt-modified PST derivatives synthesized by Qi et al. (2024), Compound 4 (Table 1) was found to exhibit optimal in vitro antimicrobial activity against Xoo, with an EC50 value of 0.28 ± 0.03 μg/mL. Analyses of the antimicrobial mechanism indicated that both Compounds 3 and 4 significantly reduced biofilm formation, EPS biosynthesis, motility, and biofilm assembly, ultimately impairing pathogenicity. Compound 3 also showed a substantial inhibitory effect on pathogenicity, other virulence factors, and bacterial extracellular enzymes. The pot trial revealed that Compound 4 possesses significant control capabilities, exhibiting a therapeutic activity of 71.4% and a protective activity of 72.6%, indicating that it can be employed for efficient in vivo control of rice leaf blight [34].
Tang et al. (2024) synthesized a series of RSV analogs by hybridizing RSV with benzoyl chloride. These compounds significantly inhibited Th2-linked cytokines and chemokines in stimulated keratinocytes through the mitogen-activated protein kinase (MAPK) and c-Jun signaling pathways. They also suppressed Th1/Th17 cytokine and chemokine expression in activated macrophages. Furthermore, these drugs can suppress p-STAT3 and, thus, prevent keratinocytes from interacting with macrophages. Topical administration of Compound 5 (Table 1), which is the most active, improved mice’s atopic dermatitis (AD)-like lesions caused by dinitrochlorobenzene (DNCB) by reducing macrophage recruitment, pro-inflammatory mediators, and epidermal thickness [35].
The BBB presents a significant obstacle to the transportation of drugs to the brain [36]. Combining medicines with glucose or its analogs is one approach to overcoming this barrier and improving brain-targeted medication delivery. These combination chemicals can use glucose transporter protein 1 (GLUT1) to penetrate the BBB, resulting in improved delivery of medication to the brain [37]. Xu et al. (2024) found that the RSV derivatives that they synthesized, incorporating glucose moieties, exhibited neuroprotective properties by mitigating H2O2-induced neurotoxicity and the production of intracellular reactive oxygen species (ROS). In addition, Compound 6 (Table 1), the most neuroprotective compound, augmented the protective efficacy against cerebral ischemia–reperfusion damage in rats [38].
Belmonte-Reche et al. (2021) created a range of O-silyl RSV derivatives by incorporating modifications like acyl, glucosyl, and carbamoyl groups. These synthesized compounds exhibited excellent neuroprotective and anti-inflammatory properties in vitro. Derivative 7 (Table 1) demonstrated optimal toxicity and neuroprotection, reducing the production of the pro-inflammatory cytokine IL-6 to a greater extent than RSV. Derivative 7 mitigated motor coordination impairment in a 3-nitropropionic acid-induced Huntington’s disease mouse model in an RSV-like manner and markedly lessened disease severity in an experimental autoimmune encephalomyelitis (EAE) multiple sclerosis mouse model [39].
Cebrián et al. (2022) synthesized silicone sulfate and silicone ester RSV derivatives, which exhibited bactericidal activity against Gram-positive bacteria comparable to that of conventional antibiotics. These compounds act by increasing membrane permeability and inducing membrane hyperpolarization, which leads to impairment of the cellular respiratory chain and ATP synthesis, ultimately leading to bacterial death. Compounds 8 and 9 (Table 1), recognized as the most effective, demonstrated activity against all evaluated Gram-positive bacterial strains. Their MICs spanned from 6.6 to 64 μM for Compound 8 and from 4 to 21.3 μM for Compound 9. In addition, these derivatives synergistically interacted with conventional antibiotics such as aminoglycosides and polymyxin B. Although these derivatives showed significant antibacterial effects on Gram-positive bacteria, they were less effective against Gram-negative bacteria, due to the fact that the outer membrane acts as a barrier to the penetration of these compounds into the inner membrane [40].
Cebrián et al. (2023) then synthesized a variety of aminoalkyl RSV derivatives using the nature of cationic amphiphilic antimicrobial peptides. Derivative 10 (Table 1) exhibited potent antibacterial properties against various anaerobic bacteria, with MICs varying from 13.3 to 64 μM for Gram-negative strains and from 3.3 to 36.7 μM for Gram-positive strains. Although it has some cytotoxicity, it has lower toxicity and hemolytic activity compared to other tested derivatives. It was found that these derivatives exert their antimicrobial effects mainly by interfering with the integrity of the bacterial membrane [41].
Szczepańska et al. (2023) synthesized lipidated RSV derivatives that exhibited superior pharmacological properties and bioavailability compared to other phenolic compounds. Notably, a mixture of the mono-RSV-oleic acid (OA) derivatives, 11 and 12, showed the strongest anticancer effects against lung cancer cells (A549), colorectal cancer cells (HT29), and pancreatic cancer cells (BxPC3), with human fibroblasts (BJ) as a control group. The compound increased apoptosis in tumor cells by altering the cystatinase activity of pro-apoptotic pathways (p21, p53, and Bax). Regarding antioxidant properties, the mixture notably elevated the expression of the antioxidant genes SOD1 (superoxide dismutase 1) and SOD2 in normal BJ cells, thus shielding these cells from heightened oxidative stress caused by excessive ROS accumulation. These findings imply that increasing lipophilic properties can boost the compound’s biological efficacy [42].

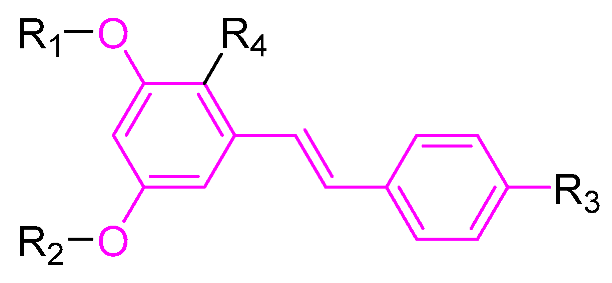
Table 1.
Molecular structure of Compounds 1–12 and their biological activities.
Table 1.
Molecular structure of Compounds 1–12 and their biological activities.
| Compound | R1 | R2 | R3 | Activity | Molecular Mechanism | In Vivo Model | Ref. |
|---|---|---|---|---|---|---|---|
| PTS | -CH3 | -CH3 | -H | - 1 | - | - | - |
| 1 | -CH3 | -CH3 | 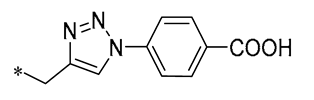 | MRSA(MIC = 1.2–2.4 μg/mL, MBC = 19.5–39 μg/mL) | Inhibiting DNA polymerase | - | [30] |
| 2 | -CH3 | -CH3 | 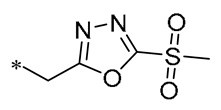 | Xoo EC50 = 4.76 ± 0.09 μg/mL Xoc EC50 = 8.85 ± 1.22 μg/mL | Suppressing the formation of bacterial biofilms Suppressing the production of EPS | Rice bacterial leaf blight Rice bacterial leaf streak | [32] |
| 3 | -CH3 | -CH3 | 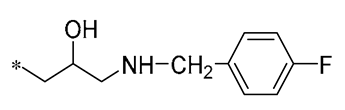 | Xoo EC50 = 0.88 ± 0.026 μg/mL Xac EC50 = 5.71 ± 0.11 μg/mL | Suppressing the formation of bacterial biofilms Suppressing the production of EPS | Rice bacterial leaf blight Citrus canker | [33] |
| 4 | -CH3 | -CH3 | 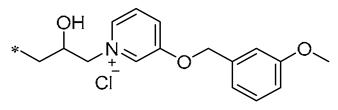 | Xoo EC50 = 0.28 ± 0.03 μg/mL | Suppressing the formation of bacterial biofilms Suppressing the production of EPS | Rice bacterial leaf blight | [34] |
| 5 | -CH3 | -CH3 | 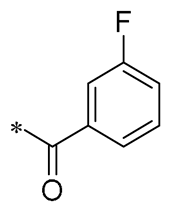 | Inhibition of Th2, Th1/Th17 expression | Impact on MAPK and c-Jun signaling pathways | DNCB-induced AD-like mice | [35] |
| 6 | 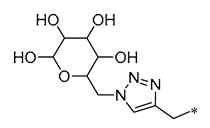 | -H | -H | Neuroprotection | Mitigating H2O2-induced neurotoxicity and the production of intracellular ROS | Cerebral ischemia–reperfusion model | [38] |
| 7 | -TIPS | --TIPS | -β-Glc-Oct | Reducing the production of IL-6 | - | EAE multiple sclerosis mouse model | [39] |
| 8 | -SO3- | 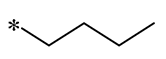 | -SO3- | Anaerobic Gram-positive bacteria (MIC = 6.6–64 μM) | Increasing membrane permeability Inducing membrane hyperpolarization | - | [40] |
| 9 | -H | -H | 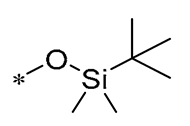 | Anaerobic Gram-positive bacteria (MIC = 4–21.3 μM) | Increasing membrane permeability Inducing membrane hyperpolarization | - | [40] |
| 10 | 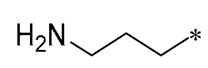 | 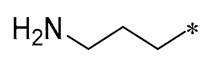 | 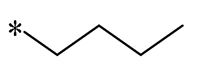 | Gram-negative (MIC = 13.3–64 μM) Gram-positive (MIC = 3.3–36.7 μM) | Suppressing the formation of bacterial biofilms | - | [41] |
| 11 | 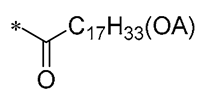 | -H | -H | Against A549, BxPC3, and HT29 cell lines | Altered cystatin activity of pro-apoptotic pathways (p21, p53, and Bax) Increased expression of SOD1 and SOD2 | - | [42] |
| 12 | -H | -H | 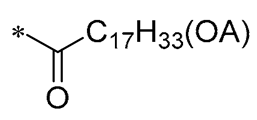 | Against A549, BxPC3, and HT29 cell lines | Altered cystatin activity of pro-apoptotic pathways (p21, p53, and Bax) Increased expression of SOD1 and SOD2 | - | [42] |
1 Not explicitly stated in the literature.
3. Structural Modification of Benzene Ring
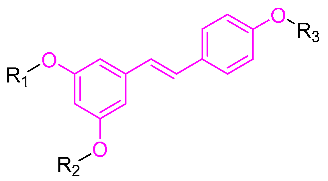
The structural modification on the benzene ring of RSV mainly focuses on the No. 2 position on ring A, such as the introduction of curcumin, chalcone, nitrovinyl, and other structures at the No. 2 position (Figure 3). This can improve its anti-inflammatory, anticancer, antimicrobial, antioxidant, and neuroprotective bioactivities, as well as enhancing the targeting properties.
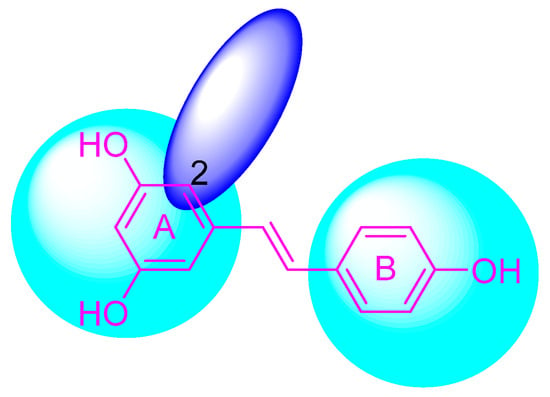
Figure 3.
Modification sites on the benzene ring of RSV.
In prior research, Wu et al. (2019) discovered that a mixture of RSV and curcumin monocarbonyl (13 and 14, Table 2) exhibited superior anti-inflammatory activity and low toxicity. Compound 13 was effective in preventing lipopolysaccharide (LPS)-induced acute lung injury in mice [43]. In subsequent research, Compound 14 demonstrated a significant reduction in lipid accumulation, liver damage, hepatic inflammation, and fibrosis caused by a high-fat diet (HFD) in a murine model. Specifically, Compound 14 notably decreased the transcription of inflammatory mediators (such as TNF-α, IL-6, IL-1β, and COX-2) and the expression of adhesion molecules (including ICAM, VCAM-1, and MCP-1) stimulated by palmitic acid (PA). Its anti-inflammatory action may be mediated by the ERK signaling pathway. The treatment involving Compound 14 has been shown to lessen body weight gain, hepatic fat accumulation, liver damage, and pathological changes in mice subjected to a high-fat diet. Compound 14 mitigated hepatic inflammation triggered by a high-fat diet by reducing the mRNA levels of inflammatory cytokines and the number of F4/80-positive cells in the liver. Additionally, it downregulated the expression of fibrotic genes like α-SMA, COL-I, COL-IV, and TGF-β, and it decelerated collagen accumulation in livers affected by an HFD. These findings indicate that 14 could be employed as a protective agent against NAFID [44]. Zhang et al. (2019) also found that 14 attenuated HFD-induced cardiac and renal inflammation and injury [45].
Ma et al. (2019) synthesized a novel piperazine-substituted chalcone RSV derivative (15, Table 2), which showed good results in inhibiting NO production, with an IC50 = 4.13 ± 0.07 μM. In addition, it demonstrated potent antiproliferative effects against three human tumor cell lines (Hela, A549, and SGC7901), with IC50s of 4.042 ± 0.16, 27.72 ± 1.45, and 3.93 ± 0.37 μM, respectively [46].
Chen et al. (2019) developed a number of novel flavonoid RSV compounds and discovered that including hydroxyl groups within the flavonoid structure improved the efficacy of suppressing NO release. Compound 16 (Table 2) proved to be the most potent in diminishing the intracellular production of NO, IL-6, and TNF-α, with respective IC50 values of 1.35, 1.12, and 1.92 μM. Preliminary mechanism studies indicated that it could inhibit the expression of TLR4 protein, resulting in activation of the NF-κB cell signaling pathway. Additionally, it significantly mitigated LPS-induced pulmonary inflammation and acute lung damage in mice [47].
Tang et al. (2021) created a series of RSV derivatives of chalcone cinnamate that were found to have anticancer activity against oral cancer cell lines. Derivative 17 (Table 2) demonstrated superior anticancer potency, registering IC50 values of 16.38 ± 0.10 µM against OECM-1 and 18.06 ± 0.05 µM against HSC-3 cell lines. The data indicated that this derivative significantly curtailed cellular proliferation and induced a G2/M cell-cycle arrest through the regulation of p21, cyclin B1, and cyclin A2. Ultimately, it enhanced apoptotic activity by reducing Bcl-2 and survival protein levels, and by increasing the cleavage of PARP and caspase-3 at elevated concentrations [48].
Fang et al. (2021) synthesized a series of new RSV derivatives by introducing an indone structure to the RSV backbone. The most potent derivative, 18 (Table 2), effectively lowered the LPS-stimulated mRNA expression of IL-1β, TNF-α, iNOS, and COX-2. The anti-inflammatory activity of Compound 18 is believed to be facilitated by the suppression of the NF-κB and MAPKs signaling pathways. Furthermore, in vivo experiments demonstrated that Compound 18 mitigated LPS-stimulated sepsis and decreased multi-organ toxicity in C57BL/6J mice [49].
Park et al. (2023) created a variety of RSV/pentadienone hybrid compounds. Compound 19 (Table 2) demonstrated the most potent suppressive impact on colony formation in MDA-MB-231 breast cancer cells, achieving a GI50 of 0.35 µM. This compound disrupted the stability of microtubule proteins and impeded the cell cycle’s advancement at the G2 phase. Additionally, computational molecular docking studies indicated that Compound 19’s binding affinity resembled that of colchicine [50].
Yang et al. (2023) synthesized Derivative 20 (Table 2), which has antioxidant and anti-inflammatory activities, by introducing chalcone and dihydropyrazole structures into the RSV parent nucleus. Derivative 20 diminishes the formation of ROS in RAW264.7 and H9C2 cells by enhancing the expression of antioxidant enzymes, including Nrf2, SOD1, catalase (CAT), and glutathione peroxidase 1 (GPX1). Furthermore, it lessens inflammation provoked by LPS or adriamycin by suppressing the NF-κB signaling pathway and reducing the expression of inflammatory factors. In a mouse model, Derivative 20 was shown to attenuate adriamycin-induced heart failure [51]. Wei et al. (2023) investigated the effects of Derivative 21 (Table 2) on inflammation regulation and vascular calcification. They found that this compound reduced the expression of phosphorylated extracellular signal-regulated kinase 1/2 (p-ERK1/2) and β-binding proteins, along with the inflammatory cytokines IL-1β, TNF-α, and IL-6, in both in vivo and in vitro settings. Derivative 21 substantially lessened calcium and lipid accumulation in the aorta and aortic root, and it markedly slowed the progression of atherosclerosis and intimal calcification in a murine model of the condition [52].
Chen et al. (2024) developed innovative RSV derivatives by integrating a thiazole molecule into the RSV framework using a pharmacophore interaction approach. Derivatives 22 and 23 (Table 2) exhibited superior activity, with IC50 values against NO of 0.7 ± 0.15 μM and 0.6 ± 0.12 μM, respectively. Both 22 and 23 were found to decrease NO and PGE2 production by suppressing the expression of iNOS and COX-2. They mitigated inflammation by reducing the phosphorylation of P65 and IκB in the NF-κB pathway and p38, ERK, and JNK in the MAPKs system. In vivo mouse studies have shown that Derivatives 22 and 23 have beneficial alleviating effects on DSS-induced acute colitis, including a decrease in body weight loss and disease index, and they exhibit a favorable safety profile in acute toxicity assessments [53,54].
Most of the novel RSV derivatives synthesized by Peng et al. (2024) containing 1,3,4-oxadiazole and amide groups exhibited excellent antimicrobial activity against Xoc and Xoo. In particular, Derivative 24 (Table 2) showed EC50 values of 4.2 ± 1.2 μg/mL and 5.0 ± 0.5 μg/mL, respectively. Preliminary mechanistic studies revealed that 24 could greatly inhibit biofilm formation, EPS production, and extracellular enzyme synthesis while also disrupting bacterial cell surface shape and lowering bacterial pathogenicity. Moreover, Derivative 24 enhanced the functionality of defense-related enzymes and modulated the expression of multiple genes associated with pathogen responses, including plant–pathogen interactions, the MAPK signaling pathway, and the biosynthesis of phenylpropanoids, which, in turn, bolstered rice’s resistance to bacterial leaf streak disease [55].
Chen et al. (2021) constructed a novel high-intensity screening model for screening small inhibitors targeting NLRP3 and identified PTS as an active scaffold. Subsequently, 50 derivatives of pterostilbene were synthesized. Compound 25 (Table 2) had the highest inhibition rate (IR) of 73.09% at 10 μM and excellent potency against IL-1β (IC50 = 0.56 ± 0.23 μM). Subsequent investigations indicated that Compound 25 modulated the assembly of NLRP3 inflammasomes by directly impacting NLRP3. In vivo bioactivity assays demonstrated that this chemical significantly alleviated DSS-triggered colitis in mice. Additionally, it was determined that the nitrovinyl substitution at position 2 is an essential active motif of PTS for NLRP3 targeting [56].
On this basis, Zhang et al. (2022, 2024) and Ruan et al. (2023) developed a variety of PTS derivatives, among which Compounds 26, 27, and 28 (Table 2) all showed good inhibitory effects on IL-1β and exhibited good safety and excellent anti-inflammatory activities in cellular assays. In addition, Compound 27 is capable of significantly lowering the release of the inflammatory cytokine IL-1β without triggering the NF-κB pathway, indicating that it possesses a distinct anti-inflammatory mode of action. Compounds 26, 27, and 28 all act directly on the NLRP3 protein, altering inflammasome assembly, decreasing NLRP3 inflammasome activation, and preventing ASC protein oligomerization. Furthermore, they all demonstrated significant therapeutic efficacy in a mouse model of DSS-induced colitis while maintaining good metabolic stability in liver microsomes, and they are all predicted to be novel medications for the treatment of Inflammatory Bowel Disease (IBD) [57,58,59].
Among the benzoylhydrazine RSV derivatives synthesized by Lu et al. (2024), Derivative 29 (Table 2) was found to have excellent scavenging of free radicals and reduction of iron ions. In the assessment of free radical scavenging abilities, Derivative 29 showed varying potencies, with IC50 values of 69.9 ± 1.8 μM for DPPH, 13 ± 0.2 μM for ABTS, and 1.1 ± 0.05 μM for FRAP, indicating its antioxidant potential across different assays. Administered at 5 μM, Derivative 29 efficiently lowered ROS generation in RAW 264.7 cells challenged with H2O2, showing similar effectiveness to RSV at a 10 μM dose. It also displayed superior suppression of NO in LPS-activated RAW 264.7 cells. Additionally, Derivative 29 fully engaged the Nrf2 signaling pathway and boosted heme oxygenase-1 (HO-1) levels under H2O2-induced oxidative conditions. Derivative 29 lessened cell damage and apoptosis by curbing the expression of pro-apoptotic proteins like cystathionase 3 and PARP. Additionally, it restrained the activation of the NF-κB, p65/iNOS, and MAPKs pathways, which contributed to its anti-inflammatory properties [60]. Lamya et al. (2024) synthesized benzoylhydrazine derivatives, specifically Derivative 30 (Table 2), which exhibited optimal cytotoxic effects, with IC50 values of 24.62 μM and 70.92 μM against the breast cancer cell lines ICF-7 and T47D, respectively [61].
Among the synthesized RSV analogs crafted by Subramanian et al. (2024), Derivative 31 (Table 2) manifested the most potent inhibition against acetylcholinesterase (AChE) and butyrylcholinesterase (BChE), registering IC50 values of 40.60 μg/mL and 40.18 μg/mL, respectively. In the DPPH radical scavenging test, Derivative 31 exhibited robust antioxidant properties, achieving an IC50 value of 46.22 μg/mL. It efficiently mitigated oxidative stress induced by β-amyloid in the DCFDA ROS assay, showing antioxidant efficacy on par with that of established medications [62].

Table 2.
Molecular structure of Compounds 13–31 and their biological activities.
Table 2.
Molecular structure of Compounds 13–31 and their biological activities.
| Compound | R1 | R2 | R3 | R4 | Activity | Mechanism | In Vivo Model | Ref. |
|---|---|---|---|---|---|---|---|---|
| 13 | -CH3 | -CH3 | -OCH3 | 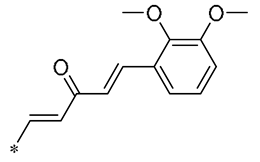 | Inhibited the LPS-induced production of IL-6 and TNF-a | Decreased LPS-induced TNF-a, IL-6, IL12, and IL-33 mRNA expression | LPS-induced acute lung injury in the in vivo mouse model | [43] |
| 14 | -CH3 | -CH3 | -OCH3 | 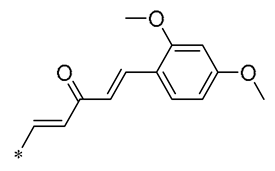 | Decreased the transcription of inflammatory mediators (such as TNF-α, IL-6, IL-1β, and COX-2) and the expression of adhesion molecules (including ICAM, VCAM-1, and MCP-1) stimulated by PA | Affects the ERK signaling pathway | HFD-induced NAFLD HFD-induced heart and kidney injury | [43,44,45] |
| 15 | -CH3 | -CH3 | -OCH3 | 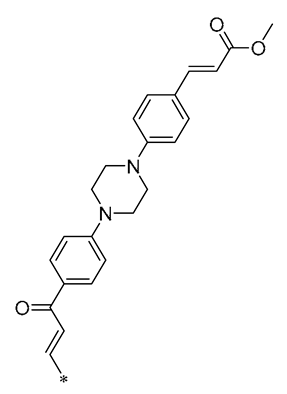 | NO IC50 = 4.13 ± 0.07 μM. Hela IC50 = 4.042 ± 0.16 μM. A549 IC50 = 27.72 ± 1.45 μM. SGC7901 IC50 = 3.93 ± 0.37 μM | - 1 | - | [46] |
| 16 | -CH3 | -CH3 | -OCH3 | 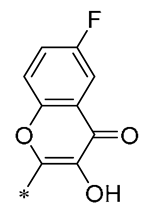 | NO IC50 = 1.35 μM IL-6 IC50 = 1.12 μM TNF-α IC50 = 1.92 μM | Inhibits the expression of TLR4 protein, resulting in activation of the NF-κB cell signaling pathway | LPS-induced acute lung injury | [47] |
| 17 | -CH3 | -CH3 | -OH | 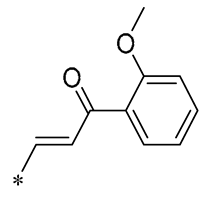 | OECM-1 IC50 = 16.38 µM HSC-3 IC50 = 18.06 µM | Inhibits cell proliferation and induces G2/M cell-cycle arrest by regulating p21, cyclin B1, and cyclin A2 Reducing Bcl-2 and survival protein levels, and increasing PARP and caspase-3 cleavage at higher concentrations | - | [48] |
| 18 | -CH3 | -CH3 |  | 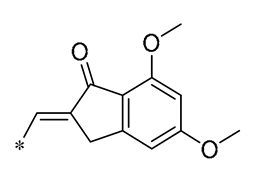 | Inhibited the LPS-induced production of IL-1β, TNF-α, iNOS, and COX-2 | Inhibition of NF-κB and MAPKs signaling pathways | LPS-induced sepsis in C57BL/6J mice and reduced multi-organ toxicity | [49] |
| 19 | -CH3 | -CH3 | -OCH3 | 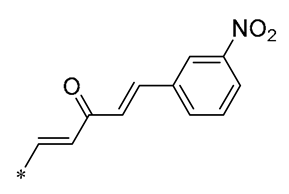 | MDA-MB-231 GI50 = 0.35 µM | Disrupted the stability of microtubule proteins | - | [50] |
| 20 | -CH3 | -CH3 | 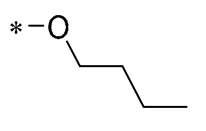 | 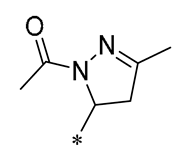 | Increased expression of Nrf2, SOD1, CAT, and GPX1 Decreased ROS generation | Inhibiting NF-κB signaling | DOX-induced heart failure | [51] |
| 21 | -CH3 | -CH3 | 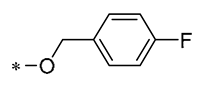 | 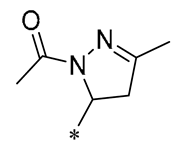 | Reduced IL-6, L-1β, and TNF-α expression | Blocks the ERK1/2 signaling pathway | Medial calcification induced by nicotine and VD3 | [52] |
| 22 | -CH3 | -CH3 | 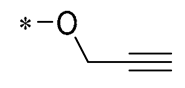 | 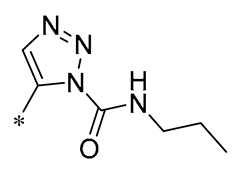 | NO IC50= 0.7 ± 0.15 μM | Inhibition of NF-κB and MAPKs signaling pathways | DSS-induced acute colitis in mice | [53] |
| 23 | -CH3 | -CH3 | 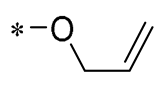 | 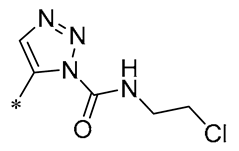 | NO IC50= 0.6 ± 0.12 μM | Inhibition of NF-κB and MAPKs signaling pathways | DSS-induced acute colitis in mice | [54] |
| 24 | -CH3 | -CH3 | -OCH3 | 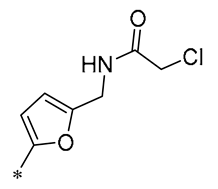 | Xoo EC50 = 4.2 ± 1.2 μg/mL Xoc EC50 = 5.0 ± 0.5 μg/mL | Suppressing the formation of bacterial biofilms | Rice bacterial leaf blight Rice bacterial leaf streak | [55] |
| 25 | -CH3 | -CH3 | 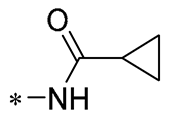 | 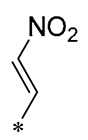 | IL-1β IC50 = 0.56 ± 0.23 μM | Influences the assembly of NLRP3 inflammatory vesicles by targeting NLRP3. | DSS-induced acute colitis in mice. | [56] |
| 26 | -CH3 | -CH3 | 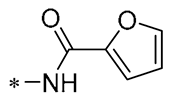 | 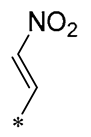 | IL-1β IC50 = 1.23 ± 0.51 μM | Influences the assembly of NLRP3 inflammatory vesicles by targeting NLRP3 | DSS-induced acute colitis in mice | [58] |
| 27 | -CH3 | -CH3 | 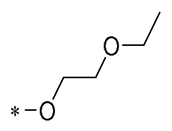 | 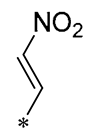 | IL-1β IC50 = 2.41 μM | Influences the assembly of NLRP3 inflammatory vesicles by targeting NLRP3 | DSS-induced acute colitis in mice | [59] |
| 28 | -CH3 | -CH3 | 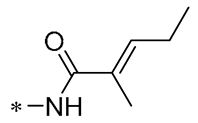 | 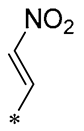 | IL-1β IC50 = 2.99 μM | Influences the assembly of NLRP3 inflammatory vesicles by targeting NLRP3 | DSS-induced acute colitis in mice | [57] |
| 29 | -H | -H | -H | 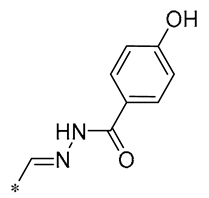 | DPPH IC50 = 69.9 ± 1.8 μM ABTS IC50 = 13 ± 0.2 μM FRAP IC50 = 1.1 ± 0.05 μM | Inhibition of NF-κB, p65/iNOS, and MAPKs pathway activation | - | [60] |
| 30 | -H | -H | -H |  | ICF-7 IC50 = 24.62 ± 4.70 μM T47D IC50 = 70.92 ± 21.28 μM | - | - | [61] |
| 31 | -CH3 | -CH3 |  | -H | AChE IC50 = 40.60 μg/mL BChE IC50 = 40.18 μg/mL | - | - | [62] |
1 Not explicitly stated in the literature.
Barbara et al. (2019) synthesized a variety of simplified RSV analogs. The cytotoxic effects of these compounds were evaluated in three pancreatic cancer cell lines with unique genetic signatures: AsPC-1, BxPC-3, and Capan-2. Compound 32 (Figure 4) showed the best cytotoxic activity [63]. Florio et al. (2023) also found that 32 significantly reduced the expression of CD133 and EpCAM markers on the surface of pancreatic cancer (PC) stem cells and restricted PC cell cloning by further investigation of 32. In AsPC-1 cancer cells, 32 was more effective than RSV at activating the DNA damage marker H2AX and promoting altered expression of cell-cycle regulatory proteins, which interfered with AsPC-1 cancer cells’ biological processes and effectively promoted apoptosis [64].
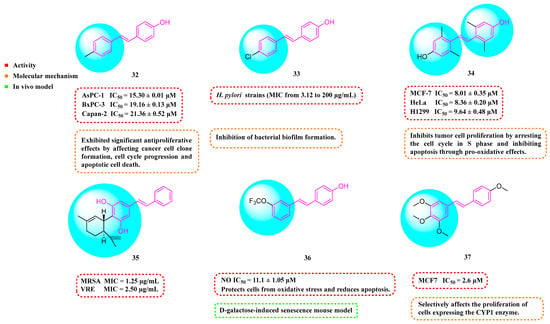
Figure 4.
Molecular structure of compounds 32–37 and their biological activities.
Fermo et al. (2020) found that both RSV and their newly synthesized RSV derivatives showed antimicrobial effects against drug-resistant H. pylori. Among them, Compound 33 (Figure 4) showed stronger antibacterial activity relative to RSV (MIC from 3.12 to 200 μg/mL). Compound 33 was shown to inhibit the formation of bacterial biofilms and reduce the clustering of H. pylori on soft agar. Additionally, Compound 33 was non-toxic to greater wax borer larvae and provided protection against H. pylori infection [65].
Xin et al. (2020) synthesized a series of hydroxy stilbene-methylated RSV analogs, among which Compound 34 (Figure 4) showed the strongest cytotoxicity against tumor cells (MCF-7, HeLa, and H1299), with IC50s of 8.01 ± 0.35, 8.36 ± 0.20, and 9.64 ± 0.48 μM, respectively. It was shown that Compound 34 inhibited the proliferation of tumor cells through the blockade of the S-phase cell cycle and inhibited apoptosis by promoting oxidation [66].
Kumarihamy et al. (2022) synthesized a series of RSV analogs. Of these analogs, 35 (Figure 4) showed significant antimicrobial activity in studies, with an MIC of 1.25 µg/mL against MRSA. The MIC against vancomycin-resistant Enterococcus faecium and E. faecalis (VRE) was 2.50 µg/mL [67].
Of the RSV derivatives synthesized by Liang et al. (2023), 36 showed inhibition of NO production (IC50 = 11.1 ± 1.05 μM), suppression of oxidative toxicity, reduction in ROS accumulation, and apoptotic activity in an in vitro oxidative stress cell model. Within a mouse model of senescence triggered by D-galactose, Derivative 36 successfully reversed liver and kidney impairment, shielded the serum, brain, and liver from oxidative stress, and bolstered the immune response in the spleen. Furthermore, Derivative 36 mitigates brain aging by safeguarding cells against oxidative stress and diminishing apoptosis [68].
Ruparelia et al. (2024) synthesized a series of methyl ether analogs of RSV. These RSV analogs were significantly more potent on cytochrome P450 1 (CYP1)-expressing breast tumor cell lines than on cell lines without CYP1 activity. Of these, Analog 37 (Figure 4) had the strongest ability to inhibit cancer cell proliferation. The compound exhibited mild-to-moderate cytotoxic effects, with an IC50 value of 2.6 μM against MCF7 cells. However, it formed highly toxic metabolites during the CYP1-mediated dealkylation reaction, showing an IC50 of 0.12 μM specifically for MCF7 cells activated by 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD). Consequently, Compound 37 selectively targets cell proliferation in cells that express the CYP1 enzyme [69].
4. Modification of Linkers Between Benzene Rings
The double bond (C=C) in RSV acts as a linker between two aromatic rings, which greatly influences the biological activity of polyphenols. Consequently, certain scientists have engineered RSV derivatives by substituting the C=C bond with either a C=N or N=N bond through a bioisosteric replacement strategy. Moreover, inflexible moieties like the C=C double bond can be swapped for more pliable groups such as amides, amines, and single carbon–carbon bonds to enhance the biological efficacy of RSV derivatives [70,71,72,73,74,75].
Iacopetta et al. (2020) developed a range of RSV imino derivatives. Their findings indicated that Derivative 38 (Figure 5) demonstrated the most effective antiproliferative properties in two breast cancer cell lines, MCF-7 and SkBr-3, with IC50 values of 12 ± 1 μM and 15 ± 1 μM, respectively. Moreover, Derivative 38 displayed enhanced bioavailability compared to the parent RSV in both gastric and small-intestinal conditions, with a 35% higher overall value relative to RSV. In addition, 38 could induce apoptotic cell death by inhibiting human topoisomerase II [76].
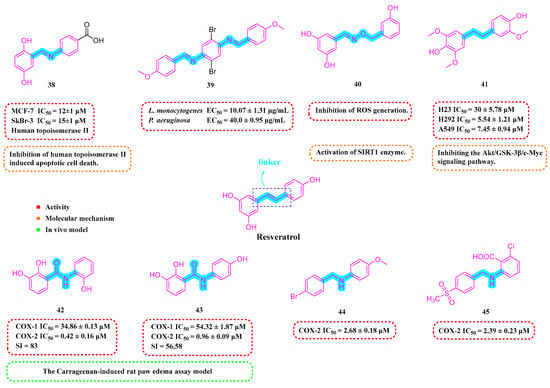
Figure 5.
Molecular structure of compounds 38–45 and their biological activities.
The most active of the Schiff base hybrid resveratrol derivatives synthesized by Sánchez-González et al. (2022) was Compound 39 (Figure 5), which showed good performance in antimicrobial activity tests against Listeria monocytogenes (LM), EC50 = 10.07 ± 1.31 µg/mL. Furthermore, Compound 39 demonstrated potent antimicrobial effects against Pseudomonas aeruginosa, with an EC50 value of 40.0 ± 0.95 µg/mL [77].
Ciccone et al. (2022) developed Compound 40 (Figure 5), which demonstrated superior antioxidant capabilities compared to RSV in the ABTS radical scavenging test. Compound 40 was able to safeguard the viability of human umbilical vein endothelial cells (HUVECs) by modulating SIRT1 and could counteract the rise in intracellular ROS triggered by H2O2 [78].
Thongsom et al. (2023) synthesized RSV Analog 41 (Figure 5), which demonstrated greater efficacy in suppressing the viability of lung cancer cell lines (H23, H292, and A549) than RSV itself. Additionally, Compound 41 can increase intracellular ROS levels and induce oxidative stress, leading to apoptosis in cancer cells. Compared to RSV, 41 exhibits a more potent capability to curb the CSC-like characteristics of lung cancer cells, achieving this by inhibiting the Akt/GSK-3β/c-Myc signaling pathway [79].
Xin et al. (2023) evaluated the inhibitory activity of synthesized RSV amide derivatives against COX-1/COX-2. The findings revealed that Compounds 42 and 43 (Figure 5) demonstrated potent inhibitory effects (IC50s of 0.42 ± 0.16 μM and 0.96 ± 0.09 μM, respectively) and marked selectivity (selectivity indices (SIs) of 83 and 56.58, respectively) for COX-2, implying that they inhibited COX-2 much more than COX-1. The compounds’ in vivo anti-inflammatory properties were further evaluated using a carrageenan-induced rat paw edema assay, with the research indicating that Compound 41 demonstrated substantial anti-inflammatory activity, achieving 45.95% inhibition of edema at three oral dosages of 50 mg/kg [80].
Wu et al. (2024) developed a range of N-substituted structurally modified RSV derivatives and assessed their anti-inflammatory and antitumor capabilities in vitro. Compounds 44 and 45 (Figure 5) strongly suppressed NO generation at 10 μm concentrations, with concentration-dependent inhibition. In addition, Compounds 44 and 45 demonstrated effective COX-2 inhibition (IC50s of 2.68 ± 0.18 and 2.39 ± 0.23 μM, respectively). Compound 45 inhibited various tumor cell lines while inducing apoptosis in MCF-7 breast cancer cells [81].
5. Resveratrol Analogs Containing Heterocycle
Hou et al. (2019) synthesized a quinolinyl-substituted RSV analog, 46 (Figure 6). It was shown that 46 reduced LPS-induced NO release, iNOS expression, ROS generation, and NADPH oxidase activation. Moreover, Compound 46 reduced the expression of TLR4 protein and hindered the activation of the MAPK and NF-κB signaling pathways triggered by LPS. In the rat model of middle cerebral artery occlusion–reperfusion (MCAO/R), Compound 45 decreased the volume of infarction and neurological deficits, improved performance on the rotarod test, and lessened the frequency of rightward turns. In the oxygen–glucose deprivation and reperfusion (OGD/R) model using primary microglial cells, Compound 46 mitigated oxidative stress and mitochondrial damage by promoting mitochondrial autophagy in SH-SY5Y cells subjected to OGD/R. Additionally, Compound 46 could lower the mRNA expression of the pro-inflammatory cytokines IL-6, TNF-α, and IL-1β, potentially alleviating neuroinflammation. Significantly, TLR4 knockdown dramatically improved 46’s anti-inflammatory activities, whereas TLR4 overexpression hindered them. Compound 46 promoted mitophagy by interacting with CK2α’, reducing damage caused by acute ischemic strokes. These insights reveal potential mechanisms through which Compound 46 could treat ischemic stroke (IS) [82,83,84].
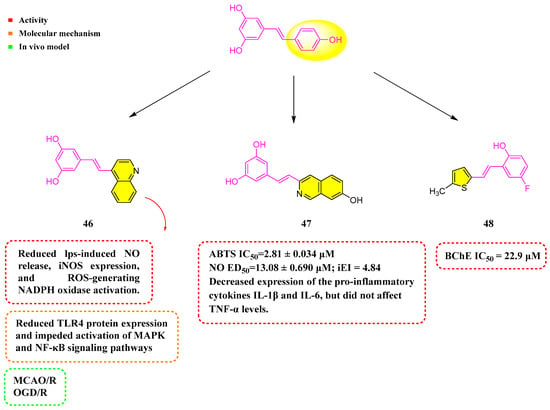
Figure 6.
Molecular structure of compounds 46–48 and their biological activities.
Nam et al. (2021) prepared a number of analogs by chemical synthesis, including structures containing naphthalene and its bioelectronic isomers. ABTS experiments showed that all compounds possessed better antioxidant activity than RSV. Among them, Analog 47 (Figure 6) showed the strongest activity, with IC50 = 2.81 ± 0.034 µM. Moreover, Compound 46 decreased the expression of the pro-inflammatory cytokines IL-1β and IL-6 in RAW 264.7 cells when stimulated by LPS, yet it did not impact TNF-α levels [85].
Mlakić et al. (2024) synthesized 14 RSV analogs using heterocyclic-containing triphenylphosphine salts and different benzaldehydes via the Wittig reaction. The compounds underwent evaluation for their potency to suppress AChE and BChE, in addition to assessing their antioxidant capabilities. Among the heteroaromatic RSV derivatives, they demonstrated greater efficacy in curbing BChE activity. Specifically, Analog 48 (Figure 6) exhibited the most pronounced inhibitory effect on BChE, registering an IC50 of 22.9 μM. In addition, these compounds showed stronger antioxidant activity than standard RSV [86].
6. Dimeric Derivatives of Resveratrol
Both resveratrol polymers and RSV are naturally occurring polyphenolic compounds that are present in various plants. These resveratrol polymers have garnered attention due to their wide range of biological activities, such as antioxidant, anti-inflammatory, antibacterial, anti-neoplastic, and neuroprotective capabilities. Predominantly, in their natural state, resveratrol polymers are found as oligomeric forms, with certain oligomeric polymers exhibiting significantly higher activity levels compared to RSV [87,88,89].
Fan et al. (2019) uncovered an RSV dimer derivative, amurensin H (49, Figure 7), exhibiting anti-inflammatory and antioxidant effects. The investigation revealed that 49 lessened inflammatory markers in lung tissues and reduced the levels of IL-6, IL-17A, TNF-α, and interferon-gamma in bronchoalveolar lavage fluid. It also substantially curtailed LPS-induced production of IL-1β, IL-6, IL-8, and TNF-α. Furthermore, 49 significantly downregulated the expression of p-Syk, NF-κB, and p-NF-κB in laboratory and animal models. Compound 49 also lessened airway inflammation in chronic obstructive pulmonary disease (COPD) [90].
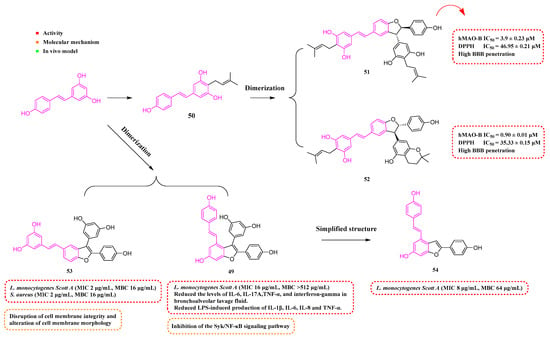
Figure 7.
Molecular structure of compounds 49–54 and their biological activities.
Tang et al. (2019) utilized RSV as a starting material and incorporated an isoprene molecule to synthesize Compound 50 (Figure 7). This compound was then subjected to a dimerization process, resulting in a range of compounds. Notably, Compounds 51 and 52 (Figure 7) exhibited potent inhibitory effects against human monoamine oxidase B (hMAO-B), with IC50 values of 3.91 ± 0.23 µM and 0.90 ± 0.01 µM, respectively. Additionally, Compounds 51 and 52 showcased superior antioxidant capabilities and low toxicity in cellular tests, while also exhibiting resistance to neurotoxicity caused by oxidative toxins such as I202, rotenone, and oligomycin-A. Compounds 51 and 52 demonstrated substantial BBB permeability. In addition, these compounds exhibited notable anti-inflammatory effects in vitro, suppressed ROS generation, mitigated apoptosis triggered by H2O2, and efficiently hindered the release of inflammatory mediators induced by LPS from BV2 cells [91].
Among the monomeric and dimeric RSV derivatives synthesized by Mattio et al. (2019), 49 and 53 (Figure 7) showed considerable antibacterial efficacy against Gram-positive bacteria. The most potent (53) showed significant antibacterial activity against LM (MIC = 2 μg/mL, MBC = 16 μg/mL). At a dosage of 100 μg/mL, 53 was able to cause severe damage to cell membranes, including loss of cell membrane potential, impairment of membrane integrity, and severe morphological changes. In addition, it was able to induce cell death in Staphylococcus aureus (S. aureus) cells at a lower concentration (16 μg/mL) [92]. Catinella et al. (2020) created a streamlined version of Compound 49, referred to as Compound 54. This derivative exhibited improved antimicrobial effects against LM, with an MIC of 8 µg/mL and an MBC of 64 µg/mL [93].
7. The Key Structural Features and Biological Activities of RSV Derivatives
Structural modifications of RSV derivatives significantly affect their biological activities. By means of different modifications of RSV, such as phenolic hydroxyl groups, benzene rings, linkages, and the introduction of heterocycles, RSV derivatives can be targeted to improve their performance in many key bioactivities, such as anti-inflammatory, anticancer, antioxidant, antimicrobial, and neuroprotective effects.
For example, PTS, a dimethoxy derivative of RSV, has a greatly enhanced lipid solubility, which makes it easier to cross the blood–brain barrier, thus exhibiting stronger antioxidant, anti-inflammatory, and anticancer activities. The stability and water solubility of RSV can be improved by introducing ester groups or long chains through esterification or etherification reactions. For example, certain esterified or etherified derivatives excel in antimicrobial activity, and these compounds have strong inhibitory effects on MRSA, xoo, xoo, xac, etc., by interfering with the integrity of bacterial membranes [30,32,33,34,35]. When RSV binds to glucose or its analogs, it can then cross the blood–brain barrier using GLUT1, enhancing its neuroprotective effects [38,39].
The introduction of heterocyclic structures such as 1,2,3-triazole and 1,3,4-oxadiazole on the benzene ring enhances the antimicrobial activity of the derivatives. For example, derivatives containing the 1,2,3-triazole structure exhibit good inhibition of MRSA [51,52,53,54]. The introduction of double or triple bonds at specific positions of the benzene ring can change the electron distribution and spatial conformation of the derivatives, thus affecting their biological activity. Certain derivatives containing a nitrovinyl structure have excellent anti-inflammatory activity and can precisely target NLRP3 [56,57,58,59].
Replacing the C=C double bond in RSV with a C=N or N=N bond can alter the biological activity of the derivatives. For example, certain RSV derivatives containing C=N bonds excel in antitumor activity [76]. Replacing the C=C double bond with a more flexible amide or single carbon–carbon bond can improve the bioavailability and activity of the derivative. For example, some derivatives containing amide bonds excel in antioxidant and anti-inflammatory activities [79,80].
The introduction of quinoline-containing groups into RSV derivatives excels in anti-inflammatory, antioxidant, and neuroprotective activities. For example, certain quinoline derivatives significantly attenuate brain damage caused by ischemic stroke. Derivatives that introduce an indole group excel in anti-inflammatory and anticancer activities [82,83,84,85]. For example, certain indolyl derivatives significantly inhibit the proliferation and migration of tumor cells [86].
Dimerized derivatives of RSV excel in a variety of biological activities, including antioxidant, anti-inflammatory, antibacterial, and anticancer activities, as well as having good blood–brain barrier permeability [90,91,92,93].
Overall, the parent structure of RSV itself is rich in biological activities. Derivatives with the same or similar structure may show good biological activities in different activity tests. The structural modification of RSV derivatives provides the possibility of their conversion between different biological activities. Through rational design and modification, new RSV derivatives with stronger bioactivity and better pharmacokinetic properties can be developed, providing new strategies and means for the treatment of related diseases.
8. Conclusions
RSV is a naturally occurring phenolic stilbene molecule that has been extensively studied for its multiple biological activities, including anti-inflammatory, anticancer, antioxidant, antimicrobial, and neuroprotective properties. However, its clinical application has been hampered by its low water solubility, low bioavailability, and potential hepatotoxicity. In the past five years, significant progress has been made in the structural modification of RSV, resulting in the development of a number of derivatives with enhanced bioactivity and better pharmacological properties. These advances have not only enriched the conformational relationships of RSV derivatives but also deepened our understanding of the drug design and pharmacological activities of RSV derivatives.
Future research should focus on developing RSV derivatives that are more selective for specific targets. For example, derivatives that selectively target the NLRP3 inflammasome could be very effective in treating IBD without causing systemic side effects. Compound 27, which significantly reduces the release of the inflammatory cytokine IL-1β without triggering the NF-κB pathway, is a promising lead in this direction [59]. The development of RSV derivatives with higher selectivity for cancer cells, especially those expressing specific biomarkers, could enhance their anticancer efficacy. For example, Compound 37 selectively targets cells expressing the CYP1 enzyme, showing potential for the development of more selective anticancer agents [69]. Combining RSV derivatives with other drugs can enhance their therapeutic effects. For example, RSV derivatives with antimicrobial properties can be used in combination with conventional antibiotics against drug-resistant bacteria. Compounds 8 and 9 synergize with aminoglycosides and polymyxin B, demonstrating the potential for combination therapy [40]. The development of RSV derivatives that target multiple pathways in the disease could provide a more comprehensive therapeutic effect. For example, compounds that simultaneously target inflammation, oxidative stress, and cell proliferation may be highly effective in treating complex diseases such as cancer and IBD.
In terms of strategies to improve bioavailability, prodrug strategies can be used to improve the bioavailability of RSV derivatives. For example, synthesizing RSV derivatives with prodrug molecules that can be cleaved in vivo to release active compounds can improve solubility and absorption. This approach has been successfully used in the development of other drugs and can be applied to RSV derivatives as well. Nanoparticle-based drug delivery systems can protect RSV derivatives from degradation and enhance delivery to target tissues. Encapsulation of RSV derivatives in liposomal or polymeric nanoparticles has been investigated to significantly improve their bioavailability and efficacy. For example, RSV derivatives encapsulated in nanoparticles showed increased stability and enhanced delivery to the brain [38].
RSV derivatives are understudied in some respects, and although some RSV derivatives have neuroprotective properties, further research is needed to investigate their mechanisms of action and potential applications in neurodegenerative diseases such as Alzheimer’s and Parkinson’s. Given the current global health challenges, exploring the antiviral activity of RSV derivatives may be a valuable direction of research. RSV has cardiovascular benefits, but more research is needed to develop derivatives specifically for cardiovascular disease. For example, Compound 20 reduces inflammation and oxidative stress and could be further investigated for its potential in the prevention and treatment of cardiovascular disease [51].
High-intensity screening and computational methods can accelerate the discovery of new RSV derivatives. These techniques can rapidly identify promising candidate compounds and predict their biological activity, thereby reducing the time and cost of drug development. For example, Compound 25 was identified as a potent inhibitor of the NLRP3 inflammasome through a high-intensity screening model [56].
Significant progress has been made in the development of new derivatives with enhanced biological activity and better pharmacological properties by structural modification of RSV. These derivatives have shown better anti-inflammatory, anticancer, antimicrobial, antioxidant, and neuroprotective activities, laying a solid foundation for further research and clinical applications. Future research should focus on developing more selective derivatives, improving bioavailability, exploring understudied bioactivities, and employing combination therapies and high-throughput screening methods. These efforts will not only expand the therapeutic potential of RSV derivatives but also contribute to the development of effective new therapies for the treatment of various diseases.
This review collates novel RSV derivatives and their various bioactivities over the past five years and outlines potential future directions, informing further research on the structural modification of RSV and unlocking the therapeutic potential of RSV derivatives.
Author Contributions
X.L., J.P., J.L., H.Z. and X.Z. (Xiaoyu Zheng) searched and organized all of the literature. B.R., X.Z. (Xingxing Zhang), and L.C. conceived and wrote the article. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Scientific Research Foundation for Advanced Talents of Hefei University (23RC27 and 22RC29) and the National Natural Science Foundation of China (Grant No. 22207028).
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| SARs | Structure–activity relationships |
| RSV | Resveratrol |
| PTS | Pterostilbene |
| BBB | Blood–brain barrier |
| MRSA | Methicillin-resistant Staphylococcus aureus |
| MIC | Minimum inhibitory concentration |
| MBC | Minimum bactericidal concentration |
| EC50 | Exhibited median effective concentration |
| Xoo | Xanthomonas oryzae pv. oryzae |
| Xoc | Xanthomonas oryzae pv. oryzicola |
| EPSs | Extracellular polysaccharides |
| MAPK | Mitogen-activated protein kinase |
| AD | Atopic dermatitis |
| DNCB | Dinitrochlorobenzene |
| GLUT1 | Glucose transporter protein 1 |
| ROS | Reactive oxygen species |
| EAE | Experimental autoimmune encephalomyelitis |
| LPS | Lipopolysaccharide |
| HFD | High-fat diet |
| TNF-α | Tumor necrosis factor-α |
| IL-6 | Interleukin-6 |
| IL-1β | Interleukin-1β |
| NF-κB | Nuclear transcription factor-κB |
| COX-2 | Cyclooxygenase-2 |
| PA | Palmitic acid |
| CAT | Catalase |
| GPX1 | Glutathione peroxidase 1 |
| p-ERK1/2 | Phosphorylated extracellular signal-regulated kinase 1/2 |
| IR | Inhibition rate |
| NLRP3 | Nucleotide-binding oligomerization domain-like receptor protein 3 |
| IBD | Inflammatory Bowel Disease |
| HO-1 | Oxygenase-1 |
| AChE | Acetylcholinesterase |
| BChE | Butyrylcholinesterase |
| PC | Pancreatic cancer |
| VRE | Vancomycin-resistant Enterococcus faecium and E. faecalis |
| CYP1 | Cytochrome P450 1 |
| TCDD | Tetrachlorodibenzo-p-dioxin |
| LM | Listeria monocytogenes |
| HUVECs | Human umbilical vein endothelial cells |
| SI | Selectivity index |
| MCAO/R | Middle cerebral artery occlusion–reperfusion |
| OGD/R | Oxygen–glucose deprivation and reperfusion |
| IS | Ischemic stroke |
| COPD | Chronic obstructive pulmonary disease |
| hMAO-B | Human monoamine oxidase B |
| STAT3 | Transducer and activator of transcription 3 |
| CDK4 | Cyclin-dependent kinase 4 |
References
- Giacomini, E.; Rupiani, S.; Guidotti, L.; Recanatini, M.; Roberti, M. The Use of Stilbene Scaffold in Medicinal Chemistry and Multi- Target Drug Design. Curr. Med. Chem. 2016, 23, 2439–2489. [Google Scholar] [CrossRef]
- Lin, W.-S.; Leland, J.V.; Ho, C.-T.; Pan, M.-H. Occurrence, Bioavailability, Anti-Inflammatory, and Anticancer Effects of Pterostilbene. J. Agric. Food Chem. 2020, 68, 12788–12799. [Google Scholar] [CrossRef]
- Intagliata, S.; Modica, M.N.; Santagati, L.M.; Montenegro, L. Strategies to Improve Resveratrol Systemic and Topical Bioavailability: An Update. Antioxidants 2019, 8, 244. [Google Scholar] [CrossRef]
- Li, Q.-S.; Li, Y.; Deora, G.S.; Ruan, B.-F. Derivatives and Analogues of Resveratrol: Recent Advances in Structural Modification. Mini Rev. Med. Chem. 2019, 19, 809–825. [Google Scholar] [CrossRef] [PubMed]
- Tsai, H.-Y.; Ho, C.-T.; Chen, Y.-K. Biological Actions and Molecular Effects of Resveratrol, Pterostilbene, and 3′-Hydroxypterostilbene. J. Food Drug Anal. 2017, 25, 134–147. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Tang, W.; Xiang, K.; Li, G. Pterostilbene in the Treatment of Inflammatory and Oncological Diseases. Front. Pharmacol. 2024, 14, 1323377. [Google Scholar] [CrossRef] [PubMed]
- Al-Khayri, J.M.; Mascarenhas, R.; Harish, H.M.; Gowda, Y.; Lakshmaiah, V.V.; Nagella, P.; Al-Mssallem, M.Q.; Alessa, F.M.; Almaghasla, M.I.; Rezk, A.A.-S. Stilbenes, a Versatile Class of Natural Metabolites for Inflammation—An Overview. Molecules 2023, 28, 3786. [Google Scholar] [CrossRef] [PubMed]
- Obrador, E.; Salvador-Palmer, R.; Jihad-Jebbar, A.; López-Blanch, R.; Dellinger, T.H.; Dellinger, R.W.; Estrela, J.M. Pterostilbene in Cancer Therapy. Antioxidants 2021, 10, 492. [Google Scholar] [CrossRef] [PubMed]
- Surien, O.; Masre, S.F.; Basri, D.F.; Ghazali, A.R. Potential Chemopreventive Role of Pterostilbene in Its Modulation of the Apoptosis Pathway. Int. J. Mol. Sci. 2023, 24, 9707. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Jin, Y.; Hofseth, A.B.; Pena, E.; Habiger, J.; Chumanevich, A.; Poudyal, D.; Nagarkatti, M.; Nagarkatti, P.S.; Singh, U.P.; et al. Resveratrol Suppresses Colitis and Colon Cancer Associated with Colitis. Cancer Prev. Res. 2010, 3, 549–559. [Google Scholar] [CrossRef]
- Orsini, F.; Verotta, L.; Klimo, K.; Gerhäuser, C. Synthesis of Resveratrol Derivatives and In Vitro Screening for Potential Cancer Chemopreventive Activities. Arch. Der Pharm. 2016, 349, 414–427. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Zhou, J.; Zhao, C.-N.; Gan, R.-Y.; Li, H.-B. Health Benefits and Molecular Mechanisms of Resveratrol: A Narrative Review. Foods 2020, 9, 340. [Google Scholar] [CrossRef] [PubMed]
- Marko, M.; Pawliczak, R. Resveratrol and Its Derivatives in Inflammatory Skin Disorders—Atopic Dermatitis and Psoriasis: A Review. Antioxidants 2023, 12, 1954. [Google Scholar] [CrossRef]
- Koh, Y.-C.; Ho, C.-T.; Pan, M.-H. Recent Advances in Health Benefits of Stilbenoids. J. Agric. Food Chem. 2021, 69, 10036–10057. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Jin, L.; Liu, H.; Hua, Z. Stilbenes: A Promising Small Molecule Modulator for Epigenetic Regulation in Human Diseases. Front. Pharmacol. 2023, 14, 1326682. [Google Scholar] [CrossRef]
- Li, F.; Han, Y.; Wu, X.; Cao, X.; Gao, Z.; Sun, Y.; Wang, M.; Xiao, H. Gut Microbiota-Derived Resveratrol Metabolites, Dihydroresveratrol and Lunularin, Significantly Contribute to the Biological Activities of Resveratrol. Front. Nutr. 2022, 9, 912591. [Google Scholar] [CrossRef]
- Grivennikov, S.I.; Greten, F.R.; Karin, M. Immunity, Inflammation, and Cancer. Cell 2010, 140, 883–899. [Google Scholar] [CrossRef] [PubMed]
- Chillemi, R.; Cardullo, N.; Greco, V.; Malfa, G.; Tomasello, B.; Sciuto, S. Synthesis of Amphiphilic Resveratrol Lipoconjugates and Evaluation of Their Anticancer Activity towards Neuroblastoma SH-SY5Y Cell Line. Eur. J. Med. Chem. 2015, 96, 467–481. [Google Scholar] [CrossRef]
- Fragopoulou, E.; Gkotsi, K.; Petsini, F.; Gioti, K.; Kalampaliki, A.D.; Lambrinidis, G.; Kostakis, I.K.; Tenta, R. Synthesis and Biological Evaluation of Resveratrol Methoxy Derivatives. Molecules 2023, 28, 5547. [Google Scholar] [CrossRef]
- Singh, D.; Mendonsa, R.; Koli, M.; Subramanian, M.; Nayak, S.K. Antibacterial Activity of Resveratrol Structural Analogues: A Mechanistic Evaluation of the Structure-Activity Relationship. Toxicol. Appl. Pharmacol. 2019, 367, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Boccellino, M.; Donniacuo, M.; Bruno, F.; Rinaldi, B.; Quagliuolo, L.; Ambruosi, M.; Pace, S.; De Rosa, M.; Olgaç, A.; Banoglu, E.; et al. Protective Effect of Piceatannol and Bioactive Stilbene Derivatives against Hypoxia-Induced Toxicity in H9c2 Cardiomyocytes and Structural Elucidation as 5-LOX Inhibitors. Eur. J. Med. Chem. 2019, 180, 637–647. [Google Scholar] [CrossRef]
- Ruan, B.-F.; Ge, W.-W.; Cheng, H.-J.; Xu, H.-J.; Li, Q.-S.; Liu, X.-H. Resveratrol-Based Cinnamic Ester Hybrids: Synthesis, Characterization, and Anti-Inflammatory Activity. J. Enzym. Inhib. Med. Chem. 2017, 32, 1282–1290. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, R.; Ebrahimzadeh, M.A. Resveratrol—A Comprehensive Review of Recent Advances in Anticancer Drug Design and Development. Eur. J. Med. Chem. 2020, 200, 112356. [Google Scholar] [CrossRef] [PubMed]
- Dvorakova, M.; Landa, P. Anti-Inflammatory Activity of Natural Stilbenoids: A Review. Pharmacol. Res. 2017, 124, 126–145. [Google Scholar] [CrossRef]
- Peñalver, P.; Belmonte-Reche, E.; Adán, N.; Caro, M.; Mateos-Martín, M.L.; Delgado, M.; González-Rey, E.; Morales, J.C. Alkylated Resveratrol Prodrugs and Metabolites as Potential Therapeutics for Neurodegenerative Diseases. Eur. J. Med. Chem. 2018, 146, 123–138. [Google Scholar] [CrossRef] [PubMed]
- Arbo, B.D.; André-Miral, C.; Nasre-Nasser, R.G.; Schimith, L.E.; Santos, M.G.; Costa-Silva, D.; Muccillo-Baisch, A.L.; Hort, M.A. Resveratrol Derivatives as Potential Treatments for Alzheimer’s and Parkinson’s Disease. Front. Aging Neurosci. 2020, 12, 103. [Google Scholar] [CrossRef]
- Jeandet, P.; Sobarzo-Sánchez, E.; Silva, A.S.; Clément, C.; Nabavi, S.F.; Battino, M.; Rasekhian, M.; Belwal, T.; Habtemariam, S.; Koffas, M.; et al. Whole-Cell Biocatalytic, Enzymatic and Green Chemistry Methods for the Production of Resveratrol and Its Derivatives. Biotechnol. Adv. 2020, 39, 107461. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; You, Y.; Lu, J.; Chen, X.; Yang, Z. Recent Advances in Synthesis, Bioactivity, and Pharmacokinetics of Pterostilbene, an Important Analog of Resveratrol. Molecules 2020, 25, 5166. [Google Scholar] [CrossRef]
- Yang, S.-C.; Tseng, C.-H.; Wang, P.-W.; Lu, P.-L.; Weng, Y.-H.; Yen, F.-L.; Fang, J.-Y. Pterostilbene, a Methoxylated Resveratrol Derivative, Efficiently Eradicates Planktonic, Biofilm, and Intracellular MRSA by Topical Application. Front. Microbiol. 2017, 8, 1103. [Google Scholar] [CrossRef]
- Tang, K.-W.; Yang, S.-C.; Tseng, C.-H. Design, Synthesis, and Anti-Bacterial Evaluation of Triazolyl-Pterostilbene Derivatives. Int. J. Mol. Sci. 2019, 20, 4564. [Google Scholar] [CrossRef]
- Chen, Y.; Luo, X.; Wang, Y.; Xing, Z.; Chen, J. Design and Synthesis Novel Amide Derivatives Containing an 1,3,4-oxadiazole Moiety as Potential Antibacterial Agents. J. Heterocycl. Chem. 2022, 59, 1160–1168. [Google Scholar] [CrossRef]
- Peng, J.; Zhang, Y.; Liu, X.; Zou, Y.; Song, H.; Wang, S.; Cai, Q.; Chen, J.; Hu, D. Design, Synthesis, Antibacterial Activity, and Mechanism of Novel Resveratrol Derivatives Containing an 1,3,4-Oxadiazole Moiety. Pestic. Biochem. Physiol. 2023, 193, 105457. [Google Scholar] [CrossRef]
- Qi, P.-Y.; Zhang, T.-H.; Feng, Y.-M.; Wang, M.-W.; Shao, W.-B.; Zeng, D.; Jin, L.-H.; Wang, P.-Y.; Zhou, X.; Yang, S. Exploring an Innovative Strategy for Suppressing Bacterial Plant Disease: Excavated Novel Isopropanolamine-Tailored Pterostilbene Derivatives as Potential Antibiofilm Agents. J. Agric. Food Chem. 2022, 70, 4899–4911. [Google Scholar] [CrossRef] [PubMed]
- Qi, P.; Zhang, T.; Yang, Y.; Liang, H.; Feng, Y.; Wang, N.; Ding, Z.; Xiang, H.; Zhou, X.; Liu, L.; et al. Beyond the β -amino Alcohols Framework: Identification of Novel β -hydroxy Pyridinium Salt-decorated Pterostilbene Derivatives as Bacterial Virulence Factor Inhibitors. Pest Manag. Sci. 2024, 80, 4098–4109. [Google Scholar] [CrossRef] [PubMed]
- Tang, K.-W.; Hsu, C.-Y.; Aljuffali, I.A.; Alalaiwe, A.; Lai, W.-N.; Gu, P.-Y.; Tseng, C.-H.; Fang, J.-Y. Skin Delivery of Synthetic Benzoyl Pterostilbenes Suppresses Atopic Dermatitis-like Inflammation through the Inhibition of Keratinocyte and Macrophage Activation. Biomed. Pharmacother. 2024, 170, 116073. [Google Scholar] [CrossRef] [PubMed]
- Terstappen, G.C.; Meyer, A.H.; Bell, R.D.; Zhang, W. Strategies for Delivering Therapeutics across the Blood–Brain Barrier. Nat. Rev. Drug. Discov. 2021, 20, 362–383. [Google Scholar] [CrossRef] [PubMed]
- Qu, B.; Li, X.; Guan, M.; Li, X.; Hai, L.; Wu, Y. Design, Synthesis and Biological Evaluation of Multivalent Glucosides with High Affinity as Ligands for Brain Targeting Liposomes. Eur. J. Med. Chem. 2014, 72, 110–118. [Google Scholar] [CrossRef]
- Xu, Y.; Yu, P.; Liang, J.; Chen, Y.; Yang, C.; Xia, C.; Deng, J.; Hai, L.; Chen, J.; Wu, Y. Synthesis and Bioactivity Evaluation of Glycosylated Resveratrol Derivatives as Antioxidative Neuroprotection Agents against Cerebral Ischemia-Reperfusion Injury. Bioorganic Chem. 2024, 153, 107791. [Google Scholar] [CrossRef] [PubMed]
- Belmonte-Reche, E.; Peñalver, P.; Caro-Moreno, M.; Mateos-Martín, M.L.; Adán, N.; Delgado, M.; González-Rey, E.; Morales, J.C. Silyl Resveratrol Derivatives as Potential Therapeutic Agents for Neurodegenerative and Neurological Diseases. Eur. J. Med. Chem. 2021, 223, 113655. [Google Scholar] [CrossRef] [PubMed]
- Cebrián, R.; Li, Q.; Peñalver, P.; Belmonte-Reche, E.; Andrés-Bilbao, M.; Lucas, R.; De Paz, M.V.; Kuipers, O.P.; Morales, J.C. Chemically Tuning Resveratrol for the Effective Killing of Gram-Positive Pathogens. J. Nat. Prod. 2022, 85, 1459–1473. [Google Scholar] [CrossRef]
- Cebrián, R.; Lucas, R.; Fernández-Cantos, M.V.; Slot, K.; Peñalver, P.; Martínez-García, M.; Párraga-Leo, A.; De Paz, M.V.; García, F.; Kuipers, O.P.; et al. Synthesis and Antimicrobial Activity of Aminoalkyl Resveratrol Derivatives Inspired by Cationic Peptides. J. Enzym. Inhib. Med. Chem. 2023, 38, 267–281. [Google Scholar] [CrossRef]
- Szczepańska, P.; Rychlicka, M.; Groborz, S.; Kruszyńska, A.; Ledesma-Amaro, R.; Rapak, A.; Gliszczyńska, A.; Lazar, Z. Studies on the Anticancer and Antioxidant Activities of Resveratrol and Long-Chain Fatty Acid Esters. Int. J. Mol. Sci. 2023, 24, 7167. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Xu, T.; Xu, F.; Zhang, Y.; Liu, Z.; Chen, W.; Fu, W.; Dai, Y.; Zhao, Y.; Feng, J.; et al. Development of Resveratrol-Curcumin Hybrids as Potential Therapeutic Agents for Inflammatory Lung Diseases. Eur. J. Med. Chem. 2017, 125, 478–491. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Xiao, Z.; Zhang, W.; Chen, H.; Liu, H.; Pan, J.; Cai, X.; Liang, G.; Zhou, B.; Shan, X.; et al. A Novel Resveratrol-Curcumin Hybrid, A19, Attenuates High Fat Diet-Induced Nonalcoholic Fatty Liver Disease. Biomed. Pharmacother. 2019, 110, 951–960. [Google Scholar] [CrossRef]
- Zhang, W.; Chen, H.; Sun, C.; Wu, B.; Bai, B.; Liu, H.; Shan, X.; Liang, G.; Zhang, Y. A Novel Resveratrol Analog PA19 Attenuates Obesity-induced Cardiac and Renal Injury by Inhibiting Inflammation and Inflammatory Cell Infiltration. Mol. Med. Rep. 2019, 19, 4770–4778. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Zheng, X.; Zhu, P.; Liu, B.; Gao, H.; Mao, Z.; Zhang, L.; Wan, C. Novel Resveratrol-Chalcone Derivatives: Synthesis and Biological Evaluation. Mini Rev. Med. Chem. 2019, 19, 424–436. [Google Scholar] [CrossRef]
- Chen, L.Z.; Yao, L.; Jiao, M.M.; Shi, J.B.; Tan, Y.; Ruan, B.F.; Liu, X.H. Novel Resveratrol-Based Flavonol Derivatives: Synthesis and Anti-Inflammatory Activity in Vitro and in Vivo. Eur. J. Med. Chem. 2019, 175, 114–128. [Google Scholar] [CrossRef]
- Tang, K.-W.; Ke, C.-C.; Tseng, C.-H.; Chen, Y.-L.; Tzeng, C.-C.; Chen, Y.-J.; Hsu, C.-C.; Tai, H.-T.; Hsieh, Y.-J. Enhancement of Anticancer Potential of Pterostilbene Derivative by Chalcone Hybridization. Molecules 2021, 26, 4840. [Google Scholar] [CrossRef] [PubMed]
- Fang, M.; Zou, T.; Yang, X.; Zhang, Z.; Cao, P.; Han, J.; Duan, Y.; Ruan, B.-F.; Li, Q.-S. Discovery of Novel Pterostilbene Derivatives That Might Treat Sepsis by Attenuating Oxidative Stress and Inflammation through Modulation of MAPKs/NF-κB Signaling Pathways. Antioxidants 2021, 10, 1333. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Koh, D.; Lee, Y.H.; Lim, Y.; Shin, S.Y. Effect of Hybrid Compounds of Stilbene and Pentadienone on Inhibition of Tubulin Polymerization. Anti-Cancer Agents Med. Chem. 2023, 23, 1156–1163. [Google Scholar] [CrossRef]
- Yang, X.; Liu, Z.; Fang, M.; Zou, T.; Zhang, Z.; Meng, X.; Wang, T.; Meng, H.; Chen, Y.; Duan, Y.; et al. Novel Pterostilbene Derivatives Ameliorate Heart Failure by Reducing Oxidative Stress and Inflammation through Regulating Nrf2/NF-κB Signaling Pathway. Eur. J. Med. Chem. 2023, 258, 115602. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Shen, Z.; Zhu, M.; Fang, M.; Wang, S.; Zhang, T.; Zhang, B.; Yang, X.; Lv, Z.; Duan, Y.; et al. The Pterostilbene-Dihydropyrazole Derivative Ptd-1 Ameliorates Vascular Calcification by Regulating Inflammation. Int. Immunopharmacol. 2023, 125, 111198. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Wang, K.; Liu, X.; Wang, L.; Zou, H.; Hu, S.; Zhou, L.; Li, R.; Cao, S.; Ruan, B.; et al. Design, Synthesis, in Vitro and in Vivo Biological Evaluation of Pterostilbene Derivatives for Anti-Inflammation Therapy. J. Enzym. Inhib. Med. Chem. 2024, 39, 2315227. [Google Scholar] [CrossRef]
- Chen, L.; Wang, K.; Wang, L.; Wang, W.; Wang, L.; Wang, W.; Li, J.; Liu, X.; Wang, M.; Ruan, B. Design and Synthesis of Pterostilbene Derivatives Bearing Triazole Moiety That Might Treat DSS-Induced Colitis in Mice through Modulation of NF-κB/MAPK Signaling Pathways. Eur. J. Med. Chem. 2024, 263, 115949. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Zhang, Y.; Yang, J.; Zhou, L.; Zhang, S.; Wu, X.; Chen, J.; Hu, D.; Gan, X. Novel Trans -Resveratrol Derivatives: Design, Synthesis, Antibacterial Activity, and Mechanisms. J. Agric. Food Chem. 2024, 72, 15561–15571. [Google Scholar] [CrossRef]
- Chen, L.Z.; Zhang, X.X.; Liu, M.M.; Wu, J.; Ma, D.; Diao, L.Z.; Li, Q.; Huang, Y.S.; Zhang, R.; Ruan, B.F.; et al. Discovery of Novel Pterostilbene-Based Derivatives as Potent and Orally Active NLRP3 Inflammasome Inhibitors with Inflammatory Activity for Colitis. J. Med. Chem. 2021, 64, 13633–13657. [Google Scholar] [CrossRef]
- Ruan, B.; Zhang, X.; Li, B.; Liu, X.; Chen, L.; Xia, C. Design, Synthesis, in Vitro and in Vivo Biological Evaluation of Pterostilbene Derivatives for the Treatment of Inflammatory Bowel Disease. Chem. Biodivers. 2024, 21, e202401081. [Google Scholar] [CrossRef]
- Zhang, X.X.; Diao, L.Z.; Chen, L.Z.; Ma, D.; Wang, Y.M.; Jiang, H.; Ruan, B.F.; Liu, X.H. Discovery of 4-((E)-3,5-Dimethoxy-2-((E)-2-Nitrovinyl)Styryl)Aniline Derivatives as Potent and Orally Active NLRP3 Inflammasome Inhibitors for Colitis. Eur. J. Med. Chem. 2022, 236, 114357. [Google Scholar] [CrossRef] [PubMed]
- Ruan, B.; Rong, M.; Ming, Z.; Wang, K.; Liu, X.; Deng, L.; Zhang, X.; Xu, K.; Shi, C.; Gao, T.; et al. Discovery of Pterostilbene Analogs as Novel NLRP3 Inflammasome Inhibitors for Potential Treatment of DSS-Induced Colitis in Mice. Bioorganic Chem. 2023, 133, 106429. [Google Scholar] [CrossRef]
- Lu, J.-X.; Wang, W.; Zhang, Q.-W.; Guo, Z.-Y.; Jin, Z.; Tang, Y.-Z. Design, Synthesis and Evaluation of Antioxidant and Anti-Inflammatory Activities of Novel Resveratrol Derivatives as Potential Multifunctional Drugs. Eur. J. Med. Chem. 2024, 266, 116148. [Google Scholar] [CrossRef] [PubMed]
- Al-lehaib, L.A.; Ali, E.M.M.; Al-Footy, K.O.; Al-Ghamdi, H.A.; Al-Zahrani, F.A.M.; Al-Amshany, Z.M.; El-Shishtawy, R.M. Resveratrol-Based Schiff Base Derivatives: Synthesis, Characterization and Cytotoxic Study. Results Chem. 2024, 7, 101516. [Google Scholar] [CrossRef]
- Subramanian, A.; Kumarasamy, V.; Sekar, M.; Subramaniyan, V.; Wong, L.S. Design, Synthesis, and Invitro Pharmacological Evaluation of Novel Resveratrol Surrogate Molecules against Alzheimer’s Disease. Chem. Biodivers. 2024, 21, e202401430. [Google Scholar] [CrossRef]
- De Filippis, B.; De Lellis, L.; Florio, R.; Ammazzalorso, A.; Amoia, P.; Fantacuzzi, M.; Giampietro, L.; Maccallini, C.; Amoroso, R.; Veschi, S.; et al. Synthesis and Cytotoxic Effects on Pancreatic Cancer Cells of Resveratrol Analogs. Med. Chem. Res. 2019, 28, 984–991. [Google Scholar] [CrossRef]
- Florio, R.; De Filippis, B.; Veschi, S.; Di Giacomo, V.; Lanuti, P.; Catitti, G.; Brocco, D.; Di Rienzo, A.; Cataldi, A.; Cacciatore, I.; et al. Resveratrol Derivative Exhibits Marked Antiproliferative Actions, Affecting Stemness in Pancreatic Cancer Cells. Int. J. Mol. Sci. 2023, 24, 1977. [Google Scholar] [CrossRef]
- Di Fermo, P.; Di Lodovico, S.; Amoroso, R.; De Filippis, B.; D’Ercole, S.; Di Campli, E.; Cellini, L.; Di Giulio, M. Searching for New Tools to Counteract the Helicobacter Pylori Resistance: The Positive Action of Resveratrol Derivatives. Antibiotics 2020, 9, 891. [Google Scholar] [CrossRef] [PubMed]
- Xin, Z.-H.; Meng, Y.-L.; Jiang, W.-J.; Li, Y.-P.; Ge, L.-P.; Zhang, C.-H.; Liu, L.-N.; Kang, Y.-F. Finding an Efficient Tetramethylated Hydroxydiethylene of Resveratrol Analogue for Potential Anticancer Agent. BMC Chem. 2020, 14, 13. [Google Scholar] [CrossRef]
- Kumarihamy, M.; Tripathi, S.; Balachandran, P.; Avula, B.; Zhao, J.; Wang, M.; Bennett, M.M.; Zhang, J.; Carr, M.A.; Lovell, K.M.; et al. Synthesis and Inhibitory Activity of Machaeridiol-Based Novel Anti-MRSA and Anti-VRE Compounds and Their Profiling for Cancer-Related Signaling Pathways. Molecules 2022, 27, 6604. [Google Scholar] [CrossRef]
- Liang, Y.; Chen, X.; Teng, Z.; Wang, X.; Yang, J.; Liu, G. Discovery of a 4-Hydroxy-3′-Trifluoromethoxy-Substituted Resveratrol Derivative as an Anti-Aging Agent. Molecules 2023, 29, 86. [Google Scholar] [CrossRef]
- Ruparelia, K.C.; Zeka, K.; Beresford, K.J.M.; Wilsher, N.E.; Potter, G.A.; Androutsopoulos, V.P.; Brucoli, F.; Arroo, R.R.J. CYP1-Activation and Anticancer Properties of Synthetic Methoxylated Resveratrol Analogues. Molecules 2024, 29, 423. [Google Scholar] [CrossRef]
- Ceramella, J.; Chimento, A.; Iacopetta, D.; De Luca, A.; Coronel Vargas, G.; Rosano, C.; Pezzi, V.; Saturnino, C.; Sinicropi, M.S. A Resveratrol Phenylacetamide Derivative Perturbs the Cytoskeleton Dynamics Interfering with the Migration Potential in Breast Cancer. Appl. Sci. 2022, 12, 6531. [Google Scholar] [CrossRef]
- Chimento, A.; Santarsiero, A.; Iacopetta, D.; Ceramella, J.; De Luca, A.; Infantino, V.; Parisi, O.I.; Avena, P.; Bonomo, M.G.; Saturnino, C.; et al. A Phenylacetamide Resveratrol Derivative Exerts Inhibitory Effects on Breast Cancer Cell Growth. Int. J. Mol. Sci. 2021, 22, 5255. [Google Scholar] [CrossRef]
- Lizard, G.; Latruffe, N.; Vervandier-Fasseur, D. Aza- and Azo-Stilbenes: Bio-Isosteric Analogs of Resveratrol. Molecules 2020, 25, 605. [Google Scholar] [CrossRef] [PubMed]
- Bellina, F.; Guazzelli, N.; Lessi, M.; Manzini, C. Imidazole Analogues of Resveratrol: Synthesis and Cancer Cell Growth Evaluation. Tetrahedron 2015, 71, 2298–2305. [Google Scholar] [CrossRef]
- Vergara, D.; De Domenico, S.; Tinelli, A.; Stanca, E.; Del Mercato, L.L.; Giudetti, A.M.; Simeone, P.; Guazzelli, N.; Lessi, M.; Manzini, C.; et al. Anticancer Effects of Novel Resveratrol Analogues on Human Ovarian Cancer Cells. Mol. BioSyst. 2017, 13, 1131–1141. [Google Scholar] [CrossRef]
- Zimmermann-Franco, D.C.; Esteves, B.; Lacerda, L.M.; Souza, I.D.O.; Santos, J.A.D.; Pinto, N.D.C.C.; Scio, E.; Da Silva, A.D.; Macedo, G.C. In Vitro and in Vivo Anti-Inflammatory Properties of Imine Resveratrol Analogues. Bioorganic Med. Chem. 2018, 26, 4898–4906. [Google Scholar] [CrossRef]
- Iacopetta, D.; Lappano, R.; Mariconda, A.; Ceramella, J.; Sinicropi, M.S.; Saturnino, C.; Talia, M.; Cirillo, F.; Martinelli, F.; Puoci, F.; et al. Newly Synthesized Imino-Derivatives Analogues of Resveratrol Exert Inhibitory Effects in Breast Tumor Cells. Int. J. Mol. Sci. 2020, 21, 7797. [Google Scholar] [CrossRef]
- Sánchez-González, R.; Leyton, P.; Aguilar, L.F.; Reyna-Jeldes, M.; Coddou, C.; Díaz, K.; Mellado, M. Resveratrol-Schiff Base Hybrid Compounds with Selective Antibacterial Activity: Synthesis, Biological Activity, and Computational Study. Microorganisms 2022, 10, 1483. [Google Scholar] [CrossRef]
- Ciccone, L.; Piragine, E.; Brogi, S.; Camodeca, C.; Fucci, R.; Calderone, V.; Nencetti, S.; Martelli, A.; Orlandini, E. Resveratrol-like Compounds as SIRT1 Activators. Int. J. Mol. Sci. 2022, 23, 15105. [Google Scholar] [CrossRef] [PubMed]
- Thongsom, S.; Racha, S.; Petsri, K.; Ei, Z.Z.; Visuttijai, K.; Moriue, S.; Yokoya, M.; Chanvorachote, P. Structural Modification of Resveratrol Analogue Exhibits Anticancer Activity against Lung Cancer Stem Cells via Suppression of Akt Signaling Pathway. BMC Complement. Med. Ther. 2023, 23, 183. [Google Scholar] [CrossRef]
- Xin, M.; Wu, H.; Du, Y.; Liu, S.; Zhao, F.; Mou, X. Synthesis and Biological Evaluation of Resveratrol Amide Derivatives as Selective COX-2 Inhibitors. Chem.-Biol. Interact. 2023, 380, 110522. [Google Scholar] [CrossRef]
- Wu, H.; Liu, L.; Song, M.; Yin, X.; Chen, M.; Lv, G.; Zhao, F.; Mou, X. Synthesis, Biological Evaluation and Docking Studies of N-Substituted Resveratrol Derivatives. Fitoterapia 2024, 174, 105872. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Mi, Y.; Meng, Q.; Liu, Y.; Wang, Y.; Zhang, Y.; Yang, Y.; Chen, G.; Liu, Y.; Hou, Y. A Quinolinyl Resveratrol Derivative Alleviates Acute Ischemic Stroke Injury by Promoting Mitophagy for Neuroprotection via Targeting CK2α’. Int. Immunopharmacol. 2024, 137, 112524. [Google Scholar] [CrossRef]
- Xu, L.; Mi, Y.; Meng, Q.; Liu, Y.; Wang, F.; Zhang, G.; Liu, Y.; Chen, G.; Hou, Y. Anti-Inflammatory Effects of Quinolinyl Analog of Resveratrol Targeting TLR4 in MCAO/R Ischemic Stroke Rat Model. Phytomedicine 2024, 128, 155344. [Google Scholar] [CrossRef]
- Hou, Y.; Zhang, Y.; Mi, Y.; Wang, J.; Zhang, H.; Xu, J.; Yang, Y.; Liu, J.; Ding, L.; Yang, J.; et al. A Novel Quinolyl-Substituted Analogue of Resveratrol Inhibits LPS-Induced Inflammatory Responses in Microglial Cells by Blocking the NF-κB/MAPK Signaling Pathways. Mol. Nutr. Food Res. 2019, 63, 1801380. [Google Scholar] [CrossRef] [PubMed]
- Nam, K.Y.; Damodar, K.; Lee, Y.; Park, L.S.; Gim, J.G.; Park, J.P.; Jeon, S.H.; Lee, J.T. Design and Synthesis of π-Extended Resveratrol Analogues and In Vitro Antioxidant and Anti-Inflammatory Activity Evaluation. Molecules 2021, 26, 646. [Google Scholar] [CrossRef] [PubMed]
- Mlakić, M.; Talić, S.; Odak, I.; Barić, D.; Šagud, I.; Škorić, I. Cholinesterase Inhibition and Antioxidative Capacity of New Heteroaromatic Resveratrol Analogs: Synthesis and Physico—Chemical Properties. Int. J. Mol. Sci. 2024, 25, 7401. [Google Scholar] [CrossRef] [PubMed]
- Huber, R.; Marcourt, L.; Quiros-Guerrero, L.-M.; Luscher, A.; Schnee, S.; Michellod, E.; Ducret, V.; Kohler, T.; Perron, K.; Wolfender, J.-L.; et al. Chiral Separation of Stilbene Dimers Generated by Biotransformation for Absolute Configuration Determination and Antibacterial Evaluation. Front. Chem. 2022, 10, 912396. [Google Scholar] [CrossRef]
- Sundin, C.; Zetterström, C.E.; Vo, D.D.; Brkljača, R.; Urban, S.; Elofsson, M. Exploring Resveratrol Dimers as Virulence Blocking Agents—Attenuation of Type III Secretion in Yersinia Pseudotuberculosis and Pseudomonas Aeruginosa. Sci. Rep. 2020, 10, 2103. [Google Scholar] [CrossRef]
- Dávid, C.Z.; Hohmann, J.; Vasas, A. Chemistry and Pharmacology of Cyperaceae Stilbenoids: A Review. Molecules 2021, 26, 2794. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Zhang, Z.; Yao, C.; Bai, J.; Yang, H.; Ma, P.; Fan, Y.; Li, S.; Yuan, J.; Lin, M.; et al. Amurensin H, a Derivative From Resveratrol, Ameliorates Lipopolysaccharide/Cigarette Smoke–Induced Airway Inflammation by Blocking the Syk/NF-κB Pathway. Front. Pharmacol. 2019, 10, 1157. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.-W.; Shi, C.-J.; Yang, H.-L.; Cai, P.; Liu, Q.-H.; Yang, X.-L.; Kong, L.-Y.; Wang, X.-B. Synthesis and Evaluation of Isoprenylation-Resveratrol Dimer Derivatives against Alzheimer’s Disease. Eur. J. Med. Chem. 2019, 163, 307–319. [Google Scholar] [CrossRef] [PubMed]
- Mattio, L.M.; Dallavalle, S.; Musso, L.; Filardi, R.; Franzetti, L.; Pellegrino, L.; D’Incecco, P.; Mora, D.; Pinto, A.; Arioli, S. Antimicrobial Activity of Resveratrol-Derived Monomers and Dimers against Foodborne Pathogens. Sci. Rep. 2019, 9, 19525. [Google Scholar] [CrossRef] [PubMed]
- Catinella, G.; Mattio, L.M.; Musso, L.; Arioli, S.; Mora, D.; Beretta, G.L.; Zaffaroni, N.; Pinto, A.; Dallavalle, S. Structural Requirements of Benzofuran Derivatives Dehydro-δ- and Dehydro-ε-Viniferin for Antimicrobial Activity Against the Foodborne Pathogen Listeria Monocytogenes. Int. J. Mol. Sci. 2020, 21, 2168. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).